Abstract
Polymorphonuclear neutrophil leukocytes (PMNs) are a critical part of innate immune defence against bacterial pathogens, and only a limited subset of microbes can escape killing by these phagocytic cells. Here we show that Neisseria meningitidis, a leading cause of septicaemia and meningitis, can avoid killing by PMNs and this is dependent on the ability of the bacterium to acquire L-glutamate through its GltT uptake system. We demonstrate that the uptake of available L-glutamate promotes N. meningitidis evasion of PMN reactive oxygen species produced by the oxidative burst. In the meningococcus, L-glutamate is converted to glutathione, a key molecule for maintaining intracellular redox potential, which protects the bacterium from reactive oxygen species such as hydrogen peroxide. We show that this mechanism contributes to the ability of N. meningitidis to cause bacteraemia, a critical step in the disease process during infections caused by this important human pathogen.
Keywords: PMNs, glutamate uptake, Neisseria meningitidis, oxidative stress
Introduction
Neisseria meningitidis is an obligate human pathogen that is an important cause of septicaemia and meningitis (van Deuren et al., 2000). The bacterium is a component of the normal flora of the human upper respiratory tract, and is present in the nasopharynx of between 5-40% of the healthy adult population (Stephens et al., 2007; Yazdankhah and Caugant, 2004). From this site, N. meningitidis can enter the endovascular compartment, where it evades immune killing and acquires nutrients enabling it to replicate to high levels (Exley et al., 2005; Vogel and Frosch, 1999). This is critical to the outcome of meningococcal sepsis as mortality from this condition is directly related to levels of bacteraemia (Brandtzaeg et al., 1995; Ovstebo et al., 2004).
Complement is a critical aspect of immunity against the meningococcus (Schneider et al., 2007; Vogel and Frosch, 1999). Individuals with genetic polymorphisms in, or deficiencies of complement factors are highly susceptible to disseminated meningococcal disease (Figueroa and Densen, 1991). The polysialic capsule and lipopolysaccharide (LPS) expressed by N. meningitidis are essential for avoidance of the complement system (Geoffroy et al., 2003; Hammerschmidt et al., 1994); both are influenced by the carbon energy sources available to the bacterium (Exley et al., 2005, and unpublished). Furthermore, the bacterium recruits factor H, a negative complement regulator, to its surface which impairs complement activation (Haralambous et al., 2006; Madico et al., 2006; Schneider et al., 2006, Schneider et al., 2009).
Much less is known about the contribution to protection against meningococcal disease of other aspects of the immune system, such as polymorphonuclear neutrophil leukocytes (PMNs). These phagocytic cells elaborate multiple mechanisms that are highly effective at killing microbes, including the production of anti-microbial peptides, degradative enzymes (such as lysosyme), and reactive oxygen species (ROS) through the oxidative burst (Urban et al., 2006). The related pathogen Neisseria gonorrhoeae is phagocytosed by PMNs, but survives within this hostile environment (Simons et al., 2005) and 2006; Veale et al., 1979). Uptake of the gonococcus is dependent on the interaction between bacterial Opa and CD66, a member of the carcinoembryonic (CEACAM) antigen family (McCaw et al., 2003). However little is known about the survival of N. meningitidis in PMNs even though the bacterium is phagocytosed by these cells (Estabrook et al., 1998; Fijen et al., 2000; McNeil and Virji, 1997), and is found within PMNs in the systemic circulation of patients with bacteraemia and in the cerebrospinal fluid (CSF) during meningitis (Guarner et al., 2004). Indeed, the meningococcus was originally named Diplococcus intracellularis meningitidis because of its characteristic association with PMNs in the CSF (Weichselbaum, 1887).
Here we show that the acquisition of L-glutamate by N. meningitidis via the GltT ABC transporter (Monaco et al., 2006) contributes to its ability to cause bacteraemic disease, consistent with previous findings (Echenique-Rivera et al., 2011). We demonstrate that uptake of L-glutamate does not enhance the expression of capsule, LPS sialylation, or resistance against complement. Instead, available L-glutamate contributes to the glutathione pool within the meningococcus, protecting it against ROS produced by PMNs, and this mechanism promotes the survival of N. meningitidis in vivo.
Results
The GltT L-glutamate transporter but not the GltS system is required for survival in vivo
The meningococcus possesses two L-glutamate uptake systems (Monaco et al., 2006), the GltT ABC-type transporter, and the GltS Na+-dependent symporter. The GltT transporter has been shown previously to be required for bacterial survival within epithelial cells (Monaco et al., 2006), and survival in human whole blood, and in a murine model (Li et al., 2009). Therefore, strains lacking essential components of these two transport systems were analysed for their ability to cause bacteraemia in the infant rat model (Sun et al., 2000), which does not require use of co-factors such as additional iron. Animals were challenged with a 1:1 ratio of the the wild-type serogroup B N. meningitidis strain, H44/76 and an gltT or gltS mutant, and the competitive index (C.I.) of the mutants was calculated by comparing the proportion of the strains in the bloodstream at later time points. The mutant lacking gltT was significantly attenuated in vivo with a C.I. of 0.08 at 20 hrs post-challenge (n = 11 infant rats, P < 0.01, Student’s t-test) and was restored in the complemented strain H44/76ΩgltT+ (C.I. 0.79, n=10), indicating that this transport system plays an important role during meningococcal bacteraemia. In contrast, the gltS mutant was not attenuated (C.I., 0.96, n = 12).
LPS sialylation and capsule expression are unaffected by L-glutamate uptake
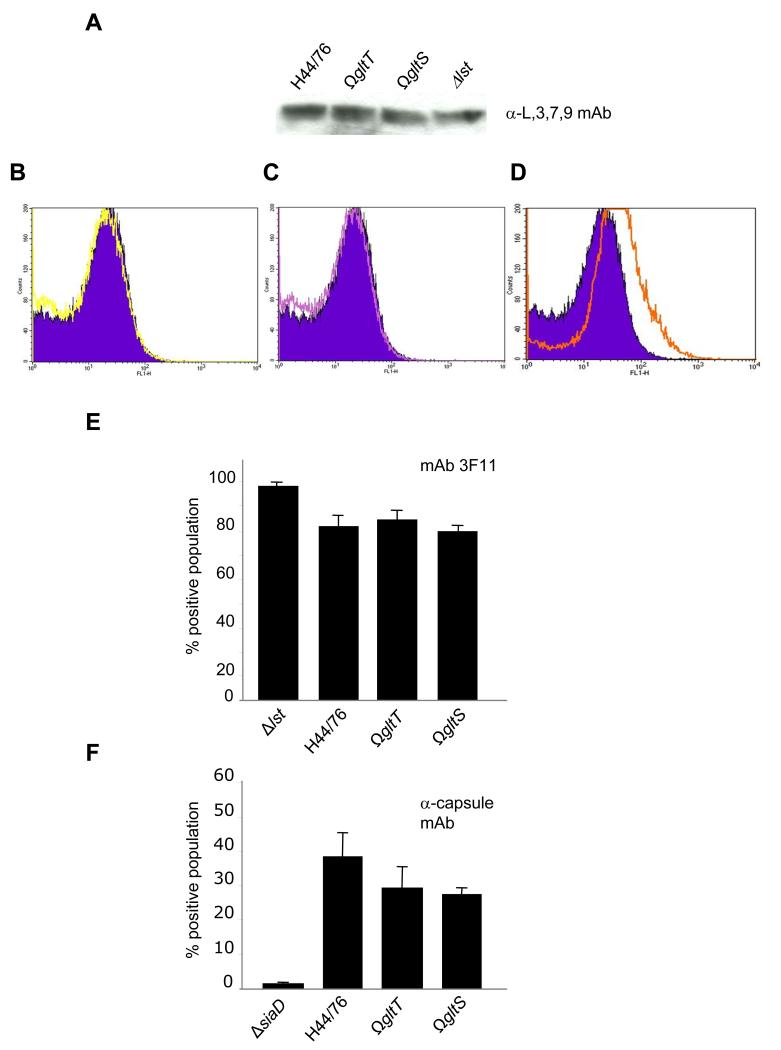
Further experiments were performed to understand the mechanisms underlying the attenuation of the gltT mutant as these have not been defined previously (Li et al., 2009). Available carbon energy sources, such as lactate, influence the extent of LPS sialylation and encapsulation of N. meningitidis (Exley et al., 2005, and unpublished data); these structures promote the virulence of the meningococcus and are required for the avoidance of complement-mediated lysis. Therefore, we examined the impact of L-glutamate, an important carbon and nitrogen source for the meningococus (Hill, 1971; Mallavia and Weiss, 1970), on LPS sialylation by Western blot and FACS analysis with mAb 3F11, which recognises unsialylated LPS (Mandrell et al., 1992), and an α-L,3,7,9 LPS immunotype mAb (Figures 1A). No significant difference was observed in the degree of sialylation of H44/76, H44/76ΩgltT, and H44/76ΩgltS. The extent of sialylation was also assessed by FACS analysis using mAb 3F11 (Figure 1B, C, D and E); a mutant lacking the LPS-specific sialyl transferase (Δlst) was included as a control. The results confirmed that there was no detectable change in LPS sialylation between the wild-type strain and those defective for glutamate acquisition, H44/76ΩgltT or H44/76ΩgltS.
