Abstract
Objective:
Passive scattering proton beam (PSPB) radiotherapy for accelerated partial-breast irradiation (APBI) provides superior dosimetry for APBI three-dimensional conformal photon radiotherapy (3DCRT). Here we examine the potential incremental benefit of intensity-modulated proton radiotherapy (IMPT) for APBI and compare its dosimetry with PSPB and 3DCRT.
Methods:
Two theoretical IMPT plans, TANGENT_PAIR and TANGENT_ENFACE, were created for 11 patients previously treated with 3DCRT APBI and were compared with PSPB and 3DCRT plans for the same CT data sets. The impact of range, motion and set-up uncertainties as well as scanned spot mismatching between fields of IMPT plans was evaluated.
Results:
IMPT plans for APBI were significantly better regarding breast skin sparing (p<0.005) and other normal tissue sparing than 3DCRT plans (p<0.01) with comparable target coverage (p=ns). IMPT plans were statistically better than PSPB plans regarding breast skin (p<0.002) and non-target breast (p<0.007) in higher dose regions but worse or comparable in lower dose regions. IMPT plans using TANGENT_ENFACE were superior to that using TANGENT_PAIR in terms of target coverage (p<0.003) and normal tissue sparing (p<0.05) in low-dose regions. IMPT uncertainties were demonstrated for multiple causes. Qualitative comparison of dose–volume histogram confidence intervals for IMPT suggests that numeric gains may be offset by IMPT uncertainties.
Conclusion:
Using current clinical dosimetry, PSPB provides excellent dosimetry compared with 3DCRT with fewer uncertainties compared with IMPT.
Advances in knowledge:
As currently delivered in the clinic, PSPB planning for APBI provides as good or better dosimetry than IMPT with less uncertainty.
Accelerated partial-breast irradiation (APBI) limits the radiation target to the volume surrounding the surgical cavity and reduces the treatment time from 3–7 weeks to 1 week or less. APBI is considered to be an appropriate alternative to whole-breast irradiation for early stage breast cancer in selected patients [1], although recent studies have highlighted the potential risks of first generation catheter-based therapies and photon-based external beam approaches [2,3]. To date, APBI therapy has been reported using multiple catheter-based approaches, using external beam conformal therapy with photons or protons and using interstitial brachytherapy techniques [4–10].
Several researchers have investigated APBI using passive scattering proton beam (PSPB) radiotherapy [11–16] in which the proton beam is typically spread out laterally via a double scattering system and longitudinally along the beam axis via a rotating modulator wheel [17]. Although PSPB has been shown to significantly reduce the radiation dose delivered to normal breast tissue, lungs and heart compared with photon three-dimensional conformal radiotherapy (3DCRT) [11–16], it has the potential disadvantage of delivering close to 100% of the prescribed dose to the skin for each beam. Indeed, higher rates of skin toxicity were reported in the literature with PSPB APBI [13], although dose, planning and delivery factors may have influenced this. Using deliberate multibeam configuration arrangements and planning, PSPB plans can render skin-sparing in high-dose regions comparable with 3DCRT plans [11,13,16] and have low reported skin toxicity [11]. This affords a highly conformal non-invasive APBI treatment that achieves comparable normal tissue sparing expected from catheter-based approaches with the homogeneity of photon-based approaches.
Although 100% skin doses using PSPB may be clinically acceptable, given a report of significant skin toxicity with this approach with a slightly different dose per fractionation than has been typically used for bid 3DCRT with photons [4 Gy (relative biological effectiveness; RBE) per fraction vs 3.85 Gy in 10 fractions], we investigated the potential benefit for reducing skin dose using APBI delivered with intensity-modulated proton radiotherapy (IMPT). IMPT is delivered via a scanning proton pencil beam, which paints the treatment target spot-by-spot, using scanning magnets to control lateral spot location and varying initial proton energy to control spot depth [18]. The scanning proton beam permits greater proximal conformity than PSPB if the target is deep enough, thereby permitting additional skin sparing compared with PSPB. In addition, the intensities and energies of all pencil beams can be optimised simultaneously in IMPT plans according to user-defined objectives that take into account target and normal-tissue constraints. As such, the dose distribution of IMPT plans can be shaped to potentially achieve higher conformity than PSPB and thereby better spare normal tissue. However, studies of clinical use of IMPT are limited and significant uncertainties must be considered and weighed against the potential benefits. To our knowledge, no investigations of IMPT for APBI have been reported thus far. In this paper, we compare IMPT with both PSPB and 3DCRT for APBI and evaluate the impact of range, motion and set-up uncertainties as well as scanned spot mismatching between fields for IMPT plans with two different beam configurations.
METHODS AND MATERIALS
CT scanning, treatment planning and dose distribution
The CT data sets for 11 patients who underwent APBI at our institution—10 who received photon 3DCRT as per the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-39/Radiation Therapy Oncology Group (RTOG) 0413 trial [19] and 1 who received photon and electron (not permitted on NSABP B-39/RTOG 0413) 3DCRT off protocol—were retrospectively selected and replanned using PSPB [16] and IMPT. The CT images were obtained at 2.5-mm slice thickness through the region of interest. All patients were in the supine position with the ipsilateral upper extremity abducted and head rotated slightly towards the contralateral side. The definition of clinical target volume (CTV) followed NSABP B-39/RTOG 0413 guidelines, i.e. uniformly expanding lumpectomy 1.5 cm but no shallower than breast skin and no deeper than the anterior chest wall and pectoralis muscles. In addition to all normal structures defined in the NSABP B-39/RTOG 0413 trial, the breast skin and ipsilateral normal (non-target) breast tissue were contoured for each patient. The “breast skin” in this analysis was defined as a rind of tissue from the ipsilateral breast surface outlined to a depth of 5 mm. The “ipsilateral normal breast” was defined as the ipsilateral breast volume (i.e. breast glandular tissue included in standard whole-breast irradiation fields) excluding the CTV to highlight the dose to the non-target breast.
The 3DCRT plans were designed using the Pinnacle (Philips Medical System, Mipitas, CA) treatment planning system (TPS). The three to five coplanar and non-coplanar beams using 6 MV and 18 MV photons were generated based on patient-specific anatomy and target location. A multileaf collimator was used to manually optimise the plan to maximise the target coverage and minimise normal tissue doses. For the mixed-modality plan, multiphoton beams were designed with an en face electron beam. The target dose and the specified normal tissue constraint followed NSABP B-39/RTOG 0413 protocol. All 3DCRT plans were reviewed and approved by seven breast cancer radiation oncologists in a routine clinical quality assurance conference and used for patient treatment.
