Abstract
Ficin (EC 3.4.22.3), a cysteine endoproteolytic protease in fig trees’ latex, has multiple isoforms. Until now, no data on autolysis of individual ficins (ficin isoforms) are available. Following purification, ficins’ autolysis was determined by HPLC chromatogram changes and ultrafiltrations at different temperatures and storage times. These results showed that the number of HPLC peaks in latex proteins purification of Ficus carica cv. Sabz varied from previous fig varieties or cultivars. Proteolytic activity of ficins was inhibited by specific cysteine protease inhibitors, confirming the participation of the cysteine residue in the active site. The zeta potential of the first two eluted peaks (I and II) was negative, while that of other peaks were positive. All ficins were susceptible to autolysis when stored at high temperatures. In contrast, only the last two ficins (B, C) were prone to autolysis at cold temperature after long storage period. The rate of degradation of the ficins was significantly increased with the increased storage time. The ficin (A) related to peak (III) had the highest and the lowest surface hydrophobic patches and ratio of autolytic to proteolytic activity, respectively.
Keywords: Ficin, Autolysis, Hydrophobic patch, Cysteine protease, Fig latex
1. Introduction
Plant proteases play pivotal roles against unfavorable conditions including water and environmental stress (García-Lorenzo, 2007). They are also involved in various physiological processes, which include protein degradation, digestion, cell maintenance, signaling, differentiation, growth, development, apoptosis, ripening, regulatory mechanisms, wound healing, germination, senescence and necrosis (Antão and Malcata, 2005; Azarkan et al., 2004; Caffini et al., 1988; Cohen, 1997; Kembhavi et al., 1993; Mantell et al., 1985; Smith et al., 1955; Thornberry et al., 1997). In addition, these proteases have commercial usages in different industries including pharmaceutical and food industry for bioactive peptides production and meat tenderization (Báez et al., 2007; Sullivan and Calkins, 2010; Salami et al., 2011).
Plant latex is a rich source for protease extraction. Some lattices contain many other proteins, for example lysozyme (Howard and Glazer, 1967; Smith et al., 1955), class-II chitinases (Azarkan et al., 1997; Huet et al., 2006) and a thaumatin-like protein (Looze et al., 2009). The name ficin is used to describe the endoproteolytic activity in latex of the genus Ficus (Jones and Glazer, 1970), and is recognized as a cysteine protease in fig trees (Liener, 1961). Other latex proteases of ornamental and industrial plant in this genus or other plants in family Moraceae are not exclusively cysteine proteases (Devaraj et al., 2008a; Lynn and Clevette-Radford, 1986; Singh et al., 2008; Yadav et al., 2006). Several ficins (ficin isoforms) from Ficus galabrata are purified as early as 1941 (Englund et al., 1968; Kortt et al., 1974; Molitor et al., 1941; Williams and Whitaker, 1969), and since then multiple ficins from Ficus carica latex are isolated (Devaraj et al., 2008b; Sgarbieri et al., 1964). The ficins may vary in their amino acid sequences, which can lead to conformational differences (Williams and Whitaker, 1969). Until now, most of the work has been carried out on the enzymes prepared from the latex of fig species, and very few studies have been carried out with the ficin isolated from the latex of specific fig cultivars (Kramer and Whitaker, 1964, 1969; Sgarbieri et al., 1964; Sugiura and Sasaki, 1974). However, previous work showed that the number and relative amounts of the components of F. carica differed among the studied cultivars or varieties (Sgarbieri et al., 1964).
One obstacle regarding ficin studies concerns with autolysis (Englund et al., 1968). In autolysis, proteolytic enzymes use neighboring native proteolytic proteins as a substrate for hydrolysis. Ficins have not been well characterized, perhaps due to their autolysis activity (Englund et al., 1968). Re-chromatography studies have shown band spreading of the purified enzymes. Thus, some ficins components may be artifacts that are produced during purification procedures. The autolysis of ficin occurs during isolation and storage (Kramer and Whitaker, 1964, 1969). Although an artifact could be produced by autolysis involving the hydrolysis of one or more peptide bonds, one study indicates that autolysis is not a serious problem during ficins extraction (Kortt et al., 1974). Disulfide bonds play a key role in the protease stability and resistant against autolysis (Bian et al., 2006). The use of reversible inhibitors of thiol group can also be another way to prevent autolysis, such as S-methyl methanethiosulfonate (MMTS) or 2,2′-dithiodipyridine (2-PDS) (Azarkan et al., 1996a, 1996b, 2011; Musu et al., 1994, 1996; Paul et al., 1994).
The number of ficins and their autolysis behavior from fig Sabz cultivar latex has not been previously studied. Here ficins were purified from fig (F. carica cv. Sabz) latex, which is one of the main fig cultivar in the world because of its dried fruit quality and rain-fed condition tolerance. The purified ficins were characterized in terms of their activity and their autolysis behavior. For further understanding of the regulation of ficin autolysis during storage, the effects of temperature and storage time on the autolysis, and subsequent changes of four selected peaks (major peaks) related to “un-retained fraction” (UF) and three ficins were investigated.
2. Results and discussion
In this study it was determined that F. carica cv. Sabz latex contains approximately 20% (v/v) white gum. The gum was removed from the aqueous solution by centrifugation. The clear, straw-colored aqueous solution contained 87.2% water and 12.8% dry materials. A previous work has shown that aqueous solution of F. carica contains between 10% and 17.5% proteins, and approximately 90% of the proteins have proteolytic activity (Sgarbieri et al., 1964). Therefore fig latex contains approximately 20% gum, 70% water, 9% ficins and 1% other dry materials (Table 1).
Table 1.
Approximate percentage of fig latex components.
| Fresh latex | |||||
|---|---|---|---|---|---|
| Calculation of percentage of fig latex components | 20% gum from latex | 80% aqueous solution from latex
|
|||
| 87.2% water from aqueous solution (69.76% water from latex) | 12.8% dry materials from aqueous solution (10.24% dry materials from latex)
|
||||
| 12.5% protein from aqueous solutiona (10% total proteins from latex)
|
0.3% other dry materials from aqueous solution (0.24% other dry materials from latex) | ||||
| 90%ficins from total proteinsa (9% ficins from latex) | 10% other proteins from total proteins (1% other proteins from latex) | ||||
| 1.24% other dry materials from latex | |||||
| Approximate percentage of fig latex components | 20% gum | 70% water | 9% ficins | 1% other dry materials | |
Data were obtained from previous work on separation of the proteolytic enzymes of Ficus carica (Sgarbieri et al., 1964).