Figure 1. L-Glutamate availability does not affect LPS sialylation or capsule expression.
A Whole cell lysates of bacteria were separated by SDS-PAGE, transferred to a nitrocellulose membranes and incubated with a mAb against L3,7,9 LPS. FACS detection of unsialylated LPS using the mAb 3F11 with the gltT (B, yellow trace), gltS (C, purple trace) or lst (D, orange trace) mutants compared the wild-type strain (filled dark blue trace, quantified in E) or the polysaccharide capsule (F). The percent positive population is shown and the strains are indicated below each lane. Error bars give the S.D. of experiments performed in triplicate on three separate occasions.
Next, mutants lacking gltS or gltT were compared with the wild-type strain H44/76 for capsule expression. Again, there was no significant alteration in capsule expression by the mutants and the wild-type strain by FACS (Figure 1F and Figure S1), while there was no detectable capsule on the surface of the siaD mutant which lacks the polysialyl transferase of serogroup B N. meningitidis.
GltT is not required for complement-mediated killing but is necessary for resistance against oxidative stress through its contribution to intracellular glutathione levels
Next we examined the sensitivity of strains to complement-mediated lysis in serum assays. All the strains with defects in glutamate uptake had wild-type levels of survival in the presence of 40% normal human serum; after a 1 hr incubation there was no significant difference between the recovery of H44/76 and H44/76ΩgltT or H44/76ΩgltS (approximately 20% for each strain, not shown), demonstrating that enhanced sensitivity to complement is not the basis of the attenuation of the gltT mutant.
To further understand the basis of the attenuation of the gltT mutant, we examined the predicted fate of L-glutamate in the meningococcus after uptake from the environment (Figure 2A, http://www.genome.jp/kegg/). L-glutamate can contribute to the TCA cycle (following conversion to 2-oxo-glutarate), and the biosynthesis of arginine, proline, glutamine, and glutathione. Glutathione is a key molecule in the control of the redox state in all living cells (Carmel-Harel and Storz, 2000), and provides a potential connection between L-glutamate acquisition and resistance against oxidative stress. In N. meningitidis, L-glutamate is predicted to be converted to L-α-glutamyl cysteine through the action of glutamate-cysteine lyase, GshA (encoded by NMB1037 (Tettelin et al., 2000), and thence to glutathione by glutathione synthase, GshB (encoded by NMB1559).
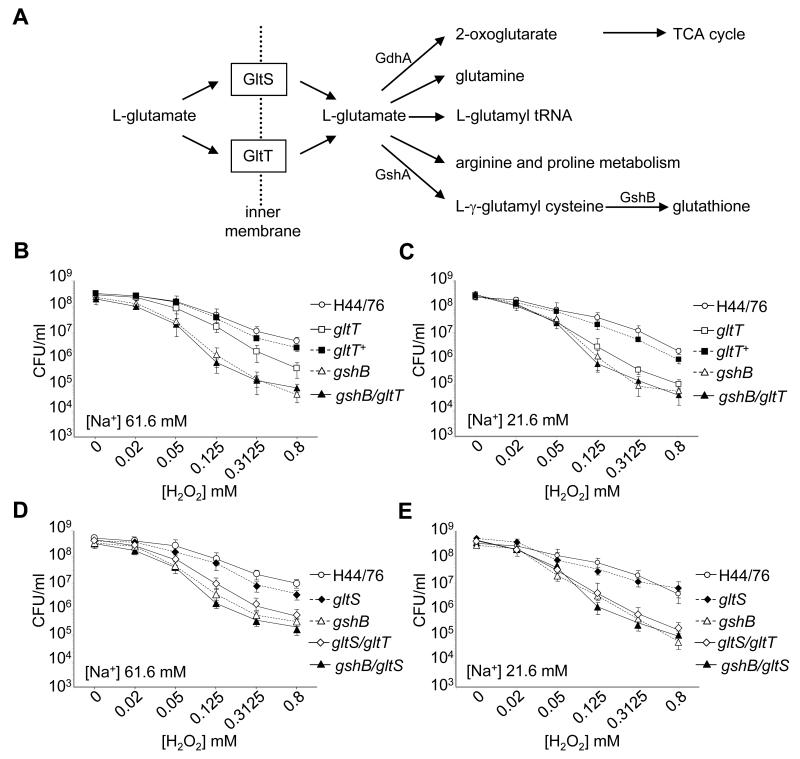
Figure 2. The fate of L-glutamate in N. meningitidis and its role in sensitivity to oxidative stress.
A Uptake systems of L-glutamate and its predicted intracellular fates in N. meningitidis. L-glutamate can be converted into glutathione by the action of GshA and GshB. The sensitivity to H2O2 of bacteria grown in medium salt (61.6 mM, MCDA-2, Panels B and D) or low sodium (21.6 mM, MCDA-1, Panels C and E) conditions. Bacteria were incubated with concentrations of H2O2 ranging from 0-0.8 mM for 20 min, and the number of surviving bacteria determined by plating to solid media. The strains are given in the key, and error bars show the S.D. of assays performed in triplicate on at least three occasions.
Therefore we assessed the resistance of strains lacking the glutamate uptake systems against exposure to hydrogen peroxide (H2O2), a membrane permeable ROS that generates oxidative stress in the bacterial cytoplasm (Seib et al., 2004). Strikingly we found that the mutant lacking GltT, but not the strain lacking GltS, was significantly more sensitive to exposure to H2O2 than H44/76. The strain H44/76ΩgltT was recovered at less than 10% of the levels of the wild-type after incubation in a range of concentrations of H2O2 (0.05-0.8 mM, Figure 2B and C, P <0.01, one tailed T test). The increased sensitivity of H44/76ΩgltT was more evident when the strains were grown under low sodium conditions (Figure 2C) in which the Na+-dependent GltS symporter is expected to be non-functional (Figure 2B and C); the survival of the complemented strain H44/76ΩgltT+ in the presence of H2O2 reverted towards wild-type levels. In contrast, the GltS symporter was dispensable for resistance to exogenous H2O2 in a medium or low sodium environment (Figure 2D and E, respectively), consistent with the lack of attenuation during bacteraemic disease of the mutant lacking GltS.
