Abstract
Intercellular adhesion molecule-1 (ICAM-1) functions in leukocyte trafficking, activation, and the formation of the immunological synapse. ICAM-1 is a member of the immunoglobulin superfamily of adhesion proteins, which share a similar structure of repeating Ig-like domains. Many genes in this family, including ICAM-1, show alternative splicing leading to the production of different protein isoforms, although little functional information is available regarding the expression patterns, ligand interactions, and functions of these isoforms, especially those arising from the ICAM-1 gene. In this study, we show using different lines of mutant mice (Icam1tm1Jcgr and Icam1tm1Bay) that alterations in the expression of the alternatively spliced ICAM-1 isoforms can significantly influence the disease course during the development of EAE. Icam1tm1Jcgr mutant mice, unlike Icam1tm1Bay mutants, do not express isoforms containing the Mac-1 binding domain and had significantly attenuated of EAE. In contrast, Icam1tm1Bay mice developed severe EAE in both active and adoptive transfer models compared to both Icam1tm1Jcgr and wild type mice. We also observed that T cells from Icam1tm1Bay mice displayed increased proliferation kinetics and produced higher levels of IFN-γ compared to Icam1tm1Jcgr and wild type mice. Thus, our investigations show that the alternatively spliced ICAM-1 isoforms are functional, and play key roles during the progression of CNS inflammation and demyelination in EAE. Furthermore, our findings suggest that these isoforms may also play key roles in controlling the development of inflammatory diseases such as multiple sclerosis, possibly through differential engagement with ICAM-1 ligands such as Mac-1.
Keywords: ICAM-1, adhesion molecules, MS/EAE, alternative splicing
1. Introduction
Intercellular adhesion molecule-1 (ICAM-1, CD54)4 is a type 1 membrane-bound glycoprotein that plays a critical role in leukocyte emigration, lymphocyte activation, and other immune and inflammatory responses (Harlan et al., 1992; Lebedeva et al., 2005; Springer, 1994). ICAM-1 is expressed on a wide variety of cell types, including most leukocyte subsets, endothelial cells, and in the central nervous system, on microglia, astrocytes, and oligodendrocytes (Bo et al., 1996; Lee and Benveniste, 1999; McMurray, 1996; Roebuck and Finnegan, 1999). Initially described as a monomeric protein, ICAM-1 is composed of repeating immunoglobulin-like (Ig) domains and is expressed as a homodimer in a “bent” conformation (Chen et al., 2007; Kirchhausen et al., 1993; Miller et al., 1995). This adhesion molecule can bind to many different ligands, including the β2 integrins LFA-1, Mac-1, and p150/95, as well as fibrinogen, and hyaluronan (Diamond et al., 1991; Languino et al., 1995; McCourt et al., 1994; Springer, 1994). ICAM-1, like other members of the immunoglobulin superfamily of adhesion molecules such as VCAM-1, PECAM-1, and MAdCAM-1, has also been shown to undergo alternative splicing and there are at least seven ICAM-1 isoforms that have now been described (Giorelli et al., 2002; King et al., 1995; Leung et al., 1997; Ochietti et al., 2002; Osborn et al., 1992; Robledo et al., 2003; van Den Engel et al., 2000; Wakatsuki et al., 1995; Werner et al., 2001; Yan et al., 1995). However, these alternatively spliced isoforms have been poorly characterized and little information exists regarding their expression patterns, ligand binding, and functions during inflammatory responses.
Several different lines of gene-targeted mutant mice have been generated that are amenable for functional analyses of these alternatively spliced ICAM-1 isoforms (Dunne et al., 2003; Sligh et al., 1993; Xu et al., 1994). The first two ICAM-1 mutants were developed in the 1990s and used replacement constructs designed to insert the neomycin selection gene into exon 4 (Icam1tm1Jcgr), which encodes for IgG domain 3, or exon 5 (Icam1tm1Bay), which encodes for IgG domain 4 of this adhesion molecule (Sligh et al., 1993; Xu et al., 1994). Although these mutations were originally thought to result in complete loss of ICAM-1 (null), it was subsequently discovered that these mice expressed different subsets of the alternatively spliced isoforms normally found in murine cells, but not the full-length ICAM-1 molecule (King et al., 1995; van Den Engel et al., 2000). ICAM-1 knockout mice completely deficient or null for all isoform expression (Icam1tm1Alb) were subsequently generated by deleting the entire coding sequence (Dunne et al., 2003). Previously, we analyzed these ICAM-1 null mutant mice in the MS model, experimental autoimmune encephalomyelitis (EAE), and observed a markedly attenuated disease phenotype (Bullard et al., 2007). These results significantly differed from earlier EAE studies involving Icam1tm1Bay mice, in which an exacerbated form of the disease was observed compared to wild type mice (Samoilova et al., 1998), even though both studies used similar induction methods and both mutations were bred onto the same inbred strain background.
To further investigate the roles of alternatively spliced ICAM-1 isoforms in regulating CNS inflammation and demyelination, we analyzed and compared the EAE phenotypes in MOG-induced EAE using Icam1tm1Jcgr and Icam1tm1Bay mice in both active and adoptive transfer EAE. We found that Icam1tm1Bay mutant T cells can induce severe EAE following adoptive transfer and that encephalitogenic T cells from these mice promote CNS inflammation and demyelination even in the absence of ICAM-1 expression on other cell types. In contrast, Icam1tm1Jcgr mice developed both active and adoptive transfer EAE with a phenotype intermediate to that of Icam1tm1Bay and Icam1tm1Alb. These findings suggest that the alternatively spliced ICAM-1 isoforms are functional, and that the full-length molecule is not necessary to promote leukocyte infiltration and activation during the development of EAE. Our studies further indicate that expression of individual ICAM-1 isoforms or specific combinations of these molecules can significantly regulate immune and inflammatory responses in the CNS and possibly other tissues.
2. Materials and Methods
2.1. Mice
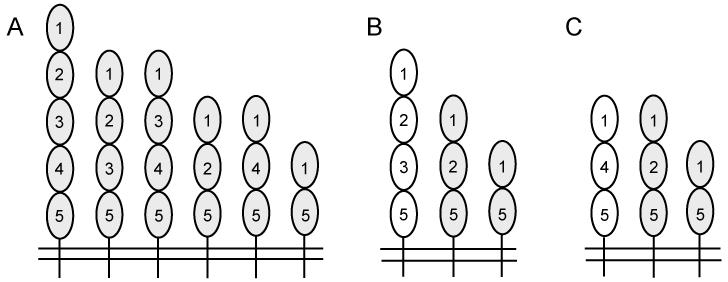
Icam1tm1Jcgr and Icam1tm1Bay C57BL/6J (N10) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and have been previously described (Sligh et al., 1993; Xu et al., 1994). Both mutant lines of mice express at least three of the ICAM-1 isoforms found in wild type mice, and two are common to each mutant strain (Fig. 1). These two mutant lines differ in at least one isoform, although both have the potential to express ICAM-1 isoforms that lack domains 3 and 4 (Figure 1B and C). ICAM-1 null mutant mice (Icam1tm1Alb) were backcrossed at least 8 generations onto C57BL/6 (Dunne et al., 2003). Only mice homozygous for the particular ICAM-1 mutation were used for these studies, and inbred C57BL/6 mice were used as controls for all experiments. All studies were performed with approval from the UAB IACUC.
