Abstract
Objective:
The purpose of our study was to validate iodine quantification in a phantom study with dual-source dual-energy CT (DECT) and to apply this technique to differentiate benign periablational reactive tissue from residual tumour in VX2 carcinoma in rabbits after radiofrequency ablation (RFA).
Methods:
We applied iodine quantification with DECT in a phantom and in VX2 carcinoma in rabbits after incomplete RFA to differentiate benign periablational reactive tissue from residual tumour and evaluated its efficacy in demonstrating response to therapeutic RFA. A series of tubes containing solutions of varying iodine concentration were scanned with DECT. The iodine concentration was calculated and compared with known true iodine concentration. Triple-phase contrast-enhanced DECT data on 24 rabbits with VX2 carcinoma were then assessed at Day 3 (n=6), 1 week (n=6), 2 weeks (n=6) and 3 weeks (n=6) after incomplete RFA independently by 2 readers. Dual-energy postprocessing was used to produce iodine-only images. Regions of interest were positioned on the iodine image over the lesion and, as a reference, over the aorta, to record iodine concentration in the lesion and in the aorta. The pathological specimens were sectioned in the same plane as DECT imaging, and the lesion iodine concentration and lesion-to-aorta iodine ratio of residual tumour and benign periablational reactive tissue were assessed.
Results:
There was excellent correlation between calculated and true iodine concentration (r=0.999, p<0.0001) in the phantom study. The lesion iodine concentration and lesion-to-aorta iodine ratio in residual tumour were significantly higher than in benign periablational reactive tissue in the 2-week group during the arterial phase (AP) (p<0.01) and in the 3-week group during both the AP (p<0.05) and the portal venous phase (p<0.05). There was no significant difference between them with respect to the lesion iodine concentration or lesion-to-aorta iodine ratio in the 3-day and 1-week groups.
Conclusion:
Iodine quantification with DECT is accurate in a phantom study and can be used to differentiate benign periablational reactive tissue from residual tumour in VX2 carcinoma in rabbits after RFA.
Advances in knowledge:
Iodine quantification with DECT may help in differentiating benign periablational reactive tissue from residual tumour in VX2 carcinoma in rabbits after RFA.
Percutaneous radiofrequency ablation (RFA) is performed for patients with hepatocellular carcinoma (HCC) worldwide as a curative local therapy because of the low rates of morbidity and mortality and high level of efficacy [1–4]. Imaging of solid tumours after RFA plays an important role in judging the completeness of the ablation, and early detection of a residual or locally recurrent tumour after RFA is critical and can facilitate successful retreatment at an early stage [5]. This assessment is commonly performed after 1 month or more with contrast-enhanced helical CT consisting of pre-contrast, arterial, portal and delayed phase scanning [6]. However, assessment of the accuracy of completeness of the RFA at immediate or <6-month follow-up CT or MRI has inevitable limitations because enhancement of inflammatory reaction to the thermal injury makes accurate evaluation difficult [7–9]. The benign periablational reactive tissue, which represents inflammatory reaction, can emerge immediately after ablation and can last as long as 6 months after ablation [8]. Fluorine-18 fludeoxyglucose (18F-FDG) position emission tomography (PET)/CT revealed the presence of local recurrence earlier than multidetector-row CT. A transient increase in 18F-FDG accumulation due to inflammatory reactions to RFA therapy occurs during the first several weeks, making assessment of therapeutic response by 18F-FDG PET less reliable. A potential time for 18F-FDG PET to evaluate treatment response is within the first 12–24 h or 6–8 weeks after RFA. The clinical utility of 18F-FDG PET for evaluating the primary effect immediately after the completion of treatment is still controversial, given the limited spatial resolution, partial volume effects for small volume disease, high inspection charges and false-positive 18F-FDG uptake in inflammatory changes [10–12]. The development of more accurate imaging tests is a challenge to evaluate RFA of solid tumours early.
Dual-source dual-energy CT (DECT) has potential for addressing some of the issues related to dual acquisition because images obtained with a primary dual-energy technique can depict the presence and amount of iodine in a single acquisition [13]. The iodine map that encodes the iodine distribution in each individual CT voxel can be generated by a post-processing algorithm based on three-material decomposition principles and can subsequently be used to subtract iodine from the image, thus resulting in a virtual non-contrast (VNC) image [14–17]. Recent advances in DECT have enhanced its use for differentiating malignant from benign pulmonary tumours, characterising adrenal nodules and evaluating treatment effects and relapses [18–20]. The iodine map may be able to provide accurate information concerning the distribution of iodine and a more direct measure of lesion vascularity and has shown good correspondence to static CT perfusion images in the lung, liver and heart [21–23]. The iodine map can also distinguish the iodine contrast material from calcium or bone and offers the potential for a range of CT tissue characterisation. To our knowledge, a study of iodine quantification with DECT for distinguishing benign periablational reactive tissue from residual tumours after RFA has not been performed. Thus, the purpose of this study was twofold: to validate iodine quantification in a phantom study with dual-source DECT and to apply this technique to differentiate benign periablational reactive tissue from residual tumour in VX2 carcinoma in rabbits after RFA.
