Abstract
Adolescence is a period of significant neurobiological change that occurs as individuals transition from childhood to adulthood. Because the nervous system is in a relatively labile state during this stage of development, it may be especially sensitive to experience-induced plasticity. One such experience that is relatively common to adolescents is the exposure to drugs of abuse, particularly alcohol and psychostimulants. In this review, we highlight recent findings on the long-lasting effects of exposure to these drugs during adolescence in humans as well as in animal models. Whenever possible, our focus is on studies that use comparison groups of adolescent- and adult-exposed subjects as this is a more direct test of the hypothesis that adolescence represents a period of enhanced vulnerability to the effects of drug-induced plasticity. Lastly, we suggest areas of future investigation that are needed and methodological concerns that should be addressed.
Keywords: adolescent, young adult, neuroanatomy, neurophysiology, psychostimulants, alcohol, rat
Adolescence, the transition from the juvenile period to adulthood, is marked by puberty and numerous physical and neural changes. In humans, adolescence begins at approximately 12 years of age and may extend to the mid-twenties (Dahl, 1994). In rats, adolescence has been conservatively defined as beginning around postnatal day (P) 28 and extending to P42 (Spear, 2000) or perhaps as late as P60 (Brenhouse and Andersen, 2011; Tirelli et al., 2003). This is based, in part, on the rise of pubertal hormones which leads to vaginal opening in female rats between P29 and 37 (Castellano et al, 2011) and preputial separation in male rats between P39-47 (Korenbrot et al, 1977). During this time, there is substantial behavioral and neural development (Sisk and Foster, 2004; Spear, 2000), with corticolimbic brain regions such as the prefrontal cortex (PFC), nucleus accumbens (NAc), and basolateral amygdala (BLA) being among the last brain circuits to fully mature in both humans and rodents (Casey et al., 2000; Brenhouse and Andersen, 2011). Because the brain is undergoing this programmed period of dramatic change, it might be especially sensitive to outside influences that have the ability to induce plasticity in the nervous system.
One such influence that is pervasive during human adolescence is exposure to drugs such as alcohol and psychostimulants. Recent data from the nationwide Monitoring the Future study (Johnston et al, 2012), which sampled from over 46,000 eighth to twelfth grade students, suggests that approximately 70% of young people have consumed alcohol by the end of the twelfth grade and 33% have been intoxicated within the last month. Nicotine (via cigarette smoking) is consumed at least once by about 40% of adolescents by the twelfth grade, and nearly 20% of twelfth graders report being current smokers. Nearly 1% and 3% of adolescents report they are current users of cocaine and amphetamines, respectively. These relatively high levels of use are of concern because these drugs are known to produce significant and long lasting changes in brain structure and function (Lüscher and Malenka, 2011) that have been linked to long-term changes in cognitive functioning and the development of addiction (Goldstein and Volkow, 2002). In this review, we will highlight recent findings on the long-lasting effects of alcohol and psychostimulant exposure during peri-adolescence. Although some evidence from humans is available, we will primarily focus on studies in animal models. In doing so, we will place a particular emphasis on those that utilize both adolescent and adult exposure groups since these directly assess the potential for age-of-exposure dependent effects.
1. Adolescence: the last developmental phase of PFC maturation
The scientific community and the general public were startled when human structural MRI studies showed that the cortex, including the prefrontal cortex (PFC), decreases in size between 11 and 22 years of age (Sowell et al., 1999; Giedd et al., 1999). Previous to this finding, most neural development in humans was thought to be completed by 12 years of age, which is approximately when overall brain volume is at adult levels (Courchesne et al, 2000). The continuation of development during adolescence suggests greater vulnerability than adults to many of the effects of environmental influences, including those produced by exposure to drugs of abuse. This is a major problem because adolescence is also a time of high novelty and sensation seeking (Spear, 2000), which often includes experimentation with drugs.
Prior to the advent of MRI studies, there were indications of cellular changes in the PFC during adolescence. Periadolescent anatomical refinement of circuitry in the primate PFC was described in both excitatory and inhibitory circuits (reviewed by Lewis, 1997; Woo et al., 1997). Synaptic density in this area was also found to decline during adolescence in both monkeys (Bourgeois et al., 1994; Anderson et al., 1995) and humans (Huttenlocher, 1979; Huttenlocher and Dabholker, 1997); however, this decrease in synaptic number would have a small effect on cortical volume, as was noted by Bourgeous and Rakic (1993). Here, we suggest that a loss of neurons could readily account for the MRI finding of volume loss in human adolescence.
Neuron number in the medial prefrontal cortex (mPFC)
The number of neurons (density x volume) during development and adolescence has not been examined in frontal cortex or any other cortical region in primates. This is due in part to the technical difficulties of parcellating regions of the cortex in large brains that have variable gyri. However, subtle declines in neuronal density between 2 and 16 years of age in human frontal cortex have been noted (Huttenlocher, 1979). The rat PFC is a more practical model than that of the primate for exploring the cellular basis for pruning during adolescence because the rat PFC is less differentiated and segregated. Furthermore, on the basis of the reciprocity of specific thalamic as well as other connections, embryological development, and electrophysiological and behavioral characteristics, rats do have a PFC that is homologous to that of the primate (Brown & Bowman, 2002; see Uylings et al, 2003 for an extensive review). Interestingly, like humans, the rat PFC undergoes a decrease in volume during the peri-adolescent period (van Eden and Uylings, 1985; Markham et al., 2007).
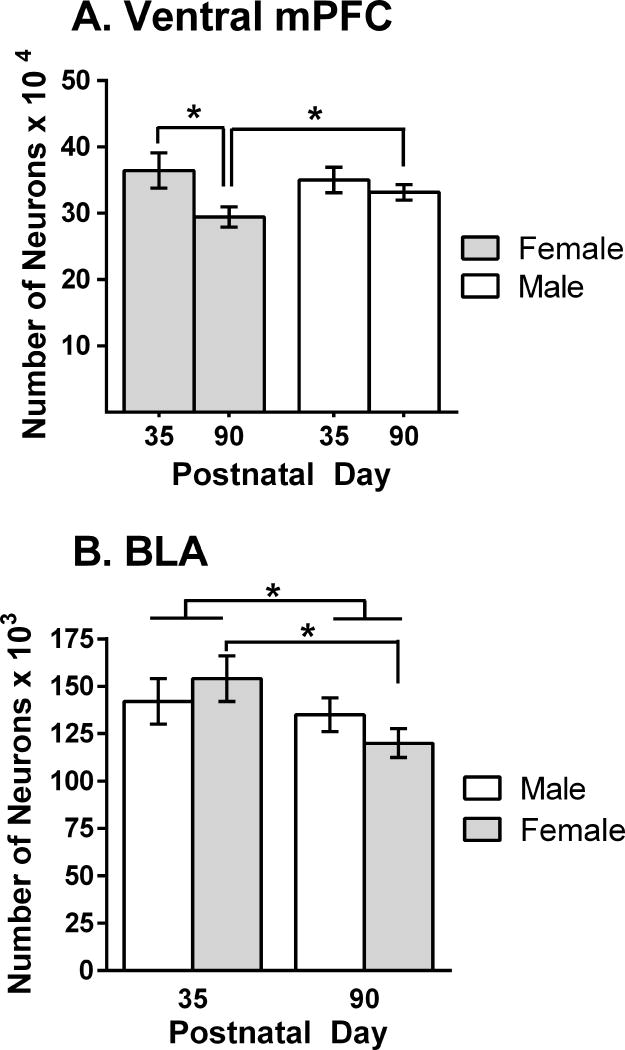
To investigate the possibility of cell loss, we (Markham et al, 2007) quantified the number of neurons in the mPFC of male and female rats that were either peripubertal (P35) or adults (P90). No sex differences were found in the number of neurons at P35, but sex differences did appear at P90. This was because females had lost more neurons between these ages (19%) than did males (5%) (Figure 1A). The mosaic of changes characteristic of volume changes in the adolescent human cortex (Giedd et al, 1999) also can be found in the rat, where the cortex adjacent to the mPFC, the anterior cingulate, does not exhibit differences in volume or number of neurons between P35 and P90 (Markham et al., 2007).
Figure 1.
(A) The number of neurons in the ventral portion of the rat mPFC at periadolescence (P35) and adulthood (P90) in both sexes. Adapted from Markham et al (2007). (B) The number of neurons in the basolateral amygdalar nucleus at periadolescence and adulthood. Adapted from Rubinow and Juraska (2009). *p<.04
Connectivity changes
There is considerable restructuring of neurotransmitters and neural connections during adolescence in the rat mPFC. Both NMDA and dopamine receptor (D1- and D2-like) density is higher in the periadolescent period than in adulthood (Insel et al, 1990; Andersen et al, 2000). Koss, Hristov and Juraska (unpublished data) have found that dendritic spines on the basilar dendrites of upper layer mPFC are higher during adolescence than they are before adolescence or in adulthood. In contrast to the losses, there appears to be a progressive increase in dopaminergic and serotonergic fiber density in the mPFC during adolescence (Benes et al, 2000). In fact, there are dissociations between a peak/fall in D1 receptors on the projecting neurons from the mPFC to the NAc and the steady increase in the cortical projection to the NAc (Brenhouse et al, 2008). There is also an increase in glutaminergic fibers from the BLA to the mPFC, at least through P65 (Cunningham et al, 2002), while the projection from the mPFC to the BLA undergoes late pruning between P45 and 90 (Cressman et al, 2010). These late changes in connectivity between the mPFC and the BLA indicate that the BLA may be changing during adolescence as well.
The basolateral amygdala
Based on its embryonic origins and cellular structure, the BLA is considered the cortical-like portion of the many nuclei of the amygdalar complex (Carlsen and Heimer, 1988; McDonald, 1998). In addition to the late adolescent growth and pruning of axons between the BLA and mPFC, increases in cholinergic innervation have been noted in the BLA until P60 (Berdel et al, 1996). While not as striking as in the mPFC, there are also indications that NMDA receptors peak in the BLA during adolescence (Insel et al, 1990). Given the sex differences in neuronal loss in the mPFC, it is unfortunate that all of these studies used only male rats. In a study that examined both males and females, Rubinow and Juraska (2009) investigated the possibility that neuronal loss in the BLA might mirror the loss of neurons in the mPFC. The number of neurons remained stable between P20 and 35, whereas 13% were lost between P35 and 90 and significantly more loss was seen in females compared to males (Figure 1B). In conclusion, the cerebral cortex and associated neural regions are changing during adolescence with losses in the number of neurons and alterations in connectivity. These cellular changes may result in increased vulnerability to the adverse consequences of repeated drug exposure, which includes deleterious effects on cognitive development and an increased susceptibility to the development of addiction.
2. Alcohol and adolescence
It is estimated that approximately 85% of people within the United States have had their first drink by the legal drinking age of 21 (Grant and Dawson, 1997). Furthermore, binge-drinking rates steadily increase from 7% to 34% in both males and females between the ages of 14 and 20 years (Johnston et al., 2012). The laboratory rat can serve as a particularly useful model for the effects of exposure to high doses of alcohol during adolescence. One of the major contributors to this endeavor, particularly in the behavioral realm, is Dr. Linda Spear (see contribution in this issue). In this review, we will concentrate on the changes in brain structure following adolescent alcohol exposure, as well as our own contribution to studies of behavior using rats of both sexes.