The proton plans were designed using the Eclipse™ (Eclipse Proton, Varian Medical Systems Inc., Palo Alto, CA) TPS and proton beam lines of the Hitachi PROBEAT™ (proton beam therapy system; Hitachi Ltd, Tokyo, Japan) at our institution. To equalise dose and planning across modalities for the sake of comparison, the prescription dose was 38.5 Gy (RBE) for 10 fractions for all planning studies [in practice PSPB patients treated on a Phase II protocol at our institution are prescribed 34 Gy (RBE) in 10 fractions]. Three to four beams were used for plans using PSPB. Each beam entrance was designed to have minimal overlap on the patient surface to reduce the skin dose when feasible. The proximal and distal margins along each beam axis were designed using Moyer's formula [20], although less may be feasible in clinical practice for breast given the tissue homogeneity and similarity to unit density. Radial margins were designed to cover 1 cm expansion of CTV (i.e. accounts for the lateral set-up uncertainties of breast irradiation using protons, followed the definition in NSABP B-39/RTOG 0413 for planning target volume (PTV)_eval for consistency). Two IMPT plans with different beam configurations were designed for each patient. One plan used two tangential beams (TANGENT_PAIR), and the other plan used one tangential beam and one en face beam (TANGENT_ENFACE). Although the correct PTV concept for proton planning is beam specific, it is technically impossible to design a single volume a priori in which to place spots that accounts for range uncertainties for multidirectional beams. As such, an approximation spot grid volume was used. The maximum and minimum energies for each beam were determined using distal and proximal margins of the 1 cm expansion of CTV. The radial margin was set to 1.0 cm. The determination of distal, proximal and radial margin of the beam followed the definition in NSABP B-39/RTOG 0413 for PTV_eval. This creates a comparable volume with the 3DCRT plans but skews the results in favour of 3DCRT as this is undoubtedly larger than needed distally for a shallow target. A 6.7-cm range shifter, as used with the Hitachi PROBEAT delivery system, was included in each beam path to ensure target coverage on the proximal edge. An inverse planning technique and a simultaneous spot optimisation algorithm was used to generate all IMPT plans. To further level the comparison between planning modalities, all plans were normalised for comparable coverage of the PTV_eval. In practice, prescribing to a PTV_eval would be inappropriate. PSPB coverage should be evaluated beam-by-beam, radially, distally and proximally. IMPT plans at this time must be evaluated in a “PTV-like” way examining the coverage of the spot grid designed to consider uncertainties within the limitations of current planning. Ultimately, evaluation of CTV coverage with IMPT will be through robustness analysis not yet available in clinical practice. This renormalisation for dosimetric comparison herein then further skews the results towards 3DCRT.
The dose distributions and dose–volume histogram (DVH) indices of targets and normal structures were calculated and collected for 3DCRT, PSPB and two IMPT plans as the following: the percentage volume receiving ≥90% of prescription dose (V90) for CTV and PTV_eval; V90, V75, V50 and V20 for non-target breast volume; V90, V75, V50, V30 and V10 for breast skin; the maximum dose of heart, contralateral breast and lungs; the mean dose, V5 Gy (percentage of volume receiving dose ≥5 Gy, V10 Gy and V20 Gy for ipsilateral lung. The DVHs of all normal structures and targets as well as dose distributions were compared among 3DCRT, PSPB and two IMPT plans.
The Friedman multigroups test with pairwise Wilcoxon-signed rank test as the post hoc test, corrected by Holm–Bonferroni approach, was used for statistical analysis with p≤0.05 as the significance level. The six pairs of data were compared and the corrected significance levels were: 1p≤0.0083, 2p≤0.010, 3p≤0.0125, 4p≤0.0167, 5p≤0.025 and 6p≤0.050, the superscript of p was the rank of six p-values, i.e. the most significant of six p-values was 1p, the second most significant of six p-values was 2p etc.
IMPT uncertainties
Numerous IMPT planning and delivery uncertainties must be taken into consideration in designing and implementing an IMPT program. Because an average of the magnitude of each effect cannot be summed across multiple effects to gauge the impact, and important outliers in specific circumstances are likely to be as important if not more important than the mean, we sought to present a demonstration of the effects to be weighed against numeric benefits demonstrated in dosimetric comparative analyses of IMPT and other approaches rather than a quantitation of the average effect size. These issues represent basic principles in IMPT planning and must be considered specifically to each case.
Patient motion and range uncertainties
We used the CT data set for a randomly selected patient from 11 patients. First, to simulate patient set-up and range uncertainties, the isocenter of proton plan (IMPT or PSPB) was shifted 5 mm in anterior, posterior, superior, inferior, right and left directions to simulate patient set-up uncertainties, and the dose distributions calculated for the stopping power ratio to CT number conversions increased and decreased 3.5% to simulate range uncertainties [16]. To simulate beamlets mismatching from scanning beams, i.e. two pencil beamlets from two separate scanning beams, which originally planned to deliver the dose to the same spot (i.e. spot matching of two beamlets) but actually deliver the dose to the different spots (i.e. spot mismatching of two beamlets), we shifted the isocenter of each beam in the IMPT plan individually 5 mm in the anterior, posterior, superior, inferior, right and left directions and calculated the dose distributions for these 12 scenarios (i.e. 12 scenarios for two beams IMPT). DVHs were calculated for all these dose distributions to quantify the variation range of dose distribution to different structures from deteriorated IMPT plans owing to various treatment uncertainties.
For each of the scenarios described above, we identified the conditions under which there was the greatest uncertainty. It should be noted that in current treatment practice, these uncertainties would persist in treatment delivery, as robustness optimisation is not in use clinically. To evaluate whether planning approaches that are under development may be able to reduce these uncertainties, we next incorporated this information into the treatment planning process using a “Worst-Case Robust Optimisation Method” [21]. The potential of this type of method is to remove the PTV concept altogether, beam specific or otherwise. In the robust optimisation method, we incorporated the effects of uncertainties (i.e. set-up uncertainties, range uncertainties and spot mismatching) on treatment planning during the optimisation of each step of inverse planning rather than analysing the impact of uncertainties after treatment planning. Compared with conventionally optimised plans, which are sensitive to various uncertainties, robustly optimised plans are more insensitive to these uncertainties. The uncertainties of the robust optimised plan were further analysed as described in the previous paragraph to illustrate the improvement of variation range of DVHs for the target and different structures.
Respiratory motion and beam geometry uncertainty
To illustrate the dosimetric effects of patient respiratory motion uncertainties for IMPT from different beam directions, two IMPT plans, each with a single proton scanning beam from the tangential and en face directions, respectively, were designed on a free-breathing CT and were applied to a deep inspiration-breath-hold CT from the same patient to demonstrate the effect on dosimetry. To illustrate the effects of range uncertainty for IMPT from different beam directions, two IMPT plans, each with either a single tangential beam or a single en face beam, were designed on one patients' CT data set with the target near the chest wall. The dose difference distributions between the original plan and the range overshoot plan (i.e. dose distribution of the plan calculated with the stopping power ratio to CT number decreasing 3.5%, which translates to 3.5% overshoot in the beam direction) [16] on the patient were calculated for two IMPT plans to show the dosimetric effect of range uncertainties using different beam directions.