MATERIALS AND METHODS
Phantom studies
A phantom study was performed with a dual-energy scanner (SOMATOM® Definition Flash; Siemens Healthcare, Forchheim, Germany). The dual-source scanner systems acquire data with two X-ray tubes with corresponding detector rings mounted on a rotating gantry. The two tubes independently operate with regard to tube voltage and tube current. A series of 10 solutions of varying iodine concentrations (range 0–10 mg ml−1) was scanned (Figure 1A). The acquisition parameters for this study were as follows: tube A, 80 kVp, 199 mAs effective; tube B, 140 kVp, 65 mAs effective; detector configuration, 32×0.6 mm; rotation time, 0.5 s; and pitch, 0.55. Tube current modulation (automatic exposure control) was used. Images were reconstructed as 1.0-mm-thick axial sections at 1-mm intervals. The DECT low-energy (80 kVp) and high-energy (140 kVp) data were postprocessed at a separate workstation to generate an iodine overlay image, which showed the distribution of iodine in the volume superimposed on the virtually unenhanced data set. The region of interest (ROI) covered about 80% of the test tube cross-section on the iodine-based material decomposition images. From the iodine-only image, the equivalent iodine concentration in milligrams per milliliter was determined. The calculated iodine concentration in the phantom was compared with known true iodine concentrations.
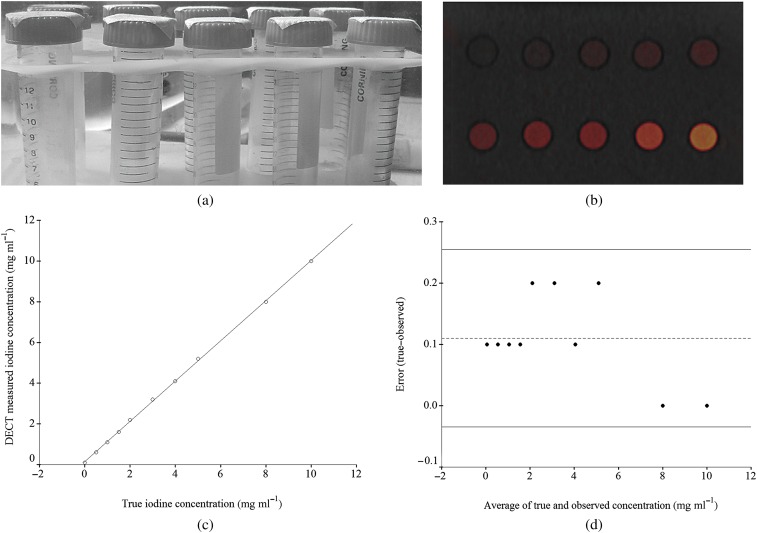
Figure 1.
Iodine phantom. (a) Photograph shows iodine phantom and test tubes consisting of 10 solutions of varying iodine concentration ranging from 0 to 10 mg I ml−1. (b) Axial dual-energy CT (DECT) iodine overlay image of the phantom with varying iodine concentration ranging from 0 to 10 mg I ml−1 produced by a three-material decomposition algorithm showing 80 kVp and 140 kVp greyscale information combined at a 50:50 ratio with iodine colour-encoded overlay. (c) Scatter plot reveals excellent correlation between the iodine concentration measured with DECT and the true iodine concentration in the phantom (r=0.999, p<0.0001). (d) Bland–Altman plot shows differences between true and observed iodine concentrations vs average of true and observed concentrations. The dashed line represents a mean error of 0.11 mg ml−1. The two solid lines represent the 25th–75th percentiles of the limits of agreement. The error in measured iodine concentration with DECT is less than the mean error for concentrations in the physiological range.
Animal preparation and tumour implantation technique
This animal experiment was in compliance with the current institutional regulations for use and care of laboratory animals. The rabbit hepatic VX2 carcinoma model was established according to previous reports [24]. The VX2 tumour tissue in the thigh of the tumour-bearing rabbit was removed and cut into pieces of 1–2 mm3 in size under sterile surgical conditions. 31 New Zealand white rabbits (males, 2.5–3.0 kg) were used throughout the experiment. They were anesthetised by ear marginal vein injection with a 3% pentobarbital solution (1 ml kg−1) before the tumour inoculation and other procedure, and their upper abdomens were shaved and depilated with an 8% Na2S solution. The rabbits were positioned supine and routinely sterilised. A median incision was made below the xiphoid process to expose the left lobe of the liver, where a hole, about 1–2 cm deep, was made through a 17-gauge trochar; then, two VX2 tumour masses and one small piece of gelatin foam were implanted into each hole through a stylet. After surgery, all rabbits were given subcutaneous injections of enrofloxacin 5–10 mg kg−1 to prevent infection.
Tumour model
2–3 weeks after the tumour inoculation, 24 of 31 rabbits (77.4%) had a hepatic nodule >2.5 cm in diameter on the CT imagings. In addition, to evaluate the histopathological changes of tumour tissue and the adjacent hepatic parenchyma after RFA, rabbits were sacrificed at different time intervals after RFA. The study comprised two phases—an acute phase and a subacute phase. In the acute phase, RFA was performed in 6 rabbits, which were sacrificed at Day 3. In the subacute phase, 18 rabbits were sacrificed at 1 week (n=6), 2 weeks (n=6) and 3 weeks (n=6) after RFA.