Fig. 1.
Schematic representation of ICAM-1 isoform structure and expression in wild type and ICAM-1 mutant mice. (A) Wild type, (B) Icam1tm1Bay, and (C) Icam1tm1Jcgr mice. Each oval represents an Ig-like domain. The numbering of each is based on the full-length isoform containing five Ig-like domains.
2.2. Induction of active and adoptive transfer EAE
For active EAE, control, Icam1tm1Jcgr and Icam1tm1Bay mice were immunized with MOG peptide35-55 as previously described (Bullard et al., 2007). MOG peptide was synthesized by standard 9-fluorenyl-methoxycarbonyl chemistry and was >95% pure as determined by reversed phase-HPLC (Biosynthesis, Lewisville, TX). Onset and progression of EAE clinical signs was monitored daily (30 days) using a standard clinical scale ranging from 0 to 6 as follows: 0, asymptomatic; 1, loss of tail tone; 2, flaccid tail; 3, incomplete paralysis of one or two hind limbs; 4, complete hind limb paralysis; 5, moribund; 6, dead. Only mice with a score of at least 2 (flaccid tail) observed for 2 or more consecutive days were judged to have onset of EAE. A cumulative disease index (CDI) was calculated from the sum of the daily clinical scores observed between day 7 and day 30. For transferred EAE, spleens of control, Icam1tm1Jcgr, or Icam1tm1Bay donors were removed two to three weeks following induction of active EAE, and prepared as previously described (Szalai et al., 2002). Adoptive transfer EAE was induced by injecting ~5×106 purified T cells into wild type or mutant recipient mice as described in the Results section. Reciprocal transfers were performed by injecting wild-type encephalitogenic T cells into Icam1tm1Jcgr, Icam1tm1Bay, or wild type mice. Mice were scored daily for 18 days using the identical system as described above.
2.3. Histopathology
Mice with actively induced EAE were sacrificed at 30 days p.i. by CO2 inhalation, and spinal columns were removed, fixed in 10% buffered-formalin and paraffin embedded. Sections (5μm thick) from the cervical, thoracic and lumbar spinal cord were cut and either stained with hematoxylin and eosin for overall lesion evaluation and characterization of inflammatory responses or with Luxol fast blue for evaluation of demyelination. The extent of inflammation and demyelination was scored based on lesion size (0-4) and lesions were evaluated for lymphocyte accumulation, neutrophil infiltration, demyelination, axonal degeneration and gliosis (0-4). Tissues were evaluated without identification as to experimental group. Severity scores were calculated as the mean over all segments of the products of the intensity scores multiplied by the extent scores for each lesion characteristic (inflammation, axonal degeneration, gliosis, and demyelination). The means of the individual lesion characteristic severity scores were summed to give the overall severity score.
2.4. T cell proliferation and cytokine and chemokine production
Antigen-specific T cell proliferation assays were performed as previously described (Szalai et al., 2002). Single cell suspensions from spleens obtained 14 days after EAE induction were cultured in triplicate in 96-well plates at 5 × 105 cells/well with increasing concentrations of MOG35-55 peptide. After 48 h, cultures were pulsed with 3H-thymidine for 18 h and incorporation of thymidine was measured. The in vitro cytokine assays were performed essentially as described for the proliferation assay. Duplicate cultures were either left untreated or stimulated with MOG peptide alone (5 μg/ml). Culture supernatants were collected at 48 hrs for use in cytokine ELISAs. ELISA kits for murine cytokines (IFN-γ, TNF-α, IL-2, IL-4, IL-10, IL-12, IL-17 and TGF-β) were purchased from R&D Systems (Minneapolis, MN). Each assay was performed according to the manufacturer’s instructions. Cytokine production by cultures of wild type, Icam1tm1Jcgr and Icam1tm1Bay cells, is reported as the percentage of wild type cytokine production from four mice per group.
2.5. T regulatory cell isolation and suppression assay
CD4+CD25+ cells were isolated or depleted from spleens and lymph nodes of wild type, Icam1tm1alb, Icam1tm1Jcgr, and Icam1tm1Bay mice using the Miltenyi regulatory T cell isolation kit, as per the manufacturer’s instructions. Isolated CD4+CD25+ populations contained greater than 95% Foxp3 expressing cells. CD4+CD25− responder T cells (5 × 104) from control mice were stimulated in round bottom 96-well plates using anti-CD3 and CD28 antibodies along with CD4+CD25+ Tregs from wild type or different ICAM-1 mutants (1 × 105) as previously described (Pandiyan et al., 2007). Cells were pulsed after 48 hr with 3H-thymidine (1μCi) for 18 hrs, harvested and thymidine incorporation determined.
2.6. Statistics
Statistical significance between control and Icam1tm1Jcgr or Icam1tm1Bay mice for active and transferred EAE experiments was calculated using the Wilcoxon signed rank test, for proliferation assays the student’s t-test was used. Results of evaluations for inflammation and demyelination were analyzed using analysis of variance for main effects and Tukey’s test for pair-wise mean comparisons.
3. Results
3.1. Active EAE induction is attenuated disease in Icam1tm1Jcgr mice
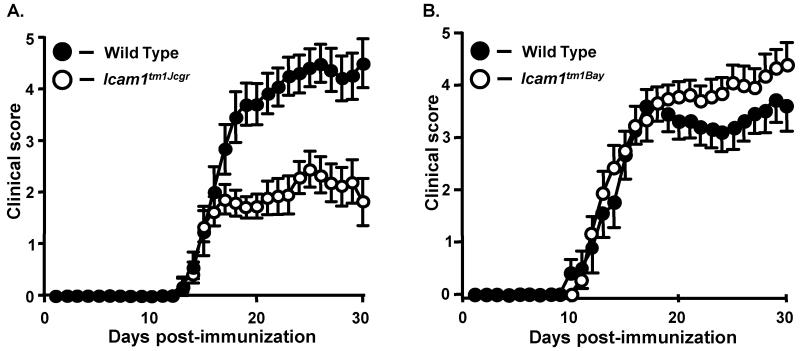
We first analyzed and compared the EAE phenotypes in mice homozygous for the Icam1tm1Jcgr and Icam1tm1Bay mutations in order to determine whether there were differences in the initiation and progression of disease. We found that the overall course of active EAE in Icam1tm1Jcgr mice was significantly attenuated compared to wild type mice (Fig. 2A and Table 1; cumulative disease index or CDI of 31 vs. 58, p=0.0004, Wilcoxon signed rank test). There was no significant difference in the onset and initial course of EAE between genotypes; however, Icam1tm1Jcgr mice showed a lack of progression to severe disease. In addition, disease-related mortality was also reduced in Icam1tm1Jcgr mice compared to wild type mice (16% versus 30%, Table 1). As reported previously (Samoilova et al., 1998), we observed that Icam1tm1Bay mice developed EAE with similar onset and severity as that of wild type mice during at early timepoints post-immunization (Fig. 2B, Table 1), and showed an overall increase in the CDI severity scores during the chronic phases (p=0.0008, Wilcoxon signed rank test). Concomitant with the severe disease, we observed a significant increase in mortality in Icam1tm1Bay mice compared to wild type controls (44% versus 20%, Table 1), nearly twice that previously reported (Samoilova et al., 1998).