RESULTS
Dose distributions
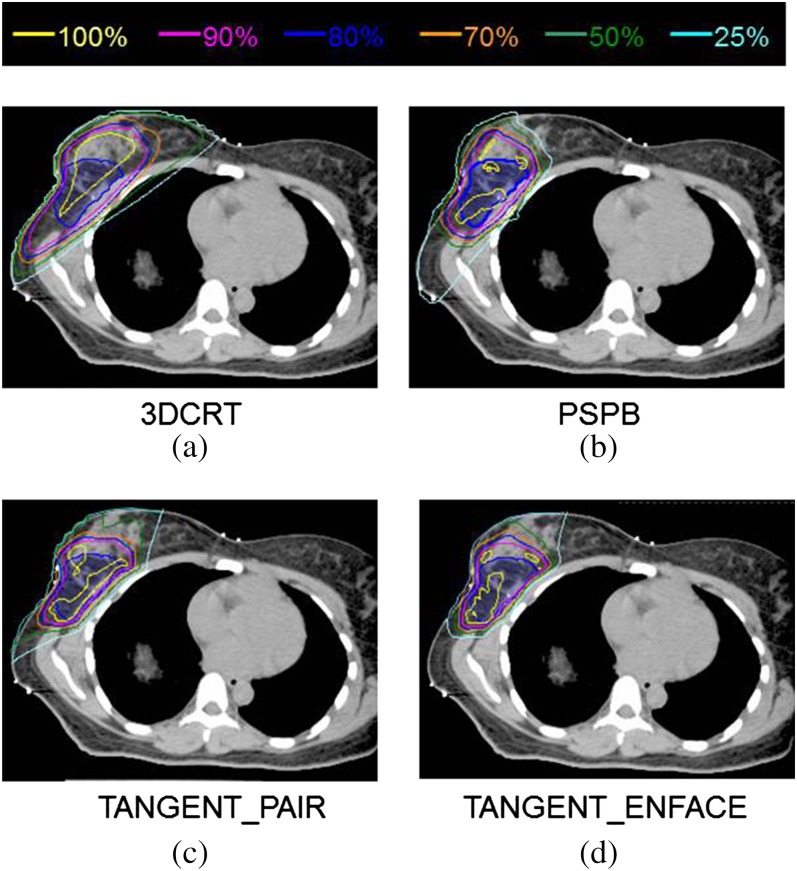
Isodose distributions are shown in Figure 1 for a representative patient for (a) 3DCRT, (b) PSPB, (c) IMPT with TANGENT_PAIR and (d) IMPT with TANGENT_ENFACE treatment plans. Similar dose distributions were observed for all patients in this study. Quantitative dose–volume results are summarised in Table 1 for 3DCRT, PSPB, IMPT with TANGENT_PAIR and IMPT with TANGENT_ENFACE treatment plans. Qualitatively, the IMPT and PSPB plans delivered lesser dose to normal structures and were more conformal to the target than the 3DCRT plans (Table 1). Compared with PSPB, the dose distributions of IMPT were statistically more conformal to the target in the higher dose regions and statistically less in the lower dose regions. There was no difference between IMPT and PSPB to heart, lungs or contralateral breast in this series. Note that the planning objective to clinically normalise planning to achieve comparable coverage to the PTV_eval for the sake of this comparison resulted in statistically significant differences in PTV_eval coverage often <1% highlighting the importance of considering the clinical significance of statistical differences.
Figure 1.
Comparison of dose distributions among (a) three-dimensional conformal photon radiotherapy (3DCRT), (b) passive scattering proton beam (PSPB), (c) intensity-modulated proton radiotherapy (IMPT) with TANGENT_PAIR and (d) IMPT with THANGENT_ENFACE treatment plans for one of the study patients.
Table 1.
Dosimetric values and comparisons for proton and three-dimensional (3D) conformal plans
| IMPT TANG_EN | IMPT TANG_PA | pa | 3DCRT | pb | pc | PSPB | pd | pe | |
| PTV_eval | |||||||||
| V90 (%) | 98.40% (95.0–100) | 97.50% (95.0–99.0) | 10.001 | 96.90% (92.0–99.4) | 20.001 | 60.375 | 96.40% (92.0–99.2) | 30.001 | 40.059 |
| Clinical target volume | |||||||||
| V90 (%) | 99.60% (99.0–100) | 99.00% (96.0–100) | 10.003 | 99.50% (97.7–100) | 60.922 | 30.064 | 99.70% (98.0–100) | 50.625 | 20.017 |
| Ipsilateral normal breast | |||||||||
| V90 (%) | 9.70% (4.00–21.00) | 9.80% (4.00–23.00) | 60.966 | 19.40% (9.20–47.50) | 10.001 | 20.001 | 12.40% (7.00–26.80) | 30.001 | 40.001 |
| V75 (%) | 17.10% (8.00–33.00) | 19.10% (10.00–38.00) | 50.010 | 30.00% (21.40–58.70) | 10.001 | 20.001 | 19.30% (10.90–36.40) | 40.007 | 60.296 |
| V50 (%) | 28.00% (12.00–48.00) | 30.50% (17.00–57.00) | 50.006 | 43.50% (28.60–68.80) | 10.001 | 20.001 | 26.90% (13.80–47.80) | 60.577 | 40.002 |
| V20 (%) | 41.90% (20.00–65.00) | 44.30% (27.00–69.00) | 50.054 | 60.20% (39.10–86.00) | 10.001 | 20.001 | 38.80% (26.50–57.40) | 60.320 | 40.010 |
| Contralateral breast | |||||||||
| Maximum dose (Gy) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 61.000 | 2.20 (0.19–10.30) | 10.001 | 20.001 | 0.12 (0.00–0.82) | 40.125 | 50.125 |
| Ipsilateral lung | |||||||||
| Mean dose (Gy) | 0.61 (0.08–1.28) | 0.81 (0.20–1.44) | 40.003 | 3.07 (0.39–6.06) | 10.001 | 20.001 | 0.84 (0.00–1.90) | 50.311 | 60.765 |
| V5Gy (%) | 3.50% (0.00–10.00) | 4.90% (0.00–10.00) | 40.007 | 18.40% (0.00–47.00) | 10.002 | 20.002 | 5.30% (0.10–12.40) | 50.278 | 60.906 |
| V10Gy (%) | 1.30% (0.00–5.00) | 2.10% (0.00–6.00) | 40.004 | 8.80% (0.00–17.00) | 10.002 | 20.002 | 2.90% (0.00–7.60) | 50.102 | 60.322 |
| V20Gy (%) | 0.10% (0.00–1.00) | 0.20% (0.00–1.00) | 60.156 | 3.50% (0.00–9.00) | 10.004 | 20.004 | 1.40% (0.00–4.20) | 30.020 | 50.105 |
| Contralateral lung | |||||||||
| Mean dose (Gy) | 0.02 (0.00–0.16) | 0.01 (0.00–0.13) | 40.500 | 0.13 (0.04–0.40) | 10.001 | 20.001 | 0.01 (0.00–0.07) | 50.875 | 60.875 |
| Heart | |||||||||
| Maximum dose (Gy) | 3.20 (0.00–16.10) | 2.90 (0.00–14.00) | 60.438 | 9.40 (0.78–37.00) | 20.01 | 30.01 | 6.00 (0.00–31.65) | 40.156 | 50.281 |
| Breast skin | |||||||||
| V90 (%) | 4.00% (0.00–11.00) | 3.70% (0.00–12.00) | 50.320 | 7.60% (1.50–22.50) | 30.005 | 40.005 | 11.50% (2.40–21.10) | 10.001 | 20.002 |
| V75 (%) | 14.50% (4.00–29.00) | 15.50% (6.00–35.00) | 60.278 | 22.70% (10.60–52.20) | 10.001 | 20.001 | 18.9% (10.00–38.20) | 30.001 | 40.002 |
| V50 (%) | 26.10% (17.00–48.00) | 30.80% (21.00–57.00) | 30.001 | 40.20% (27.90–73.80) | 10.001 | 20.001 | 27.80% (17.80–52.40) | 60.147 | 50.010 |
| V30 (%) | 37.00% (23.00–64.00) | 42.00% (31.00–70.00) | 30.001 | 52.60% (37.30–86.00) | 10.001 | 20.001 | 39.80% (28.10–59.30) | 60.067 | 50.064 |
| V10 (%) | 47.80% (31.00–77.00) | 52.90% (40.00–80.00) | 30.001 | 69.50% (47.80–92.20) | 10.001 | 20.001 | 47.30% (32.60–67.60) | 60.898 | 50.003 |
3DCRT, three-dimensional conformal photon radiotherapy; PSPB, passive scattering proton beam; TANG_EN, TANGENT_ENFACE; TANG_PA, TANGENT_PAIR.
np≤10.008, 20.010, 30.0125, 40.0167, 50.025, 60.050; the superscript is the rank of p-value. The italicised p-values are statistically significant.
