Abstract
Stereotactic ablative radiotherapy (SABR) has developed from the principles and techniques used in the stereotactic radiosurgery treatment of brain metastases. Advances in computer technology, imaging, planning and treatment delivery and evidence from retrospective analysis of single- and multi-institutional early-phase studies have established SABR in the treatment of medically inoperable early lung cancer. Effective multidisciplinary team working is crucial to safe delivery of SABR. The variation in patient selection, radiotherapy planning and delivery techniques has led to a collective approach to SABR implementation across the UK. Centres developing the technique are represented in the UK SABR Consortium, which is supported by the relevant UK professional bodies and represents a platform to develop extracranial SABR across the UK. The uptake of SABR in the UK has been slowed by workforce issues, but at least 15 centres are currently delivering treatment with over 500 patients treated using UK SABR Consortium guidance. A mentoring program is being piloted helping new centres to develop their programs, and over 30 UK centres are expected to be offering SABR treatment by the end of 2014. The use of consistent guidance for patient selection, treatment planning and delivery in the UK gives the opportunity to collect and audit toxicity and outcome across the centres, contributing to the internationally reported SABR experience. Having established this service in the UK, the development of SABR through clinical research is a priority, and with input from the Radiotherapy Trials Quality Assurance Group, the UK is developing a national study program that includes participation in international trials.
Stereotactic ablative radiotherapy (SABR), also described as stereotactic body radiotherapy (SBRT), takes the principles of intracranial stereotactic radiosurgery and applies them to extracranial sites. SABR aims to deliver an ablative radiation dose in 3–8 fractions, typically >7.5 Gy over 2–3 weeks, with high precision and accuracy of 2–3 mm. The radiobiological rationale for SABR is that in delivering a few large fractions over a short overall treatment time, a more potent biological effect is achieved [1]. However, using a high dose per fraction to treat extracranial lesions poses significant challenges owing to both the inter- and the intrafractional motion of the tumour and the organ at risk (OAR) [2].
Despite lacking modern radiotherapy planning and delivery techniques, early studies of efficacy and toxicity from SABR regimens reported local control rates comparable to surgery [3]. Modern lung SABR is characterised by use of advanced planning algorithms, with improved modelling of the heterogeneity of the lung tissue [4], and image-guided radiotherapy (IGRT) techniques that incorporate patient-specific tumour motion and ensure accurate set-up [5].
The advantage of the SABR technique has been demonstrated for early-stage lung cancer patients, who are unfit for radical surgery, with improved local control and disease-specific survival compared with conventional radiotherapy [6–8] and reported rates of serious toxicity (≥Grade 3) below 5% [9]. Despite a lack of phase III trial evidence, SABR has now become an internationally established treatment for early lung cancer based on several centres reporting large case series with consistent outcomes [10]. The strongest evidence may come from the Netherlands, where the introduction of SABR has led to an increase in radiotherapy treatments in the over-75-years population with improved survival from early lung cancer without adversely affecting surgical resection rates [11].
Within this evidence for early-stage non-small-cell lung cancer (NSCLC), where there is significant variation in patient selection and dose/fractionation, a few principles are well established: (i) a biologically equivalent dose of ≥100 Gy is needed to achieve high local control rates; (ii) increased toxicity is seen when central tumours, in close proximity to proximal airways, are treated with three fractions of 20 Gy [12]; and (iii) there does not appear to be an absolute contradiction of SABR with poor lung function.
The implementation and practice of SABR is a multidisciplinary team process that requires a high level of accuracy throughout the entire planning and treatment delivery process, for which clear clinical pathways need to be defined. This article aims to use results from the recent UK SABR Consortium survey to give an overview of the current status of SABR in the UK and discusses the impact of this technique on radiotherapy delivery at a national level.