2.1. Purification of the F. carica cv. Sabz latex ficins
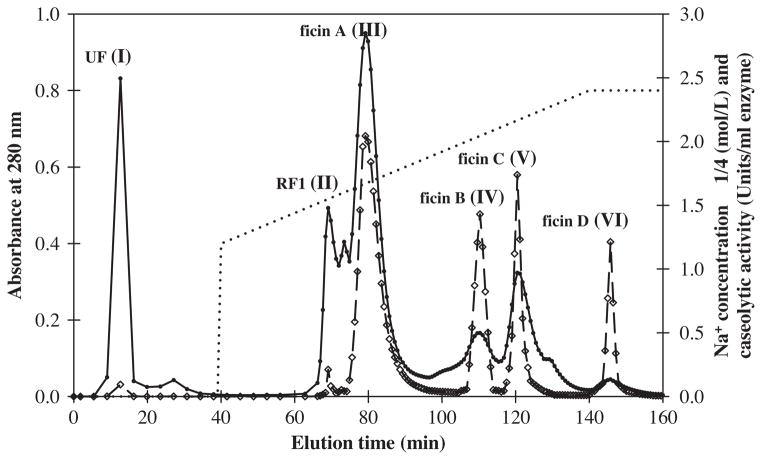
Ficins from Sabz cultivar latex were purified according to the procedure outlined below. The gum was removed from the aqueous solution by centrifugation, and the aqueous solution was then dialyzed and concentrated. The aqueous solution was applied to SP-Sepharose column, and chromatograms of the aqueous solution are illustrated in Fig. 1. These components were designated as unretained fraction (UF), retained fraction one (RF1), ficins (A, B, C and D) related to peak I, II, III, IV, V and VI in order of their elution from the column. All the fractions were assayed for proteolytic activity and subjected to SDS–PAGE in order to check their purity. Some of the peaks that were obtained upon buffer wash from the column, before salt gradient elution, contained proteins with weak proteolytic activity. However, peaks related to the salt gradient elution exhibited higher protein content with higher proteolytic activity. Among them peak III had the highest caseolytic activity and magnitude (Fig. 1). This study, as well as previous studies, indicated that fig latex contains more than one ficin (Englund et al., 1968; Sgarbieri et al., 1964; Williams and Whitaker, 1969). Sabz latex contained four ficins; while other studied cultivars or varieties in other horticultural groups had different number of ficins (Table 2).
Fig. 1.
Chromatographic separation of ficins from Ficus carica cv. Sabz latex on HPLC using a SP-Sepharose high-performance column. The column was equilibrated with 0.01 M sodium phosphate buffer at pH 7.0. Then it was eluted using gradient of 0.3–0.6 M NaCl (·········) in the same buffer, and the eluted fractions were analyzed by absorbance measurements at 280 nm (—●—) and caseolytic activity (units/ml enzyme) (—◇—).
Table 2.
Approximate latex ficins number of several cultivars or varieties of Ficus carica.
| Horticultural group | Variety or cultivar | Ficins number |
|---|---|---|
| Smyrna | Calimyrnaa | 4 |
| Smyrna | Horaishib | 4 |
| Smyrna | Sabzc | 4 |
| San Pedro | Kinga | 7 |
| Common | Adriatica | 9 |
| Common | Bealla | 6 |
| Common | Black Missiona | 6 |
| Common | Blanquettea | 5 |
| Common | California Brown Turkeya | 8 |
| Common | Kadotaa,d | 10 |
| Caprifig | Stanforda | 8 |
| Common × caprifig | Conadriaa | 6 |
Data were obtained from previous work on separation of the proteolytic enzymes of Ficus carica (Sgarbieri et al., 1964).
Data were obtained from previous work on purification of proteinases from Ficus carica.var. Horaishi (Sugiura and Sasaki, 1974).
Data from this work.
Data were obtained from previous work on nature of the conversion of Ficus carica var. Kadota ficin (Kramer and Whitaker, 1969).
2.2. Effect of cysteine protease inhibitors on ficins’ activity
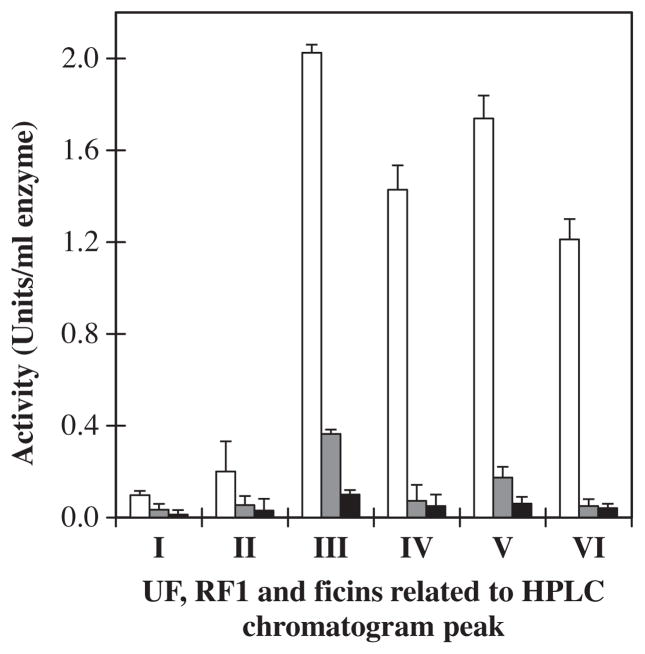
Relative activity of ficins in the presence of two inhibitors was carried out in order to establish the nature of the ficins and their characteristics. For this reason, two cysteine protease inhibitors iodoacetamide (IAA) and potassium tetrathionate (PTT) were chosen. Inhibition of enzyme activity by IAA and PTT was greater than 90% (Fig. 2), indicating that ficins are cysteine proteases. These results also confirmed the participation of the cysteine residue in the active site.
Fig. 2.