Fig. 2.
The clinical course of active EAE is attenuated in Icam1tm1Jcgr mice, but exacerbated in Icam1tm1Bay mice compared to wild type mice. Active EAE was induced with MOG35-55 peptide and clinical signs scored for 30 days as described in Materials and Methods. Results shown are the daily mean clinical score for wild type and ICAM-1 mutant mice from two to three experiments. A) Wild type (n=12) and Icam1tm1Jcgr mice (n=19). B) Wild type (n=10) and Icam1tm1Bay mice (n=16).
Table I.
EAE signs in wild-type mice, Icam1tm1Bay and Icam1tm1Jcgr mice.
| CDIA | Disease OnsetB |
Disease IncidenceC |
Mortality | |
|---|---|---|---|---|
| Wild type (n=12) | 58.3 | 16.3d | 100 | 33% |
| Icam1tm1Jcgr (n=19) | 31 | 17.3d | 95 | 16% |
| Wild type (n=10) | 56.4 | 13.4d | 100 | 20% |
| Icam1tm1Bay (n=16) | 64 | 13.7d | 100 | 44% |
Cumulative Disease Index is the mean of the sum of daily clinical scores observed between days 7 and 30.
Disease onset is defined as the first day of two consecutive days with a clinical score of two or more.
Disease incidence is defined as the percent of mice that displayed any clinical signs of disease.
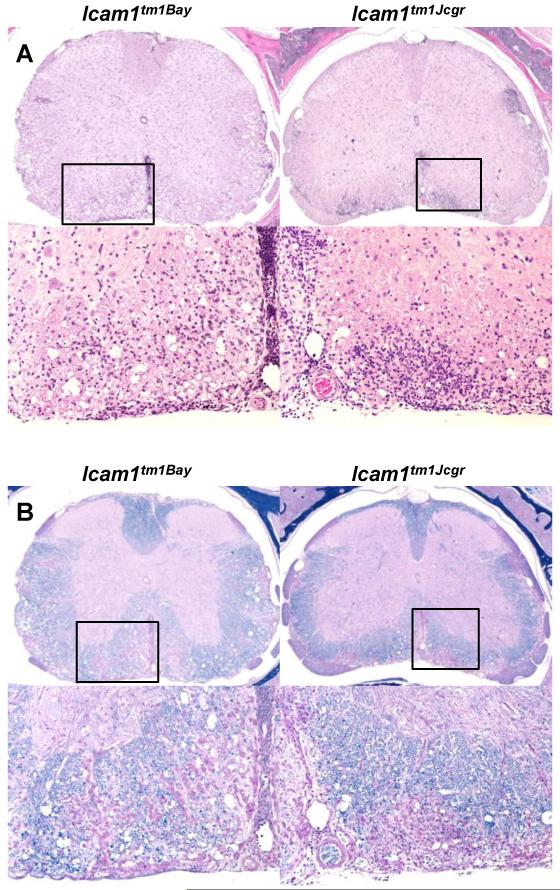
Histological evaluation of spinal cord sections taken at 30 days post-immunization (active EAE) revealed an overall increase in the severity of lesions in Icam1tm1Bay mutants compared to that of Icam1tm1Jcgr. H&E (Fig. 3A) and LFBPAS (Luxol fast blue + periodic acid-Schiff) (Fig. 3B) stained sections from Icam1tm1Bay mice showed a pattern of demyelinating leukomyelitis, most severely affecting the outer ventrolateral white matter tracts. The inflammatory cell response was mixed, and included neutrophils, macrophages, and lesser numbers of lymphocytes, as well as perivascular cuffing, primarily by lymphocytes. Within affected areas, scattered myelin sheaths were dilated and apparently empty (“moth-eaten” appearance), and a few of these contained swollen homogeneous eosinophilic (degenerating) axons. Similar manifestations were present in Icam1tm1Jcgr mice, to a lesser degree for demeylination.
Fig. 3.
Leukocyte infiltration and demyelination in Icam1tm1Jcgr mice and Icam1tm1Bay mice during EAE. Spinal cords from wild type and Icam1tm1Jcgr and Icam1tm1Bay mice (n=3 for each group) were obtained at 30 days post-immunization, fixed in 10% buffered formalin and paraffin-embedded. Sections from the cervical, thoracic and lumbar regions (5μm) were stained with H&E (A) or Luxol fast blue (B) and scored as described in the Materials and Methods. Representative sections from Icam1tm1Jcgr mice and Icam1tm1Bay stained with H&E are shown in A. In Icam1tm1Bay mice there was moderate parenchymal and meningeal infiltration while in Icam1tm1Jcgr mice infiltration was more parenchymal and widespread. The boxed region in the upper panel shows the region of magnification in the lower panel. Representative adjacent sections were stained with LFB (B). Icam1tm1Bay mice had extensive demyelination and inflammation compared to Icam1tm1Jcgr mice. The boxed region in the upper panel shows the region of magnification in the lower panel. Original magnification 4×.