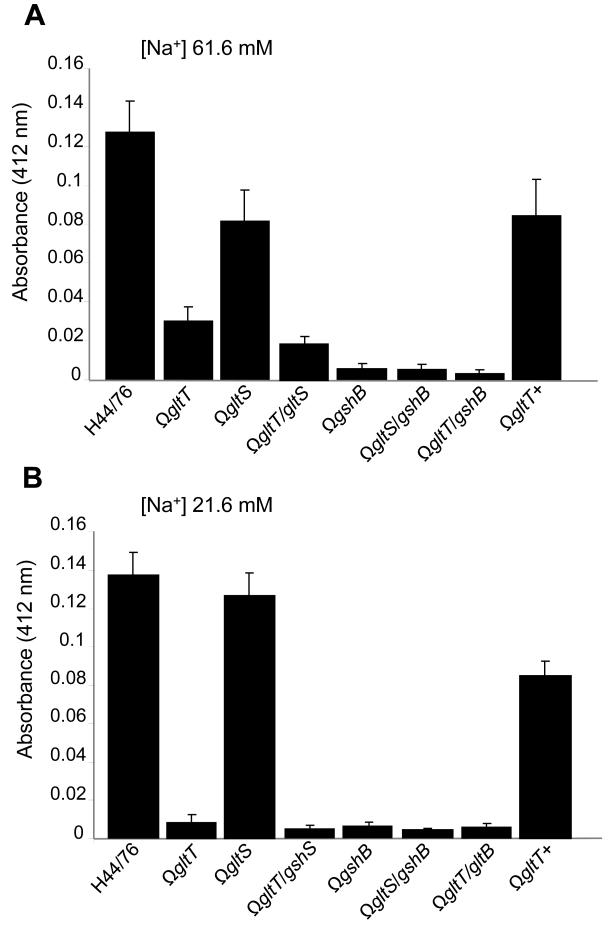
Next we constructed strains lacking the predicted meningococcal glutathione synthase, GshB (NMB1559), with or without the GltT glutamate uptake system, and analysed their sensitivity to oxidative stress. The survival of H44/76ΩgshB was dramatically impaired in the presence of H2O2 compared with the wild-type parent H44/76 (Figure 2B and C). Furthermore we analysed a mutant lacking both glutamate transport systems; survival of the gltT/gltS mutant exhibited similar levels of survival as the gshB mutant in the face of oxidative stress (Figure 2D and E). Of note, loss of GltT had no impact on resistance against H2O2 in a gshB-negative background, indicating that lack of L-glutamate uptake via GltT is linked to avoidance of oxidative stress through its contribution to glutathione synthesis, rather than through an independent pathway. This hypothesis was further examined by measuring the total intracellular pool of glutathione in strains (Figure 3). The dramatic reduction in intracellular glutathione in H44/76ΩgshB (<5% of levels in the wild-type strain) confirmed that GshB is involved in the synthesis of this thiol. Additionally, the gltT deficient mutant had less than 10 and 25 % of wild-type glutathione levels in low and medium sodium conditions respectively, consistent with reduced function of the Na+ dependent GltS system in the low salt environment. Complementation of the mutation in H44/76ΩgltT+ restored the glutathione pool towards wild-type levels (Figure 3A and B), while loss of GltS did not affect intracellular glutathione to the same extent as GltT; as expected the gltT/gltS double mutant had lower intracellular levels of glutathione than either of the single mutants. Of note, introduction of the gltT mutation into the ΩgshB strain did not result in a further decline in intracellular glutathione levels, indicating that low glutathione levels in the GltT mutant result from impaired glutathione synthesis from L-glutamate.
Figure 3. Intracellular glutathione levels.
Levels of intracellular glutathione in bacterial strains (indicated) were determined in medium (61.6 mM NaCl, Panel A) and low (21.6 mM NaCl, Panel B) sodium conditions. Strains lacking gshB and gltT have significantly reduced glutathione levels in both environments; complementation of the gltT mutant in strain gltT+ restores glutathione levels towards wild-type levels. Error bars show S.D. of assays performed in triplicate.
GltT contributes to survival of N. meningitidis against ROS produced by human PMNs
Production of ROS is a potent bactericidal mechanism employed by PMNs (Nathan, 2006; Urban et al., 2006). As the GltT-deficient mutant was sensitive to oxidative stress, we investigated interactions between N. meningitidis and PMNs. First, we confirmed that we had successfully isolated primary PMNs from healthy volunteers by examining cells for expression of the PMN markers CD45 (common leukocyte antigen), CR3 (a heterodimer of CD11b and CD18), and neutrophil elastase (Figure S2A and B). Non-opsonic association of N. meningitidis with PMNs is mediated at least in part by the CD66 receptor (Virji et al., 1996); therefore, we confirmed that the purified primary PMNs express CD66 by FACS (Figure S2C). Furthermore we demonstrated by FACS analysis that the serogroup B N. meningitidis capsule inhibits association of bacteria with primary PMNs as demonstrated previously (McNeil and Virji, 1997); the siaD mutant which does not express a polysialic acid capsule associated approximately four times more with cells than the wild-type strain (P < 0.01, Figure S3).
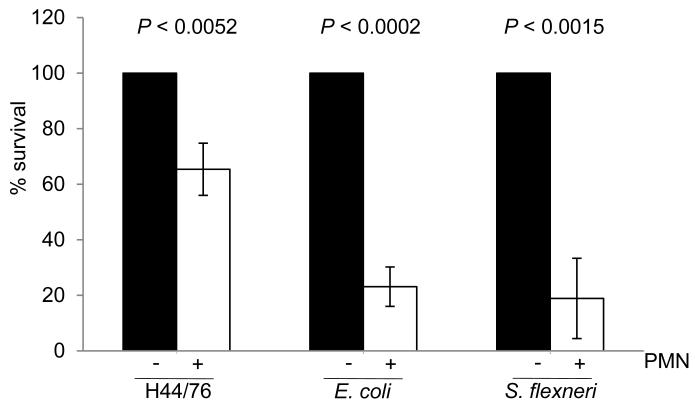
Next we determined the outcome of challenge of PMNs with wild-type N. meningitidis H44/76, and compared results obtained with Escherichia coli and Shigella flexneri. Primary human PMNs were challenged at an MOI of 2, and bacteria recovered after initial association (around 10 minutes) and 1 hr later. Exposure to PMNs led to significant amounts of bacterial killing for all three species without opsonisation (Figure 4). However similar to findings with the gonococcus (Criss and Seifert, 2008), N. meningitidis survived at significantly higher levels at the early stages of the infection compared with E. coli and S. flexneri (35% and 27% of meningococcal survival, P < 0.004 and P < 0.0017, respectively); at the later time points (T1) there was some further killing of bacteria though not to a great extent (not shown).
Figure 4. Interaction of primary PMNs with E. coli, S. flexneri and N. meningitidis.
Bacterial suspensions were added to wells with (+) or without (−) adherent human PMNs in 24-well microtiter plates at an MOI of 2. The survival of bacteria was determined by lysing cells and plating the contents of the wells to solid media. Results are shown as the percent survival compared with the number of CFU in the inocula. Both E. coli and S. flexneri survived significantly less well than H44/76 in PMNs; for H44/76, approximately 65% of the bacterial population survived following association with PMNs. Results are the means of assays performed with PMNs from four different donors on different occasions; error bars show the SD. Significance of bacterial survival with or without PMNs (indicated) was determined by the Student’s t-test.
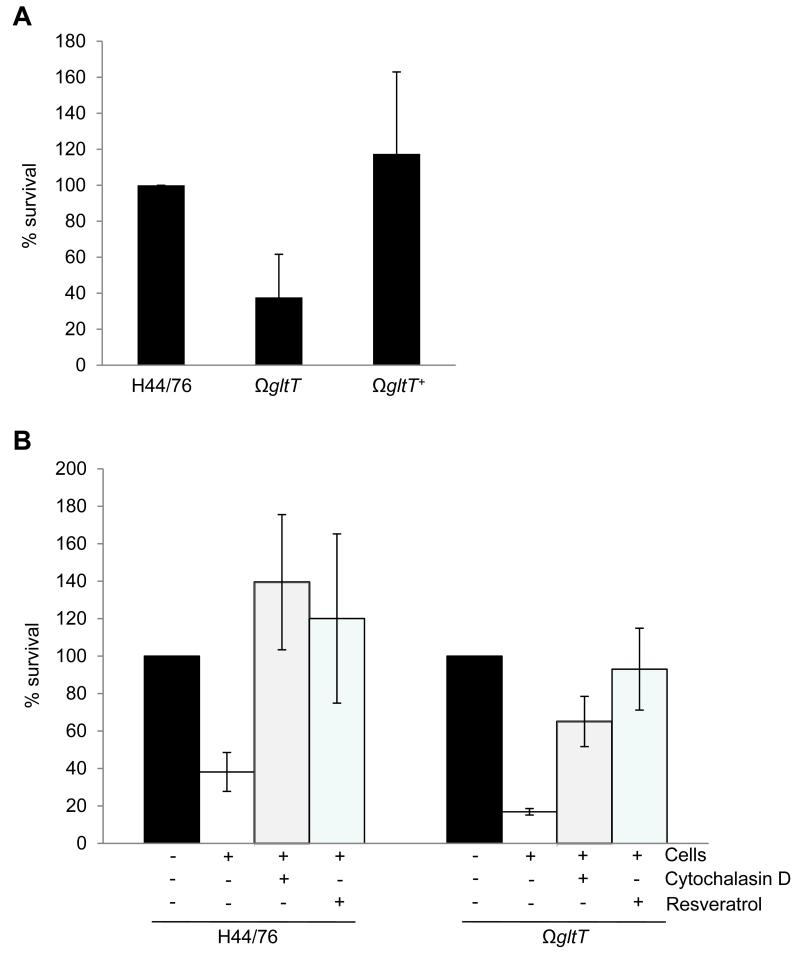
Strikingly, the survival of H44/76ΩgltT was impaired in association with primary PMNs compared with the wild-type strain with the mutant recovered on average 37% (S.D. ± 23) of the level of the wild-type strain H44/76 (P < 0.013), while the complemented mutant H44/76ΩgltT+ was recovered at the level of the wild-type (Figure 5A). This attenuation was abolished by treatment of cells with either cytochalasin D, which prevents actin polymerisation, or resveratrol, which blocks the PMN oxidative burst (Jang et al., 1999) (Figure 5B); other inhibitors of the respiratory burst proved to be toxic to N. meningitidis (not shown). Therefore GltT contributes to the survival of the meningococcus in PMNs through avoidance of the oxidative burst.