STEREOTACTIC ABLATIVE RADIOTHERAPY DELIVERY IN THE UK
The development of extracranial ablative radiotherapy in the UK has been guided by a national consortium, originally named the “National Lung SBRT Consortium” but now renamed the “UK SABR Consortium”. It was founded in 2008 to ensure safe and consistent implementation of the technique across the UK. Its remit was subsequently defined by the National Radiotherapy Implementation Group (NRIG) in 2011 [13] to develop an SABR service across the UK by maintaining evidence-based prospective treatment protocols for all body sites and producing a common data set to allow meaningful analysis of treatment outcomes.
Since its inception in 2008, the UK SABR Consortium has offered free membership to staff from all UK radiotherapy centres, and over 80% of these UK centres are now regularly represented at the twice-yearly meetings by a mixture of clinicians, radiographers, medical physicists and others involved in the delivery of SABR. Over the past year, the formal activity of the UK SABR Consortium has expanded to take on a mentorship role and assist in the provision of a safe service. We will consider various aspects of that activity, given that it reflects on the current status of SABR in the UK.
UK STEREOTACTIC ABLATIVE RADIOTHERAPY GUIDELINES
In line with the NRIG recommendation to ensure a consistently high level of accuracy throughout the entire planning and treatment delivery process, comprehensive guidelines on safe implementation have been established. These guidelines, which strive to be applicable to all planning and delivery platforms, cover the key relevant publications, patient selection criteria, quality assurance recommendations, planning guidelines and dose/fractionation schedules. Updating this guidance is part of the ongoing work of the UK SABR Consortium, and the most recent version is available online (http://actionradiotherapy.org/).
Reviewing abstracts presented at the British Thoracic Oncology Group meeting held in January 2013, it is apparent that this process of local adoption is occurring across the UK, and approximately 500 patients have received lung SABR treatment that has been planned and delivered in accordance with the national guidelines, which has been focused on the treatment of early peripheral lung cancer.
Version 4 of the UK SABR Consortium guidelines, released in January 2013 and current at the time of writing, includes new chapters containing guidance on the treatment of liver metastases and prostate with SABR based on a systematic review of the relevant literature and with the agreement of the UK SABR Consortium membership. In some instances, the recommendation in this (and future versions) will be that for a particular clinical indication SABR treatment is given as part of a clinical trial. For example, with a lack of published clinical trial data that clearly demonstrates the long-term safety and effectiveness of prostate SABR, the guidance highlights the clinical and technical issues that should be considered and recommends a minimum safe level of implementation, and concludes that the introduction of prostate SABR is done in the context of clinical trials and helps to increase the evidence base. Conversely, the level of published evidence for SABR for liver metastases, summarised in the guidelines, was felt to be sufficient to recommend its use as a routine clinical option in certain well-defined circumstances.
It is important that the guidance continues to develop, given the stated intention of several UK centres to either continue or begin treating additional clinical sites with SABR for which the UK SABR Consortium has yet to produce recommendations (see below). An example is the treatment of central lung lesions where the increasing evidence is being reviewed as part of the updating process. This process remains dependent on the ongoing enthusiasm of the UK SABR Consortium members who devote a significant amount of their time without financial reimbursement to themselves or their employers.
COMMISSIONING AND QUALITY ASSURANCE
Quality assurance (QA) is an important component of the guidelines, which gives recommendations on the commissioning of SABR. The guidelines detail dosimetry requirements, immobilisation methods, assessment of tumour motion methods, treatment planning techniques and types of algorithms which should be used, linear accelerator QA, image guidance and plan delivery techniques. All these areas are also covered in the American Association of Physicists in Medicine (AAPM) Task Report 101 [14]. The NRIG report [13] also proposed that the UK SABR Consortium should lead on the development of an interdepartmental audit program that should include a systematic test of all aspects of local SABR programs. It further recommended that within 6 months of commencing SABR, each centre should have undergone an independent external audit of its SABR processes and in-house QA.