Comparison of TANG_EN to TANG_PA.
Comparison of 3DCRT to TANG_EN.
Comparison of 3DCRT to TANG_PA.
Comparison of PSPB to TANG_EN.
Comparison of PSPB to TANG_PA.
Compared with 3DCRT, using TANGENT_PAIR absolutely reduced the average V10, V30, V50, V75 and V90 of breast skin by 16.6%, 10.6%, 9.4%, 7.2% and 3.9%, respectively; using TANGENT_ENFACE absolutely reduced the average V10, V30, V50, V75 and V90 of breast skin by 21.7%, 15.6%, 14.1%, 8.2% and 3.6%, respectively. Similarly, the average V20, V50, V75, V90 and V100 of ipsilateral normal breast were reduced by 15.9%, 13.0%, 10.9%, 9.6% and 3.1%, respectively, using TANGENT_PAIR and by 18.9%, 15.5%, 12.9%, 9.7% and 3.3%, respectively, using TANGENT_ENFACE compared with 3DCRT.
Compared with PSPB, both IMPT plans had similar dose coverage for the targets (Table 1) but more favourable V90 and V75 of breast skin (p<0.002). There were similar V50, V30 and V10 of breast skin using TANGENT_ENFACE and less favourable V50, V30 and V10 of breast skin using TANGENT_PAIR. Compared with PSPB, IMPT plans had more favourable V90 and V75 (p<0.007) and comparable V50 and V20 of ipsilateral normal breast tissue using TANGENT_ENFACE; more favourable V90 (p=0.001), comparable V75 and less favourable V50 and V20 (p<0.002) of ipsilateral normal breast tissue using TANGENT_PAIR. The IMPT plans had similar ipsilateral lung, contralateral lung, contralateral breast and heart dose compared with PSPB.
A comparison of the two different IMPT plans demonstrated that the TANGENT_ENFACE beam configuration had better target coverage than the TANGENT_PAIR configuration (Table 1). Furthermore, the TANGENT_ENFACE configuration had lower V50, V30 and V10 of breast skin; lower V75, V50 and V20 of ipsilateral normal breast and lower ipsilateral lung V10 and V5 than the TANGENT_PAIR plan. It was comparable with the TANGENT_PAIR plan for all other DVH indices.
Dosimetric variations owing to treatment uncertainties of IMPT
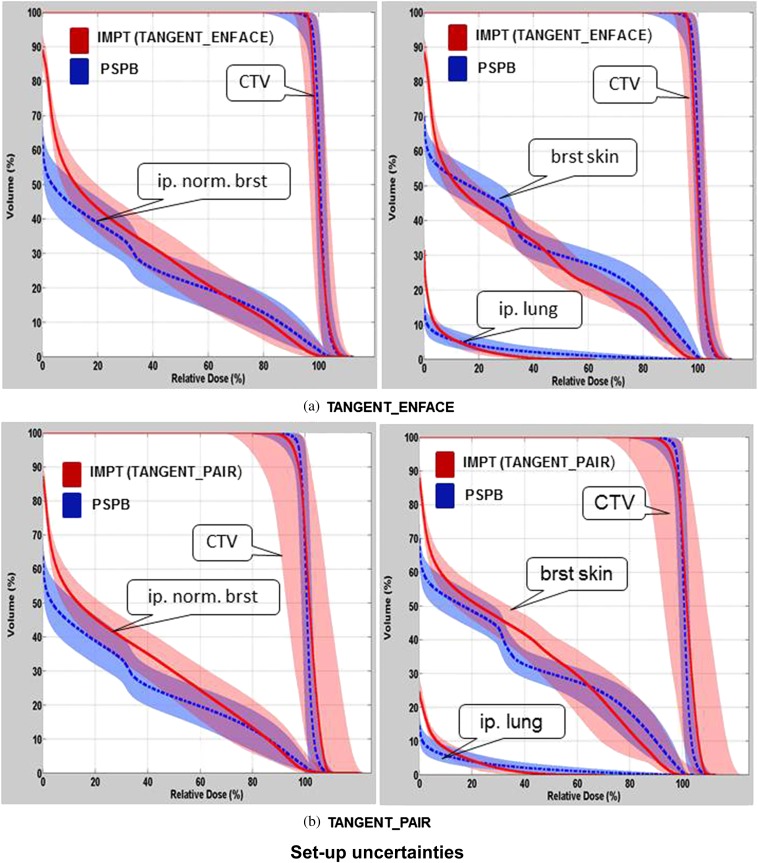
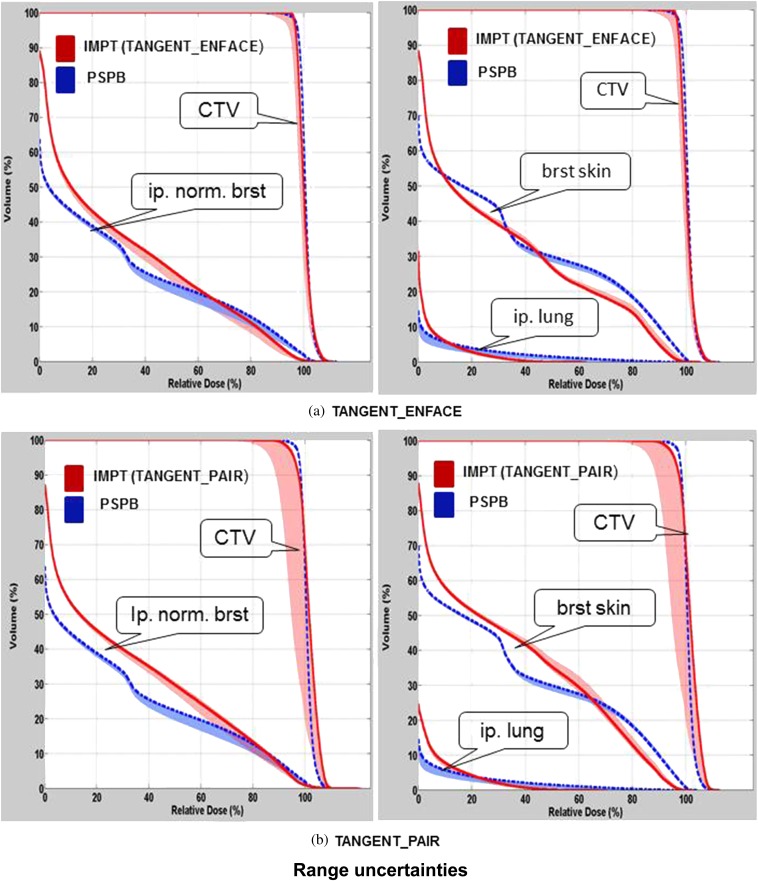
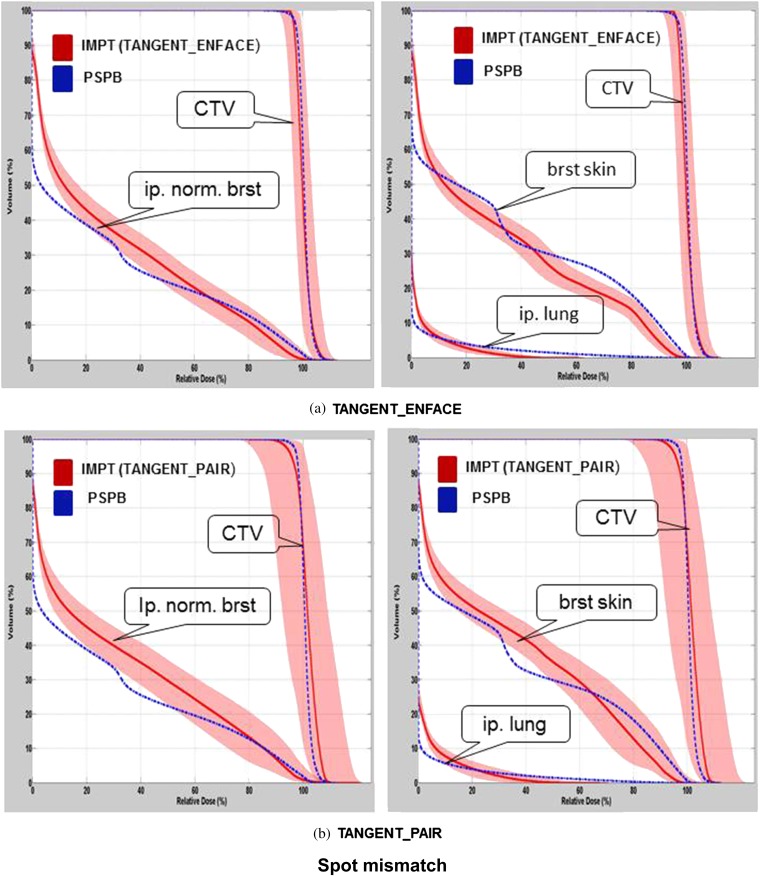
The variation in the DVHs of CTV, breast skin, ipsilateral normal breast and ipsilateral lung owing to patient set-up uncertainties, range uncertainties, for one selected patient using PSPB and IMPT, are shown in Figures 2 and 3. Figure 4 shows the variation of DVHs from spot mismatching of IMPT plans (note there is no spot mismatching uncertainty for PSPB). The DVHs using IMPT TANGENT_PAIR for set-up and spot mismatching had the largest deterioration (i.e. the widest band; Figures 2b and 4b), and the DVHs from range uncertainties had the smallest deterioration (i.e. the narrowest band; Figure 3). Considering the outer bands on the CTV, in the worst-case scenario, >90% of CTV was covered by 90% prescribed dose when accounting for set-up uncertainties, range uncertainties and spot mismatching for PSPB and IMPT TANGENT_ENFACE plans. However, only 85% of CTV was covered by 90% prescribed dose for the IMPT TANGENT_PAIR plan in the worst scenario when accounting for set-up and spot mismatching. It is worth noting this analysis for set-up uncertainty only addresses set-up uncertainty at the isocenter and not set-up uncertainty owing to breathing which will be examined in the section headed “Pros and cons of tangential vs en face beam on IMPT”.
Figure 2.