Radiofrequency ablation procedure
After the initial follow-up DECT scan, the rabbits were anesthetised by ear vein injection with 3% pentobarbital solution (1 ml kg−1) and properly prepared for surgery. The RFA system used was the Cool-tip™ RFA System (HGCF-3000, Zhuhai, China). An internally cooled 17-gauge electrode with a 2.0-cm active tip was placed into the VX2 tumour nodule under CT guidance (SOMATOM Definition Flash). During the ablation process, a temperature detector embedded in the electrode tip continuously measured local tissue temperature while a peristaltic pump was used to infuse normal saline solution at 0 °C into the lumen of the electrode at a rate of 10–25 ml min−1, which was sufficient to maintain a tip temperature of 20–25 °C. The protocols were set at 40 V, 70 °C and 2–3 min from our preliminary study to ensure RFA damage radius for two-thirds of tumour maximum radius. The incomplete tumour ablation was necessary for subsequent measurement of iodine quantification for differentiation of benign periablational reactive tissue from the residual tumour.
The RFA procedure was performed by one radiologist, assisted by three other radiologists in surgical operation.
Dual-energy CT imaging and data acquisition
The same anaesthetic regimen used for RFA was used for DECT imaging. All examinations were performed with contrast-enhanced DECT of the upper abdomen. The recipient rabbits underwent a triple-phasic contrast-enhanced CT scan [25,26]. The non-ionic-iodinated contrast material (iopromide 300 mg I ml−1) was administered at 1.5 ml s−1 and 1.5 ml kg−1 through a 21-gauge catheter placed in the ear marginal vein of the rabbits. Dual-energy data were acquired according to the following parameters: tube A: 140 kVp, 76 mAs; tube B: 80 kVp, 196 mAs; detector configuration: 32×0.6 mm; tube rotation time: 0.5 s; and pitch: 0.5–0.6.
Dual-energy data postprocessing and evaluation
The 80-kVp and 140-kVp images for the arterial phase (AP) and portal venous phase (PVP) were reconstructed with a 1.5-mm slice thickness and a 1-mm slice increment. A linear mixture of the 80-kVp and 140-kVp images was generated using the vendor default mixture ratio of 0.5 (i.e. mixing the 140-kVp and 80-kVp images at a ratio of 1:1), which is equivalent to a 120-kVp-equivalent image. Dual-energy postprocessing was performed with the liver VNC application of the dual-energy software as an image-based analysis of the low-energy and high-energy images [27] with an alleged three-material decomposition algorithm. The base materials were fat, soft tissue and iodine for this particular application. The postprocessing resulted in two volumetric data sets: a virtually unenhanced series and an iodine map showing the distribution of iodine in the volume. This iodine information was superimposed on a virtually unenhanced data set.
The dual-energy iodine-based material decomposition images were obtained just before sacrifice at certain time points and they were used for iodine quantification analysis of residual tumour and benign periablational reactive tissue after RFA. ROIs (mean pixel size, 95) were positioned by one reader (with 10 years' abdominal CT imaging experience) on iodine overlay maps to measure the iodine quantifications in the enhancing areas on the left and right sides of the lesion, corresponding to the largest residual tumour and benign periablational reactive tissue on the histological slide. To ensure consistency, all measurements were performed three times at different image levels, and the average values were obtained. Iodine concentration in the aorta was recorded by placement of a large ROI, and the ratios of inflammatory reaction to aorta and tumour to aorta were also calculated for normalised quantitative comparison.
DECT images were obtained to follow up tumour growth and monitor the changes 1 day before (n=6), and 1 week (n=6), 2 weeks (n=6) and 3 weeks (n=6) after incomplete RFA.
Histopathological analysis
RFA-treated animals were sacrificed with a lethal dose (90 mg kg−1) of intravenously administered sodium pentobarbital at Day 3, 1 week, 2 weeks and 3 weeks after RFA treatment. The gross specimens of liver were dissected in planes similar to those of the DECT. They were fixed with 10% formalin and stained with haematoxylin–eosin for the light microscopic study.
Histopathological findings of the tumour after RFA were related to DECT findings by the consensus opinion of a radiologist and a pathologist, with particular emphasis on the enhanced area of the images. They accounted for the presence of residual tumours, coagulation necrosis and benign inflammatory reaction to thermal injury, such as reactive hyperaemia and fibrosis.
Statistical analysis
Phantom study
The iodine concentration calculated with DECT was correlated to true iodine concentration by Pearson correlation. The error in measurement of iodine concentrations was determined by Bland–Altman analysis.
VX2 tumour study
SPSS® v. 11.5 statistical software (SPSS Inc., Chicago, IL) was used for analysis. With the combined data from the two observers, the mean and standard deviation (SD) were calculated for residual tumour and benign periablational reactive tissue after RFA. The paired Student’s t-test was used to compare lesion types (residual tumour, benign periablational reactive tissue) in four different time points, respectively, in terms of the lesion iodine concentration and lesion-to-aorta iodine concentration ratio. For all tests, a p-value of <0.05 was considered to indicate a statistically significant difference.
RESULTS
Phantom
The iodine concentration calculated with the dual-energy postprocessing algorithm (Figure 1b) was strongly correlated with true iodine concentration (r=0.999, p<0.0001). The scatter plot of dual-energy measured iodine concentration in relation to true iodine concentration for true iodine concentrations of 0–10 mg ml−1 is shown in Figure 1c. The calculation underestimated the iodine concentration with a mean error of 0.11 mg ml−1 for all concentrations >0 mg ml−1. The Bland–Altman plot of this error is shown in Figure 1d. All 10 spots were located in the range of the limits of agreement. The error in iodine concentration measurement was less than the mean error for concentrations in the physiological range (<10 mg mL−1).