These recommendations resulted in the UK SABR Consortium receiving around 30 requests for support to implement clinical SABR programs. These ranged from e-mail requests for advice to performing a full external audit and highlighted that the lack of funding for a nationwide QA programme has been a significant issue in implementing a national SABR program. These concerns led in April 2012 to the UK SABR Consortium forming an experienced multidisciplinary QA group who, in collaboration with the NCRI Radiotherapy Trials QA group (RTTQA) and the National Physical Laboratory (NPL), have been working to develop a fully funded national QA programme.
In 2007, prior to the UK SABR Consortium being tasked by NRIG to set up a national audit programme, Duane et al [15] suggested that such a programme should use an appropriate accredited service such as the NPL. The first part of the programme developed by the UK SABR Consortium's QA group has been the use of alanine dosimetry to check point doses across an SABR planning target volume (PTV). The NPL provided alanine to enable a simple solid-water dosimetry audit across six centres in November 2012, with centres chosen to include a variety of planning systems and delivery techniques. This initial audit was carried out to meet the immediate requests of treating centres as well as to resolve any issues before carrying out a more complex SABR audit. This is to be followed up in summer 2013 by a more extensive audit in the CIRS Lung Phantom (Computerized Imaging Reference Systems Inc., Norfolk, VA) to again include alanine dosimetry with the addition of planar dosimetry using a gafchromic film.
Other audit recommendations need to be undertaken at a local level, e.g. each centre should measure the systematic and random errors relating to their own systems of immobilisation and image guidance before the introduction of new techniques such as SABR [16]. As an example, Clatterbridge Cancer Centre reported in an evaluation of their standard lung immobilisation equipment [17] that post-treatment systematic ∑ and random σ errors were <2 mm in all directions. Similar results have been shown by a number of UK centres using standard immobilisation, and they are equivalent to values reported by centres using vacuum bag or frame devices [18,19].
A number of technical issues related to safe planning and delivery have also been discussed within the UK SABR Consortium during the evolution of the guidelines, including the choice of treatment planning algorithm and the impact of interplay effects. Although SABR PTVs are generally large enough for electronic equilibrium to be established at the centre of the tumour, the low density of the surrounding lung introduces uncertainties in the dose distribution. Therefore, the consortium guideline strongly recommends using a type-B algorithm (i.e. one that more accurately models the lack of lateral scatter). However, inhomogeneities in tissue density can reduce the good agreement seen between various treatment planning systems and measurements for SABR treatments planned with volumetric arc therapy (VMAT) [20]. A second issue is the interplay between tumour motion and multileaf collimator motion for the increasing number of patients being treated using intensity-modulated radiotherapy (IMRT) or VMAT techniques. Although there is concern that this could lead to an under- or overdose to parts of the PTV for non-gated patients, the evidence seems to suggest that this is less significant than was first thought [21,22].
UK CONSORTIUM QUESTIONNAIRES
An initial questionnaire was circulated by the UK SABR Consortium in June 2010 to assess the status of SABR in the UK. The results were reported by Baker et al [17] and identified seven treating centres with additionally six centres intending to treat using this technique in the near future.
A second nationwide questionnaire was circulated in August 2012 to update the state of SABR practice in the UK. The questionnaire was an online survey sent to 65 radiotherapy centres, took approximately 30 min to complete and aimed to ascertain the progress being made in the implementation of SABR treatment and to obtain details of current issues in centres with an active treatment programme. Questions covered several areas: current and intended number of patients being treated for each clinical site; immobilisation and motion management; four-dimensional CT; target and OAR delineation; planning; IGRT; and QA.
As of the end of November 2012, at least 15 UK centres (from the 48 that responded to the questionnaire) were treating patients with SABR. Previous national surveys from Japan and the United States suggest that despite a comparatively rapid uptake, UK SABR provision still lags behind that of other countries: approximately 56% of Japanese institutions in 2009 [23] and 64% of United States oncologists in 2011 had adopted SABR [24].