3.2. Adoptive transfer EAE studies
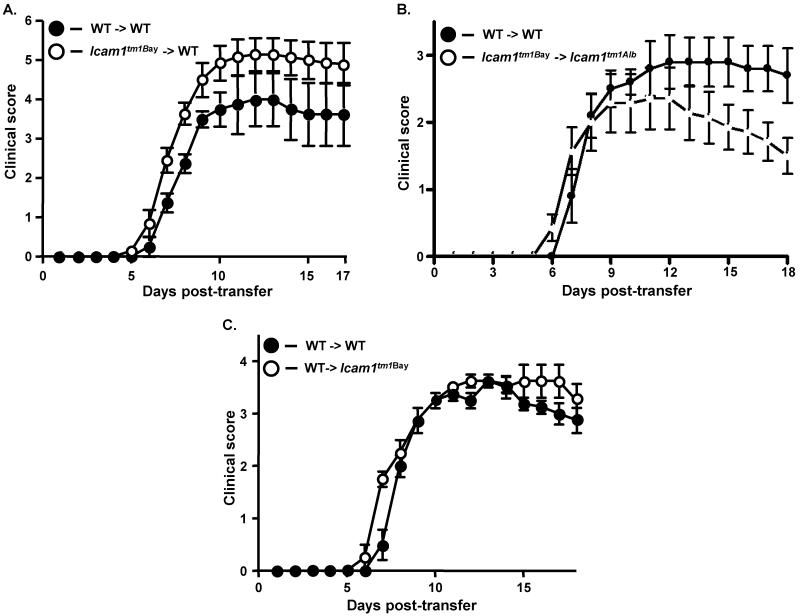
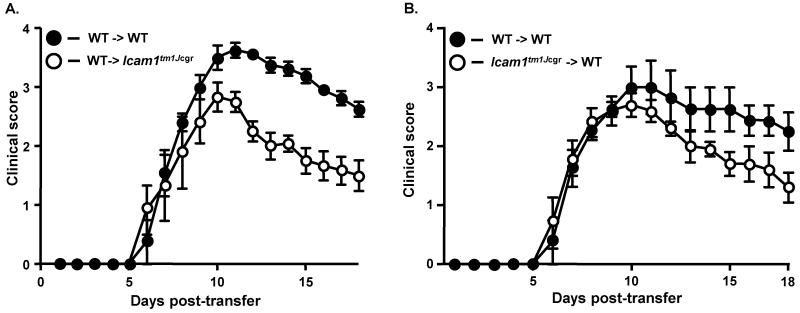
To determine if the observed phenotypes in the mutants were due to ICAM-1 isoform expression on T cells or other cell types we performed transferred EAE. Surprisingly, we found that antigen-specific Icam1tm1Bay T cells induced severe EAE in naïve wild type recipients (Fig. 4A, Table 2). The CDI was significantly elevated in Icam1tm1Bay to wild type transfers compared to wild type-to-wild type transfers (49.3 vs. 35.9, p=0.0002, Wilcoxon ranked sign test), however there were no other significant differences in the disease parameters analyzed. These data suggest that the combination of ICAM-1 isoforms expressed by Icam1tm1Bay T cells can promote severe demyelinating disease and that these cells are highly pathogenic. To further examine the potential of these cells to promote severe disease, we transferred the Icam1tm1Bay T cells into EAE-resistant Icam1tm1Alb mice that lack expression of all isoforms on APCs, endothelial cells, and other cell types (Dunne et al., 2003). We observed that Icam1tm1Bay T cells were capable of inducing CNS inflammation and demyelination in Icam1tm1Alb mice following transfer, with no significant differences in the CDI, disease onset or the acute phase course of disease compared to wild type-to-wild type transfers (Fig. 4B, Table 2). This induction is remarkable however, since transfer of wild type MOG-specific T cells into Icam1tm1Alb mice fails to produce disease, as we have previously shown (27). Next we performed the reciprocal transfer in which wild type MOG-specific T cells were transferred into naïve Icam1tm1Bay mice to investigate the roles of these isoforms on other ICAM-1 expressing cell types. We observed disease comparable to control wild type-to-wild type transfers run in parallel with no significant difference in disease onset or incidence (Fig. 4C, Table 2). These findings significantly contrast with our previous observations in which encephalitogenic wild type T cells were not capable of inducing disease in Icam1tm1Alb mice (Bullard et al., 2007).
Fig. 4.
Encephalitogenic T cells from Icam1tm1Bay mice drive the development of EAE in wild type and Icam1tm1alb mice. A, Transferred EAE was induced in wild-type (n=7) mice by injecting encephalitogenic T cells (~5 × 106) derived from Icam1tm1Bay mice with active EAE. As a control transferred EAE was induced in wild type mice (n=4) by injecting encephalitogenic T cells (~5 × 106) derived from wild-type mice with active EAE. Results shown are the daily mean clinical score from three separate experiments. B, Transferred EAE was induced in Icam1tm1alb (n=7) mice by injecting encephalitogenic T cells (~5 × 106) derived from Icam1tm1Bay mice with active EAE. Control transferred EAE in wild type mice (n=5) was run in parallel. Results shown are the daily mean clinical score from two separate experiments. C, Transferred EAE was induced in wild type (n=4) and Icam1tm1Bay mice (n=4) mice by injecting encephalitogenic T cells (~5 × 106) derived from wild-type mice with active EAE. Results shown are the daily mean clinical score from two separate experiments.
Table 2.
Transferred EAE signs in wild-type mice and Icam1tm1Bay mice.
| CDIA | Disease OnsetB |
Disease IncidenceC |
|
|---|---|---|---|
| WT > WT (n=4) | 35.9 | 7.5d | 100 |
|
| |||
| Icam1tm1Bay > WT (n=7) | 49.3 | 7d | 100 |
|
| |||
| WT > WT (n=4) | 29.5 | 8.2d | 100 |
|
| |||
|
Icam1tm1Bay > Icam1tm1Alb (n=7) |
23.8 | 7.7d | 86 |
|
| |||
| WT > WT (n=4) | 33.2 | 8d | 100 |
| WT > Icam1tm1Bay (n=4) | 37.1 | 8d | 100 |
Cumulative Disease Index is the mean of the sum of daily clinical scores observed between days 7 and 30.
Disease onset is defined as the first day of two consecutive days with a clinical score of two or more.
Disease incidence is defined as the percent of mice that displayed any clinical signs of disease.
Unlike the transferred EAE findings made in the Icam1tm1Bay mice, we found that transfer of MOG-specific T cells from wild type mice to Icam1tm1Jcgr mice resulted in significantly attenuated EAE (Fig 5A, Table 2, p=0.012, Wilcoxon ranked sign test). Differences in the disease course between the two groups of mice were observed only in the chronic phase of disease, similar to the course of active EAE in Icam1tm1Jcgr mice. Transfer of MOG-specific Icam1tm1Jcgr T cells to naïve wild type mice also induced attenuated EAE, but the difference was not statistically significant (p=0.094, Wilcoxon signed rank test; Fig. 5B, Table 2). These data indicate that the combination of ICAM-1 isoforms expressed by Icam1tm1Jcgr T cells, although capable of promoting disease development, do so with a phenotype intermediate to that of Icam1tm1Bay and Icam1tm1Alb mice (Bullard et al., 2007).
Fig. 5.
Adoptive transfer of wild type encephalitogenic T cells to Icam1tm1Jcgr mice or Icam1tm1Jcgr encephalitogenic T cells to wild type mice produces attenuated EAE. A, Transferred EAE was induced in wild type (n=4) and Icam1tm1Jcgr mice (n=6) mice by injecting encephalitogenic T cells (~5 × 106) derived from wild-type mice with active EAE. Results shown are the daily mean clinical score from two separate experiments. B, Transferred EAE was induced in wild-type (n=5) mice by injecting encephalitogenic T cells (~5 × 106) derived from Icam1tm1Jcgr mice with active EAE. As a control transferred EAE was induced in wild type mice (n=4) by injecting encephalitogenic T cells (~5 × 106) derived from wild-type mice with active EAE. Results shown are the daily mean clinical score from three separate experiments.