Inhibitory activity measurements of the UF, RF1 and ficins related to HPLC chromatogram peaks (I, II, III, IV, V and VI). Activity in presence of bovine casein substrate without inhibitor (□), activity in the presence of potassium tetrathionate (
 ) and iodoacetamide (■) inhibitor.
) and iodoacetamide (■) inhibitor.
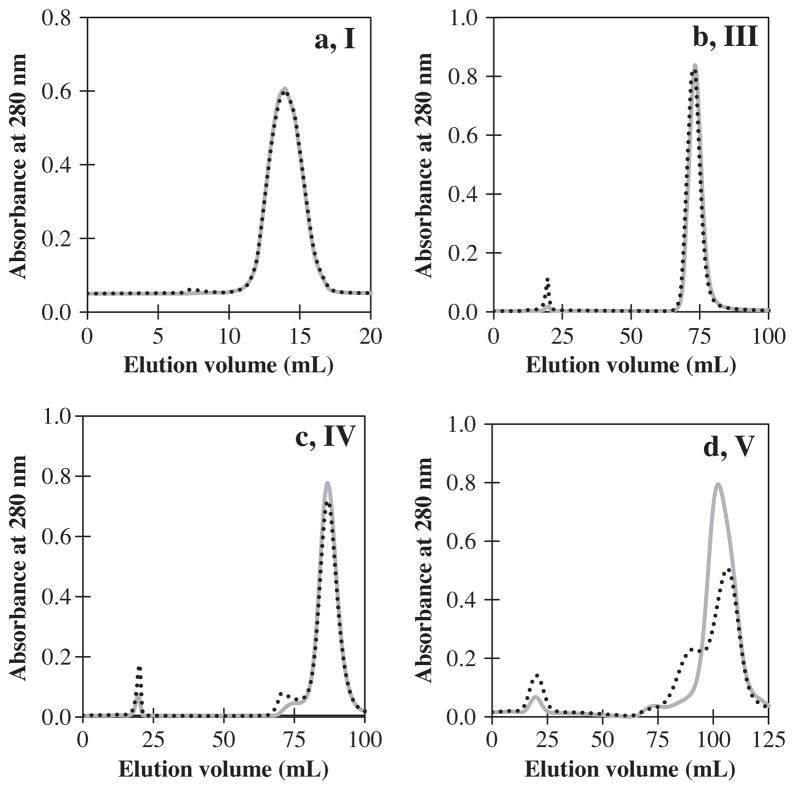
2.3. Homogeneity of the ficins
The homogeneity of peaks was evaluated by gel filtration on HPLC using a Superdex 75 column, SDS–PAGE, and chromatography on ion-exchange systems using SP-Sepharose resins. Ficin (A) related to peak III had the highest homogeneity by gel filtration and SDS–PAGE. On re-chromatography of the peaks, peak III also appeared to behave as a single component. The results of the purity criteria are shown in Fig. 3. The fractions of other peaks were heterogeneous, which suggested that these fractions may have been partially autolyzed during the purification process.
Fig. 3.
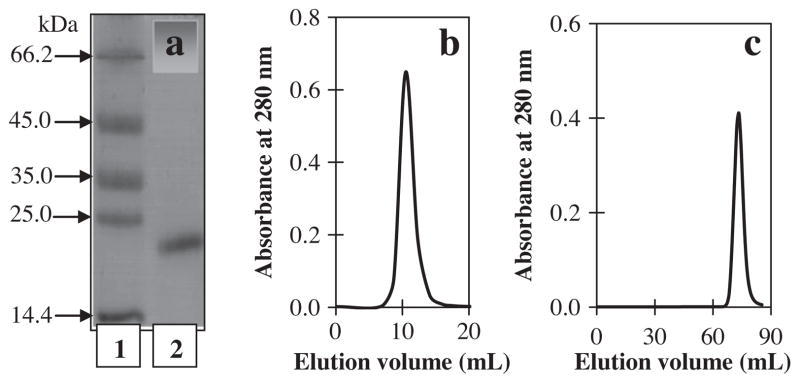
Assessment of the homogeneity of the purified ficin related to HPLC chromatogram peak (III) by (a) SDS–PAGE pattern using 15% gel. Lane 1, molecular-mass markers; lane 2, purified ficin; (b) gel filtration using Superdex 75 column; and (c) re-chromatography using SP-Sepharose column.
2.4. Zeta (ξ) potential study
The Zeta (ξ) potential is the electrostatic potential at the marginal planes of particles (protein). It is related to both protein surface charge and its local environment. Negatively charged ions or molecules decrease the surface Zeta potential, while positively charged ions increase the surface Zeta potential (Zhang et al., 2008). Fig. 4 shows the experimental results of Zeta potential measurements at pH 7.0 for the UF, RF1 and ficins related to the six peaks obtained. Although this was a cation exchange chromatography, the first two eluted peaks (I and II) had negative charge. The rest of peaks (ficins) had positive charge, and the charge intensity increased with increased elution time.
Fig. 4.
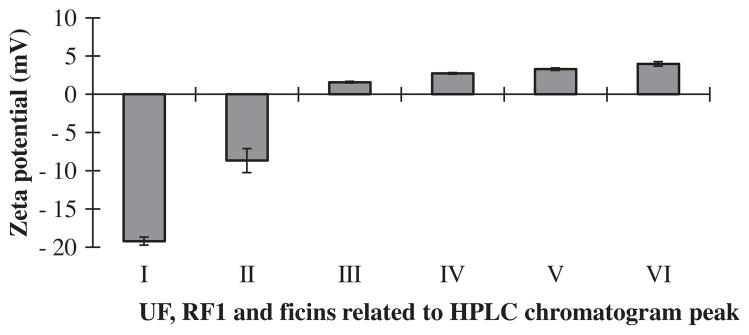
These bar graphs show the results of Zeta potential measurements for UF, RF1 and ficins related to HPLC chromatogram peaks in order of their elution time from cation-exchange column. UF related to peak (I) is before and the rest are after salt gradient elution.