Comparison of dose–volume histogram (DVH) variations for various normal structures and clinical target volume (CTV) between passive scattering proton beam (PSPB; blue band) and intensity-modulated proton radiotherapy (IMPT; red band) using (a) TANGENT_ENFACE and (b) TANGENT_PAIR owing to patient set-up uncertainties for one example patient. The shadowed band represents the DVH variation range, and the solid line represents the DVH from the original plan. brst skin, breast skin; ip. lung, ipsilateral lung; ip. norm. brst, ipsilateral normal breast.
Figure 3.
Comparison of dose–volume histogram (DVH) variations between passive scattering proton beam (PSPB; blue band) and intensity-modulated proton radiotherapy (IMPT; red band) using (a) TANGENT_ENFACE and (b) TANGENT_PAIR owing to range uncertainties for the patient shown in Figure 2. The shadowed band represents the DVH variation range, and the solid line represents the DVH from the original plan. brst skin, breast skin; CTV, clinical target volume; ip. lung, ipsilateral lung; ip. norm. brst, ipsilateral normal breast.
Figure 4.
Dose–volume histogram (DVH) variations owing to spot mismatching with intensity-modulated proton radiotherapy (IMPT; red band) using (a) TANGENT_ENFACE and (b) TANGENT_PAIR for the patient shown in Figure 2. The shadowed band represents the DVH variation range, and the solid line represents the DVH from the original plan. brst skin, breast skin; ip. lung, ipsilateral lung; ip. norm. brst, ipsilateral normal breast PSPB, passive scattering proton beam.
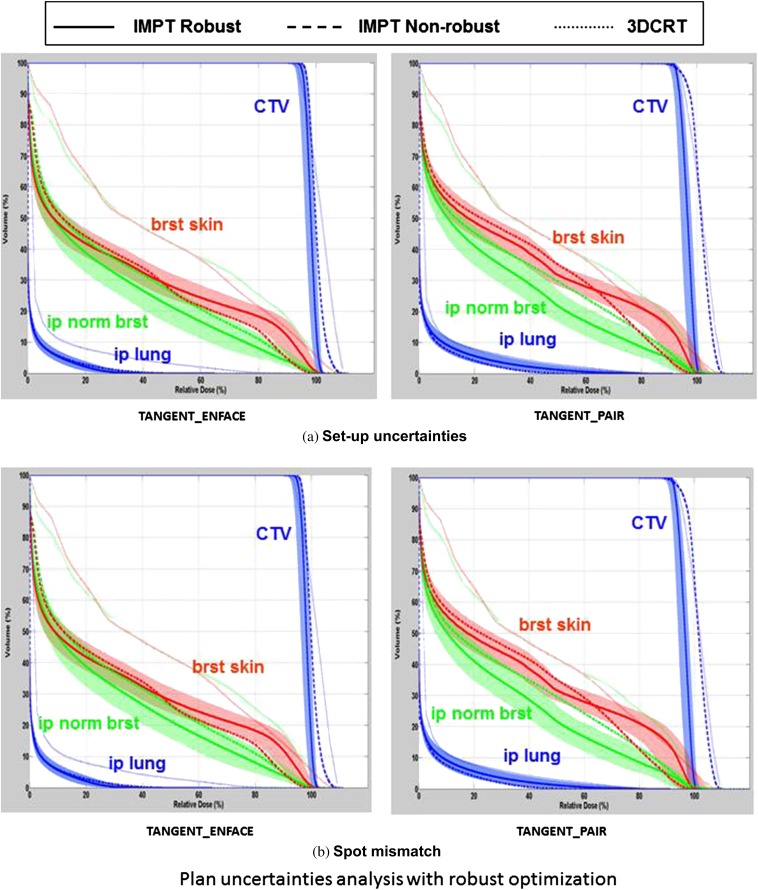
Statistics in Table 1 and DVHs in Figures 2–4 are derived from clinical IMPT planning and do not include robustness optimisation techniques. Robustness optimisation will increase or decrease the margins for uncertainty where the algorithm assessing robustness maintains robust target coverage. The variations in the DVHs of CTV, breast skin, ipsilateral normal breast and ipsilateral lung owing to patient set-up uncertainties and spot mismatching from the robust optimised IMPT plans of the same patient described in Figures 2–4 are shown in Figure 5. In this case, robust optimisation improves the normal tissue DVHs, likely because robust optimisation eliminates the unnecessary increases in coverage that were forced into the IMPT planning to create more comparable plans while maintaining the coverage. The DVH bands of the CTV were narrower than that of the non-robust plans, indicating the reduced sensitivity of the robust optimised IMPT plan to set-up uncertainties and spot mismatching for CTV coverage. Overlaying the DVHs of the 3DCRT plan and the non-robust and robust TANGENT_ENFACE and TANGENT_PAIR plans in Figure 5 demonstrates the normal tissue dose sparing for IMPT over 3DCRT is maintained even considering the wider bandwidth addressing the uncertainties. The confidence intervals (band width) on the normal tissue DVHs for robust optimisation in general encompass the DVHs from the non-robustly optimised planning, highlighting the importance of accounting for uncertainties in ascribing clinical significance to numeric DVH improvements. The target was covered more uniformly in the robust IMPT plans than in the non-robust plans and the 3DCRT plan. Of note, the maximum dose in the CTV was numerically lower than 3DCRT for both IMPT and PSPB approaches (Figure 5). The DVHs from the PSPB plan with confidence intervals generated by worst-case analysis are compared with the similar confidence intervals on the IMPT plan. The PSPB DVHs fall within the uncertainty bandwidth of IMPT, suggesting the numeric differences in DVH may be outweighed by the uncertainties.