Figure 5. Sensitivity of H44/76ΩgltT to PMN killing is dependent on bacterial uptake and the respiratory burst.
Bacterial suspensions were added to adherent human neutrophils (+) or to empty wells (−) in a 24-well microtiter plate, and survival determined by plating to solid media. (A) The gltT mutant has a significant defect for survival in the presence of PMNs (average recovery compared with the wild-type, 37%, p < 0.01) compared with the wild-type strain, whereas the complemented mutant was recovered at wild-type levels (117% p <0.02). Data are from assays performed with PMNs from 4 different donors on four occasions, and normalized according to the survival of H44/76; significance was determined by two-tailed Student’s t-test. Error bars show the SD. (B) Pre-treatment of cells with either resveratrol (100 μM) or cytochalasin (5 μM) for 30 minutes prior to challenge restored the survival of H44/76ΩgltT to wild-type levels, indicating that sensitivity of this mutant to PMN killing is dependent on actin polymerisation and ROS production. Results are the percent survival compared with the number of CFU in the inocula from assays performed with PMNs from three to five different donors on three to five independent occasions. Error bars show the SD.
The GltT transporter contributes in the protection of N. meningitidis from the PMN oxidative burst in vivo
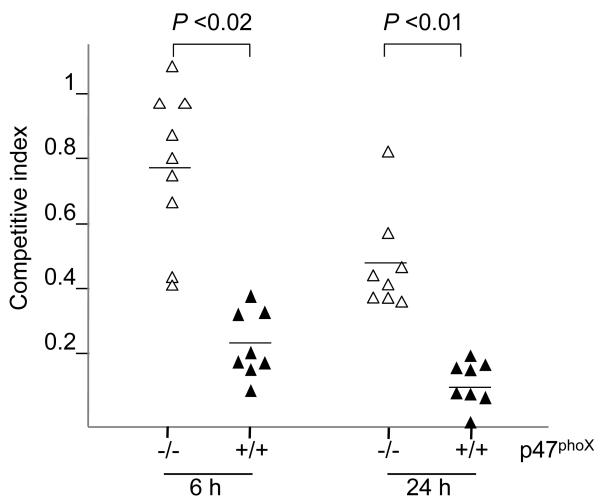
The attenuation of the H44/76ΩgltT mutant during bacteraemic infection could either be caused by a growth defect resulting from a lack of glutamate, or increased susceptibility to ROS generated by PMNs. To differentiate between these potential explanations we used the murine model of meningococcal sepsis to take advantage of available animals with specific genetic lesions, and determined the virulence of the gltT− mutant (H44/76ΩgltT) in C57BL/6 p47phox−/− mice which fail to generate an oxidative burst through absence of a component of the NADPH oxidase (Fang, 2004), and in congenic, wild-type mice. Mice were challenged with a 1:1 ratio of H44/76 and the gltT mutant and the ratio of the strains in the bloodstream determined at subsequent time points. The results (Figure 6) demonstrate that the virulence of the ΩgltT mutant is restored towards wild-type levels in vivo by the absence of the respiratory burst. The C.I. at 6 hrs post-challenge was 0.25 and 0.79 in p47phox+/+ and p47phox−/− mice, respectively (P <0.02); by 24 hrs post-challenge, the C.I. was 0.14 and 0.5 in animals with or without the NADPH-oxidase, respectively (p <0.01). Thus, within the systemic circulation GltT promotes the virulence of N. meningitidis through its effect in counteracting killing by the PMN oxidative burst.
Figure 6. The attenuation of H44/76ΩgltT in vivo is dependent on the PMN oxidative burst.
The competitive index (C.I.) of H44/76ΩgltT was compared against the wild-type strain in congenic animals with (+/+) or without (−/−) active p47phox, an essential component of the NADPH oxidase. Results for individual animals are shown by triangles at 6 and 24 hrs post challenge, and the means are the solid lines. The difference in the C.I.s observed in p47phox−/− and p47phox+/+ mice was statistically significant by the Student’s t-test.
Discussion
PMNs are critical for innate immune defence against bacterial infection. They can express multiple potent anti-microbial activities, many of which are activated upon recognition of pathogen-associated molecular patterns (PAMPs) or following phagocytosis (DeLeo et al., 1999; Hampton et al., 1998). Only a limited subset of pathogens can survive in the intracellular compartment of PMNs, including Neisseria gonorrhoeae, Streptococcus pyogenes and Helicobacter pylori (Andersen et al., 1993; Kobayashi et al., 2003; Veale et al., 1979). Here we show that L-glutamate acquisition by the meningococcus via GltT promotes survival of bacteria in association with PMNs and contributes to the ability of the meningococcus to cause bloodstream disease. L-glutamate is converted by N. meningitidis to glutathione, which has a key role in regulating the redox potential of the invading pathogen and indeed host cells. The involvement of L-glutamate in maintaining the intracellular glutathione pool protects the pathogen from ROS generated by PMNs, and contributes to the ability of the bacterium to cause systemic infection.
A characteristic feature of meningococcal infection is its rapid onset and progression to severe disseminated disease (van Deuren et al., 2000). Part of this dramatic clinical picture is a consequence of the high levels of bacteraemia and LPS in meningococcal sepsis, which are directly correlated with a poor prognosis (Brandtzaeg et al., 1995, Darton et al., 2009). It is known that carbon energy sources, in particular lactate, at specific sites during colonisation and disease contribute to the survival of N. meningitidis in the host through resistance against complement-mediated killing (Exley et al., 2005). However, we demonstrate here that the role of L-glutamate and the GltT uptake system during systemic disease is distinct from the involvement of lactate.
We considered the possibility that L-glutamate, as an important nitrogen and carbon source, could contribute to the production of phospho-enol pyruvate via the citrate cycle and thence to sialic acid biosynthesis. However the absence of any change in capsule expression, LPS sialylation or sensitivity to complement-mediated killing of strains affected in L-glutamate uptake indicates that a lack of this amino acid is likely to be compensated for by more immediate carbon sources.
Although the metabolism of L-glutamate is known to contribute to the virulence of Streptococcus pneumoniae and Helicobacter pylori (Hendriksen et al., 2008; Shibayama et al., 2007), the uptake of glutamate has not been implicated in bacterial pathogenesis aside from during meningococcal infection (Li et al., 2009). A possible explanation is that the widespread pairing of GltT and GltS homologues in bacteria that could result in functional redundancy. However a more likely explanation is that L-glutamate is not an essential amino acid for most other pathogens which can synthesise L-glutamate via glutamine synthase (Reitzer, 2003). However, the meningococcus lacks this enzyme (Parkhill et al., 2000; Tettelin et al., 2000), and so must rely on exogenous sources of L-glutamate in extra-cellular environments in vivo, where the concentration is between 30 to 90 μM (Divino Filho et al., 1998), and within cells. Although levels of L-glutamate within PMNs are unknown, these cells secrete significant amounts of this amino acid (up to 400 μM) upon activation (Collard et al., 2002).
We successfully isolated primary human PMNs (demonstrated by their expression of surface markers including CD68, CD11b, CD18 and CEACAMs, and neutrophil elastase), and showed the anti-phagocytic effect of the polysaccharide capsule of N. meningitidis as demonstrated previously (Read et al., 1996; Unkmeir et al., 2002). Interestingly our assays showed that, compared with E. coli and S. flexneri, wild-type N. meningitidis exhibits enhanced survival when in association with PMNs as does N. gonorrhoeae (Veale et al., 1979). A number of meningococcal enzymes have been characterised that contribute to resistance against oxidative stress and which may promote survival in PMNs. These include enzymes that reduce the toxic effects of ROS on the bacterium, including superoxide dismutases, glutathione peroxidase and catalase (Dunn et al., 2003; Moore and Sparling, 1996; Soler-Garcia and Jerse, 2004), or those that are responsible for DNA repair following damage through oxidative stress (Carpenter et al., 2007; Colicchio et al., 2006; Tala et al., 2008). However the role of these enzymes to meningococcal survival in association with PMNs is still to be defined.