Effects of alcohol on brain structure
Exposure to high levels of alcohol during adolescence results in neural damage. For example, binge drinking in adolescent male rats (P28-42) results in fewer neurons expressing corticotrophin releasing factor in the lateral portion of the central amygdala in adulthood (Gilpin et al, 2012). This could be due to a loss of neurons or to a phenotypic change in existing neurons. Alcohol also compromises neurogenesis in the rat hippocampal dentate gyrus in adolescents to a much greater degree than in adults (Crews and Nixon, 2003; Crews et al., 2006), as well as in adolescent rhesus monkeys (Taffe et al, 2010). Both the proliferation and survival of new dentate neurons are adversely affected by alcohol (Morris et al, 2010; Taffe et al, 2010). However, it is not clear how these alcohol-induced changes in neurogenesis relate to the drug’s long-lasting effects on cognitive function, since these were dissociated in at least one study where performance on the radial arm maze only correlated with the number of surviving hippocampal dentate neurons when both alcohol and MDMA were co-administered, not alcohol alone (Hernandez-Rabaza et al., 2010). The immune response to alcohol during adolescence, which is evident in activated microglia in the dentate gyrus (McClain et al, 2011) as well as increased levels of inflammatory cytokines in many neural areas (Pascual et al, 2007), may contribute to this vulnerability. Given that neurogenesis in the rat dentate gyrus peaks between P8-10 and continues to decrease throughout the lifespan (Schlessinger et al, 1975; Bayer et al, 1982), it is logical that the neurogenesis in the dentate gyrus would be more vulnerable during adolescence than in later adulthood when it is comparatively lower.
Along with these alcohol-induced changes in neurogenesis, silver staining, a marker of cellular stress that may lead to cell death, is increased in the olfactory tubercle, hippocampal dentate gyrus, and the piriform, perirhinal, and entorhinal cortices in rats given 4 consecutive days of exposure to a very high dose of alcohol (9–10 g/kg/day over 4 infusions/day) during adolescence (Crews et al., 2000). Aside from the dentate gyrus that was investigated with the same high dose (Obernier et al, 2002), no one has established if the silver stains inevitably indicate cell death, so that the extent of neuronal death following alcohol exposure cannot be assessed from these studies. Pascual et al. (2007) found rats exposed to 3 g/kg/day alcohol (2 out of every 3 days) from the juvenile into the adolescent period (P25-38) had evidence of increased cell death through changes in DNA fragmentation and capsase-3 activity in the neocortex, hippocampus, and cerebellum. These markers of cell death could indicate death of neurons, glia or both.
The continued loss of neurons in the cerebral cortex and BLA during adolescence might also result in these structures being particularly vulnerable to alcohol-induced neurotoxicity. During the prenatal and early postnatal period, alcohol sharply increases naturally occurring neuronal death throughout the rat brain (Goodlett and Eilers, 1997; Ikonomidou et al., 2000; Miller and Potempa, 1990), including in the mPFC (Mihalick et al., 2001). This may account for the vulnerability of the dentate granule cells, virtually all of which are generated postnatally (Schlessinger et al, 1975). Furthermore, alcohol has effects on many types of glia, including astrocytes and microglia (Evrard et al., 2006; McClain et al., 2011). The normal occurrence of neuronal death and alterations in the number of glia during adolescence (Markham et al, 2007; Rubinow and Juraska, 2009) may render the mPFC and BLA particularly vulnerable to alcohol exposure.
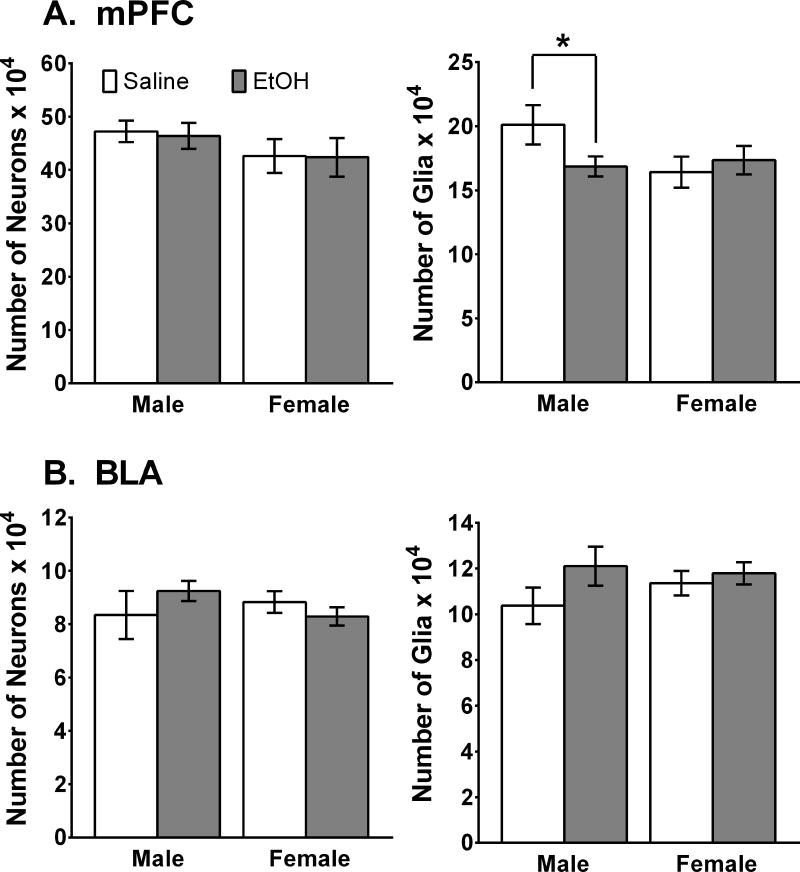
We investigated this by giving male and female rats 3g/kg alcohol (i.p.) for two out of every three days during adolescence (P35-45; Koss et al., 2012). This age range encompasses puberty and the time of neural losses for both sexes and thus is clearly within the adolescent time period. To assess the long term impact on the number of neurons and glia, we waited until the rats were adults (P100) before performing stereological quantification of cell numbers in both the ventral portion of the mPFC and the BLA. As shown in Fig. 2, the number of neurons was not altered in either of these neural areas in either sex. The number of glia, however, was reduced in the mPFC in male rats that had been exposed to alcohol as adolescents. This was not found in females or in the BLA of either sex. Markham et al (2007) found that the number of glia in the mPFC increased between P35 and P90 in males but not in females and that there were no indications of glial proliferation in the BLA (Rubinow and Juraska, 2009). Thus, only the dividing cells in the mPFC and BLA were vulnerable to the effects of alcohol, as in the hippocampal dentate gyrus (Nixon and Crews, 2002) and in the adult olfactory bulb where neuronal proliferation continues through adulthood (Hansson et al, 2010). It is also possible that the increasing levels of estrogen during adolescence may be neuroprotective, which is true in the case of hypoxia-ischemic episodes (Wise and Dubal, 2000; Zhu et al., 2006). The functional implications of the loss of glia remain to be elucidated. Since glia are now known to be involved in synaptic stabilization (Eroglu & Barres, 2010), the loss of these cells might have implications for the synaptic connectivity changes in the mPFC during male adolescence.
Figure 2.
The number of neurons and glia in the mPFC and BLA in adults that have been exposed to binge levels of alcohol during adolescence. (A) There were no effects of alcohol on the number of neurons in the mPFC in either sex, but exposure to alcohol resulted in fewer glial cells (14%) in males (*p < 0.02), but not in females compared to saline-exposed controls. (B) There were no differences in neurons or glia in either sex in the BLA. Adapted from Koss et al (2012).
Effects of alcohol on neurophysiology
In light of these effects of alcohol exposure during adolescence on brain structure, it is not surprising that multiple influences on neurophysiology have also been reported. Much of this work has focused on the hippocampus, especially with regard to lasting alterations in synaptic excitability, though some studies also report effects in the frontal cortex and amygdala. For example, early work suggested that exposing adolescent male rats to alcohol vapor for either 5 or 10 days from P30-40 induced changes in the EEG of the parietal cortex and hippocampus that persisted up to seven weeks (Slawecki et al., 2001). In a follow-up study (Slawecki, 2002), rats exposed to alcohol vapor from P35-P40 were less sensitive to subsequent challenges with 1.5 g/kg ethanol as adults. Specifically, ethanol-exposed rats failed to exhibit the increases in 4–6 Hz power in the hippocampus and parietal cortex that were evident in control rats given the ethanol challenge. These rats were also rated by observers as less intoxicated compared to controls, suggesting that the lack of change in EEG power after ethanol is indicative of a reduced sensitivity to the sedative properties of the drug. The mechanism for these effects of alcohol exposure was not identified, but subsequent studies suggested a potential role for changes in NMDA receptor expression and/or function. Rats exposed to ethanol vapor for 12 h/day as juveniles through late adolescence/young adulthood (P24-P60) exhibited significant changes in both cortical and hippocampal EEG following a challenge with the NMDA receptor antagonist MK-801 (Criado et al., 2008). Additionally, expression of NR1 and NR2A subunits in the hippocampus were increased and decreased following 24-hr and 2-week withdrawal, respectively, from alcohol vapor exposure that occurred for 14 h/day starting in the juvenile period (P23-P37) (Pian et al., 2010). This study also showed no change in subunit expression in frontal cortex after either withdrawal period.
The effects of alcohol on synaptic excitability have been well documented in studies of in vitro brain slice preparations, though most of this work has been done in rodents exposed during adulthood. For example, acute exposure to ethanol in vitro typically reduces long term potentiation (LTP) in the adult hippocampus (Sinclair and Lo, 1986; Taube and Schwartzkroin, 1986; Blitzer et al., 1990) and mPFC (Kroener et al., 2012). In the adult NAc, acute ethanol decreases long term depression (LTD), whereas ethanol pre-exposure leads to an absence of LTD and an emergence of LTP (Jeanes et al., 2011). In the adolescent brain, where studies focusing on alcohol’s effects on excitability have been much less numerous, certain drug effects are enhanced while others are diminished compared to those seen in adults. For example, LTP in hippocampal slices taken from rats at P30 was enhanced relative to that observed in slices taken from adults (P90). Subsequent application of ethanol (10–30 mM) resulted in a blockade of LTP in the adolescent, but not the adult, hippocampus (Pyapali et al., 1999). Contributing factors to the acute effects of ethanol on hippocampal LTP in adolescents relative to adults include a greater sensitivity to ethanol’s inhibitory effects on NMDA-mediated excitation (Swartzwelder et al., 1995), its ability to enhance GABA-receptor mediated inhibition (Fleming et al., 2007), and its ability to enhance the activity of GABAergic interneurons (Yan et al., 2009, 2010). In contrast to this enhanced sensitivity to ethanol in the adolescent hippocampus, there appears to be a decreased sensitivity in the cerebellum. Using in vivo electrophysiology, Van Skike et al. (2010) showed that the inhibitory effect of 1.5 g/kg (i.p.) ethanol on the activity of cerebellar Purkinje neurons was evident in adult, but not adolescent, rats. Together, the relatively limited number of studies that have directly compared the acute effects of ethanol on synaptic excitability suggest a heightened sensitivity of adolescents to ethanol-induced decreases in LTP in the hippocampus but a decreased sensitivity to this effect the cerebellum.
Repeated alcohol exposure beginning in adolescence (Roberto et al., 2002) or adulthood (Durand and Carlen, 1984; Fujii et al., 2008) also reduces hippocampal LTP, and this effect persists for up to two months of withdrawal (Durand and Carlen, 1984). In rats exposed to alcohol during early (P28-36), but not late (P45-50), adolescence, there is an enhancement of a unique, NMDA receptor-independent form of hippocampal LTP (Sabeti and Gruol, 2007). This effect, which was dependent on activation of sigma receptors, was observed when brain slices were taken 24 hours following the last ethanol exposure (i.e., during acute withdrawal). Sabeti (2011) further found these changes in LTP following early adolescent exposure to ethanol are accompanied by changes in the intrinsic excitability of CA1 pyramidal neurons that likely develop during withdrawal. Using a binge-like method for chronic ethanol exposure, Fleming et al. (2012) recently demonstrated that the GABA receptor-mediated inhibitory tone is reduced in the hippocampus of adolescent-exposed adult rats compared to saline-treated controls. Thus, the emerging picture from these studies of ethanol effects on the adolescent brain is that many, though certainly not all, of the effects of drug exposure on neurophysiology are greater and, potentially, longer lasting than those seen when exposure occurs during adulthood.