Figure 5.
Dose–volume histogram (DVH) variation range (wide band) owing to (a) patient set-up uncertainties and (b) spot mismatching using the worst-case robust optimisation method and DVH comparison among intensity-modulated proton radiotherapy (IMPT) with robust optimisation (solid line), IMPT with non-robust optimisation (dashed line), and 3DCRT (dotted line) for TANGENT_PAIR and TANGENT_ENFACE, for the patient shown in Figure 2. 3DCRT, three-dimensional conformal photon radiotherapy; brst skin, breast skin; CTV, clinical target volume; ip lung, ipsilateral lung; ip norm brst, ipsilateral normal breast.
Pros and cons of tangential vs en face beam on IMPT
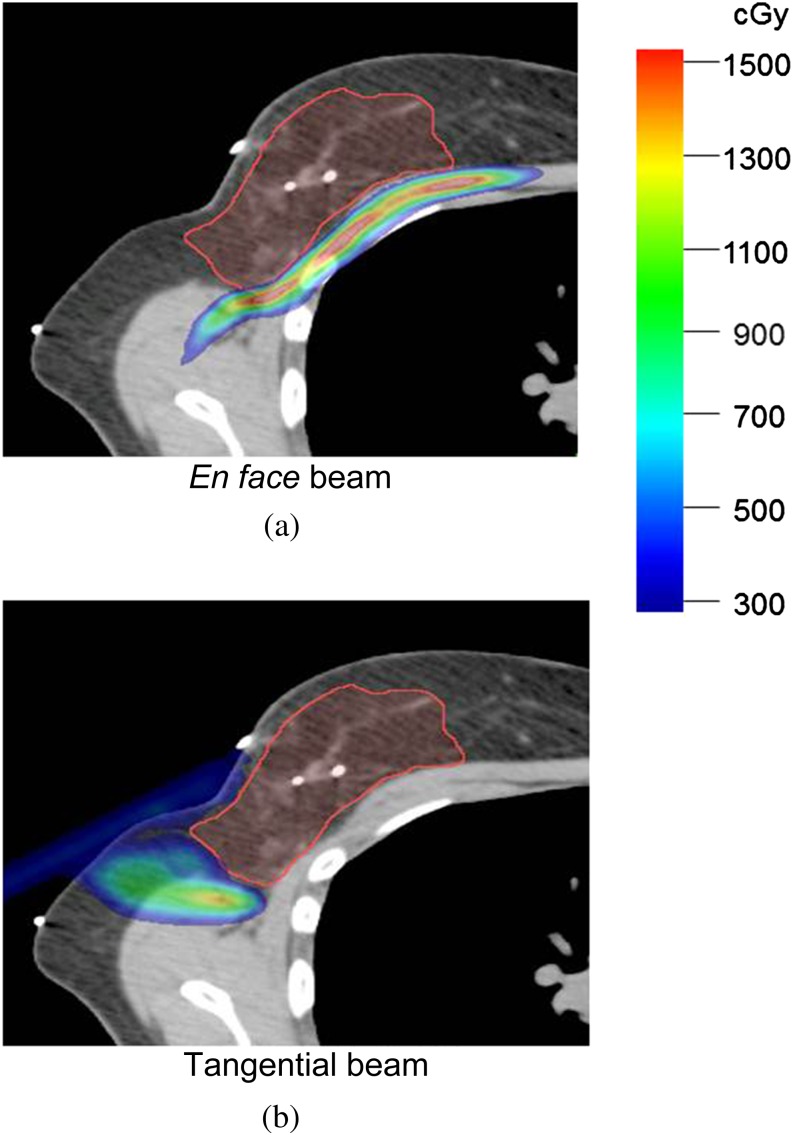
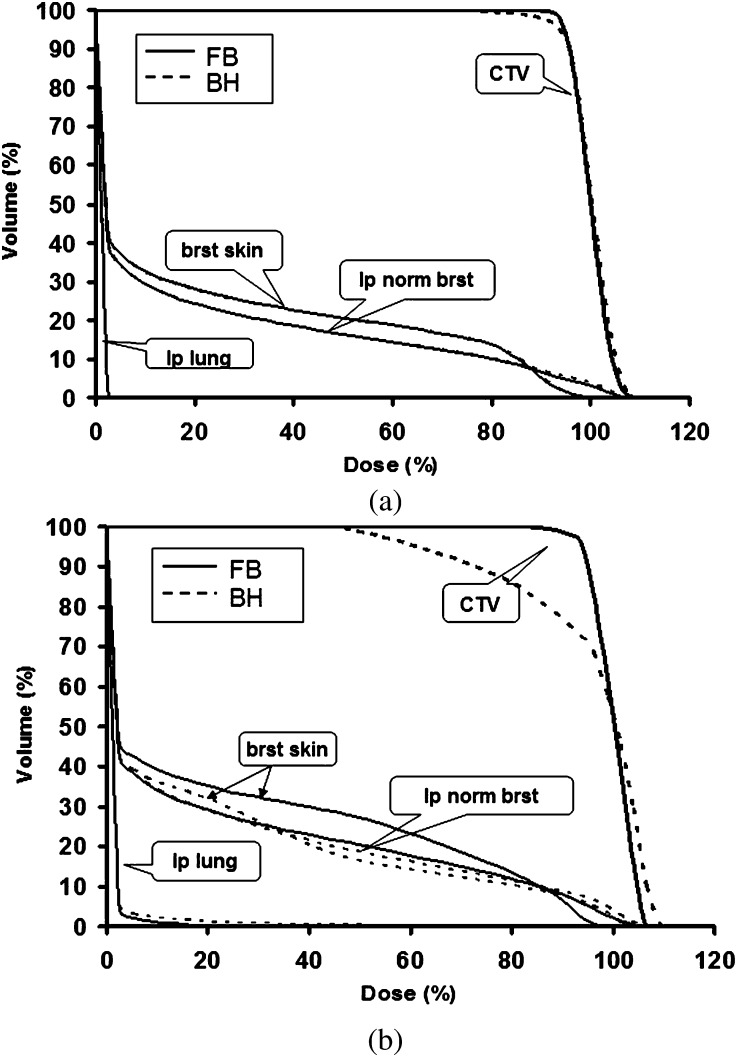
Figures 2–4 also demonstrate the importance of choosing beam angles to minimise uncertainty with large uncertainty bands on the CTV in the TANGENT_PAIR IMPT plan. Figure 6 shows the dose difference distributions of the original and range overshoot IMPT plans using the single en face beam (Figure 6a) vs the single tangential beam (Figure 6b). There were regions near the chest wall with >15 Gy dose difference for the plan using an en face beam and fewer regions with dose differences for the plan with single tangential beam. The DVHs of IMPT plans using single tangential beam and single en face beam based on free-breathing CT and deep-breath-hold CT are shown in Figure 7. The plan with single tangential beam was very sensitive to respiratory motion; the CTV V90 was <80% (Figure 7b). The plan with single en face beam had less deterioration of CTV dose coverage with deep inspiration breath-hold (Figure 7a). These data illustrate basic principles and inform clinical planning principles to use multiple beams to spread out the risk of range out onto the ribs and potential rib fracture related to higher RBE at the end of range as well as to minimise the use of tangential beams that are highly sensitive to range uncertainties.