The function of the GltS uptake system is dependent on available sodium and is dispensable for virulence, presumably through the presence of GltT and relatively low sodium conditions found in vivo. In contrast, GltT-mediated uptake is sodium independent and can therefore operate in low sodium environments such as those present intracellularly (Monaco et al., 2006). We show that this transporter is required for resistance against oxidative stress, survival within PMNs and during bacteraemic disease. Mutants lacking GltT were preferentially killed by PMN in an actin- and respiratory burst-dependent mechanism, reflecting its sensitivity to ROS such as H2O2. While this is not the only ROS produced in vivo (which also include hypochlorous acid and superoxides), it provides an in vitro measure of susceptibility to this aspect of innate immunity (Imlay, 2008). This is likely to be caused by the link between L-glutamate uptake and the biosynthesis of glutathione which has been described previously in E. coli and Salmonella (Bouter et al., 1988). Glutathione is a low molecular weight thiol that is critical in maintaining the redox potential in cells. While some bacteria can acquire exogenous glutathione (Thomas, 1984), the meningococcus has no uptake system and must synthesise its own glutathione (Tettelin et al., 2000). This molecule functions as a potent anti-oxidant, and is necessary for preserving the reduced state of the cytoplasm of prokaryotic and eukaryotic cells, and for protection against ROS. Interestingly, we previously found that a N. meningitidis glutaredoxin mutant (grx3) is attenuated during bacteraemic infection (Sun et al., 2000), and we will investigate the result mechanism of attenuation of this and the gshB mutant in further studies. Alternatively, the effect of glutathione might be indirect, and be involved in signalling the response of the bacterium to oxidative stress or modify the export of antimicrobial peptides (Tzeng et al., 2005).
To determine the pathophysiological relevance of the findings obtained with isolated human PMNs, we examined the impact of PMN ROS on the virulence of strains lacking GltT. Mice with a defect in p47phox−/− are unable to assemble a functional NADPH oxidase which is necessary for production of intravacuolar superoxide, H2O2 and hydroxyl radicals via the oxidative burst. These ROS induce damage in bacterial DNA, proteins and lipids, and may be potentiated by other antibacterial activities such as peptides and enzymes present at sublethal concentrations (Fang, 2004). Mutations in p47phox−/− are found in patients with chronic granulomatous disease (CGD) who are susceptible to pathogens such as Staphylococcus aureus, Candida albicans, and Apsergillus spp. but not to meningococcal disease (Segal, 1996), which may reflect the inherent resistance of the meningococcus to killing by PMNs as well as the rarity of CGD. Strikingly the virulence of the gltT mutant reverted towards the wild-type in animals lacking the oxidative burst, with the C.I. at 22 hr post-challenge in wild-type animals of 0.137, compared with 0.5 in p47phox−/− mice (P < 0.01). This restoration of virulence of the gltT mutant is likely to reflect the lack of ROS production in the PMNs. However, the NADPH oxidase has other functions in PMNs such as the formation of neutrophil extracellular traps (NETs) (Fuchs et al., 2007), and it is possible that the gltT mutant is sensitive to these mechanisms of PMN killing. The residual attenuation in p47phox−/− animals may reflect the sensitivity of the mutant to non-NADPH oxidase dependent oxidative damage (such as through myeloperoxidase (Hampton et al., 1998), or a growth defect of the mutant that cannot be rescued by manipulation of host innate immune responses.
Microarray analysis of a mutant with an inactivation in a gene adjacent to GltT suggests that loss of this glutamate uptake system might result in marked changes in gene expression in the meningococcus (Li et al., 2009). However the role of this gene in L-glutamate transport was not defined, and it is not clear if these alterations in transcriptional profile actually affect the behaviour of N. meningitidis or its surface structure as proposed. Although the strain lacking GltT is affected for growth in specific defined media in vitro (Figure S4), this does not make a major contribution during bacteraemic disease as this would have been evident in the p47phox−/− mice (Figure 6).
Overall our findings demonstrate that acquisition of L-glutamate from the host by N. meningitidis promotes its virulence by enhancing resistance against ROS generated by human PMN. This serves to emphasise that metabolism and virulence are not separate activities within bacteria, but are often interlinked through biosynthetic pathways in which acquisition of key nutrients available in vivo serve to increase the fitness of the pathogen by enhancing its virulence through the avoidance of host innate immunity. It is possible that a new class of anti-microbials and vaccines could be designed that block non-essential metabolic pathways and render the bacterium more sensitive to innate immune killing.
Experimental procedures
Bacterial strains and growth
H44/76 is a serogroup B N. meningitidis isolate which expresses immunotype L3,7,9 LPS, while capsule-minus (ΔsiaD) mutants have been described previously (Exley et al., 2005). The bacterium was grown on GC agar supplemented with 1% (v/v) Polyvitox (Oxoid) or on Brain-Heart Infusion (BHI, Oxoid) agar with 5% Levanthal’s supplement at 37°C in 5% CO2. The number of CFU of N. meningitidis was calculated by measuring the O.D. A260 of a 1:50 dilution of bacterial suspensions in 200 mM NaOH/1% SDS; an O.D. of 1.8 is equivalent of 108 CFU ml−1. For growth in liquid media, bacteria were harvested into phosphate buffered saline (PBS) after an overnight incubation on solid media, and used to inoculate BHI media at an OD600 of 0.15. For growth in defined media, bacteria were inoculated into MCDA-1 or MCDA-2 media (Monaco et al., 2006), or Hanks Balanced Saline Solution (HBSS) containing lactate (1 mM) and pyruvate (10 μM) as necessary. MCDA-1 and MCDA-2 were formulated on the basis of MCDA media (Catlin, 1973) and differed in their sodium ion concentrations, which were 21.6 mM and 61.6 mM, respectively. E. coli (XL1-blue, Invitrogen) was propagated in LB media (Difco). Antibiotics were added as required at the following concentrations: kanamycin (kan), 75 μg ml−1 and 100 μg ml−1; erythromycin (ery), 2 μg ml−1 and 200 μg ml−1; chloramphenicol (cat), 5 μg ml−1 and 25 μg ml−1 for N. meningitidis and E. coli respectively. To fix bacteria, strains were grown on solid media overnight, harvested to PBS and fixed in 3% paraformaldehyde (PFA) for 15 mins, then washed extensively with PBS. E. coli DH5α and S. flexneri M90T were grown on LB and TCS with 0.1% Congo Red, respectively.
DNA manipulation and construction of strains
Chromosomal and plasmid DNA was isolated by standard methods. PCRs included 1 pmol μl−1 of each primer, 1.5 mM MgCl2, 200 μM dNTPs, 2 units of Taq DNA polymerase (Sigma) and 0.1 volumes of x10 PCR buffer (Gibco BRL) in a 25 μl final volume. PCRs were performed in a GeneAMp 2400 cycler (Perkin Elmer). PCR fragments were ligated into pCR2.1 TOPO (Invitrogen) according to the manufacturer’s protocol. Other ligations were performed with T4 DNA ligase at 16°C overnight.
The Neisseria-E. coli shuttle vector pDEX (Pagliarulo et al., 2004) was used to construct pDE-gltS and pDE-gshB. For pDE-gltS, DNA corresponding to the central segment of the gltS gene was amplified using primers gltS1 (5′-GGGTTTGGATCCGCTCATCGGTCTGATTAC-3′) and gltS2 (5′-CCGGTCGGATCCCACAGTTTCAAATTCAGCAACG-3′), and the BamHI-restricted 558 bp PCR product (sites underlined) was ligated into the BamHI site of pDEX, harbouring a functional erythromycin-resistance cassette. An analogous strategy was used to obtain pDE-gshB with the primers gsh-for (5′-GAGCGGGGATCCGTCTGTAAACGGCGG-3′) and gsh-rev (5′-TGCCGCAAGCTTGCCGCGTGTTTCGCC-3′) amplifying a BamHI/HindIII-restricted 592 bp PCR product. This product was ligated into the BamHI/HindIII digested pACYC184 which harbours a chloramphenicol resistance cassette, to generate pACYC-gshB. H44/76ΩgltT (corresponding to H44/76ΩNMB1965) was obtained as described previously (Monaco et al., 2006). The gltS and gshB genes were insertionally inactivated in H44/76 by single cross-over using pDE-gltS or pDE-gshB, respectively. The double mutants H44/76ΩgltSΩgshB and H44/76ΩgltTΩgshB were obtained by insertional inactivation of gshB in the relevant genetic backgrounds, using pACYC-gshB.