Effects of alcohol on neurochemistry
Several neurochemical systems, and their associated receptors, are altered by alcohol exposure during adolescence. Both voluntary alcohol intake (Sahr et al, 2004) and exposure through i.p. injection (Badanich et al, 2007; Pascual et al, 2009; Philpot et al, 2009) induce changes in NAc dopamine in adulthood. Specifically, rats exposed to alcohol in adolescence have higher basal levels of dopamine in the NAc compared to unexposed controls (Sahr et al., 2004; Badanich et al., 2007) and adult-exposed rats (Pascual et al., 2009). However, adolescents exhibit attenuations in ethanol-induced increases in dopamine overflow following a challenge injection (Philpot et al., 2009). Using in vivo voltammetry to measure rapid changes in dopamine concentrations as rats engaged in a risk-based decision making task, Nasrallah et al. (2011) showed that adult rats who self-administered ethanol from P30-P49 had a greater release of NAc dopamine following presentation of a lever associated with a risky choice. These rats also made more risky choices compared to controls.
Glutamate in the NAc is also affected as mice pre-exposed to ethanol during adolescence respond to an ethanol challenge (1.8 g/kg, i.p.) with 200% increases in glutamate concentrations (Carrara-Nascimento et al., 2011). Mice in this study that were pre-exposed to ethanol in adulthood, in contrast, had 50% reductions in NAc glutamate in response following the challenge. The mechanism for this age of exposure-dependent divergence in glutamate response to ethanol challenge is not apparent, though it has been shown that NMDA receptors are upregulated (Sircar and Sircar, 2006), whereas the function of the NR2B subunit is reduced (Pascual et al, 2009), in the PFC of adolescent-exposed rats. In addition, a recent whole-brain analysis of ethanol-induced changes in neurotransmitter-related gene expression revealed widespread downregulation in the brains of adolescent-exposed mice, compared to controls (Coleman et al., 2011). This included significant changes in the expression of genes coding for peptides, GABAA receptor subunits, nicotinic acetylcholine receptors, and dopamine receptors. It is not clear if alcohol-induced decreases in NMDA receptor expression and function or changes in neurotransmitter-related gene expression are directly responsible for the changes in neurotransmitter levels demonstrated in studies of adolescent alcohol exposure, but it is notable that many of the effects reported by Coleman et al. were in addition to the changes in gene expression evident in control mice assessed in adolescence compared to adulthood. Thus, alcohol’s ability to alter the normal trajectory of development in neurotransmitter systems appears to play an important role in its long-lasting effects on neurochemistry. Interestingly, some of the effects of alcohol exposure may be more prominent when exposure occurs in adulthood. For example, alcohol-induced decreases in mRNAs for corticotropin releasing hormone (CRH), GABAA and α1-adrenergic receptors were decreased in the BLA of adult-, but not adolescent-, exposed rats (Falco et al., 2009).
Given the important role of experience in the development of the hypothalamo-pituitary-adrenal (HPA) axis and the response to stress (Romeo, 2010), it is not surprising that exposure to alcohol during adolescence has long-lasting consequences on glucocorticoids, For example, in adolescent rats, ethanol significantly elevates plasma corticosterone levels relative to untreated or saline-injected controls, though this effected is attenuated with repeated exposure and is more significant in females compared to males (Przybycien-Szymanska et al., 2010). This change in corticosterone response is associated with an increase in CRH gene expression in the hypothalamus of male, but not female, rats exposed repeatedly to ethanol. Subsequent studies revealed this sex difference was due to a protective effect of estradiol on ethanol-induced upregulation of gene expression in females (Przybycien-Szymanska et al., 2012). When tested during adulthood, male rats pre-exposed to ethanol during adolescence, compared to saline-treated controls, exhibit reductions in basal corticosterone levels and exaggerated increases in corticosterone and CRH gene expression following ethanol challenge (Przybycien-Szymanska et al., 2011). In male rats given alcohol vapor exposure during adolescence and challenged with 4.5 g/kg ethanol during late adolescence/early adulthood, CRH gene expression and c-fos expression was shown to be reduced and unaffected, respectively, compared to air-exposed controls (Allen et al., 2011). A significant reduction in CRH immunoreactive cells in the central nucleus of the amygdala has also been found in rats that self-administered alcohol from P27 through sacrifice at P42 (Allen et al., 2011). Although more investigations of the interacting effects of stress and alcohol exposure during adolescence are needed, it is clear from the currently available studies that alcohol can have a long-lasting impact on the neurochemistry of the stress response system and that some of these effects may contribute to sex differences in alcohol-induced behavior.
Behavioral effects of alcohol during adolescence
Alcohol exposure during adolescence has been shown to have consequences for behavior later in life, with alcohol self-administration receiving a significant amount of research attention. The literature is somewhat equivocal, however, as there is published evidence for both increases and decreases in drinking behavior when exposure begins in adolescence. On the one hand, chronic exposure to alcohol via systemic injection (Pascual et al, 2007, 2009; Maldonado-Devincci et al., 2010), voluntary drinking (Rodd-Henricks et al, 2002; Walker and Ehlers, 2009; Strong et al., 2010; O’Tousa et al., 2013) or forced consumption (Blizard et al., 2004) has been shown to increase drinking behavior in adulthood. Using similar methods of ethanol pre-exposure, however, the opposite or no consistent effect on alcohol drinking in adulthood has been reported (Lancaster et al, 1996; Slawekci and Betancourt, 2002; Slawecki et al, 2004; Siegmund et al, 2005; Broadwater et al., 2011; Gilpin et al., 2012). In our own lab (Sherrill et al., 2011b), we found that alcohol drinking was modestly increased in both male and female rats who were pre-exposed in a “binge-like” fashion to 3 g/kg ethanol (i.p.) during adolescence. Interestingly, pre-pubertal gonadectomy potentiated the pre-exposure effect, but only in females. Thus, estrogen appeared to protect females from the long-term effects of alcohol exposure during adolescence. In a parallel study (Sherrill et al., 2011a), we found that adult males develop a more robust ethanol-induced conditioned taste aversion compared to females. In addition, males, but not females, exhibited an attenuated taste aversion in adulthood following pre-exposure to ethanol during adolescence. Changes in alcohol’s aversive properties might contribute to the ability of adolescent alcohol exposure to modulate alcohol drinking in adulthood. The reasons for the equivocal findings in these studies of alcohol pre-exposure during adolescence are not entirely clear, although differences in exposure methods and procedures for assessing drinking behavior certainly play a role.
Investigations of the effects of adolescent alcohol exposure on cognitive behavior typically demonstrate adverse consequences. For example, ethanol-induced impairments in auditory fear conditioning (Bergstrom et al, 2006) and memory (Markwiese et al, 1998; White et al, 2000; Land and Spear, 2004; Silvers et al, 2003, 2006) are more pronounced in adolescent compared to adult rats. Interestingly, this is true even though adolescents are known to be less sensitive to ethanol’s hypothermic, anxiolytic, and motor impairing effects (Spear and Varlinskaya, 2005). Following chronic, intermittent exposure to alcohol vapor during adolescence (P35-40) (Slawecki et al, 2004) or free access to 10% ethanol for 5 weeks starting in the juvenile period (3–8 weeks of age) (Salimov et al, 1996), rats exhibit increases in anxiety- and depression-like behaviors when they are tested in adulthood. A recent study found that only those rats with prior binge drinking experience restricted to adolescence, rather than earlier in adulthood, showed increases in open-arm entries on an elevated plus maze (Gilpin et al., 2012). This result was interpreted as being consistent with either decreased anxiety or increased impulsivity. The latter possibility is consistent with data from adult humans performing delayed reward discounting tasks, which assess impulsive choice behavior. In these studies, those with a history of adolescent alcohol exposure have higher levels of impulsivity than controls (Rogers et al, 2010). Data from laboratory animals are inconsistent with this interpretation, however. In adult rats trained to resist the impulse to respond during a premature phase of a five-choice serial reaction time task, there were no observed effects of repeated exposure to 5 g/kg (i.g.) ethanol every 8 hours from P33-P36 on baseline measures of attention, impulsivity or cognitive flexibility. Somewhat surprisingly, adolescent-exposed rats appeared to maintain a lasting tolerance to ethanol as they were less sensitive to ethanol-induced disruptions of task performance following a challenge with 1.5 or 3.0 g/kg ethanol (Semenova, 2012).
Additional evidence for alcohol-induced changes in decision making comes from studies in rats that were allowed to consume a sweetened gelatin and ethanol mixture from P30-49 and were then tested in a probability discounting task when they were adults. In this task, where rats are given the choice between small and certain or large but uncertain rewards, those exposed to alcohol during adolescence behaved in a more risky fashion than controls (Nasrallah et al., 2009). This preference for risk seems to be due to an imbalance in learning of better-than-expected outcomes over those that are worse than expected (Clark et al., 2012). Together, studies of the effects of alcohol exposure during adolescence have often, though not always, suggested a long-lasting impairment in cognitive function that is evident well into adulthood. Much of this work has not included comparison groups of adult-exposed subjects, however, so it is not yet clear if adolescents are uniquely sensitive compared to adults.
3. Psychostimulants and adolescence
The use of the psychostimulant drugs nicotine, cocaine and the amphetamines is relatively high among adolescents, particularly in comparison to other age groups. The most recent National Survey of Drug Use and Health (SAMHSA, 2012) suggests that individuals 18 to 25 years old have the highest rates of current tobacco use (39.5%) compared to those 12 to 17 (10.0%) and adults who are 26 or older (26.3%). For individuals in the 12 to 25 year old range, that represents approximately 21 million individuals. Although considerably lower, the use of more difficult to obtain drugs like cocaine and the amphetamines is nonetheless significant in young people (Johnston et al., 2012). Cocaine use by 12th grade students, which peaked in the mid-1980’s at about 13%, is now estimated to be at about 3%. The non-therapeutic use of amphetamines, which are more widely available due in part to diversion of prescriptions for medical conditions such as attention deficit hyperactivity disorder (ADHD), is nearly three times higher, with approximately 9% of 12th graders reporting use in the previous year. The use of these drugs is estimated to be even higher in those who are in the latter stages of adolescence. For those 18 to 25, it is estimated that over 1.5 million have used cocaine in the previous year, whereas nearly 1.3 million used amphetamines and other non-tobacco stimulants (SAMHSA, 2012). Clearly, adolescents are using these drugs and it is therefore critical to develop a full understanding of the potential for psychostimulants to induce adaptations in the brain and behavior that may persist and lead to adverse consequences, even after drug taking has ceased.
Effects of psychostimulants on brain structure
Similar to what has been seen in adult-exposed rats (Robinson and Kolb, 1997, 1999; Brown and Kolb, 2001), psychostimulant exposure during adolescence has been reported to alter neuronal morphology, with most of the changes reported to date in the PFC for amphetamine, cocaine and nicotine, along with additional effects reported for nicotine in the NAc, hippocampus and BLA. For example, the length and branching of basilar dendrites of pyramidal neurons in the mPFC of 48 day-old rats was increased by twice daily injections of 0.5 mg/kg amphetamine from P22-34 (Diaz Heijtz et al., 2003). This study also showed that these structural changes were associated with an upregulation in the expression of calcium/calmodulin-dependent protein kinase II (CaMKII) and tyrosine hydroxylase in slices of the mPFC taken from a separate group of rats given the same treatments. Long-lasting changes in the response of dendritic spines in the orbitofrontal cortex have also been reported. In this study (Gourley et al., 2012), mice were pre-exposed to 10 mg/kg cocaine, once per day from P31-36 and were then given a challenge injection of 10 mg/kg cocaine on P63. When spine morphology was assessed 24 hours later, it was discovered that there was decreased spine density, but increased spine head size, in cocaine pre-exposed compared to saline pre-exposed mice.