3.3. Icam1tm1Bay T cells have increased proliferative capacity and an altered IFN-γ production
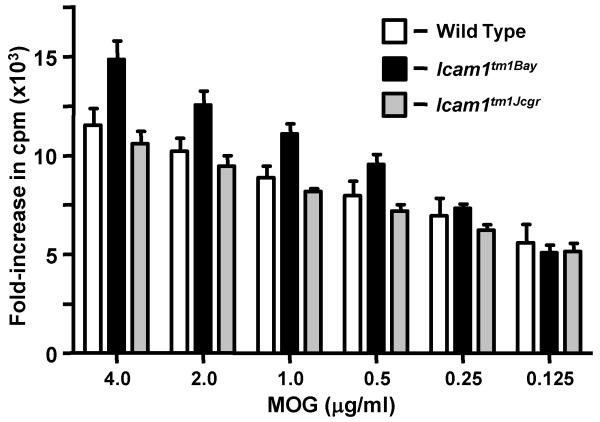
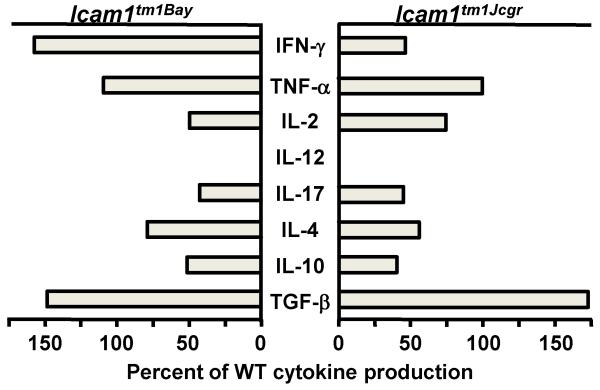
We next performed T cell in vitro proliferation assays to test whether the EAE phenotypes observed in the Icam1tm1Bay and Icam1tm1Jcgr mice were associated with alterations in the proliferative capacity of these cells. T cells from Icam1tm1Bay mice showed significantly enhanced proliferation capacity following MOG stimulation when compared to wild type T cells (p=0.04, paired t-test). Conversely, T cells from Icam1tm1Jcgr mice showed similar proliferation kinetics as those derived from wild type mice with EAE (p=0.28, ns: Fig. 6). To determine if there were major shifts in the production of several key T cell cytokines between Icam1tm1Bay, Icam1tm1Jcgr, and wild type T cells, we examined for expression differences in splenic-derived T cells from all three genotypes. Surprisingly, the only significant change observed was in the production of IFN-γ between Icam1tm1Bay and Icam1tm1Jcgr mice relative to wild type controls (Fig. 7). IFN-γ production by Icam1tm1Bay mice was elevated 50% over that of wild type mice, but reduced (<50% of wild type mice) in Icam1tm1Jcgr mice. Icam1tm1Bay mice also had modest elevations of TNF-α and TGF-β compared to wild type mice, however the remaining cytokines we examined were either reduced (IL-2, IL-4, IL-10, IL-17) or similar to that of wild type mice (IL-12). These data suggest that differences in IFN-γ production may play a dominant role in regulating disease severity in these ICAM-1 mutants, but we cannot rule out the role of other cytokines based on these studies.
Fig. 6.
Icam1tm1Bay T cells proliferate comparably compared to wild type T cells. Encephalitogenic T cells enriched by nylon-wool adherence from the spleens of wild type, Icam1tm1Bay mice and Icam1tm1Jcgr mice (n=4, all groups) undergoing active EAE, or T cells from healthy controls (naïve cells), were co-cultured with irradiated splenic APCs plus MOG peptide (0.125 - 4 μg/ml). The cells were pulsed with 3H-thymidine and harvested at 18 h for determination of radioisotope incorporation. The results shown are expressed as the mean + SEM of fold-induction of wild type, Icam1tm1Bay and Icam1tm1Jcgr T cell proliferation relative to background proliferation.
Fig. 7.
Splenic Icam1tm1Bay T cells produce elevated levels of IFN-γ compared to wild type and Icam1tm1Jcgr mice during EAE. Encephalitogenic T cells enriched by nylon-wool adherence from the spleens of wild type, Icam1tm1Bay and Icam1tm1Jcgr mice (n=4 all groups) undergoing active EAE (day 15), were co-cultured with irradiated splenic APCs from naïve donors and stimulated with MOG peptide (1μg/well). Supernatants were collected 48 hours after stimulation and assayed by ELISA to quantitate production of each cytokine. Cytokine production is shown as percent of wild type cytokine production.
3.4. Analysis of T regulatory cell function
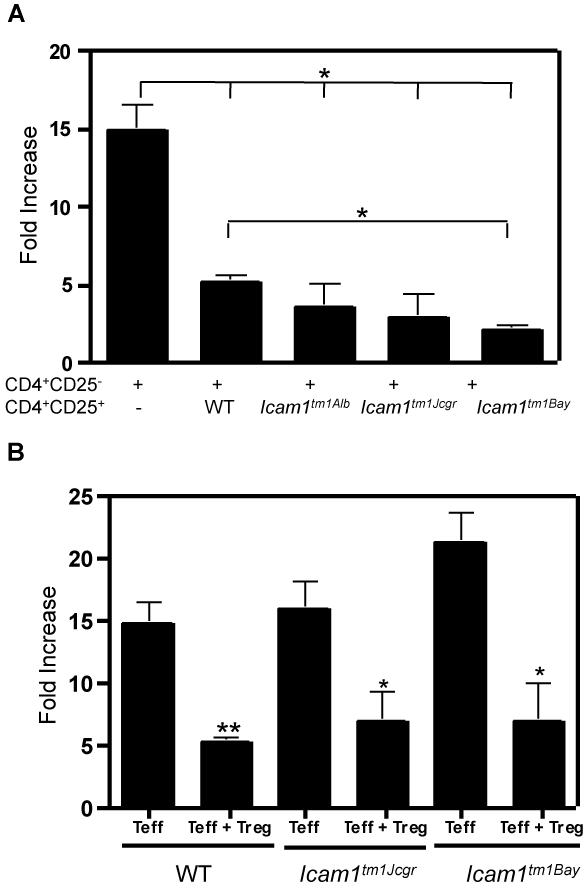
Regulatory T cells (Tregs) have been shown to play an important role in modulating the severity of EAE (Anderton and Liblau, 2008). The expression of the unique subsets of ICAM-1 isoforms in Icam1tm1Bay and Icam1tm1Jcgr could potentially alter the suppressive functions Tregs and contribute to the unique disease phenotypes seen in these mice. To assess this possibility, we performed in vitro Treg assays using Tregs isolated from different ICAM-1 mutant mice. As expected, wild type Tregs readily suppressed effector T cell proliferation in response to antigen-independent stimulation using anti-CD3 and anti-CD28 antibodies. We also found that Icam1tm1Alb and Icam1tm1Jcgr Tregs were able to suppress wild type effector T cells with comparable efficiency to wild type Tregs (Fig. 8A). In contrast, Icam1tm1Bay CD4+CD25+ cells suppress wild type effector T cell proliferation with significantly greater efficiency than wild type Tregs (Fig. 8A) indicating that the exacerbated disease seen in the Icam1tm1Bay mice is not due to a deficiency in regulatory T cell function. To determine if alterations in ICAM-1 isoform expression on responder T cells (CD4+CD25−) influence the ability of these cells to undergo Treg-mediated suppression, we stimulated Icam1tm1Jcgr and Icam1tm1Bay effector T cells, in vitro, in the presence of wild type Tregs and analyzed proliferation levels. Wild type Tregs were capable of suppressing both Icam1tm1Jcgr and Icam1tm1Bay effector T cells (Fig. 8B), These studies collectively, at least at the in vitro level, suggest that ICAM-1 isoforms expressed on Tregs or responders cells is not critical for suppressive activity of these cells.