For complementation, gltT was amplified from H44/76 using primers gltT-MluI (5′-AGTTGAACGCGTCTGAAACCGTTGAAC-3′) and gltT-BamHI (5′-ATCCGAGGATCCGCGTTTCGTCTTGAG-3′). The 838 bp product was introduced into the MluI and BamHI sites in pYHS1882, downstream of an opa promoter, generating pYHS1882ΩgltT. The pYHS1882 vector contains the promoter, multiple cloning site and kanamycin resistance cassette flanked by fragments of NMB0102 and NMB0103. The construct pYHS1882ΩgltT was used to transform H44/76ΩgltT and integration in the intergenic region between NMB0102 and NMB0103 was confirmed by Southern hybridization. For GFP expression, strains were transformed with plasmid pEGFP (kind gift from Dr Myron Christodoulides, (Christodoulides et al., 2000). To generate the H44/76ΩgltSΩgltT mutant, the BamHI digested 482 bp PCR product, corresponding to the central segment of NMB1965 (Monaco et al., 2006) was ligated into the corresponding site in pACYC18. The resulting construct pACYC-gtlT, harbouring a chloramphenicol resistance cassette, was using to insertionally inactivate gltT in H44/76ΩgltS.
Measurement of the intracellular glutathione pool
To analyse the intracellular glutathione pool, bacteria were grown in MCDA-2 (Monaco et al., 2006) to an O.D. A600 of 1.0, then, about 109 cells were washed twice in H2O, centrifuged at 1000 ×g for 10 mins. Next, three volumes of 5% sulphosalicylic acid were added to the cells which were treated with lysozyme (1 μg μl−1), freeze thawed twice in liquid nitrogen, then left for 5 mins at 4°C. Finally, samples were centrifuged at 13,000 ×g for 10 mins and glutathione concentrations measured in the supernatants with the Glutathione Assay Kit (Sigma) according to manufacturer’s instructions. For the analysis in MCDA-1 (containing 21.6 mM NaCl), the bacterial cultures were grown in medium salt conditions (MCDA-2) and then shifted to low salt conditions (MCDA-1) for 30 mins at 37°C before washing with water.
Sensitivity to complement-mediated lysis and hydrogen peroxide
For complement-mediated bacteriolysis assays, the cells were harvested by centrifugation at 20,000 g, and then 100 μl aliquots containing 104 CFU were incubated with serial dilutions of normal human sera for 1 h at 37°C. The number of bacteria in the inoculum and after incubation with serum was determined by plating to solid media; assays were performed in duplicate and at least on three independent occasions. Heat-inactivated sera were used in control assays. The Student’s t-test was performed to detect statistically significant differences.
Sensitivity of meningococcal strains to hydrogen peroxide was determined as previously described (Tala et al., 2008). Bacteria were grown overnight on solid media at 37°C in the presence of 5% CO2, and re-suspended in MCDA-1. Strains were grown in 10 ml of MCDA-2 until the O.D. A600 reached 0.6 and then re-suspended in 1 ml of the same medium. The bacterial suspension was split into two tubes containing 50 ml of MCDA-2 or MCDA-1 and incubated at 37°C with vigorous shaking for 30 mins. After this time, different amounts of H2O2 were added and incubated for 20 mins. The number of surviving bacteria was determined by plating serial dilutions to GC agar plates.
Protein and Western blot analysis
Samples were prepared by harvesting 109 CFU of bacterial strains into PBS following overnight growth on solid media. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out as previously (Exley et al., 2005), and 12% gels were run in SDS buffer (200 mM glycine, 248 mM Tris, 34 mM SDS) at 120 V for 1-2 hrs. Samples were heated at 90°C for 10 mins before loading, and proteins were visualised using Coomassie Blue.
For Western blot analysis, samples separated by SDS-PAGE were transferred to PVDF membranes (Millipore) by wet transfer at 70 V for 1 hr. Membranes were blocked in 5% milk-PBS (PBS-M) at 4°C overnight with gentle shaking. All further incubations were carried out in PBS-TM (PBS, 0.05% Tween 20, 0.5% milk). Membranes were washed three times, incubated with the primary antibody (α-L,3,7,9 LPS antibody at a 1:2,000 final dilution, NIBSC ref. No. 01/412; 3F11 α-unsialylated LPS at a 1:10 final dilution, kind gift, M. Apicella) for 2 hrs, and washed again, before incubation with a secondary antibody conjugated to horseradish peroxidise (HRP) for 1 hr. Antibody binding was detected with the ECL Plus kit (Amersham).
Isolation and infection of primary human PMN
Heparinised peripheral venous blood was diluted with the same volume of PBS (without calcium and magnesium). Subsequently, diluted blood was layered carefully onto the Histopaque/Ficoll gradient and centrifuged at 396 ×g for 20 mins at RT. The buffy coat was re-suspended in RPMI (Gibco) containing 25 mM Hepes, L-glutamate and 0.05 % human serum albumin and then spun at 249 ×g for 10 mins at 4° C. The cells were resuspended in 9 ml of cold water and mixed vigorously. The hyperosmotic shock was stopped after 40 s by adding 1 ml of 10 times Dulbecco’s PBS (without calcium and magnesium; Mediatech).
Cells were washed in RPMI (Gibco) containing 25 mM Hepes, L-glutamate and 0.05 % human serum albumin and centrifuged at 249 ×g for 10 mins at 4° C. The pellet containing purified PMNs was resuspended in the cold RPMI medium and the number of cells was determined. PMN preparations routinely contained > 95% granulocytes, assessed by FACS, and were > 99% viable, monitored by trypan blue exclusion.
To measure bacterial survival in PMNs, cells were resuspended in RPMI (Gibco BRL) containing 25 mM Hepes, L-glutamate and 0.05 % human serum albumin at 2.5 × 106 PMN ml−1. A 400 μl aliquot of the suspension containing 106 cells was seeded into 24-well tissue culture plates at 37°C for 30 min to allow the cells to adhere to plastic coverslips (Sarstedt) at the bottom of each well. During that time, PMNs were treated with cytochalasin D (5 μM) or resveratrol (100 μM) as required. Bacterial suspensions were prepared in RPMI (Gibco BRL) containing 25 mM HEPES, L-glutamate and 0.05 % human serum albumin. Bacteria were added to each well at an MOI of 2 and the plate was centrifuged at 400 × g for 4 min at 12°C. Cells were washed once and the media replaced with either 1 % saponin to lyse cells (at T0) or pre-warmed RPMI and incubated at 37°C in 5% CO2 for 1 hr (T1). After incubation, cells were washed once and the lysed with saponin to release bacteria which were enumerated by plating to solid media. Survival of bacteria was expressed as a ratio of the CFU recovered after incubation with PMNs with results following incubation without PMNs. Data are expressed as the mean ± SEM of at least three replicate wells, and experiments were repeated at least three times. Depending on the assay, significance was determined by the one-sample t test or two-tailed Student’s t-test.
Analysis of the expression of surface markers was performed by incubating bacteria (1 × 108 cells ml−1) or PMNs (2.5 × 106 cells ml−1) with primary antibodies at the following dilutions: ZM51 α-serogroup B capsule at a 1:100 final dilution (Oxoid); 3F11 as above; α-CD45 at 1:1,000 final dilution (Dako); α-CD18 at a 1:1,000 final dilution (BD Pharmingen) α-CD11b at a 1:1,000 final dilution (BD Pharmingen). Cells were then washed with PBS-T (PBS, 0.05% Tween 20), then incubated with a secondary antibody conjugated to FITC (1:400 final dilution). Samples were analysed using a FACS Calibur (Becton Dickinson), and at least 20,000 events counted. Expression was quantified by the percentage total positive population. All assays were performed in triplicate on at least three independent occasions.
Virulence studies
Litters of 5-day-old rats (Wistar, Harlan U.K.) received 106 N. meningitidis in PBS by the intra-peritoneal (I.P.) route in a volume of 100 μl of PBS. The virulence of mutants was compared directly with the wild-type strain in animals receiving a 1:1 ratio of wild-type to mutant. The proportion of mutant and wild-type bacteria recovered from animals at 20 hrs following challenge was established obtaining venous blood by plating samples of venous blood to media with (to recover the mutant) or without (to recover the mutant and wild-type strain) antibiotics, and the Competitive Index (C.I.) calculated as the ratio of mutant to wild-type bacteria recovered from animals divided by the ratio in the inoculum (Sun et al., 2000).