Nicotine-induced structural plasticity has been demonstrated in the NAc, BLA, hippocampus and mPFC, with some effects appearing to be dependent on the age of exposure. In rats that were sacrificed for anatomical analysis on P144 following continuous exposure to nicotine from the juvenile period to young adulthood (P22-69) via an osmotic minipump (2 mg/kg/day), medium spiny neurons of the NAc had more and longer dendritic segments compared to saline-exposed controls (McDonald et al., 2005). Subsequently, this same group demonstrated that nicotine-induced increases in dendritic length and branch number in medium spiny neurons was selectively increased in rats exposed from P29-43, but not in those exposed from P80-94 (McDonald et al., 2007). In the mPFC, and more specifically pyramidal cells of the prelimbic cortex, continuous exposure to nicotine during adolescence (P29-43) increases the length of basilar dendrites in cells classified as “complex” because of their large dendritic arbor. In rats exposed to nicotine in adulthood (P80-94), there was an increase in dendritic length and number of branches, but only in cells classified as “simple” because of their relatively small arbors (Bergstrom et al., 2008). This effect of nicotine on mPFC might be somewhat selective for the prelimbic region as nicotine failed to alter dendrites in the infralimbic region when it was given to adolescent (P32-46) and adult (P61-75) rats via an intermittent exposure method (six subcutaneous injections of 0.5 mg/kg; Bergstrom et al., 2010). This intermittent-exposure study also showed that nicotine increased dendritic length in principal neurons of the BLA, though this effect was dependent on both age of exposure and on brain hemisphere. Specifically, adult nicotine exposure induced an increase in dendritic complexity and, in the right hemisphere only, an increase in dendritic length. In adolescent-exposed rats, there was also an increase in complexity relative to saline injected controls, but the increase in length was not observed (Bergstrom et al., 2010). An important issue that remains to be fully addressed is the duration of these effects of nicotine on brain structure and whether or not they are fully reversed following withdrawal. A recent study in adolescent mice (P30-45) that were chronically exposed to nicotine in their drinking water suggests most observed effects were reversed by the time animals reached young adulthood (Oliveira-da-Silva et al., 2010).
Effects of psychostimulants on neurophysiology
Numerous studies have used neurophysiological measures to investigate psychostimulant-induced plasticity in adolescent-exposed rodents, though most of these have only assessed adaptations following relatively short withdrawal periods – a few days to 3 weeks. For example, studies in juvenile to adolescent rats or mice (typically between P21 and 41) that were sacrificed soon after their last injection of nicotine, cocaine or amphetamine have demonstrated drug-induced changes in synaptic excitability in the ventral tegmental area (Mansvelder and McGehee, 2000; Ungless et al., 2001; Saal et al., 2003), hippocampus (Perez et al., 2010), amygdala (Huang et al., 2003; Pollandt et al., 2006) and mPFC (Huang et al., 2007; Goriounova and Mansvelder, 2012). In the hippocampus of late adolescent/young adult mice (P63), the induction of LTP was enhanced by once daily exposure to 3 mg/kg amphetamine from P28-P37, compared to that observed in saline-treated controls (Gramage et al., 2013). This neural adaptation was associated with a significant, albeit transient, increase in anxiety-related behavior and memory impairments, as measured by deficits in passive avoidance and Y-maze performance, respectively.
In the NAc, pre-exposure to cocaine during adolescence tends to decrease excitability (Thomas et al., 2001), though this effect may be dependent on the method of drug exposure (experimenter- versus self-administered; see Jacobs et al., 2003) and the duration of withdrawal (Mu et al., 2010). In addition, a recent study (Huang et al., 2011) suggested the effects of cocaine on accumbal LTD may be dependent on the subregion that is analyzed. In this report, five days of exposure to cocaine (15 mg/kg/day) starting between P26 and 28 led to a decrease in LTD in the NAc shell, but not core, that lasted for up to 28 days following withdrawal (P56). Kourrich and Thomas (2009) also showed that unique effects in the shell compared to the core may depend on the duration of withdrawal from cocaine or amphetamine. In at least one study (Li and Kauer, 2004), amphetamine exposure during adolescence had no effect on the induction of LTP by high-frequency activation of glutamatergic afferents. However, when brain slices were subsequently exposed to amphetamine, which causes a dopamine-dependent attenuation of LTP induction in saline pre-treated rats, there was a reduced sensitivity to the inhibitory effect of acute amphetamine exposure on LTP (Li and Kauer, 2004). An important, and as yet unanswered question, is whether or not these effects differ between males and females. Sex differences were not assessed in any of these studies and nearly all of them utilized male rats or mice (or sex was not specified).
Although the neurophysiological effects of repeated psychostimulant exposure in adulthood have been extensively studied (for recent reviews, see Bowers et al., 2010; Luscher and Malenka, 2011), the paucity of studies using comparison groups of adolescent- and adult-exposed subjects makes it difficult to make direct comparisons and, in turn, assess whether adolescents are differentially sensitive to drug-induced plasticity. In the small number of studies that have used comparison groups of different ages, there is evidence that the adolescent brain is more sensitive to some drug effects. In one study of repeated nicotine exposure, rats given nicotine (0.4 mg/kg, three times per day) from P34-43 had decreases in short-term depression of evoked excitatory postsynaptic currents (eEPSCs) in layer V pyramidal cells of the mPFC. This effect, which was measured 5 weeks after the last nicotine injection (starting at P78), was not observed in saline-treated controls or in rats exposed to nicotine during late adolescence/young adulthood (P60-69) (Counotte et al., 2011). Additionally, parallel studies revealed that these long-lasting changes in synaptic function were associated with decreases in mGluR2 expression and function in the mPFC, as well as deficits in behavioral measures of attention and inhibitory control (Counotte et al., 2009, 2011). Additional support for the important role of nicotine-induced adaptations in mGluR2 comes from a report showing alternations in LTP in the mPFC of adult rats exposed to nicotine from P34-43 (Goriounova and Mansvelder, 2012). In this study, decreases in LTP were observed in the adolescent mPFC following bath application of nicotine (10 μM) and during the first 4 days following the last nicotine injection (on P43). However, when LTP was assessed following a 5-week withdrawal period (after P78), nicotine pre-exposed rats exhibited enhanced LTP compared to saline-treated controls. These short- and long-term adaptations, which were both linked to impairments in mGluR2 signaling in the mPFC, were not present in rats exposed to nicotine from P60-69 (Goriounova and Mansvelder, 2012).
Recently, we have found that repeated exposure to amphetamine during adolescence leads to changes in the function of mPFC neurons that persist for ~3 months following the last drug injection (Paul, Kang, Cox, and Gulley, unpublished observations). In this study, rats were given saline or 3 mg/kg amphetamine (i.p.), every other day during adolescence (P27-45) or adulthood (P85-103). When rats were between P125 and P140, we prepared brain slices at the level of the mPFC and performed whole-cell recordings of excitatory layer V pyramidal cells and fast-spiking inhibitory interneurons using methods similar to those described previously (Paul and Cox, 2012). We found no differences in the basic cellular properties of pyramidal neurons between the controls and those exposed to amphetamine. However, application of amphetamine (25 μm) or dopamine (50 μm) increased the frequency of spontaneous inhibitory postsynaptic currents (sIPSC) in controls while having no significant effects in amphetamine pre-exposed rats. These effects, which were not dependent on age of exposure, were also apparent in a comparison group of rats that was studied at approximately P66 (i.e., the same ~3-week withdrawal period used in the adult-exposed rats). Furthermore, we found that the excitability of interneurons, as measured by number and frequency of action potentials to depolarizing pulses, was significantly reduced in amphetamine-exposed compared to control rats. Thus, it appears that that the reduction of spontaneous inhibitory activity on layer V pyramidal neurons in amphetamine-exposed animals is due to the reduced excitability of fast spiking interneurons. Moreover, the effects of amphetamine exposure during adolescence are measurable at both short and long withdrawal periods.
Effects of psychostimulants on neurochemistry
Given their potent pharmacological effects and the continuing development of monoamine systems during adolescence, it is not surprising that psychostimulants have the potential to induce unique changes in dopamine system function in the young brain. Following a single injection, cocaine and amphetamine have been shown to increase extracellular dopamine concentrations in the dorsal striatum to a greater extent in adolescent compared to adult male rats (Walker and Kuhn, 2008; Walker et al., 2010) while rats in the juvenile period have a significantly lower dopamine response to cocaine (Chen et al., 2010). Additionally, inconsistent results have been found in the NAc, where cocaine-induced dopamine overflow was found to be either greater in adolescents (Badanich et al., 2006) or not different as a function of age following cocaine (Frantz et al., 2007) or amphetamine (Silvagni et al., 2008). A likely mechanism for the age-dependent differences that have been observed is differences in the expression and/or function of dopamine transporters. Indeed, both are increased in the striatum of adolescents compared to adults (Volz et al., 2009). The function of the vesicular monoamine transporter, which is responsible for sequestering monoamines into vesicles, is also higher in adolescents compared to adults (Volz et al., 2009). These developmental differences in dopamine transporters, along with those reported for D1 and D2 receptor expression (Anderson et al., 2000; Brenhouse et al., 2008) and tyrosine hydroxylase (Mathews et al., 2009), provide an opportunity for enhanced vulnerability to the effects of repeated drug exposure.
Chronic treatment with nicotine, cocaine or amphetamine leads to enhanced responsiveness, or sensitization, to the behavioral and neurobiological effects of these drugs (Berridge and Robinson, 1993; Vanderschuren and Kalivas, 2000). Some evidence suggests this drug-induced plasticity may be enhanced when exposure occurs during adolescence. For example, in rats given 2 or 10 mg/kg amphetamine daily for three days and challenged with 2 mg/kg amphetamine after a 5-day withdrawal period, it was shown that amphetamine-stimulated dopamine release in the striatum was significantly higher in those exposed during adolescence (P33-43) compared to young adulthood (P61-71; Laviola et al., 2001). Following a similar treatment protocol, but a significantly longer withdrawal period of 28 days, adolescent-exposed rats continue to exhibit a sensitized behavioral response that was associated with heightened neural activation in the striatum and amygdala (McPherson and Lawrence, 2006). A potential mechanism for these effects is a lasting change in the responsiveness of monoamine neurons to subsequent drug challenges. Labonte et al. (2012) used in vivo electrophysiology to demonstrate that daily exposure to amphetamine from P30-50 led to significant increases in the firing rate of dopamine neurons in the ventral tegmental area and 5-HT neurons in the dorsal raphe, compared to neurons recorded from these brain regions in saline-exposed controls.
Nicotine’s effects on dopamine, which are indirect via the drug’s activation of nicotinic acetylcholine receptors (nAChRs) located on dopamine terminals (Livingstone and Wonnacott, 2009), have generally been shown to be different in adolescents compared to adults, but the direction of this effect has varied. In the NAc, a single injection of 0.3 mg/kg nicotine was reported to elevate dopamine levels to a greater extent in adolescent (~P28) compared to adult (P63-84) rats (Shearman et al., 2008), whereas 0.6 mg/kg nicotine reportedly increased dopamine in adults (P60), but not adolescents (P35 or P45; Badanich and Kirstein, 2004). Consistent with the former result is a study showing elevations in nicotine-stimulated [3H]dopamine from NAc synaptosomes prepared from adolescent rats, compared to those taken from adults (Azam et al., 2007). With repeated exposure, nicotine induces adaptations in the dopamine system such that dopamine concentrations in the NAc are decreased during precipitated withdrawal in both adolescents and adults. Interestingly, the magnitude of this decrease is significantly lower in adolescent-exposed rats (Natividad et al., 2010). This effect was shown to be mediated by age-dependent differences in nicotine-induced adaptations in the interacting glutamatergic and GABAergic systems of the ventral tegmental area (Natividad et al, 2012). Stimulated dopamine release and receptor-mediated signaling are also known to be elevated in adult rats exposed to nicotine during adolescence (Trauth et al., 2001; Abreu-Villaca et al., 2003; Dickson et al., 2011). Although many of these studies of chronic nicotine effects have utilized continuous exposure techniques that result in high-dose exposure for relatively long periods of time, intermittent exposure to low or moderate doses during adolescence also has the potential to produce lasting effects. For example, daily injections of 0.4 mg/kg nicotine from P30-36 results in increases and decreases in the expression of dopamine and serotonin transporters, respectively, that were not evident in rats exposed from P60-66 (Collins et al., 2004). After only 4 days of intravenous injections with 0.06 mg/kg nicotine, serotonin transporters are elevated in the PFC and the BLA of adolescent- (P32) but not adult-exposed (P90) rats (Dao et al., 2011).