2.5. Autolysis study
Most proteolytic enzymes undergo autolysis (autodigestion), which dependents on protein concentration, temperature, storage time, inhibitor and other experimental conditions. The autolysis behavior of the UF and three ficins related to four HPLC chromatogram peaks (I, III, IV, and V) were monitored under different conditions. They were selected for autolysis study because of their partial purity and magnitude. The RF1 and ficin (D) were not selected because of impurity and lower amounts, respectively. Despite the weak amidase activity of the UF related to peak (I), its autolysis activity was also studied, because of its high concentration in Sabz cultivar. All autolysis experiments were conducted in the absence of substrate and inhibitor. The extent of autolysis was expressed in terms of the percent of protein or peptide concentration and chromatogram profile.
2.5.1. Protein/peptide concentration conversions
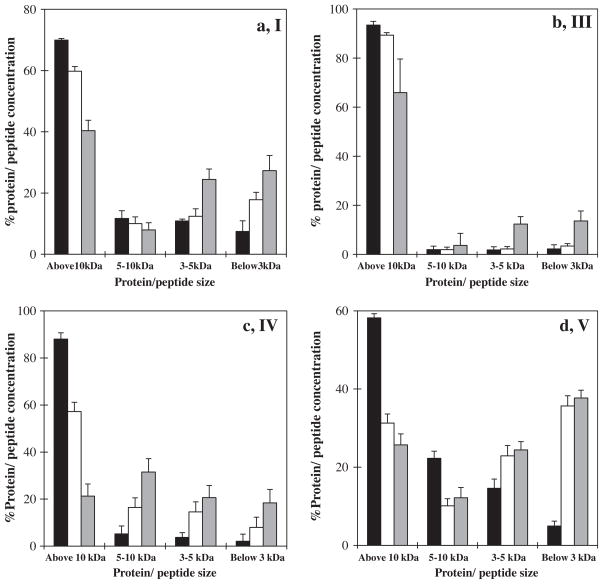
The protease autolysis was studied by using ultrafiltration membranes (3, 5, 10 kDa) for the first time. The peptides were separated according to their size. The permeate and retentate were collected after each stage of autolysis. The protein and peptide concentration of the permeate and retentate were then determined. The changes in the protein and peptide concentration of the retentate were correlated with the autolysis process. As the autolysis rate increased the concentration of protein and peptides in the retentate decreased. Effects of storage time on the autolysis of the UF and three ficins related to peaks (I, III, IV and V) are illustrated in Fig. 5a–d, respectively. Autolysis rate of ficins (B and C) were higher than the UF and ficin (A) in the absence of substrate.
Fig. 5.
Effects of storage time on the autolysis of UF and ficins related to HPLC chromatogram peaks (I), a; (III), b; (IV), c; and (VI), d; with period (■) 1, (□) 5, and (
 ) 10 days storage by using ultrafiltration membranes in absence of substrate and inhibitor.
) 10 days storage by using ultrafiltration membranes in absence of substrate and inhibitor.
2.5.1.1. Effects of storage time
Our result showed that the concentration of peptides produced during the autolysis of the UF and ficins (A, B, C) related to peaks (I, III, IV and V) was increased with increasing storage time from 1 to 10 days. However, the increasing rate was different for each ficin. Fig. 5 shows that all of them were susceptible to autolysis. However, unlike the last two ficins (Fig. 5c and d), the UF and ficin (A), (Fig. 5a and b) had lower autolytic activity.
2.5.2. Chromatogram profile changes
Here we developed a cation-exchange chromatography technique to monitor the autolysis process of ficins. The autolysis process was investigated under two conditions: storage at 37 °C for 24 h and storage at 4 °C for 20 days. When the autolyzed solutions were subjected to cation exchange column, the main peak of the elution pattern, coinciding with the band of native ficin, was forwarded by one or several peptide peaks. These results confirmed the autolysis of ficins. The chromatogram profiles of UF and all three ficins changed after incubation at 37 °C for 24 h, which indicated that they were susceptible to autolysis at this temperature (Fig. 6). Peptide peak of ficins (B and C) had higher absorbance than UF and ficin (A) (Fig. 6).
Fig. 6.
Chromatogram profile before (
 ) and after (·········) incubation at 37 °C for 24 h. When the incubated solution was subjected to cation exchange column, the main peak of the elution pattern, coinciding with the band of native ficin, was forwarded by one peptide peak produced or increased from autolysis of the UF and ficins related to HPLC chromatogram peaks; (I), a; (III), b; (IV), c; and (V), d in absence of substrate and inhibitor.
) and after (·········) incubation at 37 °C for 24 h. When the incubated solution was subjected to cation exchange column, the main peak of the elution pattern, coinciding with the band of native ficin, was forwarded by one peptide peak produced or increased from autolysis of the UF and ficins related to HPLC chromatogram peaks; (I), a; (III), b; (IV), c; and (V), d in absence of substrate and inhibitor.
The chromatogram profiles of ficins related to peaks (IV and V) changed after storage at 4 °C for 20 days (Fig. 7), which indicated that these two ficins were prone to autolysis under these conditions. Thus, many of the ficins which have high activity, and yet there are little or no information on their properties and structures, undergo considerable autolysis. The chromatogram profiles of the UF and ficin (A) did not change, leading to a conclusion that the UF and ficin (A) were resistant to autolysis under these conditions.
Fig. 7.
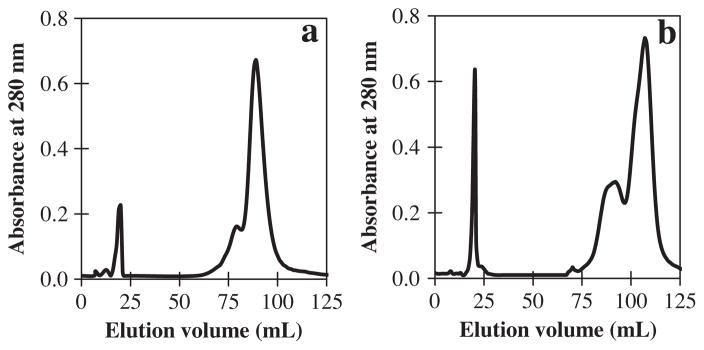
Chromatogram profiles changes after incubation at 4 °C for 20 days. When the autolyzed solution was subjected to cation exchange column, the main peak of the elution pattern, coinciding with the band of native ficin, was forwarded by several peptide peak produced from autolysis of ficin related to HPLC chromatogram peaks; (IV), a; and (V), b in absence of substrate and inhibitor.