For murine challenge, bacteria were grown overnight on solid media then made up to 5 × 107 C.F.U. in 250 μl of BHI/5% iron dextran (vol./vol., Sigma) which was administered to via the I.P. route (Exley et al., 2005). Mice were between 6-8 weeks old C57BL/6 or C57BL/6 p47phox−/− congenic mice (Harlan), and blood was collected from animals by tail vein bleeds at 6 and 24 hr post-challenge. Bacteria were recovered by plating to solid media as above. All procedures were conducted in accordance with Home Office guidelines.
Statistical methods
Statistical significance in the C.I. from different groups of animals was examined by the Student’s t-test.
Supplementary Material
Acknowledgements
Work in CMT’s laboratory is supported by the Wellcome Trust, the Medical Research Council and the Meningitis Research Foundation. This work was partly supported by MIUR PRIN to PA. AMC was an MRC student and RME was a Leverhulme Research Fellow. We are very grateful to Bärbel Raupach and Constantin Urban for help with PMN assays.
References
- Andersen LP, Blom J, Nielsen H. Survival and ultrastructural changes of Helicobacter pylori after phagocytosis by human polymorphonuclear leukocytes and monocytes. Apmis. 1993;101:61–72. [PubMed] [Google Scholar]
- Bouter S, Kerklaan PR, Zoetemelk CE, Mohn GR. Biochemical characterization of glutathione-deficient mutants of Escherichia coli K12 and Salmonella strains TA1535 and TA100. Biochem Pharmacol. 1988;37:577–581. doi: 10.1016/0006-2952(88)90128-1. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Ovstebo R, Kierulf P. Bacteremia and compartmentalization of LPS in meningococcal disease. Prog Clin Biol Res. 1995;392:219–233. [PubMed] [Google Scholar]
- Carmel-Harel O, Storz G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu Rev Microbiol. 2000;54:439–461. doi: 10.1146/annurev.micro.54.1.439. [DOI] [PubMed] [Google Scholar]
- Carpenter EP, Corbett A, Thomson H, Adacha J, Jensen K, Bergeron J, Kasampalidis I, Exley R, Winterbotham M, Tang C, Baldwin GS, Freemont P. AP endonuclease paralogues with distinct activities in DNA repair and bacterial pathogenesis. Embo J. 2007;26:1363–1372. doi: 10.1038/sj.emboj.7601593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin BW. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973;128:178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Christodoulides M, Everson JS, Liu BL, Lambden PR, Watt PJ, Thomas EJ, Heckels JE. Interaction of primary human endometrial cells with Neisseria gonorrhoeae expressing green fluorescent protein. Mol Microbiol. 2000;35:32–43. doi: 10.1046/j.1365-2958.2000.01694.x. [DOI] [PubMed] [Google Scholar]
- Colicchio R, Pagliarulo C, Lamberti F, Vigliotta G, Bruni CB, Alifano P, Salvatore P. RecB-dependent mutator phenotype in Neisseria meningitidis strains naturally defective in mismatch repair. DNA Repair (Amst) 2006;5:1428–1438. doi: 10.1016/j.dnarep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Colicchio R, Ricci S, Lamberti F, Pagliarulo C, Pagliuc C, Braione V, Braccini T, Talà A, Montanaro D, Tripodi S, Cintorino M, Troncone G, Bucci C, Pozzi G, Bruni CB, Alifano P, Salvatore P. The meningococcal ABC-Type L-gutamate transporter GltT is necessary for the development of experimental meningitidis in mice. Infect Immun. 2009;77:3578–87. doi: 10.1128/IAI.01424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard CD, Park KA, Montalto MC, Alapati S, Buras JA, Stahl GL, Colgan SP. Neutrophil-derived glutamate regulates vascular endothelial barrier function. J Biol Chem. 2002;277:14801–14811. doi: 10.1074/jbc.M110557200. [DOI] [PubMed] [Google Scholar]
- Criss AK, Seifert HS. Neisseria gonorrhoeae suppresses the oxidative burst of human polymorphonuclear leukocytes. Cell Microbiol. 2008;10:2257–2270. doi: 10.1111/j.1462-5822.2008.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darton T, Guiver M, Naylor S, Jack DL,, Kaczmarski EB, Borrow R, Read RC. Severity of meningococcal disease associated with genomic bacterial load. Clin Infect Dis. 2008;48:587–94. doi: 10.1086/596707. [DOI] [PubMed] [Google Scholar]
- DeLeo FR, Allen LA, Apicella M, Nauseef WM. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- Divino Filho JC, Hazel SJ, Furst P, Bergstrom J, Hall K. Glutamate concentration in plasma, erythrocyte and muscle in relation to plasma levels of insulin-like growth factor (IGF)-I, IGF binding protein-1 and insulin in patients on haemodialysis. J Endocrinol. 1998;156:519–527. doi: 10.1677/joe.0.1560519. [DOI] [PubMed] [Google Scholar]
- Dunn KL, Farrant JL, Langford PR, Kroll JS. Bacterial [Cu,Zn]-cofactored superoxide dismutase protects opsonized, encapsulated Neisseria meningitidis from phagocytosis by human monocytes/macrophages. Infect Immun. 2003;71:1604–1607. doi: 10.1128/IAI.71.3.1604-1607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echenique-Rivera H, Muzzi A, Del Tordello E, Seib KL, Francois P, Rappuoli R, Pizza M, Serruto D. Transcriptome Analysis of Neisseria meningitidis in Human Whole Blood and Mutagenesis Studies Identify Virulence Factors Involved in Blood Survival. PloS Pathog. 2011;7:e1002027. doi: 10.1371/journal.ppat.1002027. doi:10.1371/journal.ppat.1002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook MM, Zhou D, Apicella MA. Nonopsonic phagocytosis of group C Neisseria meningitidis by human neutrophils. Infect Immun. 1998;66:1028–1036. doi: 10.1128/iai.66.3.1028-1036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley RM, Shaw J, Mowe E, Sun YH, West NP, Williamson M, Botto M, Smith H, Tang CM. Available carbon source influences the resistance of Neisseria meningitidis against complement. J Exp Med. 2005;201:1637–1645. doi: 10.1084/jem.20041548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–395. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijen CA, Bredius RG, Kuijper EJ, Out TA, De Haas M, De Wit AP, Daha MR, De Winkel JG. The role of Fcgamma receptor polymorphisms and C3 in the immune defence against Neisseria meningitidis in complement-deficient individuals. Clin Exp Immunol. 2000;120:338–345. doi: 10.1046/j.1365-2249.2000.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy MC, Floquet S, Metais A, Nassif X, Pelicic V. Large-scale analysis of the meningococcus genome by gene disruption: resistance to complement-mediated lysis. Genome Res. 2003;13:391–398. doi: 10.1101/gr.664303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner J, Greer PW, Whitney A, Shieh WJ, Fischer M, White EH, Carlone GM, Stephens DS, Popovic T, Zaki SR. Pathogenesis and diagnosis of human meningococcal disease using immunohistochemical and PCR assays. Am J Clin Pathol. 2004;122:754–764. doi: 10.1309/A7M2-FN2T-YE6A-8UFX. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt S, Birkholz C, Zahringer U, Robertson BD, van Putten J, Ebeling O, Frosch M. Contribution of genes from the capsule gene complex (cps) to lipooligosaccharide biosynthesis and serum resistance in Neisseria meningitidis. Mol Microbiol. 1994;11:885–896. doi: 10.1111/j.1365-2958.1994.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Haralambous E, Dolly SO, Hibberd ML, Litt DJ, Udalova IA, O’Dwyer C, Langford PR, Simon Kroll J, Levin M. Factor H, a regulator of complement activity, is a major determinant of meningococcal disease susceptibility in UK Caucasian patients. Scand J Infect Dis. 2006;38:764–771. doi: 10.1080/00365540600643203. [DOI] [PubMed] [Google Scholar]