Dopamine and other monoamines are not the only neurochemical systems to exhibit age-dependent differences in psychostimulant-induced adaptations. In the adolescent brain, cholinergic systems are a primary target for nicotine-induced plasticity (O’Dell, 2009). This may be due in part to the numerous changes in expression and function of the nicotinic receptor during normal adolescent development (Slotkin, 2002). Recent studies have also highlighted a potential role for adaptations in cannabinoid and opiate receptors in the long-lasting behavioral effects of adolescent nicotine exposure (Marco et al., 2007; Mateos et al., 2011).
Behavioral effects of psychostimulants during adolescence
Much of the work on the behavioral effects of adolescent psychostimulant exposure has focused on long-lasting changes in either the locomotor stimulant properties of the drugs or their ability to influence reward seeking behavior. With regards to the former, some studies report greater amphetamine- or cocaine-induced sensitization in adolescent-exposed rodents (Adriani et al., 1998; Schramm-Sapyta et al., 2004; Caster et al., 2005; Mathews et al., 2010, 2011; Kameda et al., 2011), whereas others indicate greater effects in adults (Frantz et al., 2007; Zakharova et al., 2009; Good and Radcliffe, 2011; Richetto et al., 2013; Sherrill et al., 2013) or no difference between age groups (Niculescu et al., 2005; Good and Radcliffe, 2011). Studies with nicotine have also reported inconsistent results for age-dependent differences in locomotor sensitization (Belluzzi et al., 2004; Schochet et al., 2004; Cruz et al., 2005). Between- and within-study methodological differences contribute to some of these discrepant findings, with key factors being age of exposure (e.g., early vs. late adolescence), drug dose and the aspect of drug-induced behavior that is measured (e.g., locomotion or stereotypy). For example, at lower doses of amphetamine (< 1.5 mg/kg), adolescents tend to show an attenuated response to the first injection but enhanced locomotor sensitization relative to adults (Mathews and McCormick, 2007; Mathews et al., 2009; Zakharova et al., 2009; Bolanos et al., 1998). With higher doses (> 2 mg/kg), however, age-dependent differences in initial responsiveness diminish and repeated exposure produces robust stereotypy and reduced locomotor activity, particularly in adults (Adriani et al., 1998; Adriani and Laviola, 2002; Sherrill et al., 2013). Thus, adolescents appear to have a higher threshold for the psychomotor-activating effects of cocaine and amphetamine, but once activated their response is similar to that seen in adults.
Studies of age-dependent differences in the rewarding properties of psychostimulants have generally been more consistent – rodents exposed during adolescence tend to exhibit heightened reward-related behavior later in life compared to those exposed during adulthood. For example, nicotine-induced conditioned place preference is enhanced in adolescents compared to adults (Vastola et al., 2002; Belluzzi et al., 2004; Torrella et al., 2004; Shram et al., 2006; Shram and Le, 2010; Kota et al., 2008, 2011; Brielmaier et al., 2007; Torres et al., 2008, 2009). Adolescent rats also exhibit reduced conditioned place aversion to nicotine compared to adults (O’Dell et al., 2007). A similar increase in sensitivity to cocaine and amphetamine reward in adolescents has been demonstrated (Badanich et al., 2006; Brenhouse and Andersen, 2008; Brenhouse et al., 2008; Zakharova et al., 2008), with adolescents exhibiting a potent resistance to extinction of drug-environment associations (Brenhouse et al., 2010). There are, however, several studies showing that adults are relatively more sensitive (Adriani and Laviola, 2003; Aberg et al., 2007; Vidal-Infer et al., 2012) or no age-dependent differences (Campbell et al., 2000; Schramm-Sapyta et al., 2004; Mathews and McCormick, 2007; Mathews et al., 2010). Moreover, there are robust sex differences in these effects. For example, in a study of cocaine-induced conditioned place preference (Zakharova et al., 2008), it was demonstrated that the female sex and adolescent exposure independently resulted in higher sensitivity to the rewarding effects of cocaine in adults. Studies showing changes in psychostimulant self-administration behavior following pre-exposure to the drugs in adolescence or adulthood, which have been reviewed in detail elsewhere (Shahbazi et al., 2008; Schramm-Sapyta et al., 2009; Anker et al., 2011), suggest that adolescents have a more “addiction vulnerable” phenotype. Specifically, they exhibit heightened motivation for the drug, reduced extinction and greater relapse of drug-seeking behavior, and have reduced withdrawal responses.
Of great clinical interest is the potential for long-lasting changes in cognition that might result from adolescent exposure to psychostimulant drugs. Human stimulant abusers, who usually initiate drug use in adolescence, have been shown to exhibit significant cognitive deficits that vary in magnitude depending on the duration of drug exposure (Bolla et al., 1998; Verdejo-Garcia et al., 2006). In adult rats exposed to psychostimulants during adolescence, there is evidence of enduring deficits in cognitive tasks that assess attention, fear learning, memory, decision making, and impulse control (Harvey et al., 2009; Santucci and Rabidou, 2011; Sillivan et al., 2011; Hankosky and Gulley, 2012). However, in these studies, it is difficult to ascertain if adolescents are relatively more sensitive to these effects because comparison groups of adult-exposed subjects were rarely utilized.
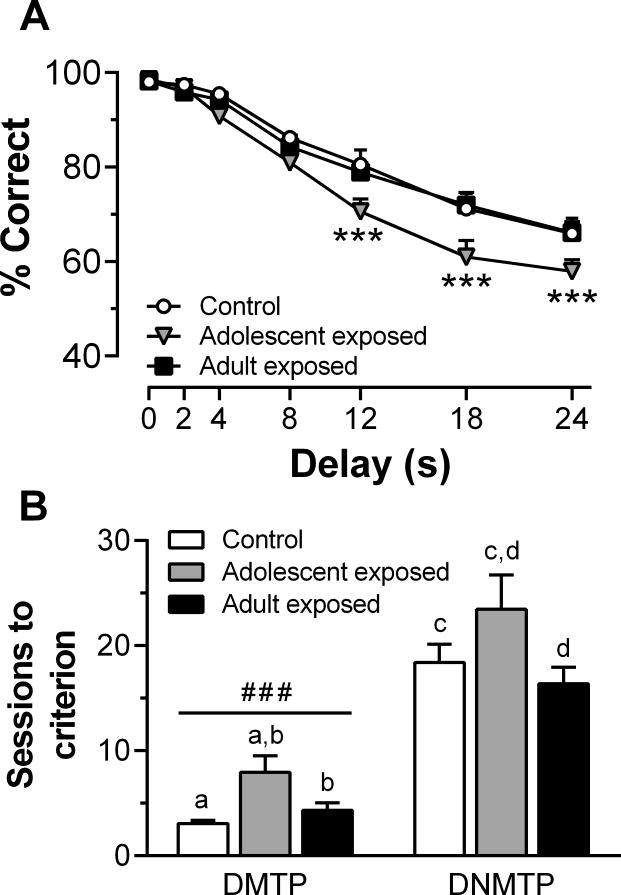
In studies that directly assess the age-dependence of psychostimulant-induced cognitive deficits, there is evidence that adolescents may be more vulnerable. For example, nicotine exposure during adolescence, but not adulthood, increases impulsive action and enhances electrically stimulated dopamine release in vitro from slices of the mPFC (Counotte et al. 2009). In addition, there are unique effects of cocaine on memory processes when the onset of drug exposure occurs in adolescence compared to adulthood (Harvey et al., 2009). Recently, we found that adult rats that were exposed to 3 mg/kg amphetamine during adolescence displayed delay-dependent deficits in choice accuracy in an operant-based working memory task (Sherrill et al., 2013). In addition, they required more sessions to optimize performance and learn task rules, and they were more susceptible to proactive interference, compared to control and adult-exposed groups (Fig 3). Interestingly, we also found that amphetamine-induced locomotor sensitization was enhanced in adult- compared to adolescent-exposed rats, suggesting that drug-induced changes in cognition were dissociable from amphetamine’s lasting effects on sensitivity to its motor activating effects. These findings of heightened vulnerability of adolescents to the psychostimulant-induced cognitive deficits are not without exception, however. Repeated cocaine exposure has been reported to have no specificity or even greater effects in adults on an amygdala-sensitive maze task (Kerstetter and Kantak, 2007).
Figure 3.
The effects of pre-exposure to amphetamine on working memory in a delay matching to position (DMTP) and delay non-match to position (DNMTP) task. (A) Mean choice accuracy (% correct) within each delay block averaged across the first two training sessions. (B) Mean number of sessions to reach a performance criterion (STC) of ≥ 85% correct choices for two consecutive sessions. Matching letters indicate p < 0.001; ***p < 0.001 vs control and adult-exposed groups within delay; ###p < 0.001 vs DNMTP, collapsed across exposure group. Adapted from Sherrill et al. (2013).
4. Future challenges
There is mounting evidence from the rat model that adolescence is a particularly vulnerable time for both behavioral and neural effects of alcohol and psychostimulant exposure. Nevertheless, more studies are needed that directly compare adolescent and adult exposures to firmly establish the unique sensitivity of the adolescent to drug-induced plasticity and its associated consequences. Until recently, the majority of the work in this area has focused on one age group or the other (McCutcheon and Marinelli, 2009). More studies are also needed to understand the discrepancies in results that exist. Many of the inconsistencies are at least partially attributable to obvious factors such as drug dose and method of exposure. These are aspects of experimental design that influence the generality of findings and ultimately contribute to the translational impact of observed results. There are other factors, however, that need to be more closely controlled since their variation can lead to results that are confounded and otherwise difficult to interpret. For example, the ages that are considered adolescence are often defined too broadly. If a rise in gonadal hormones is an essential marker of adolescence, which it is in humans at approximately 12 years old, then the earliest age at which rats should be considered adolescent is P28. This is especially true for male rats where overt signs of puberty are not found until after P38. Exposures that are started before this age are modeling drug intake during the juvenile period that continues into adolescence. Observed effects could thus be due to the juvenile exposure, per se. Moreover, including juveniles in these studies is addressing separate issues since it is not modeling the experimentation with drugs and alcohol that occur during human adolescence in our society.
The inclusion of females in experimental designs is also needed. As we have discussed above, there are numerous studies in the literature and from our own laboratories that demonstrate different effects of drug exposure in male compared to female adolescents. Clearly, the inclusion of both sexes adds complexity to experimental design, analysis, and interpretation of results, but the lack of female subjects in most experiments to date limits their generalizability.