VX2 tumour after radiofrequency ablation and iodine quantification
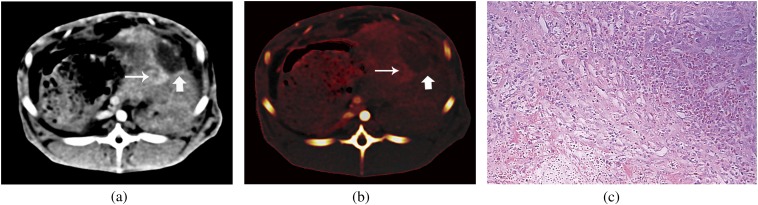
Before RFA, the maximal diameter of the VX2 carcinoma in rabbits ranged from 2.1 to 3.5 cm (mean 2.7±0.2 cm). Soon after RFA, rim enhancement on contrast-enhanced images became prominent and clearly defined on images at Day 3 after RFA. After 1–3 weeks of RFA, the rim enhancement along the ablated tumour became less prominent and nodular enhancing abnormalities were noted at the periphery of the ablated tumour (Figure 2a,b) in all cases. At histopathological analysis, the rim enhancement corresponded to the compound composed of inflammatory reaction with hyperaemia and granulation tissue in the acute group, the mixture of inflammatory granulation tissue and early fibrosis in the 1-week group (Figure 2c), and almost fibrosis in the 2-week and 3-week groups, whereas areas of peripheral nodular and irregular enhancement corresponded to the residual tumour.
Figure 2.
Images of a rabbit with VX2 tumour after radiofrequency ablation (RFA). Contrast-enhanced dual-energy CT image (a) and iodine map (b) show residual tumour (thin arrow) and the inflammation tissue (thick arrow) after RFA. (c) Photomicrograph shows viable tumour, mixture of inflammatory granulation tissue and necrotic areas (haematoxylin–eosin stain, ×200).
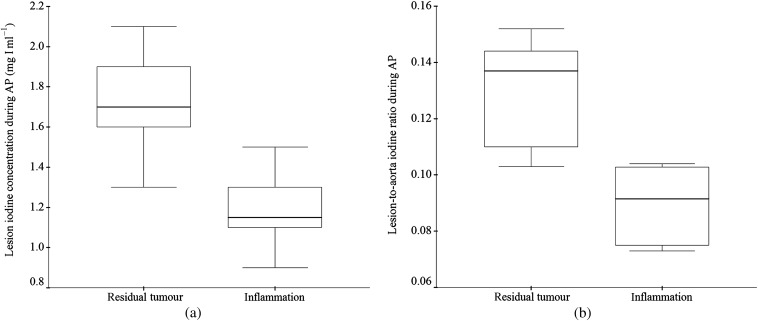
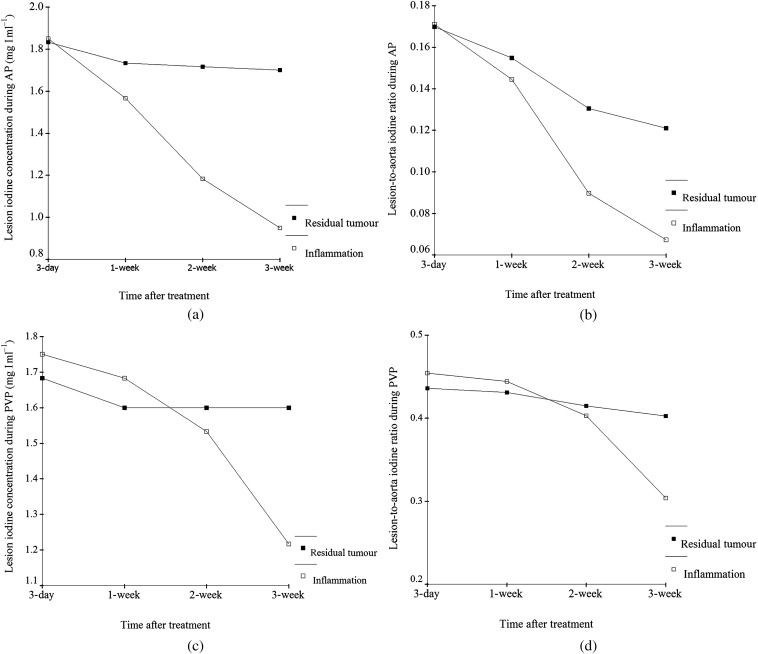
The mean and SD of the lesion iodine concentration and lesion-to-aorta iodine concentration ratio for residual tumour and benign periablational reactive tissue are shown in Tables 1 and 2. The lesion iodine concentration of residual tumour was significantly higher than that of benign periablational reactive tissue in the 2-week group during the AP (1.72±0.27 mg I ml−1 vs 1.18±0.20, p<0.01; Figure 3a) and in the 3-week group during both the AP (1.70±0.14 mg I ml−1 vs 0.95±0.19, p<0.05) and the PVP (1.60±0.14 mg I ml−1 vs 1.22±0.15, p<0.05). Similarly, the lesion-to-aorta iodine ratio was significantly higher in the residual tumour than in the benign periablational reactive tissue in the 2-week group during the AP (13.1±2.0% vs 9.0±1.3%, p<0.01; Figure 3b) and in the 3-week group in both the AP (12.1±1.1% vs 6.7±1.2%, p<0.05) and the PVP (40.3±4.5% vs 30.4±1.2%, p<0.05). There was no significant difference between them with respect to the lesion iodine concentration (p>0.05) or the lesion-to-aorta iodine ratio (p>0.05) in the 3-day and 1-week groups, respectively. The changes between them obtained from Day 3 to Week 3 after RFA are shown in Figure 4.