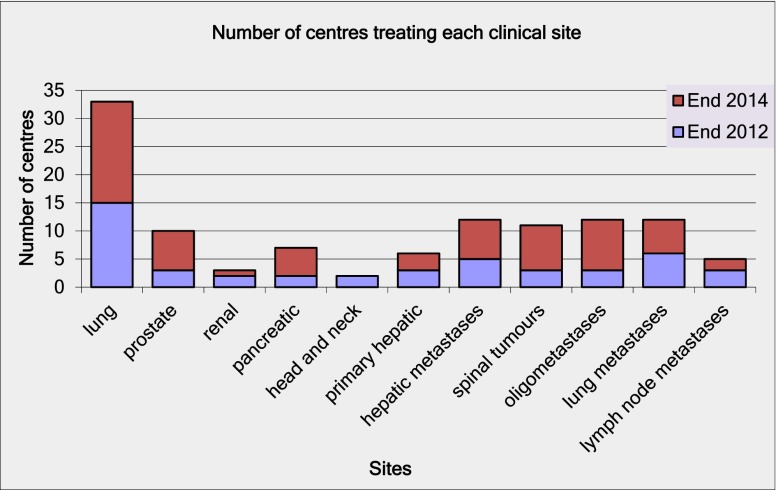
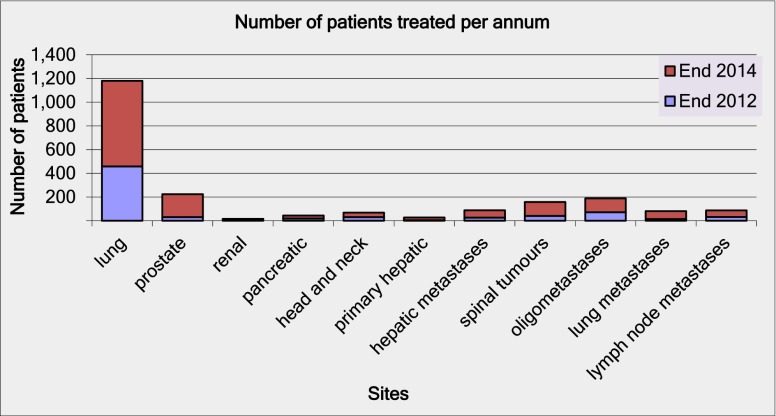
However, the questionnaire responses shown in Figure 1 suggest that this rapid early uptake is expected to continue, with a projected doubling of the number of UK centres providing SABR by the end of 2014. The responses in Figure 2 indicate that this could lead to over 1000 patients being treated with SABR annually in the UK using standardised protocols based on the UK SABR Consortium guidelines. This data may represent an optimistic projection of future uptake but would suggest that UK SABR provision will be broadly in line with international practice within 2 years. However, this projection is still at the lower end of the NRIG guidance for commissioners, which indicates that 1000–3000 patients with inoperable lung cancer should be treated with SABR [13].
Figure 1.
Results from the 2012 survey indicating the number of actively treating UK centres (including sites of treatment) and centres planning to open a stereotactic ablative radiotherapy program within 2 years.
Figure 2.
Projected increase in number of patients receiving stereotactic ablative radiotherapy treatment per year by the end of 2014.
RESOURCE IMPLICATIONS
The projected numbers from the questionnaire are only an indication of future UK provision and demand for SABR. However, a comparison of the 2010 and 2012 questionnaires suggests that rapid expansion is happening and would be expected to continue. SABR techniques place a considerably heavy workload on all members of the multidisciplinary team, which includes extra clinician outlining, complex planning, physicist QA and longer treatment sessions. The AAPM task group report suggests that “additional physics resources will be needed to implement and maintain an SABR program for most centers” [14]. However, in the current financial climate, this may not always be possible, so collaborative working is more important than ever. The success of the multidiscipliany team approach is demonstrated with many of the more experienced centres moving to a radiographer-led service.