2.5.3. Comparison of ficins autolysis
Peptides produced from ficins autolysis showed that the ficin (C) related to peak (V) had the highest autolysis activity (37%), while the UF and ficin (A) had low autolytic activity 0.9% and 1.4%, respectively, at 37 °C for 24 h (Fig. 8). However, the UF related to peak (I) also had the lowest proteolytic activity (Fig. 1). Thus, the ratios of autolytic activity to proteolytic activity were calculated in order to compare their autolysis.
Fig. 8.
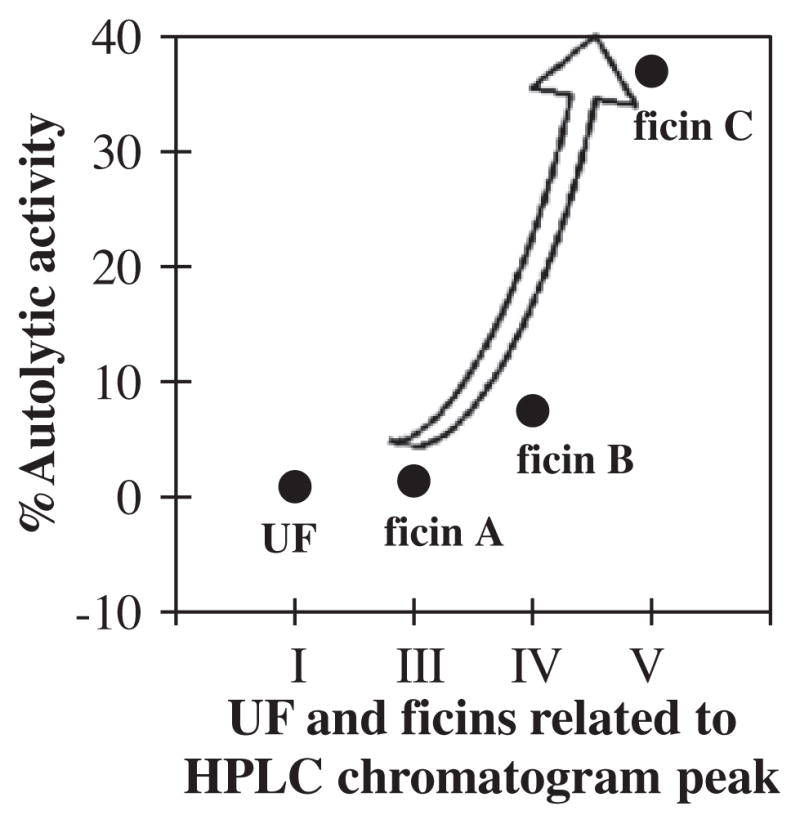
Autolytic activity (% produced peptides from autolysis/UF or ficin used before autolysis) of the UF and three ficins related to HPLC chromatogram peaks (I, III, IV and V) at 37 °C for 24 h in absence of substrate and inhibitor.
2.5.4. Effect of surface hydrophobic patches on ficins’ autolysis
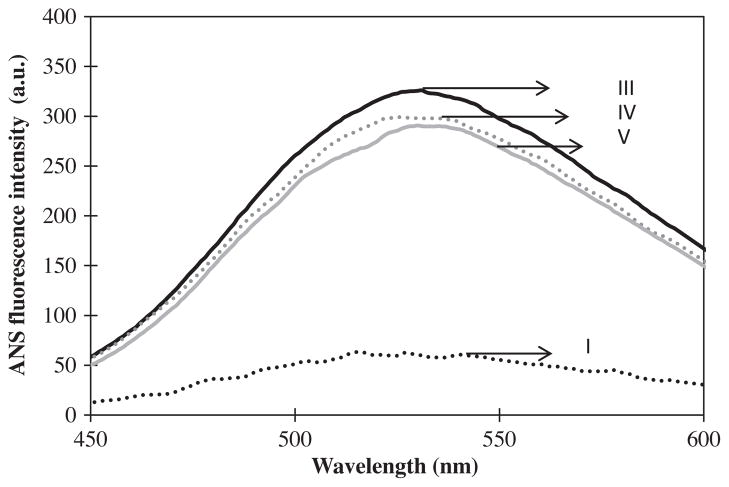
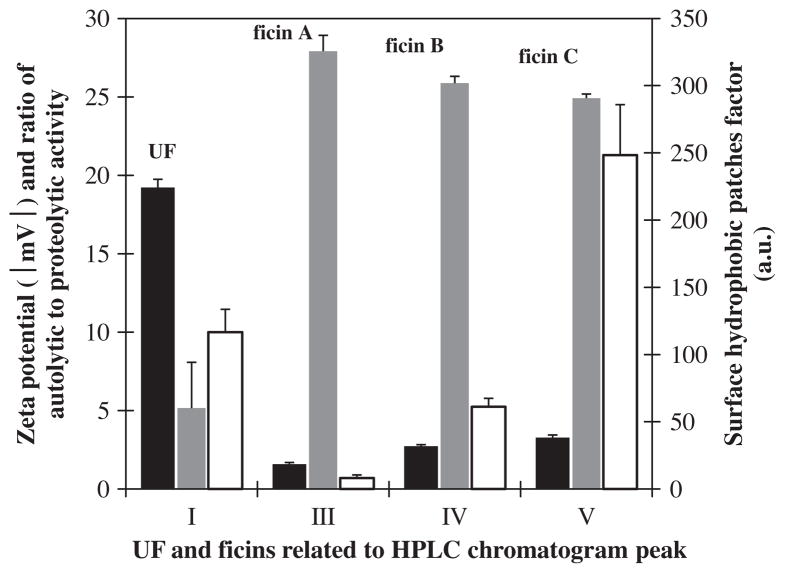
Extrinsic (ANS) fluorescence spectra and surface hydrophobic patches factor for the UF and three ficins related to peaks (I, III, IV, and V) are illustrated in Figs. 9 and 10, respectively. Extrinsic fluorescence shows the amount of surface hydrophobic patches. Ficin (A) had the highest surface hydrophobic patches factor and the lowest ratio of autolytic to proteolytic activity, and absolute value (modulus) of zeta potential among studied ficins (Fig. 10). It is resistant against autolysis because of proportionate surface hydrophobic patches. For this reason, when other ficins surface hydrophobic patches slightly decreased their autolysis activity very increased. We suggested that the slight changes in ficins surface were caused severe difference in their autolysis behavior.