Table 1.
Lesion iodine concentration of residual tumour and benign periablational reactive tissue
| Group | AP (mg I ml−1) | PVP (mg I ml−1) | ||||
| Residual tumour | Periablational reactive tissue | p-value | Residual tumour | Periablational reactive tissue | p-value | |
| 3-day (n=6) | 1.83±0.21 | 1.85±0.36 | 0.876 | 1.68±0.18 | 1.75±0.14 | 0.286 |
| 1-week (n=6) | 1.73±0.21 | 1.57±0.43 | 0.175 | 1.60±0.42 | 1.68±0.48 | 0.698 |
| 2-week (n=6) | 1.72±0.27 | 1.18±0.20 | <0.01 | 1.60±0.30 | 1.53±0.49 | 0.781 |
| 3-week (n=6) | 1.70±0.14 | 0.95±0.19 | <0.01 | 1.60±0.14 | 1.22±0.15 | <0.01 |
AP, arterial phase; PVP, portal venous phase.
Table 2.
Lesion-to-aorta iodine concentration ratio (%) of residual tumour and benign periablational reactive tissue
| Group | AP (%) | PVP (%) | ||||
| Residual tumour | Periablational reactive tissue | p-value | Residual tumour | Periablational reactive tissue | p-value | |
| 3-day (n=6) | 17.0±4.3 | 17.1±5.0 | 0.913 | 43.6±4.4 | 45.4±4.6 | 0.236 |
| 1-week (n=6) | 15.5±3.7 | 14.2±5.0 | 0.171 | 43.1±21.6 | 44.4±16.7 | 0.826 |
| 2-week (n=6) | 13.1±2.0 | 9.0±1.3 | <0.01 | 41.5±10.1 | 40.3±15.8 | 0.850 |
| 3-week (n=6) | 12.1±1.1 | 6.7±1.2 | <0.01 | 40.3±4.5 | 30.4±1.2 | <0.01 |
AP, arterial phase; PVP, portal venous phase.
Figure 3.
Box plots of the lesion iodine concentration (a) and lesion-to-aorta iodine concentration ratio (b) in residual tumours and inflammation tissues in the 2-week group during the arterial phase. The top and bottom of the boxes represents the 25th–75th percentiles of the data values. The line in each box represents the median value.
Figure 4.
Changes in iodine quantification of residual tumours and inflammation tissues obtained from Day 3 to Week 3 after radiofrequency ablation (RFA). The lesion iodine concentration and lesion-to-aorta iodine ratio of the residual tumour were significantly higher than that of the benign periablational reactive tissue in the 2-week and 3-week groups during the arterial phase (a,b). The lesion iodine concentration and lesion-to-aorta iodine ratio of residual tumour were significantly higher than that of the benign periablational reactive tissue in the 3-week group during the portal venous phase (c,d). There was no significant difference between them in the 3-day and 1-week groups, respectively. Enhancement of inflammatory reaction appeared to be time-dependent and showed a declining trend.
DISCUSSION
In recent years, percutaneous RFA has been increasingly applied in the local treatment of malignant hepatic tumours with the further development of ablation devices [28]. The heat-sink effect of nearby blood vessels in the liver may be responsible for dissipation of the heat and often results in incomplete ablation and local residual tumour [29]. Early detection of a residual tumour after RFA of HCC is important and can facilitate successful retreatment at an early stage. The purpose of diagnostic follow-up after RFA is to detect the presence of residual tumour when it is as small as possible to minimise the false-positive diagnosis that results in unnecessary biopsy or additional treatment. Currently, contrast-enhanced helical CT has been widely used and remains the primary modality in the evaluation of residual or recurrent tumours. However, short-term follow-up CT performed within 3 months after RFA treatment is a not reliable method to precisely determine therapeutic effectiveness, owing to the complexity of the peripheral enhancing tissue [30,31]. Peripheral rim enhancement, representing inflammatory reaction to the thermal injury, frequently occurred within 1–6 months after RFA treatment. Thus, with conventional contrast-enhanced CT and MRI, estimation of response to RFA can only be made relatively late following treatment and with evidence that tumour regrowth has not occurred [32]. Relative to conventional anatomical imaging, such as CT and MRI, 18F-FDG PET can monitor response to cancer treatment by exploiting changes in tumour glucose metabolism. Whether delayed imaging in 18F-FDG PET can distinguish residual tumour from inflammatory reaction or not is controversial, given that some authors reported that 18F-FDG uptake in inflammatory lesion remains stable over time, but other authors have thought that it increases with time [33]. PET tracers targeting angiogenes as a promising application might be appropriate for differentiating between residual tumour and RFA-induced inflammation [10,34]. Deficiencies, such as limited spatial resolution and high inspection charges for repeated follow-up, limit extensive clinical application of 18F-FDG PET.
The former main basis of identification was the shape of the lesions and the density changes; however, CT attenuation values are easily affected by various internal and external conditions [35], such as beam-hardening artefacts, etc., and heterogeneous attenuation after RFA on pre-contrast images interferes with the evaluation of the presence of enhancement within the treated area.