The rapid expansion of SABR provision in this era of limited radiotherapy resources raises concerns about assuring the quality of the treatment delivered across the many centres that are involved in the UK. In response, the multidisciplinary QA group developed a mentorship program that secured limited government funding early this year to support the next three centres starting their SABR programs. This program includes a scoping visit to the recipient centre by mentors, a visit to the supporting centre by the recipient SABR team, remote support from mentors to assist with setting up local protocols, a mentor physicist visit to the recipient centre to support QA and planning of the first clinical patient, a mentor radiographer to be present for the first treatment to assist with IGRT, and remote support for subsequent patients for further 3 months. Finally, each of the mentored centres will participate in the CIRS Lung Phantom dosimetry audit.
It is hoped that this program will serve as a template for future SABR mentoring by establishing standards against which centres wanting to offer SABR can be assessed. In future, it may also be the template for the adoption of other advanced radiation technologies, such as multimodality imaging, adaptive planning or treatment of novel clinical SABR sites.
WORKFORCE DEVELOPMENT
Owing to the high technical demands of SABR, the American Society of Therapeutic Radiation Oncology (ASTRO)/American College of Radiologists (ACR) groups have published practice guidelines that detail recommendations for staffing levels and staff responsibilities for this technique [25].
Prior to the implementation of an SABR service, it has been shown to be advantageous, in all treating centres, to establish a multiprofessional team to develop and implement the technique. This team should be site specific, although some of the personnel may be involved in all anatomical site groups as there will be some crossover of expertise. It is important for the group to review their local processes using the UK SABR Consortium guidelines as a basis for this. Each step in the process needs to be discussed and considered carefully.
For lung SABR treatments, the image guidance process is a logical progression following the recent increase in the use of image guidance for lung cancer patients as suggested in the NRIG IGRT report [13]. The experience gained for routine conformal treatments will lead to a safe delegation of responsibility to competent radiographers.
Initially, the process for SABR treatments may involve the clinician and physicist leads being present at each treatment fraction to approve the soft-tissue match on the CBCT and any necessary isocentre corrections. As confidence builds, individual departments may feel that it is appropriate to change the SABR IGRT process to be radiographer led, with the SABR clinician and physicist planner available at the verification (day 0) appointment before handing over to the SABR radiographer team for subsequent treatments. This approach would utilise the physicist's knowledge of the treatment plan to guide the expert radiographer's image guidance methods for subsequent fractions to make the appropriate decision for required isocentre corrections.
The next step could be the establishment of consultant radiographer posts specialising in SABR with the technical expertise to take responsibility for soft-tissue match during treatment and to co-ordinate the SABR radiographer team of advanced imaging practitioners required for this technique. They would have their own workload of SABR patients to provide on-treatment review and follow-up clinics and would assist in the development of national SABR guidelines, training and research projects.
SERVICE DEVELOPMENT
One of the barriers to implementation of SABR in the UK is the lack of an appropriate tariff to recognise the extra planning effort required for this technique. How do we convince commissioners to ensure correct payment? Tariffs are usually based on the number of treatment fractions rather than complexity of the planning and treatment process. The use of image guidance, for example, is not currently recognised within the tariff system. This is also an issue that has been highlighted in the UK provision of inverse planned IMRT treatments [26]. Mayles discusses the need for financial recognition of the additional planning effort required for IMRT, an argument equally applicable to SABR treatments. Unless a compensating increase in payment for the treatment preparation and data collection methods is implemented, it is unlikely that the desired level of SABR provision will be achieved in the UK.
At the recent “Britain Against Cancer” 2011 conference, the Health Secretary at that time, the Right Honourable Andrew Lansley, outlined plans to develop a range of tariffs to reward high-quality, cost-effective services. Hopefully, this will help to encourage innovation and the early adoption of new techniques such as SABR.
STEREOTACTIC ABLATIVE RADIOTHERAPY RESEARCH IN THE UK
A major advantage of UK centres starting SABR within the context of the UK SABR Consortium guidelines has meant that there is little variation in patient selection, radiotherapy planning and dose fractionation across the UK centres delivering SABR. As a result, this collaboration has collected a standard set of data and has accumulated outcome and toxicity data very quickly. The first SABR patients were treated in 2009 in the UK and the total number treated now exceeds 500, with a median follow-up of around 18 months. Although any data will not be mature enough to report survival and late toxicity, there are plans to start analysing the multicentre data for early side effects and local control.