Fig. 9.
Extrinsic (ANS) fluorescence spectra of the UF and three ficins related to HPLC chromatogram peaks; I, (·········); III, (—); IV, (
 ); and V, (
); and V, (
 ).
).
Fig. 10.
Correlation between zeta potential modulus (■), surface hydrophobic patches factor (
 ), and ratio of autolytic activity to proteolytic activity (□) of the UF and three ficin related to HPLC chromatogram peaks (I, III, IV and V).
), and ratio of autolytic activity to proteolytic activity (□) of the UF and three ficin related to HPLC chromatogram peaks (I, III, IV and V).
3. Concluding remarks
The latex ficins number of F. carica cv. Sabz differed from previously studied fig varieties or cultivars in other horticultural groups, and had different autolysis rate. Ficin autolysis directly and inversely correlated with surface charges and hydrophobic patches, respectively. Ficin (A) had the lowest autolysis, and can be easily used in experimental and industrial works without using any autolysis inhibitors.
4. Experimental
4.1. Materials
Iodoacetamide (IAA), potassium tetrathionate (PTT), acrylamide, bisacrylamide, sodium dodecyl sulfate (SDS), 2-mercaptoethanol, cysteine hydrochloride, ammonium persulfate, N,N,N′,N′-tetramethylethylenediamine (TEMED), 8-anilinonaphtalene-1-sulfonic acid (ANS) were from Sigma–Aldrich Chemie Gmbh (Munich, Germany). Trichloroacetic acid (TCA) was from Merck (Germany). All other chemicals used were of analytical grade (Sigma–Aldrich). All solutions were prepared using deionized Water and stored at 4 °C. For casein preparations, bovine milk was warmed to 37 °C and skimmed immediately by centrifugation (5000g, 15 min). Acidity of skim milk was adjusted to pH 4·6 with 1 N HCl. The solution was mixed at 37 °C for 30 min, and caseins were precipitated and separated from whey proteins by centrifugation (5860g, 60 min, 4 °C), washed three times with deionized water, lyophilized, and stored at −20 °C until use.
4.2. Methods
4.2.1. Collection of latex
Fresh latex was collected from fig (Ficus carica cv. Sabz) trees growing in Estahban fig research station by separation of immature green fruits from tree shoots. All the latex samples used here were collected in middle of September. The latex fluid was poured into 15 ml tubes and was immediately stored at −20 °C.
4.2.2. Removal of gum
The frozen latex was thawed at to 4 °C and was centrifuged at 100,000g at 0 °C for 20 min to remove gum and other debris (Sgarbieri et al., 1964). The clarified extract was lyophilized and stored at −20 °C.
4.2.3. Sample preparation
Approximately 20 g of lyophilized latex powder was dissolved in 0.01 M sodium phosphate buffer at pH 7.0. The solution was dialyzed (membrane cut off: 10,000 Da) against 0.01 M sodium phosphate buffer at pH 7.0 at 4 °C for 24 h and was centrifuged (40,000g, 4 °C, 30 min). The insoluble material was discarded and the supernatant was immediately divided into 30 equal volumes and stored at −20 °C for further analysis.
4.2.4. Cation-exchange chromatography on SP-Sepharose fast flow
Each supernatant aliquot was loaded onto a SP-Sepharose fast flow column (51.2 ml bed volume), pre-equilibrated with 0.01 M sodium phosphate buffer at pH 7.0. The column was washed with the same buffer until no protein was detected in the eluent. Then it was eluted using a gradient of 0.3–0.6 M NaCl in the same buffer at a flow rate 1 ml/min. To determine the protein content of each fraction the absorbance at 280 nm was measured using UV–vis spectrophotometer. Fractions of peak I–VI were pooled, dialyzed against deionized water, lyophilized and stored at −20 °C for further analysis.
4.2.5. Enzymatic activity measurements
Ficin activity was assayed at 37 °C using bovine casein (2% w/v) as substrate in a 100 mM potassium phosphate buffer pH 7.6 containing 250 mM EDTA and 250 mM L-cysteine at enzyme concentrations of 0.25 mg/ml for each ficin. The hydrolyzing activity of ficin was based on the method described by Kunitz with some modifications using denatured casein as substrate (Kunitz, 1947). In activity measurements, 0.12 ml enzyme in 1 M potassium phosphate buffer at pH 7.0 was incubated at 37 °C for 20 min prior to the assay. The reaction was stopped by the addition of 1.8 ml of 5% TCA. For the blank, the substrate was added after the enzyme was first inactivated by TCA. The resulting precipitate was removed by centrifugation at 10,000g for 20 min, and the absorbance of TCA soluble peptides in the supernatant was measured at 280 nm.
4.2.6. Protein and peptide concentration
Protein concentration was determined using Bradford method with bovine serum albumin (BSA) as the standard (Bradford, 1976; Marshall and Williams, 1993). The peptide concentration was quantified spectrophotometrically with the OPA (o-phthaldialdehyde) method using peptone as standard (Church et al., 1983).
4.2.7. SDS–PAGE experiment
SDS–PAGE was carried out on slab gels prepared according to Laemmli (Laemmli, 1970). The separating (pH 8.8, 15% acrylamide) and stacking (pH 6.8, 4% acrylamide) gels were run at a constant voltage (100 V). The electrophoresis chambers contained Tris–glycine buffer (pH 8.3) with 0.1% SDS. Before loading onto the gel, the ficins solutions were diluted with a buffer containing 25% Tris–HCl 1 M, pH 6.8, 2% SDS, 25% glycerol and 1% bromophenol blue and heated at 95 °C for 5 min. The gels were stained with 0.25% Coomassie Blue R250 dissolved in methanol (45%), water (45%) and glacial acetic acid (10%) solution. The destaining solution contained methanol, glacial acetic acid and water (1:1:8) (Ahmed, 2005). The molecular mass (kDa) marker used contained: Lysozyme (14400), REase Bsp981 (25000), lactate dehydrogenase (35000), ovalbumin (45000) and bovine serum albumin (66200).
4.2.8. Zeta potential
Zeta potential measurements were performed with a Brookhaven Instruments ZetaPALS at a controlled temperature of 25 °C. Zeta potential was used as an indication of ficin conformational change and its surface charge. Deionized water was used as background electrolyte. The number of runs for each sample was set at 10 and the number of cycles for each run was set at 10.