Another key factor that has been largely ignored is the effect of differential rearing environments and early-life stress that are introduced when experimental animals are shipped from commercial vendors to research facilities when they are in utero or around weaning (~P22). This is of particular concern for many of the studies discussed in this review as rearing environment and early-life stress have been shown to have significant effects on PFC development and cognitive processes mediated by this brain region (Liston et al., 2006; Cerqueira et al., 2007; Green et al., 2012; Yuen et al., 2012). Moreover, there are numerous reports demonstrating the long-lasting effects of shipping stress and rearing conditions on behavior and neurobiology (Prager et al., 2011), including the response to drugs (Bardo et al., 2001; Adriani and Laviola, 2002; Ogawa et al., 2007; Wiley and Evans, 2009; Mogi et al., 2012; Martini and Valverde, 2012). There is also ample evidence that the effects of stress during adolescence are different and often more prolonged in their duration compared to those seen when stress is induced in adulthood (see Romeo in this volume). It could be argued that all subjects (i.e., both “experimental” and “control”) experience the same early-life stress. However, it is likely that these effects can interact with later drug exposures. Such interactions, along with the additional influence of the animal’s sex, have been demonstrated previously (Ogawa et al., 2007; Wiley and Evans, 2009; Mogi et al., 2012). All of these potential interactions can be avoided if animals are bred and housed within the research facility where experiments will occur. This approach has the additional advantage of allowing for the control of litter effects that are prevalent in multiparous research animals like rats and mice (Zorilla, 1997), which would make the distinction between effects in adolescents and adults clearer.
Adolescence is a period of significant neurobiological and behavioral change
Adolescence is a time when individuals first use drugs and alcohol
Adolescents may be especially sensitive to drug-induced plasticity
We review evidence suggesting adolescence is a period of high vulnerability
Acknowledgments
Studies from the author’s laboratories were supported in part by grants from National Institute on Alcohol Abuse and Alcoholism (AA017354) and the National Institute on Drug Abuse (DA029815). We thank Wendy Koss for assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg M, Wade D, Wall E, Izenwasser S. Effect of MDMA (ecstasy) on activity and cocaine conditioned place preference in adult and adolescent rats. Neurotoxicol Teratol. 2007;29:37–46. doi: 10.1016/j.ntt.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Seidler FJ, Slotkin TA. Impact of adolescent nicotine exposure on adenylyl cyclase-mediated cell signaling: enzyme induction, neurotransmitter-specific effects, regional selectivities, and the role of withdrawal. Brain Res. 2003;988:164–172. doi: 10.1016/s0006-8993(03)03368-7. [DOI] [PubMed] [Google Scholar]
- Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behav Neurosci. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Spontaneous novelty seeking and amphetamine-induced conditioning and sensitization in adult mice: evidence of dissociation as a function of age at weaning. Neuropsychopharmacology. 2002;27:225–236. doi: 10.1016/S0893-133X(02)00300-7. [DOI] [PubMed] [Google Scholar]
- Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain Behav Immun. 2011;25(Suppl 1):S50–60. doi: 10.1016/j.bbi.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Rivier CL, Lee SY. Adolescent alcohol exposure alters the central brain circuits known to regulate the stress response. Neuroscience. 2011;182:162–168. doi: 10.1016/j.neuroscience.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Conde F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Zlebnik NE, Navin SF, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology (Berl) 2011;215:785–799. doi: 10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Kirsteina CL. Nicotine administration significantly alters accumbal dopamine in the adult but not in the adolescent rat. Ann N Y Acad Sci. 2004;1021:410–417. doi: 10.1196/annals.1308.054. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. Chronic ethanol exposure during adolescence increases basal dopamine in the nucleus accumbens septi during adulthood. Alcohol Clin Exp Res. 2007;31:895–900. doi: 10.1111/j.1530-0277.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl ) 2001;155:278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Belluzzi J, Lee A, Oliff H, Leslie F. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl ) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Berdel B, Morys J, Maciejewska B, Narkiewicz O. Acetylcholinesterase activity as a marker of maturation of the basolateral complex of the amygdaloid body in the rat. Int J Dev Neurosci. 1996;14:543–549. [PubMed] [Google Scholar]
- Bergstrom HC, McDonald CG, French HT, Smith RF. Continuous nicotine administration produces selective, age-dependent structural alteration of pyramidal neurons from prelimbic cortex. Synapse. 2008;62:31–39. doi: 10.1002/syn.20467. [DOI] [PubMed] [Google Scholar]
- Bergstrom HC, McDonald CG, Smith RF. Alcohol exposure during adolescence impairs auditory fear conditioning in adult Long-Evans rats. Physiol Behav. 2006;88:466–472. doi: 10.1016/j.physbeh.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Bergstrom HC, Smith RF, Mollinedo NS, McDonald CG. Chronic nicotine exposure produces lateralized, age-dependent dendritic remodeling in the rodent basolateral amygdala. Synapse. 2010;64:754–764. doi: 10.1002/syn.20783. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Gil O, Landau EM. Long-term potentiation in rat hippocampus is inhibited by low concentrations of ethanol. Brain Res. 1990;537:203–208. doi: 10.1016/0006-8993(90)90359-j. [DOI] [PubMed] [Google Scholar]
- Blizard DA, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34:177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Cadet JL, London ED. The neuropsychiatry of chronic cocaine abuse. J Neuropsychiatry Clin Neurosci. 1998;10:280–289. doi: 10.1176/jnp.10.3.280. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Dumais K, Andersen SL. Enhancing the salience of dullness: behavioral and pharmacological strategies to facilitate extinction of drug-cue associations in adolescent rats. Neuroscience. 2010;169:628–636. doi: 10.1016/j.neuroscience.2010.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier JM, McDonald CG, Smith RF. Immediate and long-term behavioral effects of a single nicotine injection in adolescent and adult rats. Neurotoxicol Teratol. 2007;29:74–80. doi: 10.1016/j.ntt.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic Intermittent Ethanol Exposure in Early Adolescent and Adult Male Rats: Effects on Tolerance, Social Behavior, and Ethanol Intake Alcohol. Clin Exp Res. 2011;35:1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, Kolb B. Nicotine sensitization increases dendritic length and spine density in the nucleus accumbens and cingulate cortex. Brain Res. 2001;899:94–100. doi: 10.1016/s0006-8993(01)02201-6. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25:340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Carlsen J, Heimer L. The basolateral amygdaloid complex as a cortical-like structure. Brain Res. 1988;441:377–380. doi: 10.1016/0006-8993(88)91418-7. [DOI] [PubMed] [Google Scholar]
- Carrara-Nascimento PF, Griffin WC, 3rd, Pastrello DM, Olive MF, Camarini R. Changes in extracellular levels of glutamate in the nucleus accumbens after ethanol-induced behavioral sensitization in adolescent and adult mice. Alcohol. 2011;45:451–460. doi: 10.1016/j.alcohol.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Sanchez-Garrido MA, Ruiz-Pino F, Romero M, Garcia-Galiano D, Aguilar E, Pinilla L, Dieguez C, Mikkelsen JD, Tena-Sempere M. Early metabolic programming of puberty onset: impact of changes in postnatal feeding and rearing conditions on the timing of puberty and development of the hypothalamic kisspeptin system. Endocrinology. 2011;152:3396–3408. doi: 10.1210/en.2010-1415. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YI, Choi JK, Xu H, Ren J, Andersen SL, Jenkins BG. Pharmacologic Neuroimaging of the Ontogeny of Dopamine Receptor Function. Dev Neurosci. 2010;32:125–138. doi: 10.1159/000286215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JJ, Nasrallah NA, Hart AS, Collins AL, Bernstein IL, Phillips PE. Altered risk-based decision making following adolescent alcohol use results from an imbalance in reinforcement learning in rats. PLoS One. 2012;7:e37357. doi: 10.1371/journal.pone.0037357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Jr, He J, Lee J, Styner M, Crews FT. Adolescent Binge Drinking Alters Adult Brain Neurotransmitter Gene Expression, Behavior, Brain Regional Volumes, and Neurochemistry in Mice. Alcoholism: Clinical and Experimental Research. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Wade D, Ledon J, Izenwasser S. Neurochemical alterations produced by daily nicotine exposure in periadolescent vs. adult male rats. Eur J Pharmacol. 2004;502:75–85. doi: 10.1016/j.ejphar.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer AN, Smit AB, Mansvelder HD, Pattij T, Spijker S. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci. 2011;14:417–419. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518:2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Alcohol, neural stem cells, and adult neurogenesis. Alcohol Res Health. 2003;27:197–204. [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Liu T, Ehlers CL, Mathe AA. Prolonged chronic ethanol exposure alters neuropeptide Y and corticotropin-releasing factor levels in the brain of adult Wistar rats Pharmacol. Biochem Behav. 2011;99:104–111. doi: 10.1016/j.pbb.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Electrophysiological effects of dizocilpine (MK-801) in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2008;32:1752–1762. doi: 10.1111/j.1530-0277.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- Cruz FC, Delucia R, Planeta CS. Differential behavioral and neuroendocrine effects of repeated nicotine in adolescent and adult rats. Pharmacol Biochem Behav. 2005;80:411–417. doi: 10.1016/j.pbb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dao JM, McQuown SC, Loughlin SE, Belluzzi JD, Leslie FM. Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology. 2011;36:1319–1331. doi: 10.1038/npp.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? Eur J Neurosci. 2003;18:3394–3399. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- Dickson PE, Rogers TD, Lester DB, Miller MM, Matta SG, Chesler EJ, Goldowitz D, Blaha CD, Mittleman G. Genotype-dependent effects of adolescent nicotine exposure on dopamine functional dynamics in the nucleus accumbens shell in male and female mice: a potential mechanism underlying the gateway effect of nicotine. Psychopharmacology (Berl) 2011;215:631–642. doi: 10.1007/s00213-010-2159-2. [DOI] [PubMed] [Google Scholar]
- Durand D, Carlen PL. Impairment of long-term potentiation in rat hippocampus following chronic ethanol treatment. Brain Res. 1984;308:325–332. doi: 10.1016/0006-8993(84)91072-2. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–231. doi: 10.1038/nature09612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard SG, Duhalde-Vega M, Tagliaferro P, Mirochnic S, Caltana LR, Brusco A. A low chronic ethanol exposure induces morphological changes in the adolescent rat brain that are not fully recovered even after a long abstinence: an immunohistochemical study. Exp Neurol. 2006;200:438–459. doi: 10.1016/j.expneurol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Falco AM, Bergstrom HC, Bachus SE, Smith RF. Persisting changes in basolateral amygdala mRNAs after chronic ethanol consumption. Physiol Behav. 2009;96:169–173. doi: 10.1016/j.physbeh.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol. 2007;97:3806–3811. doi: 10.1152/jn.00101.2007. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the Rat, Chronic Intermittent Ethanol Exposure During Adolescence Alters the Ethanol Sensitivity of Tonic Inhibition in Adulthood. Alcoholism: Clinical and Experimental Research. 2012;36:279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Fujii S, Yamazaki Y, Sugihara T, Wakabayashi I. Acute and chronic ethanol exposure differentially affect induction of hippocampal LTP. Brain Res. 2008;1211:13–21. doi: 10.1016/j.brainres.2008.02.052. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilpin NW. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol. 2012;46:329–337. doi: 10.1016/j.alcohol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent Binge Drinking Leads to Changes in Alcohol Drinking, Anxiety, and Amygdalar Corticotropin Releasing Factor Cells in Adulthood in Male Rats. PLoS ONE. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good RL, Radcliffe RA. Methamphetamine-induced locomotor changes are dependent on age, dose and genotype. Pharmacol Biochem Behav. 2011;98:101–111. doi: 10.1016/j.pbb.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21:738–744. [PubMed] [Google Scholar]