However, there are logistical challenges in trying to pool data across the centres such as confidentiality and the manpower required to analyse the available data. One of the initial aims of the UK SABR Consortium was to develop a strong research base for SABR in the UK and a research subgroup has been active in reviewing research ideas and helping support funding applications. At present, the only multicentre trial open in the UK is the industry-sponsored SABR trial in prostate cancer (PACE, UKCRN ID 12628). However, funding submissions are being submitted for research proposals for SABR in patients with early lung cancer of borderline fitness for surgery, pancreatic carcinoma and oligometastatic disease arising from breast, colorectal, lung and other primary sites. In addition, the UK SABR Consortium is seeking support to run the European Organisation for Research and Treatment of Cancer (EORTC) phase II study for SABR in central tumours (LUNG TECH, NCT01795521) in the UK.
The UK SABR Consortium's QA program will have established that the centres offering SABR in the UK will be able to participicate in these multicentre studies. An important aspect of the development of multicentre SABR trails across the UK will be a strong trials QA program co-ordinated by NCRI RTTQA, which will ensure that best practice across centres delivering SABR is maintained.
Having safely established SABR as standard of care in medically inoperable peripheral early lung cancer, the questions being asked by the international community pertain to (i) the efficacy of SABR in peripheral early operable lung cancers against surgery, (ii) the efficacy of SABR in larger and central lung cancer [Radiation Therapy Oncology Group (RTOG) 0813 and the EORTC study) and (iii) the role of SABR in oligometastatic disease. Some early institution-based data are promising. RTOG 0915, a randomised phase II trial, compared two radiotherapy schedules in medically inoperable Stage I lung cancer patients with peripheral tumours: 34 Gy/1# and 48 Gy/4# [27]. It recruited 94 patients and completed accrual in March 2011. The study is yet to report on its primary end point of >grade 3 toxicity between the two schedules at 1 year. Secondary end points include 1-year local control rates, survival, progression-free survival and other translational end points such as fludeoxyglucose (FDG) changes, pulmonary function and biomarker analysis.
The main pattern of relapse following SABR appears to be distant metastases that can be as high as 20% [28]. Trials are being proposed by the RTOG and Cancer and Leukaemia Group B (CALGB) subgroups to evaluate the role of adjuvant chemotherapy in tumours larger than T1b. Obtaining histological confirmation will be crucial to the success of these studies. Combining SABR with targeted agents against mechanisms of radiation resistance is an approach being proposed by some in the international community [29].
Defining local recurrence can be difficult following SABR owing to the changes in pulmonary parenchyma because of radiotherapy. The resulting radiological change post SABR, which is common and can be progressive, has been described well by Dahele et al [30]. Additionally since 18-FDG PET scans may not be reliable, given that standardised uptake values can be seen even in post-radiotherapy changes, where possible, histological confirmation should be sought. However, the difficulty of this process means that expert follow-up is recommended. Studies are being undertaken to develop algorithms to better distinguish local recurrence from radiation-induced changes [31].
SUMMARY
SABR for small NSCLC tumours is increasing local control rates and significantly improving the patient's quality of life. It seems likely that similar advantages are possible for other tumour sites; therefore, we expect a growth in hypofractionated techniques in the UK. The provision of SABR should be a patient-focused service with a clear vision to be able to offer all patients the most appropriate treatment. The collective approach taken to SABR implementation in the UK aims to ensure equitable nationwide access.
Teamwork, national integration and collaboration are fundamental for the rapid and safe implementation of this complex, continuously evolving technique. Specialised teams may be established with consultant radiographer roles created in this area.
It is important to acknowledge that, both in the UK and internationally, funding is needed to support both QA in the implementation of this new technology and timely data collection to ensure that the outcome of and toxicities from SABR are brought rapidly into the public domain.
Are we ready for the challenge?
ACKNOWLEDGMENTS
We would like to thank the UK SABR Consortium members for their input to this article.