In this study, dual-source DECT postprocessing was performed as an imaging-based approach with a three-material decomposition algorithm. The use of a monochromatic X-ray beam in DECT would eliminate the beam-hardening artefacts, averaging attenuation effects and deformation or non-linear motion during imaging acquisition. Therefore, DECT scanning images would enable more objective and easier assessment of the therapeutic response of RFA. The dual-energy technique is based on the fact that attenuation of different materials and tissues varies with the tube potential, and therefore the X-ray spectrum, they are scanned with. The spread and ratio of attenuation values at a high and low tube voltage will expand with a bigger difference in the X-ray spectrum to a certain point and can be characteristic for certain materials and tissues [36]. Chae et al [19] have already demonstrated in a pilot study that pulmonary nodules could be differentiated by DECT as benign or malignant with a sensitivity, specificity and accuracy of 92%, 70% and 82.2%, respectively. Furthermore, iodine distribution maps have been used qualitatively in the evaluation of pulmonary perfusion [21] and the assessment of treatment response in gastrointestinal stromal tumour [37]. Another study has indicated that iodine-related attenuation correlates with the maximum standardised uptake value of 18FDG-PET, which has a predictive value for patient outcome to treatment [38]. DECT has brought the option for material and tissue differentiation into clinical routine [14]. Although there have been only a limited number of studies demonstrating the diagnostic value of VNC images or the iodine map of DECT in the abdomen [38,39], only Lee et al [18] have already shown that the iodine map could improve the conspicuity of the ablation zone more than linearly blended images because of its excellent internal homogeneity and sharp ablative margin in evaluating the therapeutic response of liver malignancies to RFA. To our knowledge, no study of iodine quantification with DECT for distinguishing periablational reactive tissue from residual tumours after RFA has been performed.
We initially validated our ability to quantify the iodine concentration in a phantom study by measuring correlation between calculated and true iodine concentrations. True iodine concentration was overestimated with a mean error of 0.11 mg ml−1, but this error was small for concentrations in the physiological range. The results show a good consistency between calculated and true iodine concentrations. To eliminate individual differences for precise quantitative information, we also normalised the results. The ratio of iodine concentration in the lesion to that in the aorta was therefore used to compare the degree of enhancement between lesions.
According to our study results, we ascertained that the iodine concentration and lesion-to-aorta iodine ratio could not differentiate the enhanced soft tissue at enhanced CT using dual energy in the 3-day and 1-week groups. Rapid and strong contrast enhancement is bound up with high vascularity in tumours and interstitial accumulation of contrast material via increased permeability of tumour capillaries. These features are often present in malignant tumours [40]. However, increased blood flow and capillary permeability in acute and early subacute inflammations are factors that possibly affect obvious contrast enhancement, even with a higher enhancement than the viable tumour in light of the histological findings, which was the reason for our finding in the 3-day group.
In our study, the evaluation of tumour vascularity by measuring the iodine concentration and lesion-to-aorta iodine ratio has proved to be helpful for distinguishing the residual tumour after RFA from the inflammation tissue using dual energy in the 2-week group during the AP and in the 3-week group during both the AP and the PVP. This may have been owing to the time dependence of the enhancement of the inflammatory reaction. During the middle–late subacute stage of inflammation, fibrosis gradually increased with time, and the peripheral rim enhancement indicating reactive inflammation became weaker and partly showed high- or isoattenuation during the PVP. Enhancement of inflammatory reaction appeared to be time-dependent and showed a declining trend. DECT offers the potential for a range of CT tissue characterisation [14]. DECT imaging has the potential to greatly expand the clinical application of tissue differentiation [41]. The iodine quantification and lesion-to-aorta iodine ratio with DECT in the residual tumour were significantly higher than those in benign periablational reactive tissue. This finding agrees with the results of an earlier experimental study about the clinical utility of DECT in the evaluation of solitary pulmonary nodules [19]. Both the density of the lesion and the vascularity of the inflammatory reaction can change with time, and simply measuring the attenuation on conventional contrast-enhanced images fails to capture these complex changes. DECT with an iodine quantification technique can potentially tease out perfusion changes and might be a better monitor method of response to RFA therapy. In addition, it has already been confirmed that the DECT technique can decrease the radiation dosage by omitting true non-contrast images [19,36,39].
Our study had several limitations. First, the results obtained in this study are from a single type of tumour in rabbits and may not represent the general enhancement pattern of all HCCs, although the animal model afforded very similar conditions to the clinical situation. Second, the number of rabbits was relatively small, which may limit the statistical power of the experiment study. However, we believed that the obtained data were sufficient to achieve our aims. The feasibility of evaluation of therapeutic effects of RFA in HCC with an iodine map requires further study.
In conclusion, the results obtained suggest that iodine quantification with DECT is accurate in a phantom and for differentiating benign periablational reactive tissue from residual tumour in VX2 carcinoma in rabbits after RFA; enhancement of inflammatory reaction appears to be time-dependent, and the data suggest that, clinically, iodine quantification with DECT should be performed 2–3 weeks or more after RFA. This should be tested in future experimental and clinical investigations.
FUNDING
The study was supported by the Research Foundation of Health Bureau of Hebei Province, China (number 20090152).