- Goriounova NA, Mansvelder HD. Nicotine exposure during adolescence leads to short- and long-term changes in spike timing-dependent plasticity in rat prefrontal cortex. J Neurosci. 2012;32:10484–10493. doi: 10.1523/JNEUROSCI.5502-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Olevska A, Warren MS, Taylor JR, Koleske AJ. Arg Kinase Regulates Prefrontal Dendritic Spine Refinement and Cocaine-Induced Plasticity. J Neurosci. 2012;2012:2314–2323. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramage E, Del Olmo N, Fole A, Martin YB, Herradon G. Periadolescent amphetamine treatment causes transient cognitive disruptions and long-term changes in hippocampal LTP depending on the endogenous expression of pleiotrophin. Addiction Biology. 2013;18:19–29. doi: 10.1111/j.1369-1600.2011.00362.x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long–Evans rats. Dev Psychobiol. 2012 doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- Hankosky ER, Gulley JM. Performance on an impulse control task is altered in adult rats exposed to amphetamine during adolescence. Dev Psychobiol. 2012;54 doi: 10.1002/dev.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Nixon K, Rimondini R, Damadzic R, Sommer WH, Eskay R, Crews FT, Heilig M. Long-term suppression of forebrain neurogenesis and loss of neuronal progenitor cells following prolonged alcohol dependence in rats. Int J Neuropsychopharmacol. 2010;13:583–593. doi: 10.1017/S1461145710000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RC, Dembro KA, Rajagopalan K, Mutebi MM, Kantak KM. Effects of self-administered cocaine in adolescent and adult male rats on orbitofrontal cortex-related neurocognitive functioning. Psychopharmacology (Berl) 2009;206:61–71. doi: 10.1007/s00213-009-1579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Navarro-Mora G, Velazquez-Sanchez C, Ferragud A, Marin MP, Garcia-Verdugo JM, Renau-Piqueras J, Canales JJ. Neurotoxicity and persistent cognitive deficits induced by combined MDMA and alcohol exposure in adolescent rats. Addict Biol. 2010;15:413–423. doi: 10.1111/j.1369-1600.2010.00259.x. [DOI] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Navarro-Mora G, Velazquez-Sanchez C, Ferragud A, Marin MP, Garcia-Verdugo JM, Renau-Piqueras J, Canales JJ. Neurotoxicity and persistent cognitive deficits induced by combined MDMA and alcohol exposure in adolescent rats. Addiction Biology. 2010;15:413–423. doi: 10.1111/j.1369-1600.2010.00259.x. [DOI] [PubMed] [Google Scholar]
- Hoorn EJ, McCormick JA, Ellison DH. High tail-cuff blood pressure in mice 1 week after shipping: the need for longer acclimation. Am J Hypertens. 2011;24:534–536. doi: 10.1038/ajh.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-, Lin H-, Hsu K- Repeated Cocaine Administration Promotes Long-Term Potentiation Induction in Rat Medial Prefrontal. Cortex Cerebral Cortex. 2007;17:1877–1888. doi: 10.1093/cercor/bhl096. [DOI] [PubMed] [Google Scholar]
- Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH, Hsu KS. Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci. 2011;31:4194–4203. doi: 10.1523/JNEUROSCI.5239-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Wang SJ, Chiou LC, Gean PW. Mediation of amphetamine-induced long-term depression of synaptic transmission by CB1 cannabinoid receptors in the rat amygdala. J Neurosci. 2003;23:10311–10320. doi: 10.1523/JNEUROSCI.23-32-10311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. J Pharmacol Exp Ther. 2011;336:155–164. doi: 10.1124/jpet.110.171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. J Pharmacol Exp Ther. 2011;336:155–164. doi: 10.1124/jpet.110.171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975–2011. Volume II: College students and adults ages. 2012;19:50–314. [Google Scholar]
- Kameda SR, Fukushiro DF, Trombin TF, Procopio-Souza R, Patti CL, Hollais AW, Calzavara MB, Abilio VC, Ribeiro RA, Tufik S, D’Almeida V, Frussa-Filho R. Adolescent mice are more vulnerable than adults to single injection-induced behavioral sensitization to amphetamine. Pharmacol Biochem Behav. 2011;98:320–324. doi: 10.1016/j.pbb.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Kantak KM. Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology (Berl) 2007;194:403–411. doi: 10.1007/s00213-007-0852-6. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Koss WA, Sadowski RN, Sherrill LK, Gulley JM, Juraska JM. Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res. 2012;1466:24–32. doi: 10.1016/j.brainres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Damaj MI. Age-dependent differences in nicotine reward and withdrawal in female mice. Psychopharmacology (Berl) 2008;198:201–210. doi: 10.1007/s00213-008-1117-8. [DOI] [PubMed] [Google Scholar]
- Kota D, Sanjakdar S, Marks MJ, Khabour O, Alzoubi K, Damaj MI. Exploring behavioral and molecular mechanisms of nicotine reward in adolescent mice. Biochem Pharmacol. 2011;82:1008–1014. doi: 10.1016/j.bcp.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroener S, Mulholland PJ, New NN, Gass JT, Becker HC, Chandler LJ. Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLoS One. 2012;7:e37541. doi: 10.1371/journal.pone.0037541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte B, McLaughlin RJ, Dominguez-Lopez S, Bambico FR, Lucchino I, Ochoa-Sanchez R, Leyton M, Gobbi G. Adolescent amphetamine exposure elicits dose-specific effects on monoaminergic neurotransmission and behaviour in adulthood. Int J Neuropsychopharmacol. 2012;15:1319–1330. doi: 10.1017/S1461145711001544. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Land C, Spear NE. Ethanol impairs memory of a simple discrimination in adolescent rats at doses that leave adult memory unaffected. Neurobiol Learn Mem. 2004;81:75–81. doi: 10.1016/j.nlm.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Pieretti S. Striatal dopamine sensitization to D-amphetamine in periadolescent but not in adult rats. Pharmacol Biochem Behav. 2001;68:115–124. doi: 10.1016/s0091-3057(00)00430-5. [DOI] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of hippocampal GABAA receptor-mediated IPSCS to ethanol. Alcohol Clin Exp Res. 2003;27:2017–2022. doi: 10.1097/01.ALC.0000108390.62394.71. [DOI] [PubMed] [Google Scholar]
- Li Y, Kauer JA. Repeated exposure to amphetamine disrupts dopaminergic modulation of excitatory synaptic plasticity and neurotransmission in nucleus accumbens. Synapse. 2004;51:1–10. doi: 10.1002/syn.10270. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone PD, Wonnacott S. Nicotinic acetylcholine receptors and the ascending dopamine pathways. Biochem Pharmacol. 2009;78:744–755. doi: 10.1016/j.bcp.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav. 2010;96:476–487. doi: 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-Term Potentiation of Excitatory Inputs to Brain Reward Areas by Nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Marco EM, Granstrem O, Moreno E, Llorente R, Adriani W, Laviola G, Viveros MP. Subchronic nicotine exposure in adolescence induces long-term effects on hippocampal and striatal cannabinoid-CB1 and mu-opioid receptors in rats. Eur J Pharmacol. 2007;557:37–43. doi: 10.1016/j.ejphar.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Martini M, Valverde O. A single episode of maternal deprivation impairs the motivation for cocaine in adolescent mice. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2385-2. [DOI] [PubMed] [Google Scholar]
- Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Castelli M, Viveros MP. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB1 cannabinoid receptors. J Psychopharmacol. 2011;25:1676–1690. doi: 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Kelly H, McCormick CM. Low doses of amphetamine lead to immediate and lasting locomotor sensitization in adolescent, not adult, male rats. Pharmacol Biochem Behav. 2011;97:640–646. doi: 10.1016/j.pbb.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, McCormick CM. Female and male rats in late adolescence differ from adults in amphetamine-induced locomotor activity, but not in conditioned place preference for amphetamine. Behav Pharmacol. 2007;18:641–650. doi: 10.1097/FBP.0b013e3282effbf5. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Morrissey MD, McCormick CM. Individual differences in activity predict locomotor activity and conditioned place preference to amphetamine in both adolescent and adult rats. Pharmacol Biochem Behav. 2010;95:63–71. doi: 10.1016/j.pbb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Waters P, McCormick CM. Changes in hyporesponsiveness to acute amphetamine and age differences in tyrosine hydroxylase immunoreactivity in the brain over adolescence in male and female rats. Dev Psychobiol. 2009;51:417–428. doi: 10.1002/dev.20381. [DOI] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011;25(Suppl 1):S120–8. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald CG, Dailey VK, Bergstrom HC, Wheeler TL, Eppolito AK, Smith LN, Smith RF. Periadolescent nicotine administration produces enduring changes in dendritic morphology of medium spiny neurons from nucleus accumbens. Neurosci Lett. 2005;385:163–167. doi: 10.1016/j.neulet.2005.05.041. [DOI] [PubMed] [Google Scholar]
- McDonald CG, Eppolito AK, Brielmaier JM, Smith LN, Bergstrom HC, Lawhead MR, Smith RF. Evidence for elevated nicotine-induced structural plasticity in nucleus accumbens of adolescent rats. Brain Res. 2007;1151:211–218. doi: 10.1016/j.brainres.2007.03.019. [DOI] [PubMed] [Google Scholar]
- McPherson CS, Lawrence AJ. Exposure to amphetamine in rats during periadolescence establishes behavioural and extrastriatal neural sensitization in adulthood. Int J Neuropsychopharmacol. 2006;9:377–392. doi: 10.1017/S1461145705005845. [DOI] [PubMed] [Google Scholar]
- Mihalick SM, Crandall JE, Langlois JC, Krienke JD, Dube WV. Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicol Teratol. 2001;23:453–462. doi: 10.1016/s0892-0362(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Miller MW, Potempa G. Numbers of neurons and glia in mature rat somatosensory cortex: effects of prenatal exposure to ethanol. J Comp Neurol. 1990;293:92–102. doi: 10.1002/cne.902930108. [DOI] [PubMed] [Google Scholar]
- Mogi K, Shimokawa Y, Nagasawa M, Kikusui T. Effects of sex and rearing environment on imipramine response in mice. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2821-y. [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA, Schluter OM, Dong Y. Exposure to Cocaine Dynamically Regulates the Intrinsic Membrane Excitability of Nucleus Accumbens Neurons. Journal of Neuroscience. 2010;30:3689–3699. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL. Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. Proc Natl Acad Sci U S A. 2011;108:5466–5471. doi: 10.1073/pnas.1017732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TW, Bernstein IL. Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proc Natl Acad Sci U S A. 2009;106:17600–17604. doi: 10.1073/pnas.0906629106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Tejeda HA, Torres OV, O’Dell LE. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64:136–145. doi: 10.1002/syn.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Parsons LH, Torres OV, O’Dell LE. Adolescent rats are resistant to adaptations in excitatory and inhibitory mechanisms that modulate mesolimbic dopamine during nicotine withdrawal. J Neurochem. 2012;123:578–588. doi: 10.1111/j.1471-4159.2012.07926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu M, Ehrlich ME, Unterwald EM. Age-specific behavioral responses to psychostimulants in mice. Pharmacol Biochem Behav. 2005;82:280–288. doi: 10.1016/j.pbb.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Obernier JA, Bouldin TW, Crews FT. Binge ethanol exposure in adult rats causes necrotic cell death. Alcohol Clin Exp Res. 2002;26:547–557. [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol. 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology. 2009;56:263–278. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Kuwagata M, Hori Y, Shioda S. Valproate-induced developmental neurotoxicity is affected by maternal conditions including shipping stress and environmental change during early pregnancy. Toxicol Lett. 2007;174:18–24. doi: 10.1016/j.toxlet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Oliveira-da-Silva A, Manhaes AC, Cristina-Rodrigues F, Filgueiras CC, Abreu-Villaca Y. Hippocampal increased cell death and decreased cell density elicited by nicotine and/or ethanol during adolescence are reversed during drug withdrawal. Neuroscience. 2010;167:163–173. doi: 10.1016/j.neuroscience.2010.01.060. [DOI] [PubMed] [Google Scholar]