REFERENCES
- 1.Hadziahmetovic M, Loo BW, Timmerman RD, Mayr NA, Wang JZ, Huang Z, et al. Stereotactic body radiation therapy (stereotactic ablative radiotherapy) for stage I non-small cell lung cancer—updates of radiobiology, techniques, and clinical outcomes. Discov Med 2010;9:411–17 [PubMed] [Google Scholar]
- 2.Martin A, Gaya A. Stereotactic body radiotherapy: a review. Clin Oncol 2010;22:157–72 10.1016/j.clon.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 3.Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I non-small cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:162331 10.1002/cncr.20539 [DOI] [PubMed] [Google Scholar]
- 4.Lax I, Panettieri V, Wennberg B, Amor Duch M, Naslund I, Baumann P, et al. Dose distributions in SBRT of lung tumors: comparison between two different treatment planning algorithms and Monte-Carlo simulation including breathing motions. Acta Oncol 2006;45:978–88 [DOI] [PubMed] [Google Scholar]
- 5.Timmerman R, Heinzerling J, Abdulrahamn R, Choy H, Meyer JL. Stereotactic body radiation therapy for thoracic cancers: recommendations for patient selection, setup and therapy. Front Radiat Ther Oncol 2011;43:395–411 10.1159/000322503 [DOI] [PubMed] [Google Scholar]
- 6.Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;70:685–92 10.1016/j.ijrobp.2007.10.053 [DOI] [PubMed] [Google Scholar]
- 7.Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427 10.1016/j.ijrobp.2005.05.034 [DOI] [PubMed] [Google Scholar]
- 8.Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003:124:1946. [DOI] [PubMed] [Google Scholar]
- 9.Haasbeek CJ, Senan S, Smit EF, Paul MA, Slotman BJ, Lagerwaard FJ. Critical review of nonsurgical treatment options for stage I non-small cell lung cancer. Oncologist 2008;13:309–19 10.1634/theoncologist.2007-0195 [DOI] [PubMed] [Google Scholar]
- 10.Nagata Y, Wulf J, Lax I, Timmerman R, Zimmermann F, Stojkovski I, et al. Stereotactic radiotherapy of primary lung cancer and other targets: results of consultant meeting of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys 2011;79:660–9 10.1016/j.ijrobp.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 11.Palma D, Visser O, Lagerwaard FJ, Belderbos J, Slotman BJ, Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153–9 10.1200/JCO.2010.30.0731 [DOI] [PubMed] [Google Scholar]
- 12.Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833 10.1200/JCO.2006.07.5937 [DOI] [PubMed] [Google Scholar]
- 13.National Radiotherapy Implementation Group Stereotactic body radiotherapy: guidance for clinicians, providers and commissioners. London, UK: NRIG; 2010.
- 14.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078–101 [DOI] [PubMed] [Google Scholar]
- 15.Duane S, Palmans H, Sharpe P, Vynckier S. Traceability and absorbed dose standards for small fields, IMRT and helical tomotherapy. Workshop on “Absorbed dose and air kerma primary standards”; 9–11 May 2007; Paris, France: Paris, France: NPL; 2007. Available from: http://www.nucleide.org/ADAKPS_WS/Session%20G%20-%20Special%20Standards/G1_Or-Duane.pdf [Google Scholar]
- 16.The Royal College of Radiologists, Institute of Physics and Engineering in Medicine, Society and College of Radiographers On target: ensuring geometric accuracy in radiotherapy. London, UK: The Royal College of Radiologists; 2008. Available from: http://www.rcr.ac.uk/docs/oncology/pdf/BFCO(08)5_On_target.pdf [Google Scholar]
- 17.Baker A, Appleton L, Scott A, Jain P. Current and future impact of stereotactic body radiotherapy on UK oncology services. Imaging Oncol 2011;14–22 [Google Scholar]