Fig. 8.
Alterations in ICAM-1 isoform expression do not affect regulatory T cell function. In vitro suppression assays were performed as described in the Materials and Methods using wild type, Icam1tm1Alb, Icam1tm1Jcgr, or Icam1tm1Bay CD4+CD25− responder or CD4+CD25+ regulatory T cells. Cultures were stimulated with soluble anti-CD3 and anti-CD28 antibodies for 72 hours and were pulsed with 3H-thymidine for the last 18 hours. A, The results are shown as fold-increase over baseline controls for WT CD4+CD25− cells alone (n=6), control CD4+CD25− cells with wild type CD4+CD25+ cells (n=7), wild type CD4+CD25− cells with Icam1tm1Alb CD4+CD25+ cells (n=5), wild type CD4+CD25− cells with Icam1tm1Jcgr CD4+CD25+ cells (n=4), and wild type CD4+CD25− cells with Icam1tm1Bay CD4+CD25+ cells (n=4). B, wild type Treg cells are able to suppress proliferation of Icam1tm1Jcgr and Icam1tm1Bay CD4+CD25− T cells. The results shown are fold-increase over baseline controls for wild type CD4+CD25− cells alone (n=6), wild type CD4+CD25− cells with wild type CD4+CD25+ cells (n=7), Icam1tm1Jcgr CD4+CD25− cells alone (n=3), Icam1tm1Jcgr CD4+CD25− cells with wild type CD4+CD25+ cells (n=4), Icam1tm1Bay CD4+CD25− cells alone (n=4), Icam1tm1Bay CD4+CD25− cells with wild type CD4+CD25+ cells (n=4). Statistical analysis was performed using Anova (*, p≤0.1 and ***, p≤0.001).
4. Discussion
Multiple forms of ICAM-1 exist in mammals due to alternative RNA splicing, and current evidence suggests that these ICAM-1 variants can differ in their expression levels, ligand binding, and possibly in their functions during CNS inflammatory processes (Giorelli et al., 2002; King et al., 1995; Ochietti et al., 2002; Robledo et al., 2003; van Den Engel et al., 2000; Werner et al., 2001). Our analyses of different ICAM-1 mutant mice in the EAE model suggests that specific ICAM-1 isoforms expressed on T cells and other cell types, despite low levels of expression, can significantly contribute to disease development independent of the expression of the full-length molecule. Alternatively spliced isoforms are not unique to the ICAM-1 gene, and have been described for many different genes in the immune system, including other adhesion molecules, CTLA-4, and CD45 (Lynch, 2004; Tchilian and Beverley, 2006). Furthermore, studies to date suggest that many of these genes are functionally active, and may in some cases promote the development of EAE, MS, and other inflammatory diseases (Dawes et al., 2006; Tchilian and Beverley, 2006; Ueda et al., 2003).
Our findings in the Icam1tm1Bay mice suggest that specific ICAM-1 isoforms expressed on T cells and other cell types can significantly contribute to disease development independent of the expression of the full-length molecule. In active EAE experiments, our results mimicked those of Samoilova et al (Samoilova et al., 1998), and transfer of wild type encephalitogenic T cells into Icam1tm1Bay recipients induced a similar disease course to that wild type control transfers. Unexpectedly, severe disease was also induced when Icam1tm1Bay encephalitogenic T cells were transferred into wild type or Icam1tm1Alb recipients. These results indicate that specific alternatively spliced ICAM-1 isoforms on T cells alone, in the absence of ICAM-1 expression on other cell types, can readily drive disease development. In contrast to these findings we observed dramatically different EAE phenotypes in Icam1tm1Jcgr mice. Active EAE in Icam1tm1Jcgr mice was markedly attenuated compared to wild type mice, although disease severity in this ICAM-1 mutant mouse was increased compared to Icam1tm1Alb mice, which we previously showed develop only mild or no disease (Bullard et al., 2007). Transferred EAE experiments involving these mutant mice showed phenotypes similar to that observed in active EAE (Fig. 5).
There are many possible mechanisms that could contribute to the phenotypic differences observed in between ICAM-1 mutant and wild type mice. Our current and previous findings suggest an important role for ICAM-1 on T cells and, thus differential isoform expression may alter the proliferative capacity of these cells during the course of EAE (Bullard et al., 2007). Indeed, proliferation of T cells from Icam1tm1Bay mice was modestly elevated on restimulation with MOG peptide, which may at least partially explain increased disease severity. In contrast, T cells from Icam1tm1Jcgr mutants proliferated comparably to wild type mice, while our previous studies of ICAM-1 null mice in identical assays showed that complete loss of ICAM-1 isoforms significantly attenuates MOG-induced restimulation (Bullard et al., 2007). This suggests that isoforms expressed by Icam1tm1Jcgr mutants represent a minimal complement of ICAM-1 molecules required for T cell proliferation.
We also examined for differences in T cell effector functions between Icam-1tm1Jcgr and Icam1tm1Bay mice in terms of T cell-produced cytokines and regulatory T cell function. Neither group of mice produced a cytokine profile consistent with any T helper cell subset that matched their respective disease phenotypes. Although the proinflammatory cytokines IFN-γ, TNF-α, IL-12 and IL-17 in Icam-1tm1Jcgr mice with EAE were reduced relative to those of control mice, the levels of anti-inflammatory cytokines including IL-4 and IL-10 were also reduced, while TGF-β levels were essentially identical to that seen in Icam1tm1Bay mice. In contrast, IFN-γ was highly elevated during EAE in Icam1tm1Bay mice compared to wild type mice, while in Icam1tm1Jcgr mice, Ievels of this cytokine were markedly reduced. In the absence of a cytokine profile that provides a clear mechanistic explanation for the phenotypic differences between the ICAM-1 mutant mice, we investigated whether Treg numbers or suppressive functions were different. Similar to Icam1tm1Alb mice, both mutant strains had normal numbers of Tregs (data not shown) and were fully capable of suppressing T cell activation (Figure 8). These data indicate that ICAM-1 plays little to no role in Treg function, although we cannot rule out that the expression of other combinations of ICAM-1 isoforms on Tregs may be important. It is interesting to note that LFA-1, a receptor that frequently pairs with ICAM-1 in mediating many leukocyte functions, is critical for Treg function but does so independent of ICAM-1 expression on the responder T cells (Wohler et al., 2009).