Figure 6.
Impact of range overshoot on the chest wall and lung for intensity-modulated proton radiotherapy with (a) en face beams and (b) tangential beams. The red colour wash represents the area with dose difference >15 Gy.
Figure 7.
Dose–volume histograms (DVHs) on free-breathing (FB) CT (solid line) and breath-hold (BH) CT (dashed line) for (a) en face beams and (b) tangential beams. brst skin, breast skin; CTV, clinical target volume; ip lung, ipsilateral lung; ip norm. brst, ipsilateral normal breast.
DISCUSSION
Recent studies, including a Phase III clinical trial comparing whole-breast irradiation to external beam photon APBI, demonstrate unacceptable rates of poor cosmesis using photon-based APBI [22]. Given the relative infancy of APBI and the evolving understanding of potential risks and benefits of APBI [23], meticulous target coverage that preserves the non-target sparing is of the utmost importance. PSPB APBI eliminates the excess dose to non-target breast tissue seen with photons while preserving the dose homogeneity in the target typical of photon-based approaches [11,13,16,24,25]. Here we examine the potential incremental benefit of IMPT for APBI over PSPB. Like PSPB, IMPT significantly reduced the dose to normal tissue compared with 3DCRT; however, modest statistically significant improvements in dose to normal tissue over PSPB appear to be offset by uncertainties in IMPT planning.
Although studies have indicated that IMPT using scanning beams produces superior dose distribution for multiple disease sites [26,27], IMPT is sensitive to delivery uncertainties. Intrafractional motion, including respiratory motion, could cause two pencil beamlets from two scanning beams to be mismatched. Interfractional motion, from patient set-up uncertainties, could cause two matching beamlets to deliver the dose to an untargeted area. If IMPT plans meet only minimum clinical requirements for target coverage and normal tissue constraints, the large variation in dose from these uncertainties can result in underdosing the target or overdosing normal structures. The benefit for IMPT over 3DCRT in normal tissue sparing was clearly demonstrated, even considering uncertainties. However, it means the risk of underdosage to the CTV in plans targeting the CTV with proton appropriate margins may be larger than measured here. Breast tissue is relatively homogeneous that makes IMPT for APBI relatively insensitive to range uncertainties. For set-up and spot mismatching uncertainties, however, when reviewing the DVH bands to examine the possible worst-case scenarios, significant decrements are seen in CTV coverage particularly related to set-up uncertainty.
Although patient set-up uncertainties and uncertainties caused by spot mismatching are random uncertainties and can be averaged out by repainting dose and fractionating treatments, these data argue for caution using IMPT without significant attention to minimise set-up uncertainty.
Technology advances in IMPT may further reduce the uncertainty regarding CTV coverage. We demonstrate that the worst-case robust optimisation method can be used to overcome uncertainties and improve the validity of the treatment plan for CTV coverage. This method does not necessarily sacrifice target coverage or normal tissue sparing [21]. Uncertainties regarding normal structures remain large however. Using this approach, we only consider maximum dose uncertainties for normal structures, thus the uncertainty bands of DVHs of normal structures were not narrowed in the robust optimised plan. Unlike CTVs, there are no minimum DVHs for normal structures which can be used to control the lower range of uncertainty band. This is desired because we should not penalise the normal structures that might achieve better normal tissue sparing. Since most commercial TPSs have not offered the capability of robust optimisation, the results shown in Figure 5 obtained from an in-house developed research system clearly demand the clinical urgency to implement the robust optimisation to truly maximising the potential of proton therapy. Currently, the IMPT technique is still in its infancy period of technology development in terms of hardware and software. This work clearly demonstrates that if (1) we can reduce the set-up uncertainties, (2) have more confidence on the range uncertainties and (3) have better optimisation algorithms such as robust optimisation adopted in clinical treatment, the IMPT technique will be the preferred method for APBI treatment.
The advantages and disadvantages of the different beam orientations with regard to the uncertainties of scanning beam delivery are very similar to those observed in PSPB [16]. Because breathing motion is parallel to the beam axis, en face beams are less sensitive to breathing motion uncertainties but potentially range onto ribs and lungs, which may increase the risk for toxicity in these structures. Conversely, the tangential beams are highly sensitive to uncertainties related to breathing motion and set-up inaccuracy, and, as such, could lead to underdosing the target or overdosing the normal breast tissue. Overall, IMPT is much more sensitive to uncertainties than PSPB [16], especially motion uncertainties, regardless of the beam configuration used. This is related to the interplay between position variability and actively scanned beamlets in IMPT plans. Figure 7 shows that the target was severely underdosed using the free-breathing designed IMPT plan on a deep-breath hold CT data set that represents the worst-case scenario regarding breathing motion. The respiration motion of free breathing was usually small (<2 mm) and might be negligible for many free-breathing patients in a supine position with ipsilateral shoulder abducted and the chest fully extended. The uncertainty analysis for IMPT discussed above assumed that interfractional variation is of rigid body type and does not consider deformations and changes in positions of anatomic structures relative to each other as might occur with soft tissue swelling or seroma resolution during treatment. It only showed the dosimetric effects of each uncertainty individually. In reality, multiple uncertainties may exist simultaneously, highlighting the importance of careful planning as well as reproducible positioning and target volume monitoring from planning CT to treatment.
Protons for APBI provide clearly superior dosimetry to 3DCRT. IMPT is another advance in proton radiotherapy technology. IMPT dose distributions may be painted and optimised with highly modulated beamlet intensities and energies to reduce the dose to normal tissues. Although careful planning with PSPB also reduces normal tissue dose, compared with PSPB, the scanning proton beam without compensators and blocks delivers less unnecessary secondary scattering to the patient and requires no in-room block changes. With the better and better understanding of IMPT uncertainties and advanced techniques to limit its uncertainties, IMPT may have real advantages over PSPB. At the current state of the art, however, PSPB appears as good as, if not better than, IMPT dosimetrically and more robust.