4.2.9. Autocatalysis treatments
Here the effects of storage time (1, 5, 10 and 20 days), and temperature (4, 20, 37 °C) on autolysis were studied. The UF and three ficins related to the four peaks (I, III, IV, and V) at same concentration (0.05 mg/ml) in 0.01 M sodium phosphate buffer pH 7.0 were incubated under indicated conditions. Previous work showed that the protein is susceptible to autodigestion at concentrations below 0.1 mg/ml (Yadav et al., 2006). The RF1 and ficin (D) related to peaks (II and VI) were not selected for this study because of high impurity and low magnitude, respectively.
4.2.10. Autolysis product measurements
After each treatment, intact ficin was separated from its autolysis products (peptides) using ultrafiltration membranes (Amicon Ultra-15, Millipore, cutoff of 10, 5, and 3 kDa) by centrifugation for 60 min at 290g at 4 °C. Permeates of each stage of filtration were collected. Peptide concentration was quantified by the OPA (o-phthaldialdehyde) method. Ion-exchange chromatography was also performed to fractionate and study the autolysis products.
4.2.11. Autolytic activity
Produced peptides from ficin autolysis were determined with subtracting chromatogram peak area related to autolyzed ficin (A2) at 37°C for 24 h from the chromatogram peak area related to UF or ficin used before autolysis (A1).
The percent of produced peptides was considered as an indicator of autolytic activity of ficins in this study.
4.2.12. Surface hydrophobic patches factor
Surface hydrophobic patches factor was determined with extrinsic (ANS) fluorescence emission measurement. Fluorescence emissions were recorded using a Varian spectrofluorimeter, model Cary Eclipse. For extrinsic (ANS) fluorescence emission measurements, excitation was set at 387 nm and emission spectra were recorded in the range of 400–600 nm using excitation and emission slit widths of 10 and 20 nm, respectively.
Acknowledgments
The support of University of Tehran and Center of Excellence in Biothermodynamics (CEBiotherm) and Iran National Science Foundation (INSF) are gratefully acknowledged.
References
- Ahmed H. Principles and Reactions of Protein Extraction, Purification, and Characterization. CRC Press; New York: 2005. [Google Scholar]
- Antão CM, Malcata FX. Plant serine proteases: biochemical, physiological and molecular features. Plant Physiol Biochem. 2005;43:637–650. doi: 10.1016/j.plaphy.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Azarkan M, Maes D, Bouckaert J, Thi MHD, Wyns L, Looze Y. Thiol pegylation facilitates purification of chymopapain leading to diffraction studies at 1.4 Å resolution. J Chromatogr A. 1996a;749:69–72. [Google Scholar]
- Azarkan M, Wintjens RT, Smolders N, Nijs M, Looze Y. S-pegylthiopapain, a versatile intermediate for the preparation of the fully active form of the cysteine proteinase archetype. J Chromatogr A. 1996b;724:185–192. [Google Scholar]
- Azarkan M, Amrani A, Nijs M, Vandermeers A, Zerhouni S, Smolders N, Looze Y. Carica papaya latex is a rich source of a class II chitinase. Phytochemistry. 1997;46:1319–1325. doi: 10.1016/s0031-9422(97)00469-x. [DOI] [PubMed] [Google Scholar]
- Azarkan M, Wintjens R, Looze Y, Baeyens-Volant D. Detection of three wound-induced proteins in papaya latex. Phytochemistry. 2004;65:525–534. doi: 10.1016/j.phytochem.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Azarkan M, Matagne A, Wattiez R, Bolle L, Vandenameele J, Baeyens-Volant D. Selective and reversible thiol-pegylation, an effective approach for purification and characterization of five fully active ficin (iso)forms from Ficus carica latex. Phytochemistry. 2011;72:1718–1731. doi: 10.1016/j.phytochem.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Báez R, Lopes MT, Salas CE, Hernández M. In vivo antitumoral activity of stem pineapple (Ananas comosus) bromelain. Planta Med. 2007;73:1377–1383. doi: 10.1055/s-2007-990221. [DOI] [PubMed] [Google Scholar]
- Bian Y, Liang X, Fang N, Tang XF, Tang B, Shen P, Peng Z. The roles of surface loop insertions and disulfide bond in the stabilization of thermophilic WF146 protease. FEBS letters. 2006;580:6007–6014. doi: 10.1016/j.febslet.2006.09.068. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caffini N, Lopez L, Natalucci C, Priolo N. Proteases of higher plants. General features, physiological roles and applications. Acta Farm Bonaerense. 1988;7:195–213. [Google Scholar]
- Church FC, Swaisgood HE, Porter DH, Catignani GL. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J Dairy Sci. 1983;66:1219–1227. [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj K, Gowda LR, Prakash V. An unusual thermostable aspartic protease from the latex of Ficus racemosa (L.) Phytochemistry. 2008a;69:647–655. doi: 10.1016/j.phytochem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Devaraj KB, Kumar PR, Prakash V. Purification, characterization, and solvent-induced thermal stabilization of ficin from Ficus carica. J Agric Food Chem. 2008b;56:11417–11423. doi: 10.1021/jf802205a. [DOI] [PubMed] [Google Scholar]
- Englund PT, King TP, Craig LC, Walti A. Ficin I. Its isolation and characterization. Biochemistry. 1968;7:163–175. doi: 10.1021/bi00841a021. [DOI] [PubMed] [Google Scholar]
- García-Lorenzo M. The Role of Proteases in Plant Development. VMC-KBC, Umeå University; Umeå, Sweden: 2007. [Google Scholar]
- Howard JB, Glazer A. Studies of the physicochemical and enzymatic properties of papaya lysozyme. J Biol Chem. 1967;242:5715–5723. [PubMed] [Google Scholar]