- O’Tousa DS, Matson LM, Grahame NJ. Effects of Intoxicating Free-Choice Alcohol Consumption During Adolescence on Drinking and Impulsivity During Adulthood in Selectively Bred High-Alcohol Preferring Mice. Alcoholism: Clinical and Experimental Research. 2013;37:141–149. doi: 10.1111/j.1530-0277.2012.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Paul K, Cox CL. Age-dependent Actions of Dopamine on Inhibitory Synaptic Transmission in Superficial Layers of Mouse Prefrontal. Cortex J Neurophysiol. 2012 doi: 10.1152/jn.00756.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MF, Gabach LA, Almiron RS, Carlini VP, De Barioglio SR, Ramirez OA. Different chronic cocaine administration protocols induce changes on dentate gyrus plasticity and hippocampal dependent behavior. Synapse. 2010;64:742–753. doi: 10.1002/syn.20788. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int J Dev Neurosci. 2009;27:805–815. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Milner R, Ehlers CL. N-methyl-D-aspartate receptor subunit expression in adult and adolescent brain following chronic ethanol exposure. Neuroscience. 2010;170:645–654. doi: 10.1016/j.neuroscience.2010.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Prager EM, Bergstrom HC, Grunberg NE, Johnson LR. The importance of reporting housing and husbandry in rat research. Front Behav Neurosci. 2011;5:38. doi: 10.3389/fnbeh.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Gillespie RA, Pak TR. 17beta-Estradiol is required for the sexually dimorphic effects of repeated binge-pattern alcohol exposure on the HPA axis during adolescence. PLoS One. 2012;7:e32263. doi: 10.1371/journal.pone.0032263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Mott NN, Paul CR, Gillespie RA, Pak TR. Binge-pattern alcohol exposure during puberty induces long-term changes in HPA axis reactivity. PLoS One. 2011;6:e18350. doi: 10.1371/journal.pone.0018350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybycien-Szymanska MM, Rao YS, Pak TR. Binge-pattern alcohol exposure during puberty induces sexually dimorphic changes in genes regulating the HPA axis. Am J Physiol Endocrinol Metab. 2010;298:E320–8. doi: 10.1152/ajpendo.00615.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–111. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Richetto J, Feldon J, Riva MA, Meyer U. Comparison of the long-term consequences of withdrawal from repeated amphetamine exposure in adolescence and adulthood on information processing and locomotor sensitization in mice. European Neuropsychopharmacology. 2013;23:160–170. doi: 10.1016/j.euroneuro.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Roberto M, Nelson TE, Ur CL, Gruol DL. Long-term potentiation in the rat hippocampus is reversibly depressed by chronic intermittent ethanol exposure. J Neurophysiol. 2002;87:2385–2397. doi: 10.1152/jn.2002.87.5.2385. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Moeller FG, Swann AC, Clark L. Recent research on impulsivity in individuals with drug use and mental health disorders: implications for alcoholism. Alcohol Clin Exp Res. 2010;34:1319–1333. doi: 10.1111/j.1530-0277.2010.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52:244–53. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of Abuse and Stress Trigger a Common Synaptic Adaptation in Dopamine. Neurons Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sabeti J. Ethanol exposure in early adolescence inhibits intrinsic neuronal plasticity via sigma-1 receptor activation in hippocampal CA1 neurons. Alcohol Clin Exp Res. 2011;35:885–904. doi: 10.1111/j.1530-0277.2010.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Gruol DL. Emergence of NMDAR-independent long-term potentiation at hippocampal CA1 synapses following early adolescent exposure to chronic intermittent ethanol: role for sigma-receptors. Hippocampus. 2008;18:148–168. doi: 10.1002/hipo.20379. [DOI] [PubMed] [Google Scholar]
- Sahr AE, Thielen RJ, Lumeng L, Li TK, McBride WJ. Long-lasting alterations of the mesolimbic dopamine system after periadolescent ethanol drinking by alcohol-preferring rats. Alcohol Clin Exp Res. 2004;28:702–711. doi: 10.1097/01.alc.0000125344.79677.1c. [DOI] [PubMed] [Google Scholar]
- Salimov RM, McBride WJ, McKinzie DL, Lumeng L, Li TK. Effects of ethanol consumption by adolescent alcohol-preferring P rats on subsequent behavioral performance in the cross-maze and slip funnel tests. Alcohol. 1996;13:297–300. doi: 10.1016/0741-8329(95)02060-8. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Rabidou D. Residual performance impairments in adult rats trained on an object discrimination task subsequent to cocaine administration during adolescence. Addiction Biology. 2011;16:30–42. doi: 10.1111/j.1369-1600.2009.00200.x. [DOI] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Schochet TL, Kelley AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacology (Berl) 2004;175:265–273. doi: 10.1007/s00213-004-1831-9. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Pratt AR, Winder DG. Effects of periadolescent versus adult cocaine exposure on cocaine conditioned place preference and motor sensitization in mice. Psychopharmacology (Berl) 2004;173:41–48. doi: 10.1007/s00213-003-1696-3. [DOI] [PubMed] [Google Scholar]
- Semenova S. Attention, impulsivity, and cognitive flexibility in adult male rats exposed to ethanol binge during adolescence as measured in the five-choice serial reaction time task: the effects of task and ethanol challenges. Psychopharmacology (Berl) 2012;219:433–442. doi: 10.1007/s00213-011-2458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacology (Berl) 2008;196:71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- Shearman E, Fallon S, Sershen H, Lajtha A. Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Res Bull. 2008;76:626–639. doi: 10.1016/j.brainresbull.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behav Brain Res. 2011;225:104–109. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Koss WA, Foreman ES, Gulley JM. The effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on ethanol drinking in adult male and female rats. Behav Brain Res. 2011;216:569–575. doi: 10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Stanis JJ, Gulley JM. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behav Brain Res. 2013;242:84–94. doi: 10.1016/j.bbr.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Le AD. Adolescent male Wistar rats are more responsive than adult rats to the conditioned rewarding effects of intravenously administered nicotine in the place conditioning procedure. Behav Brain Res. 2010;206:240–244. doi: 10.1016/j.bbr.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl ) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Sillivan SE, Black YD, Naydenov AV, Vassoler FR, Hanlin RP, Konradi C. Binge cocaine administration in adolescent rats affects amygdalar gene expression patterns and alters anxiety-related behavior in adulthood. Biol Psychiatry. 2011;70:583–92. doi: 10.1016/j.biopsych.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvagni A, Barros VG, Mura C, Antonelli MC, Carboni E. Prenatal restraint stress differentially modifies basal and stimulated dopamine and noradrenaline release in the nucleus accumbens shell: an ’in vivo’ microdialysis study in adolescent and young adult rats. Eur J Neurosci. 2008;28:744–758. doi: 10.1111/j.1460-9568.2008.06364.x. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, O’Buckley T, Morrow AL, Matthews DB. Chronic intermittent ethanol exposure during adolescence reduces the effect of ethanol challenge on hippocampal allopregnanolone levels and Morris water maze task performance. Alcohol. 2006;39:151–158. doi: 10.1016/j.alcohol.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Sinclair JG, Lo GF. Ethanol blocks tetanic and calcium-induced long-term potentiation in the hippocampal slice. Gen Pharmacol. 1986;17:231–233. doi: 10.1016/0306-3623(86)90144-8. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Repeated ethanol treatment in adolescent rats alters cortical NMDA receptor. Alcohol. 2006;39:51–58. doi: 10.1016/j.alcohol.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2002;26:246–254. [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Ann N Y Acad Sci. 2004;1021:448–452. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol. 2002;24:369–384. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm Behav. 2010;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Differential Sensitivity of NMDA Receptor-Mediated Synaptic Potentials to Ethanol in Immature Versus Mature Hippocampus. Alcoholism: Clinical and Experimental Research. 1995;19:320–323. doi: 10.1111/j.1530-0277.1995.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder NA, Louise Risher M, Abdelwahab SH, D’Abo A, Rezvani AH, Levin ED, Wilson WA, Swartzwelder HS, Acheson SK. Effects of ethanol, Delta(9)-tetrahydrocannabinol, or their combination on object recognition memory and object preference in adolescent and adult male rats. Neurosci Lett. 2012 doi: 10.1016/j.neulet.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Schwartzkroin PA. Ineffectiveness of organic calcium channel blockers in antagonizing long-term potentiation. Brain Res. 1986;379:275–285. doi: 10.1016/0006-8993(86)90781-x. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Torrella TA, Badanich KA, Philpot RM, Kirstein CL, Wecker L. Developmental differences in nicotine place conditioning. Ann N Y Acad Sci. 2004;1021:399–403. doi: 10.1196/annals.1308.052. [DOI] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O’Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 2009;206:303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90:658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Res. 2001;892:269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Postnatal volumetric development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241:268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- Van Skike CE, Botta P, Chin VS, Tokunaga S, McDaniel JM, Venard J, Diaz-Granados JL, Valenzuela CF, Matthews DB. Behavioral effects of ethanol in cerebellum are age dependent: potential system and molecular mechanisms. Alcohol Clin Exp Res. 2010;34:2070–2080. doi: 10.1111/j.1530-0277.2010.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A, Recknor EC, Perez-Garcia M. Executive dysfunction in substance dependent individuals during drug use and abstinence: an examination of the behavioral, cognitive and emotional correlates of addiction. J Int Neuropsychol Soc. 2006;12:405–415. doi: 10.1017/s1355617706060486. [DOI] [PubMed] [Google Scholar]
- Vidal-Infer A, Arenas MC, Daza-Losada M, Aguilar MA, Minarro J, Rodriguez-Arias M. High novelty-seeking predicts greater sensitivity to the conditioned rewarding effects of cocaine. Pharmacol Biochem Behav. 2012;102:124–132. doi: 10.1016/j.pbb.2012.03.031. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, Rowley SD, Hanson GR, Fleckenstein AE. Age-dependent differences in dopamine transporter and vesicular monoamine transporter-2 function and their implications for methamphetamine neurotoxicity. Synapse. 2008;63:147–151. doi: 10.1002/syn.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behav Neurosci. 2009;123:926–935. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Morris SE, Arrant AE, Nagel JM, Parylak S, Zhou G, Caster JM, Kuhn CM. Dopamine uptake inhibitors but not dopamine releasers induce greater increases in motor behavior and extracellular dopamine in adolescent rats than in adult male rats. J Pharmacol Exp Ther. 2010;335:124–132. doi: 10.1124/jpet.110.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Kuhn CM. Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicol Teratol. 2008;30:412–418. doi: 10.1016/j.ntt.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]
- Wiley JL, Evans RL. To breed or not to breed? Empirical evaluation of drug effects in adolescent rats. Int J Dev Neurosci. 2009;27:9–20. doi: 10.1016/j.ijdevneu.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Dubal DB. Estradiol protects against ischemic brain injury in middle-aged rats. Biol Reprod. 2000;63:982–985. doi: 10.1095/biolreprod63.4.982. [DOI] [PubMed] [Google Scholar]
- Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Yan H, Li Q, Fleming R, Madison RD, Wilson WA, Swartzwelder HS. Developmental sensitivity of hippocampal interneurons to ethanol: involvement of the hyperpolarization-activated current, Ih. J Neurophysiol. 2009;101:67–83. doi: 10.1152/jn.90557.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Li Q, Madison R, Wilson WA, Swartzwelder HS. Differential sensitivity of hippocampal interneurons to ethanol in adolescent and adult rats. J Pharmacol Exp Ther. 2010;335:51–60. doi: 10.1124/jpet.110.168450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes-Rapozo V, de Moura EG, da Silva Lima N, Barradas PC, Manhaes AC, de Oliveira E, Lisboa PC. Early weaning is associated with higher neuropeptide Y (NPY) and lower cocaine- and amphetamine-regulated transcript (CART) expressions in the paraventricular nucleus (PVN) in adulthood. Br J Nutr. 2012:1–10. doi: 10.1017/S0007114512000487. [DOI] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]