CONCLUSIONS
PSPB APBI provides excellent homogeneous target coverage and superior normal tissue sparing compared with 3DCRT. Uncertainties in IMPT planning, dominated by uncertainties influenced by set-up, limit the robustness of IMPT for APBI using current delivery and planning technology. Further, the tighter penumbra of PSPB offsets the potential benefit of IMPT for this relatively superficial target.
FUNDING
UT MD Anderson Cancer Center.
REFERENCES
- 1.Smith BD, Arthur DW, Buchholz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). Int J Radiat Oncol Biol Phys 2009;74:987–1001 [DOI] [PubMed] [Google Scholar]
- 2.Smith GL, Xu Y, Buchholz TA, Giordano SH, Jiang J, Shih Y-CT, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA 2012;307:1827–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whelan TJ, Olivotto I, Parpia S, Berrang T, Kim D, Kong I, et al. Interim toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation (APBI) using 3D conformal external beam radiation therapy (3DCRT). Int J Radiat Oncol Biol Phys 2013;85:21–2 [DOI] [PubMed] [Google Scholar]
- 4.Rusthoven KE, Carter DL, Howell K, Kercher JM, Henkenberns P, Hunter KL, et al. Accelerated partial-breast intensity-modulated radiotherapy results in improved dose distribution when compared with three-dimensional treatment-planning techniques. Int J Radiat Oncol Biol Phys 2008;70:296–302 [DOI] [PubMed] [Google Scholar]
- 5.Wazer DE. Point: brachytherapy for accelerated partial breast irradiation. Brachytherapy 2009;8:181–3 10.1016/j.brachy.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 6.Bensaleh S, Bezak E, Borg M. Review of MammoSite brachytherapy: advantages, disadvantages and clinical outcomes. Acta Oncol 2009;48:487–94 10.1080/02841860802537916 [DOI] [PubMed] [Google Scholar]
- 7.Orecchia R, Veronesi U. Intraoperative electrons. Semin Radiat Oncol 2005;15:76–83 [DOI] [PubMed] [Google Scholar]
- 8.Polgár C, Strnad V, Major T. Brachytherapy for partial breast irradiation: the European experience. Semin Radiat Oncol 2005;15:116–22 [DOI] [PubMed] [Google Scholar]
- 9.Vaidya JS, Tobias JS, Baum M, Wenz F, Kraus-Tiefenbacher U, D'Souza D, et al. TARGeted intraoperative radiotherapy (TARGIT): an innovative approach to partial-breast irradiation. Semin Radiat Oncol 2005;15:84–91 [DOI] [PubMed] [Google Scholar]
- 10.Vicini FA, Arthur DW. Breast brachytherapy: North American experience. Semin Radiat Oncol 2005;15:108–15 [DOI] [PubMed] [Google Scholar]
- 11.Bush DA, Slater JD, Garberoglio C, Do S, Lum S, Slater JM. Partial breast irradiation delivered with proton beam: results of a phase II trial. Clin Breast Cancer 2011;11:241–5 10.1016/j.clbc.2011.03.023 [DOI] [PubMed] [Google Scholar]
- 12.Fogliata A, Bolsi A, Cozzi L. Critical appraisal of treatment techniques based on conventional photon beams, intensity modulated photon beams and proton beams for therapy of intact breast. Radiother Oncol 2002;62:137–45 [DOI] [PubMed] [Google Scholar]
- 13.Kozak KR, Smith BL, Adams J, Kornmehl E, Katz A, Gadd M, et al. Accelerated partial-breast irradiation using proton beams: initial clinical experience. Int J Radiat Oncol Biol Phys 2006;66:691–8 10.1016/j.ijrobp.2006.06.041 [DOI] [PubMed] [Google Scholar]
- 14.Moon SH, Shin KH, Kim TH, Yoon M, Park S, Lee D-H, et al. Dosimetric comparison of four different external beam partial breast irradiation techniques: three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, helical tomotherapy, and proton beam therapy. Radiother Oncol 2009;90:66–73 10.1016/j.radonc.2008.09.027 [DOI] [PubMed] [Google Scholar]
- 15.Taghian AG, Kozak KR, Katz A, Adams J, Lu HM, Powell SN, et al. Accelerated partial breast irradiation using proton beams: initial dosimetric experience. Int J Radiat Oncol Biol Phys 2006;65:1404–10 [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Amos RA, Zhang X, Taddei PJ, Woodward WA, Hoffman KE, et al. External-beam accelerated partial breast irradiation using multiple proton beam configurations. Int J Radiat Oncol Biol Phys 2011;80:1464–72 10.1016/j.ijrobp.2010.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koehler AM, Schneider RJ, Sisterson JM. Flattening of proton dose distributions for large-field radiotherapy. Med Phys 1977;4:297–301 [DOI] [PubMed] [Google Scholar]
- 18.Kanai T, Kawachi K, Kumamoto Y, Ogawa H, Yamada T, Matsuzawa H, et al. Spot scanning system for proton radiotherapy. Med Phys 1980;7:365–9 [DOI] [PubMed] [Google Scholar]
- 19.NSABP B-39/RTOG 0413 protocol. Philadelphia, PA: Radiation Therapy Oncology Group; 2009. Available from: www.oncuview.tv/portals/0/linkedfiles/NSABP%20Protocol%20B-39,%20RTOG%20Protocol%200413.pdf [Google Scholar]
- 20.Moyers MF, Miller DW, Bush DA, Slater JD. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys 2001;49:1429–38 [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Zhang X, Li Y, Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys 2012;39:1079 10.1118/1.3679340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagsi R, Ben-David MA, Moran JM, Marsh RB, Griffith KA, Hayman JA, et al. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys 2010;76:71–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith GL, Xu Y, Buchholz TA, Giordano SH, Smith BD. Partial breast brachytherapy is associated with inferior effectiveness and increased toxicity compared with whole breast irradiation in older patients. SABCS 2011;71:S2–1 [Google Scholar]
- 24.Bush DA, Slater JD, Garberoglio C, Yuh G, Hocko JM, Slater JM. A technique of partial breast irradiation utilizing proton beam radiotherapy: comparison with conformal X-ray therapy. Cancer J 2007;13:114–18 10.1097/PPO.0b013e318046354b [DOI] [PubMed] [Google Scholar]
- 25.Taghian AG, Kozak KR, Doppke KP, Katz A, Smith BL, Gadd M, et al. Initial dosimetric experience using simple three-dimensional conformal external-beam accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys 2006;64:1092–9 [DOI] [PubMed] [Google Scholar]
- 26.Ares C, Khan S, Gruber G, MacArtain AM, Lutters G, Heuberger J, et al. Intensity-modulated proton-versus photon radiotherapy for locoregional, left sided breast cancer: a dose-comparison to heart and ipsilateral lung. EJC Suppl 2007;5:190 [Google Scholar]
- 27.Welsh J, Gomez D, Palmer MB, Riley BA, Mayankkumar AV, Komaki R, et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: a dosimetric study. Int J Radiat Oncol Biol Phys 2011;81:1336–42 [DOI] [PMC free article] [PubMed] [Google Scholar]