Different ICAM-1 isoform expression may modulate the course of not only EAE but other biological process through alterations in ligand binding or intracellular signaling on endothelial cells, APCs or T cells. Previous studies have shown that LFA-1 binds to Ig-like domain 1, while Mac-1 interacts with Ig-like domain 3 (Casasnovas et al., 1998; Diamond et al., 1991). Interestingly, all isoforms identified to date retain domain 1, while only a few contain the domain 3 (King et al., 1995); (Giorelli et al., 2002; Ochietti et al., 2002; Robledo et al., 2003; van Den Engel et al., 2000; Werner et al., 2001) (Wakatsuki et al., 1995). Icam1tm1Jcgr mice express the three smallest ICAM-1 isoforms (Fig. 1) and do not have any known binding sites for Mac-1, and this may account for the dramatic phenotypic differences observed (Diamond et al., 1991). It is interesting to note that several ICAM-1 isoforms expressed in Icam1tm1Bay mice retain the Mac-1 binding domain (Fig. 1). This raises the possibility that Mac-1/ICAM-1 interactions may serve as an activation rheostat on certain cell types and alter intracellular signaling in T cells and/or APCs. Nevertheless, it is not yet clear how this particular set of ICAM-1 isoforms expressed in the Icam1tm1Bay mice mediates severe demyelinating disease. Interpretation of these studies as well as others is complicated by our poor understanding of ICAM-1 isoform biology. For example, it is not known in any mammalian species whether all ICAM-1 isoforms are similarly expressed on different cell types under both homeostatic and inflammatory conditions, or if specific isoforms have distinct functions. Further studies of alternatively spliced ICAM-1 isoforms with respect to their expression patterns, ligand interactions and signaling capabilities are necessary to address these many questions.
Adhesion molecules have long been therapeutic targets in animal models of demyelinating disease (Engelhardt and Briskin, 2005; Miller et al., 2003; von Andrian and Engelhardt, 2003). Our results argue that an ICAM-1-mediated therapeutic approach warrants significant but cautious investigation. Exacerbated disease in Icam1tm1Bay mice suggests that enhanced disease could be a possible therapeutic side effect in MS patients treated with anti-ICAM-1 antibodies. Despite this possibility, anti-ICAM-1 therapy may have advantages over other adhesion molecule immunotherapeutics. For example, since ICAM-1 is not a ligand for α4-integrins, the serious clinical side effects associated with Tysabri, the anti-α4-integrin antibody may be less of a concern (Engelhardt and Briskin, 2005; Miller et al., 2003; von Andrian and Engelhardt, 2003). Furthermore, unlike LFA-1, ICAM-1 does not appear to be critical for Treg function (Wohler et al., 2009), therefore anti-ICAM-1 antibody therapy may be less immunosuppressive in modulating demyelinating disease.
Table 3.
Transferred EAE signs in wild-type mice and Icam1tm1JCgr mice.
| CDIA | Disease OnsetB |
Disease IncidenceC |
|
|---|---|---|---|
| WT > WT (n=4) | 34.9 | 8d | 100 |
| WT > Icam1tm1Jcgr (n=6) | 24.2 | 8d | 100 |
|
| |||
| WT > WT (n=4) | 29.2 | 7.8d | 100 |
| Icam1tm1Jcgr > WT (n=5) | 26.4 | 7d | 100 |
Cumulative Disease Index is the mean of the sum of daily clinical scores observed between days 7 and 30.
Disease onset is defined as the first day of two consecutive days with a clinical score of two or more.
Disease incidence is defined as the percent of mice that displayed any clinical signs of disease.
Acknowledgements
This work was supported by grants from the National Multiple Sclerosis Society (RG-3437-A-6) and from the NIH (NS46032) to S.R. Barnum and RR017009 to D.C. Bullard. Histologic services for this project were supported in part by the Gnotobiotic and Genetically-Engineered Mouse Core of the UAB Mucosal HIV and Immunobiology Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors have no financial conflicts.
References
- Anderton SM, Liblau RS. Regulatory T cells in the control of inflammatory demyelinating diseases of the central nervous system. Curr Opin Neurol. 2008;21:248–54. doi: 10.1097/WCO.0b013e3282febf58. [DOI] [PubMed] [Google Scholar]
- Bo L, Peterson JW, Mork S, Hoffman PA, Gallatin WM, Ransohoff RM, Trapp BD. Distribution of immunoglobulin superfamily members ICAM-1, -2, -3, and the beta 2 integrin LFA-1 in multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:1060–72. [PubMed] [Google Scholar]
- Bullard DC, Hu X, Schoeb TR, Collins RG, Beaudet AL, Barnum SR. Intercellular adhesion molecule-1 expression is required on multiple cell types for the development of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:851–7. doi: 10.4049/jimmunol.178.2.851. [DOI] [PubMed] [Google Scholar]
- Casasnovas JM, Stehle T, Liu JH, Wang JH, Springer TA. A dimeric crystal structure for the N-terminal two domains of intercellular adhesion molecule-1. Proc Natl Acad Sci U S A. 1998;95:4134–9. doi: 10.1073/pnas.95.8.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Kim TD, Carman CV, Mi LZ, Song G, Springer TA. Structural plasticity in Ig superfamily domain 4 of ICAM-1 mediates cell surface dimerization. Proc Natl Acad Sci U S A. 2007;104:15358–63. doi: 10.1073/pnas.0707406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes R, Petrova S, Liu Z, Wraith D, Beverley PC, Tchilian EZ. Combinations of CD45 isoforms are crucial for immune function and disease. J Immunol. 2006;176:3417–25. doi: 10.4049/jimmunol.176.6.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Staunton DE, Marlin SD, Springer TA. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglubulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell. 1991;65:961–971. doi: 10.1016/0092-8674(91)90548-d. [DOI] [PubMed] [Google Scholar]
- Dunne JL, Collins RG, Beaudet AL, Ballantyne CM, Ley K. Mac-1, but not LFA-1, uses intercellular adhesion molecule-1 to mediate slow leukocyte rolling in TNF-alpha-induced inflammation. J Immunol. 2003;171:6105–11. doi: 10.4049/jimmunol.171.11.6105. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Briskin MJ. Therapeutic targeting of alpha 4-integrins in chronic inflammatory diseases: tipping the scales of risk towards benefit? Eur J Immunol. 2005;35:2268–73. doi: 10.1002/eji.200535195. [DOI] [PubMed] [Google Scholar]