REFERENCES
- 1.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005;103:1201–9 10.1002/cncr.20892 [DOI] [PubMed] [Google Scholar]
- 2.Lee MW, Kim YJ, Park SW, Hwang JH, Jung SI, Jeon HJ, et al. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: a preliminary study of combined treatment with transarterial chemoembolisation. Br J Radiol 2009;82:908–15 [DOI] [PubMed] [Google Scholar]
- 3.Wood BJ, Ramkaransingh JR, Fojo T, Walther MM, Libutti SK. Percutaneous tumor ablation with radiofrequency. Cancer 2002;94:443–51 10.1002/cncr.10234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montella L, Addeo R, Caraglia M, Faiola V, Guarrasi R, Vincenzi B, et al. Vascular endothelial growth factor monitoring in advanced hepatocellular carcinoma patients treated with radiofrequency ablation plus octreotide: a single center experience. Oncol Rep 2008;20:385–90 [PubMed] [Google Scholar]
- 5.Choi H, Loyer EM, DuBrow RA, Kaur H, David CL, Huang S, et al. Radio-frequency ablation of liver tumors: assessment of therapeutic response and complications. Radiographics 2001;21:S41–54 [DOI] [PubMed] [Google Scholar]
- 6.Dromain C, de Baere T, Elias D, Kuoch V, Ducreux M, Boige V, et al. Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow-up. Radiology 2002;223:255–62 [DOI] [PubMed] [Google Scholar]
- 7.Raman SS, Lu DSK, Vodopich DJ, Sayre J, Lassman C. Creation of radiofrequency lesions in a porcine model: correlation with sonography, CT, and histopathology. AJR Am J Roentgenol 2000;175:1253–8 10.2214/ajr.175.5.1751253 [DOI] [PubMed] [Google Scholar]
- 8.Goldberg SN, Charboneau JW, Dodd GD, 3rd, Dupuy DE, Gervais DA, Gillams AR, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology 2003;228:335–45 10.1148/radiol.2282021787 [DOI] [PubMed] [Google Scholar]
- 9.Hiraoka A, Hirooka M, Koizumi Y, Hidaka S, Uehara T, Ichikawa S, et al. Modified technique for determining therapeutic response to radiofrequency ablation therapy for hepatocellular carcinoma using US-volume system. Oncol Rep 2010;23:493–7 [PubMed] [Google Scholar]
- 10.Avril N. 18F-FDG PET after radiofrequency ablation: is timing everything? J Nucl Med 2006:47:1235–7 [PubMed] [Google Scholar]
- 11.Kubota K. From tumor biology to clinical PET: a review of positron emission tomography (PET) in oncology. Ann Nucl Med 2001;15:471–86 [DOI] [PubMed] [Google Scholar]
- 12.Travaini LL, Trifirò G, Ravasi L, Monfardini L, Vigna PD, Bonomo G, et al. Role of [18F] FDG-PET/CT after radiofrequency ablation of liver metastases: preliminary results. Eur J Nucl Med Mol Imaging 2008;35:1316–22 10.1007/s00259-008-0748-7 [DOI] [PubMed] [Google Scholar]
- 13.Chandarana H, Megibow AJ, Cohen BA, Srinivasan R, Kim D, Leidecker C, et al. Iodine quantification with dual-energy CT: phantom study and preliminary experience with renal masses. AJR Am J Roentgenol 2011;196:W693–700 10.2214/AJR.10.5541 [DOI] [PubMed] [Google Scholar]
- 14.Graser A, Johnson TR, Chandarana H, Macari M. Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur Radiol 2009;19:13–23 10.1007/s00330-008-1122-7 [DOI] [PubMed] [Google Scholar]
- 15.Fletcher JG, Takahashi N, Hartman R, Guimaraes L, Huprich JE, Hough DM, et al. Dual-energy and dual-source CT: is there a role in the abdomen and pelvis? Radiol Clin North Am 2009;47:41–57 10.1016/j.rcl.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 16.Holmes DR, 3rd, Fletcher JG, Apel A, Huprich JE, Siddiki H, Hough DM, et al. Evaluation of non-linear blending in dual-energy computed tomography. Eur J Radiol 2008;68:409–13 10.1016/j.ejrad.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrendt FF, Schmidt B, Plumhans C, Keil S, Woodruff SG, Ackermann D, et al. Image fusion in dual energy computed tomography: effect on contrast enhancement, signal-to-noise ratio and image quality in computed tomography angiography. Invest Radiol 2009;44:1–6 10.1097/RLI.0b013e31818c3d4b [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Lee JM, Kim KW, Klotz E, Kim SH, Lee JY, et al. Dual-energy computed tomography to assess tumor response to hepatic radiofrequency ablation: potential diagnostic value of virtual noncontrast images and iodine maps. Invest Radiol 2011;46:77–84 10.1097/RLI.0b013e3181f23fcd [DOI] [PubMed] [Google Scholar]
- 19.Chae EJ, Song JW, Seo JB, Krauss B, Jang YM, Song KS. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: initial experience. Radiology 2008;249:671–81 10.1148/radiol.2492071956 [DOI] [PubMed] [Google Scholar]
- 20.Gupta RT, Ho LM, Marin D, Boll DT, Barnhart HX, Nelson RC. Dual-energy CT for the characterization of adrenal nodules: initial experience. AJR Am J Roentgenol 2010;194:1479–83 10.2214/AJR.09.3476 [DOI] [PubMed] [Google Scholar]