- Huet J, Wyckmans J, Wintjens R, Boussard P, Raussens V, Vandenbussche G, Ruysschaert J, Azarkan M, Looze Y. Structural characterization of two papaya chitinases, a family GH19 of glycosyl hydrolases. Cell Mol Life Sci. 2006;63:3042–3054. doi: 10.1007/s00018-006-6320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IK, Glazer A. Comparative studies on four sulfhydryl endopeptidases (“ficins”) of Ficus glabrata latex. J Biol Chem. 1970;245:2765–2772. [PubMed] [Google Scholar]
- Kembhavi A, Buttle D, Knight C, Barrett A. The two cysteine endopeptidases of legume seeds: purification and characterization by use of specific fluorometric assays. Arch Biochem Biophys. 1993;303:208–213. doi: 10.1006/abbi.1993.1274. [DOI] [PubMed] [Google Scholar]
- Kortt AA, Hamilton S, Webb EC, Zerner B. Ficins (EC 3.4. 22.3) Purification and characterization of the enzymic components of the latex of Ficus glabrata. Biochemistry. 1974;13:2023–2028. doi: 10.1021/bi00707a004. [DOI] [PubMed] [Google Scholar]
- Kramer DE, Whitaker JR. Ficus Enzymes. II Properties of the proteolytic enzymes from the latex of Ficus carica variety kadota. J Biol Chem. 1964;239:2178–2183. [PubMed] [Google Scholar]
- Kramer DE, Whitaker JR. Nature of the conversion of Ficus carica variety Kadota ficin component D to component C. Some physicochemical properties of components C and D. Plant Physiol. 1969;44:1566–1573. doi: 10.1104/pp.44.11.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitz M. Crystalline soybean trypsin inhibitor. J Gen Physiol. 1947;30:291–310. doi: 10.1085/jgp.30.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liener IE. A study of the number and reactivity of the sulfhydryl groups of ficin. Biochim Biophys Acta. 1961;53:332–342. doi: 10.1016/0006-3002(61)90445-0. [DOI] [PubMed] [Google Scholar]
- Looze Y, Boussard P, Huet J, Vandenbussche G, Raussens V, Wintjens R. Purification and characterization of a wound-inducible thaumatin-like protein from the latex of Carica papaya. Phytochemistry. 2009;70:970–978. doi: 10.1016/j.phytochem.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Lynn K, Clevette-Radford N. Ficin E, a serine-centred protease from Ficus elastica. Phytochemistry. 1986;25:1559–1561. [Google Scholar]
- Mantell SH, Matthews J, McKee R. Principles of Plant Biotechnology: An Introduction to Genetic Engineering in Plants. Blackwell Scientific Publications; Oxford, UK: 1985. [Google Scholar]
- Marshall T, Williams KM. Bradford protein assay and the transition from an insoluble to a soluble dye complex: effects of sodium dodecly sulphate and other additives. J Biochem Biophys Methods. 1993;26:237–240. doi: 10.1016/0165-022x(93)90047-r. [DOI] [PubMed] [Google Scholar]
- Molitor H, Mushett CW, Kuna S. Some toxicological and pharmacological properties of the proteolytic enzyme, ficin. J Pharmacol Exp Ther. 1941;71:20–29. [Google Scholar]
- Musu T, Azarkan M, Brygier J, Paul C, Vincentelli J, Baeyens-Volant D, Guermant C, Nijs M, Looze Y. Reversible modification of thiol-containing polypeptides with poly (ethylene glycol) through formation of mixed disulfide bonds. Appl Biochem Biotechnol. 1996;56:243–263. doi: 10.1007/BF02786956. [DOI] [PubMed] [Google Scholar]
- Musu T, Brygier J, Vincentelli J, Guermant C, Paul C, Baeyens-Volant D, Looze Y. Easy purification of ananain through reversible pegylation. Int J Bio-Chromatogr. 1994;1:17–27. [Google Scholar]
- Paul C, Vincentelli J, Brygier J, Musu T, Baeyens-Volant D, Guermant C, Looze Y. Preparation and characterization of a S-monomethoxypoly-(ethylene glycol) thioderivative of papain. Phytochemistry. 1994;35:1413–1417. [Google Scholar]
- Salami M, Moosavi-Movahedi AA, Moosavi-Movahedi F, Ehsani MR, Yousefi R, Farhadi M, Niasari-Naslaji A, Saboury AA, Chobert JM, Haertlé T. Biological activity of camel milk casein following enzymatic digestion. J Dairy Res. 2011;78:471–478. doi: 10.1017/S0022029911000628. [DOI] [PubMed] [Google Scholar]
- Sgarbieri VC, Gupte SM, Kramer DE, Whitaker JR. Ficus Enzymes. I Separation of the proteolytic enzymes of Ficus carica and Ficus glabrata latices. J Biol Chem. 1964;239:2170–2177. [PubMed] [Google Scholar]
- Singh VK, Patel AK, Moir A, Jagannadham MV. Indicain, a dimeric serine protease from Morus indica cv. K2 Phytochemistry. 2008;69:2110–2119. doi: 10.1016/j.phytochem.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Smith EL, Kimmel JR, Brown DM, Thompson EO. Isolation and properties of a crystalline mercury derivative of a lysozyme from papaya latex. J Biol Chem. 1955;215:67–89. [PubMed] [Google Scholar]
- Sugiura M, Sasaki M. Studies on proteinases from Ficus carica var. Horaishi V Purification and properties of a sugar-containing proteinase (Ficin S) Biochim Biophys Acta Enzymol. 1974;350:38–47. doi: 10.1016/0005-2744(74)90200-9. [DOI] [PubMed] [Google Scholar]
- Sullivan GA, Calkins C. Application of exogenous enzymes to beef muscle of high and low-connective tissue. Meat Sci. 2010;85:730–734. doi: 10.1016/j.meatsci.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP. A combinatorial approach defines specificities of members of the caspase family and granzyme B. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- Williams DC, Whitaker JR. Multiple molecular forms of Ficus glabrata Ficin. Their separation and relative physical, chemical, and enzymatic properties. Plant Physiol. 1969;44:1574–1583. doi: 10.1104/pp.44.11.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav SC, Pande M, Jagannadham M. Highly stable glycosylated serine protease from the medicinal plant Euphorbia milii. Phytochemistry. 2006;67:1414–1426. doi: 10.1016/j.phytochem.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang M, Portney NG, Cui D, Budak G, Ozbay E, Ozkan M, Ozkan CS. Zeta potential: a surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed Microdevices. 2008;10:321–328. doi: 10.1007/s10544-007-9139-2. [DOI] [PubMed] [Google Scholar]