- Giorelli M, De Blasi A, Defazio G, Avolio C, Iacovelli L, Livrea P, Trojano M. Differential regulation of membrane bound and soluble ICAM 1 in human endothelium and blood mononuclear cells: effects of interferon beta-1a. Cell Commun Adhes. 2002;9:259–72. doi: 10.1080/15419060216305. [DOI] [PubMed] [Google Scholar]
- Harlan JM, Winn RK, Vedder NB, Doerschuk CM, Rice CL. Adhesion: Its Role in Inflammatory Disease. W. H. Freeman and Company; New York: 1992. [Google Scholar]
- King PD, Sandberg ET, Selvakumar A, Fang P, Beaudet AL, Dupont B. Novel isoforms of murine intercellular adhesion molecule-1 generated by alternative RNA splicing. Journal of Immunology. 1995;154:6080–6093. [PubMed] [Google Scholar]
- Kirchhausen T, Staunton DE, Springer TA. Location of the domains of ICAM-1 by immunolabeling and single-molecule electron microscopy. J Leukoc Biol. 1993;53:342–6. doi: 10.1002/jlb.53.3.342. [DOI] [PubMed] [Google Scholar]
- Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC. Regulation of leukocyte-endothelium interaction and leukocyte transendothelial migration by intercellular adhesion molecule 1-fibrinogen recognition. Proc Natl Acad Sci USA. 1995;92:1505–1509. doi: 10.1073/pnas.92.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva T, Dustin ML, Sykulev Y. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr Opin Immunol. 2005;17:251–8. doi: 10.1016/j.coi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Benveniste EN. Adhesion molecule expression and regulation on cells of the central nervous system. J Neuroimmunol. 1999;98:77–88. doi: 10.1016/s0165-5728(99)00084-3. [DOI] [PubMed] [Google Scholar]
- Leung E, Berg RW, Langley R, Greene J, Raymond LA, Augustus M, Ni J, Carter KC, Spurr N, Choo KH, Krissansen GW. Genomic organization, chromosomal mapping, and analysis of the 5′ promoter region of the human MAdCAM-1 gene. Immunogenetics. 1997;46:111–9. doi: 10.1007/s002510050249. [DOI] [PubMed] [Google Scholar]
- Lynch KW. Consequences of regulated pre-mRNA splicing in the immune system. Nat Rev Immunol. 2004;4:931–40. doi: 10.1038/nri1497. [DOI] [PubMed] [Google Scholar]
- McCourt PAG, Ek B, Forsberg N, Gustafson S. Intercellular adhesion molecule-1 is a cell surface receptor for hyaluronan. The Journal of Biological Chemistry. 1994;269:30081–30084. [PubMed] [Google Scholar]
- McMurray RW. Adhesion molecules in autoimmune disease. Sem Arthr Rheum. 1996;25:215–233. doi: 10.1016/s0049-0172(96)80034-5. [DOI] [PubMed] [Google Scholar]
- Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, Dustin ML. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J Exp Med. 1995;182:1231–41. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochietti B, Lemieux P, Kabanov AV, Vinogradov S, St-Pierre Y, Alakhov V. Inducing neutrophil recruitment in the liver of ICAM-1-deficient mice using polyethyleneimine grafted with Pluronic P123 as an organ-specific carrier for transgenic ICAM-1. Gene Ther. 2002;9:939–45. doi: 10.1038/sj.gt.3301716. [DOI] [PubMed] [Google Scholar]
- Osborn L, Vassallo C, Benjamin CD. Activated endothelium binds lymphocytes through a novel binding site in the alternately spliced domain of vascular cell adhesion molecule-1. J Exp Med. 1992;176:99–107. doi: 10.1084/jem.176.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–62. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- Robledo O, Papaioannou A, Ochietti B, Beauchemin C, Legault D, Cantin A, King PD, Daniel C, Alakhov VY, Potworowski EF, St-Pierre Y. ICAM-1 isoforms: specific activity and sensitivity to cleavage by leukocyte elastase and cathepsin G. Eur J Immunol. 2003;33:1351–60. doi: 10.1002/eji.200323195. [DOI] [PubMed] [Google Scholar]
- Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–88. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- Samoilova EB, Horton JL, Chen Y. Experimental autoimmune encephalomyelitis in intercellular adhesion molecule-1-deficient mice. Cell Immunol. 1998;190:83–9. doi: 10.1006/cimm.1998.1395. [DOI] [PubMed] [Google Scholar]
- Sligh JE, Ballantyne CM, Rich SS, Hawkins HK, Smith CW, Bradley A, Beaudet AL. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. 1993;90:8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–7. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- Tchilian EZ, Beverley PC. Altered CD45 expression and disease. Trends Immunol. 2006;27:146–53. doi: 10.1016/j.it.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RC, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, Ronningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SC. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- van Den Engel NK, Heidenthal E, Vinke A, Kolb H, Martin S. Circulating forms of intercellular adhesion molecule (ICAM)-1 in mice lacking membranous ICAM-1. Blood. 2000;95:1350–5. [PubMed] [Google Scholar]
- von Andrian UH, Engelhardt B. Alpha4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348:68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- Wakatsuki T, Kimura K, Kimura F, Shinomiya N, Ohtsubo M, Ishizawa M, Yamamoto M. A distinct mRNA encoding a soluble form of ICAM-1 molecule expressed in human tissues. Cell Adhes Commun. 1995;3:283–92. doi: 10.3109/15419069509081014. [DOI] [PubMed] [Google Scholar]
- Werner A, Martin S, Gutierrez-Ramos JC, Raivich G. Leukocyte recruitment and neuroglial activation during facial nerve regeneration in ICAM-1-deficient mice: effects of breeding strategy. Cell Tissue Res. 2001;305:25–41. doi: 10.1007/s004410100393. [DOI] [PubMed] [Google Scholar]
- Wohler J, Bullard D, Schoeb T, Barnum S. LFA-1 is critical for regulatory T cell homeostasis and function. Mol Immunol. 2009;46:2424–8. doi: 10.1016/j.molimm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Gonzalo JA, St Pierre Y, Williams IR, Kupper TS, Cotran RS, Springer TA, Gutierrez-Ramos JC. Leukocytosis and resistance to septic shock in intercellular adhesion molecule 1-deficient mice. J Exp Med. 1994;180:95–109. doi: 10.1084/jem.180.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HC, Baldwin HS, Sun J, Buck CA, Albelda SM, DeLisser HM. Alternative splicing of a specific cytoplasmic exon alters the binding characteristics of murine platelet/endothelial cell adhesion molecule-1 (PECAM-1) J Biol Chem. 1995;270:23672–80. doi: 10.1074/jbc.270.40.23672. [DOI] [PubMed] [Google Scholar]