- 21.Zhang LJ, Zhao YE, Wu SY, Yeh BM, Zhou CS, Hu XB, et al. Pulmonary embolism detection with dual-energy CT: experimental study of dual-source CT in rabbits. Radiology 2009; 252:61–70 10.1148/radiol.2521081682 [DOI] [PubMed] [Google Scholar]
- 22.Zhang LJ, Wu S, Wang M, Lu L, Chen B, Jin L, et al. Quantitative dual energy CT measurements in rabbit VX2 liver tumors: comparison to perfusion CT measurements and histopathological findings. Eur J Radiol 2012;81:1766–75 10.1016/j.ejrad.2011.06.057 [DOI] [PubMed] [Google Scholar]
- 23.Zhang LJ, Yang GF, Zhao YE, Zhou CS, Lu GM. Detection of pulmonary embolism using dual-energy computed tomography and correlation with cardiovascular measurements: a preliminary study. Acta Radiol 2009;50:892–901 10.1080/02841850903095393 [DOI] [PubMed] [Google Scholar]
- 24.Li Q, Du J, Yu M, He G, Luo W, Li H, et al. Transmission electron microscopy of VX2 liver tumors after high-intensity focused ultrasound ablation enhanced with SonoVue. Adv Ther 2009;26:117–25 10.1007/s12325-008-0126-7 [DOI] [PubMed] [Google Scholar]
- 25.Liang XM, Tang GY, Cheng YS, Zhou B. Evaluation of a rabbit rectal VX2 carcinoma model using computed tomography and magnetic resonance imaging. World J Gastroenterol 2009;15:2139–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KW, Lee JM, Kim JH, Klotz E, Kim HC, Han JK, et al. CT color mapping of the arterial enhancement fraction of VX2 carcinoma implanted in rabbit liver: comparison with perfusion CT. AJR Am J Roentgenol 2011;196:102–8 10.2214/AJR.09.3971 [DOI] [PubMed] [Google Scholar]
- 27.Petersilka M, Bruder H, Krauss B, Stierstorfer K, Flohr TG. Technical principles of dual source CT. Eur J Radiol 2008;68:362–8 10.1016/j.ejrad.2008.08.013 [DOI] [PubMed] [Google Scholar]
- 28.Solbiati L, Goldberg SN, Ierace T, Livraghi T, Meloni F, Dellanoce M, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology 1997;205:367–73 [DOI] [PubMed] [Google Scholar]
- 29.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, III, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 2005;235:728–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, et al. Hepatocellular carcinoma treated with percutaneous radio-frequency ablation: evaluation with follow-up multiphase helical CT. Radiology 2001;221:447–54 [DOI] [PubMed] [Google Scholar]
- 31.Antoch G, Vogt FM, Veit P, Freudenberg LS, Blechschmid N, Dirsch O, et al. Assessment of liver tissue after radiofrequency ablation: findings with different imaging procedures. J Nucl Med 2005;46:520–5 [PubMed] [Google Scholar]
- 32.Kim CK, Choi D, Lim HK, Kim SH, Lee WJ, Kim MJ, et al. Therapeutic response assessment of percutaneous radiofrequency ablation for hepatocellular carcinoma: utility of contrast-enhanced agent detection imaging. Eur J Radiol 2005;56:66–73 [DOI] [PubMed] [Google Scholar]
- 33.Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med 2001;42:1412–170 [PubMed] [Google Scholar]
- 34.Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ, et al. Biodistribution and pharmaco kinetics of the alphavbeta3-selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333–41 [PubMed] [Google Scholar]
- 35.Platt JF, Reige KA, Ellis JH. Aortic enhancement during abdominal CT angiography: correlation with test injections, flow rates, and patient demographics. AJR Am J Roentgenol 1999;172:53–6 10.2214/ajr.172.1.9888738 [DOI] [PubMed] [Google Scholar]
- 36.Zatz LM. The effect of the kVp level on EMI values. Radiology 1976;119:683–8 [DOI] [PubMed] [Google Scholar]
- 37.Apfaltrer P, Meyer M, Meier C, Henzler T, Barraza JM, Jr, Dinter DJ, et al. Contrast-enhanced dual-energy CT of gastrointestinal stromal tumors is iodine-related attenuation a potential indicator of tumor response? Invest Radiol 2012;47:65–70 10.1097/RLI.0b013e31823003d2 [DOI] [PubMed] [Google Scholar]
- 38.Schmid-Bindert G, Henzler T, Chu TQ, Meyer M, Nance JW, Schoepf UJ, et al. Functional imaging of lung cancer using dual energy CT: how does iodine related attenuation correlate with standardized uptake value of 18FDG-PET-CT? Eur Radiol 2012;22:93–103 10.1007/s00330-011-2230-3 [DOI] [PubMed] [Google Scholar]
- 39.Graser A, Johnson TR, Hecht EM, Becker CR, Leidecker C, Staehler M, et al. Dual-energy CT in patients suspected of having renal masses: can virtual nonenhanced images replace true nonenhanced images? Radiology 2009;252:433–40 10.1148/radiol.2522080557 [DOI] [PubMed] [Google Scholar]
- 40.Abramovitch R, Marikovsky M, Meir G, Neeman M. Stimulation of tumour angiogenesis by proximal wounds: spatial and temporal analysis by MRI. Br J Cancer 1998;77:440–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson NG, Butler AP, Scott NJ, Cook NJ, Butzer JS, Schleich N, et al. Spectroscopic (multi-energy) CT distinguishes iodine and barium contrast material in MICE. Eur Radiol 2010;20:2126–34 10.1007/s00330-010-1768-9 [DOI] [PubMed] [Google Scholar]