- 18.Josipovic M, Persson GF, Logadottir A, Smulders B, Westmann G, Bangsgaard JP. Translational and rotational intra- and inter-fractional errors in patient and target position during a short course of frameless stereotactic body radiotherapy. Acta Oncol 2012;51:610–17 10.3109/0284186X.2011.626448 [DOI] [PubMed] [Google Scholar]
- 19.Li W, Purdie TG, Taremi M, Fung S, Brade A, Cho BC, et al. Effect of immobilization and performance status on intrafraction motion for stereotactic lung radiotherapy: analysis of 133 patients. Int J Radiat Oncol Biol Phys 2011;81:1568–75 10.1016/j.ijrobp.2010.09.035 [DOI] [PubMed] [Google Scholar]
- 20.Fakir H, Gaede S, Mulligan M, Chen JZ. Development of a novel ArcCHECK(™) insert for routine quality assurance of VMAT delivery including dose calculation with inhomogeneities. Med Phys 2012;39:4203–8 10.1118/1.4728222 [DOI] [PubMed] [Google Scholar]
- 21.Ong C, Verbakel WF, Cuijpers JP, Slotman BJ, Senan S. Dosimetric impact of interplay effect on RapidArc lung stereotactic treatment delivery. Int J Radiat Oncol Biol Phys 2011;79:305–11 10.1016/j.ijrobp.2010.02.059 [DOI] [PubMed] [Google Scholar]
- 22.Rao M, Wu J, Cao D, Wong T, Mehta V, Shepard D, et al. Dosimetric impact of breathing motion in lung stereotactic body radiotherapy treatment using image-modulated radiotherapy and volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 2012;83:e251–6 10.1016/j.ijrobp.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 23.Nagata Y, Hiraoka M, Mizowaki T, Narita Y, Matsuo Y, Norihisa Y, et al. Survey of stereotactic body radiation therapy in Japan by the Japan 3-d Conformal External Beam Radiotherapy Group. Int J Radiat Oncol Biol Phys 2009;75:343–47 10.1016/j.ijrobp.2009.02.087 [DOI] [PubMed] [Google Scholar]
- 24.Pan H, Simpson DR, Mell LK, Mundt AJ, Lawson JD. A Survey of stereotactic radiation therapy in the United States. Cancer 2011;117:4566–72 10.1002/cncr.26067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potters L, Kavanagh B, Galvin JM, Hevezi JM, Janjan NA, Larson DA, et al. American Society for Therapeutic Radiology and Oncology; American College of Radiology. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326–32 [DOI] [PubMed] [Google Scholar]
- 26.Mayles WPM. Survey of the availability and use of advanced radiotherapy technology in the UK. Clin Oncol (R Coll Radiol) 2010;22:636–42 10.1016/j.clon.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 27.rtog.org [homepage on the internet]. Philadelphia, PA: Radiation Therapy Oncology Group; c2010 [cited 25 March 2013]. Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0915
- 28.Chi A, Liao Z, Nguyen NP, Xu J, Stea B, Komaki R. Systemic review of the patterns of failure following stereotactic body radiation therapy in early stage non-small-cell lung cancer: clinical implications. Radiother Oncol 2010;94:1–11 10.1016/j.radonc.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 29.Brown JM, Diehn M, Loo BW., Jr Stereotactic ablative radiotherapy should be combined with a hypoxic cell radiosensitizer. Int J Radiat Oncol Biol Phys 2010;78:323–7 10.1016/j.ijrobp.2010.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahele M, Palma D, Lagerwaard F, Slotman B, Senan S. Radiological changes after stereotactic radiotherapy for stage I lung cancer. J Thorac Oncol 2011;6:1221–8 10.1097/JTO.0b013e318219aac5 [DOI] [PubMed] [Google Scholar]
- 31.Senan S, Huang K, Senthi S, Spoelstra F, Warner A, Slotman BJ, et al. Blinded assessment of radiological changes after stereotactic ablative radiotherapy (SABR) for early-stage lung cancer: local recurrences versus fibrosis. J Clin Oncol 2013;31:suppl; abstr 7520 [Google Scholar]