- Hendriksen WT, Kloosterman TG, Bootsma HJ, Estevao S, de Groot R, Kuipers OP, Hermans PW. Site-specific contributions of glutamine-dependent regulator GlnR and GlnR-regulated genes to virulence of Streptococcus pneumoniae. Infect Immun. 2008;76:1230–1238. doi: 10.1128/IAI.01004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JC. Effect of glutamate on exogenous citrate catabolism of Neisseria meningitidis and of other species of Neisseria. J Bacteriol. 1971;106:819–823. doi: 10.1128/jb.106.3.819-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang DS, Kang BS, Ryu SY, Chang IM, Min KR, Kim Y. Inhibitory effects of resveratrol analogs on unopsonized zymosan-induced oxygen radical production. Biochem Pharmacol. 1999;57:705–712. doi: 10.1016/s0006-2952(98)00350-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi SD, Braughton KR, Whitney AR, Voyich JM, Schwan TG, Musser JM, DeLeo FR. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc Natl Acad Sci U S A. 2003;100:10948–10953. doi: 10.1073/pnas.1833375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MS, Chow NY, Sinha S, Halliwell D, Finney M, Gorringe AR, Watson MW, Kroll JS, Langford PR, Webb SA. A Neisseria meningitidis NMB1966 mutant is impaired for invasion of respiratory epithelial cells, survival in human blood and for virulence in vivo. Med Microbiol Immunol. 2009;198:57–67. doi: 10.1007/s00430-008-0105-2. [DOI] [PubMed] [Google Scholar]
- Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallavia LP, Weiss E. Catabolic activities of Neisseria meningitidis: utilization of glutamate. J Bacteriol. 1970;101:127–132. doi: 10.1128/jb.101.1.127-132.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell RE, McLaughlin R, Aba Kwaik Y, Lesse A, Yamasaki R, Gibson B, Spinola SM, Apicella MA. Lipooligosaccharides (LOS) of some Haemophilus species mimic human glycosphingolipids, and some LOS are sialylated. Infect Immun. 1992;60:1322–1328. doi: 10.1128/iai.60.4.1322-1328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaw SE, Schneider J, Liao EH, Zimmermann W, Gray-Owen SD. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol Microbiol. 2003;49:623–637. doi: 10.1046/j.1365-2958.2003.03591.x. [DOI] [PubMed] [Google Scholar]
- McNeil G, Virji M. Phenotypic variants of meningococci and their potential in phagocytic interactions: the influence of opacity proteins, pili, PilC and surface sialic acids. Microb Pathog. 1997;22:295–304. doi: 10.1006/mpat.1996.0126. [DOI] [PubMed] [Google Scholar]
- Monaco C, Tala A, Spinosa MR, Progida C, De Nitto E, Gaballo A, Bruni CB, Bucci C, Alifano P. Identification of a meningococcal L-glutamate ABC transporter operon essential for growth in low-sodium environments. Infect Immun. 2006;74:1725–1740. doi: 10.1128/IAI.74.3.1725-1740.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TD, Sparling PF. Interruption of the gpxA gene increases the sensitivity of Neisseria meningitidis to paraquat. J Bacteriol. 1996;178:4301–4305. doi: 10.1128/jb.178.14.4301-4305.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Ovstebo R, Brandtzaeg P, Brusletto B, Haug KB, Lande K, Hoiby EA, Kierulf P. Use of robotized DNA isolation and real-time PCR to quantify and identify close correlation between levels of Neisseria meningitidis DNA and lipopolysaccharides in plasma and cerebrospinal fluid from patients with systemic meningococcal disease. J Clin Microbiol. 2004;42:2980–2987. doi: 10.1128/JCM.42.7.2980-2987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarulo C, Salvatore P, De Vitis LR, Colicchio R, Monaco C, Tredici M, Tala A, Bardaro M, Lavitola A, Bruni CB, Alifano P. Regulation and differential expression of gdhA encoding NADP-specific glutamate dehydrogenase in Neisseria meningitidis clinical isolates. Mol Microbiol. 2004;51:1757–1772. doi: 10.1111/j.1365-2958.2003.03947.x. [DOI] [PubMed] [Google Scholar]
- Parkhill J, Achtman M, James KD, Bentley SD, Churcher C, Klee SR, Morelli G, Basham D, Brown D, Chillingworth T, Davies RM, Davis P, Devlin K, Feltwell T, Hamlin N, Holroyd S, Jagels K, Leather S, Moule S, Mungall K, Quail MA, Rajandream MA, Rutherford KM, Simmonds M, Skelton J, Whitehead S, Spratt BG, Barrell BG. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- Read RC, Zimmerli S, Broaddus C, Sanan DA, Stephens DS, Ernst JD. The (alpha2-->8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect Immun. 1996;64:3210–3217. doi: 10.1128/iai.64.8.3210-3217.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L. Nitrogen assimilation and global regulation in Escherichia coli. Annu Rev Microbiol. 2003;57:155–176. doi: 10.1146/annurev.micro.57.030502.090820. [DOI] [PubMed] [Google Scholar]
- Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, Tang CM. Functional Significance of Factor H Binding to Neisseria meningitidis. J Immunol. 2006;176:7566–7575. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- Schneider MC, Exley RM, Ram S, Sim RB, Tang CM. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 2007;15:233–240. doi: 10.1016/j.tim.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, Lea SM. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009;16:890–3. doi: 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal AW. The NADPH oxidase and chronic granulomatous disease. Mol Med Today. 1996;2:129–135. doi: 10.1016/1357-4310(96)88723-5. [DOI] [PubMed] [Google Scholar]
- Seib KL, Tseng HJ, McEwan AG, Apicella MA, Jennings MP. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: distinctive systems for different lifestyles. J Infect Dis. 2004;190:136–147. doi: 10.1086/421299. [DOI] [PubMed] [Google Scholar]
- Shibayama K, Wachino J, Arakawa Y, Saidijam M, Rutherford NG, Henderson PJ. Metabolism of glutamine and glutathione via gamma-glutamyltranspeptidase and glutamate transport in Helicobacter pylori: possible significance in the pathophysiology of the organism. Mol Microbiol. 2007;64:396–406. doi: 10.1111/j.1365-2958.2007.05661.x. [DOI] [PubMed] [Google Scholar]
- Simons MP, Nauseef WM, Griffith TS, Apicella MA. Neisseria gonorrhoeae delays the onset of apoptosis in polymorphonuclear leukocytes. Cell Microbiol. 2006;8:1780–1790. doi: 10.1111/j.1462-5822.2006.00748.x. [DOI] [PubMed] [Google Scholar]
- Simons MP, Nauseef WM, Apicella MA. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect Immun. 2005;73:1971–7. doi: 10.1128/IAI.73.4.1971-1977.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Garcia AA, Jerse AE. A Neisseria gonorrhoeae catalase mutant is more sensitive to hydrogen peroxide and paraquat, an inducer of toxic oxygen radicals. Microb Pathog. 2004;37:55–63. doi: 10.1016/j.micpath.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- Sun YH, Bakshi S, Chalmers R, Tang CM. Functional genomics of Neisseria meningitidis pathogenesis. Nat Med. 2000;6:1269–1273. doi: 10.1038/81380. [DOI] [PubMed] [Google Scholar]
- Tala A, De Stefano M, Bucci C, Alifano P. Reverse transcriptase-PCR differential display analysis of meningococcal transcripts during infection of human cells: up-regulation of priA and its role in intracellular replication. BMC Microbiol. 2008;8:131. doi: 10.1186/1471-2180-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelin H, Saunders NJ, Heidelberg J, Jeffries AC, Nelson KE, Eisen JA, Ketchum KA, Hood DW, Peden JF, Dodson RJ, Nelson WC, Gwinn ML, DeBoy R, Peterson JD, Hickey EK, Haft DH, Salzberg SL, White O, Fleischmann RD, Dougherty BA, Mason T, Ciecko A, Parksey DS, Blair E, Cittone H, Clark EB, Cotton MD, Utterback TR, Khouri H, Qin H, Vamathevan J, Gill J, Scarlato V, Masignani V, Pizza M, Grandi G, Sun L, Smith HO, Fraser CM, Moxon ER, Rappuoli R, Venter JC. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- Thomas EL. Disulfide reduction and sulfhydryl uptake by Streptococcus mutans. J Bacteriol. 1984;157:240–246. doi: 10.1128/jb.157.1.240-246.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YL, Ambrose KD, Zughaier S, Zhou X, Miller YK, Shafer WM, Stephens DS. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J Bacteriol. 2005;187:5387–5396. doi: 10.1128/JB.187.15.5387-5396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkmeir A, Kammerer U, Stade A, Hubner C, Haller S, Kolb-Maurer A, Frosch M, Dietrich G. Lipooligosaccharide and polysaccharide capsule: virulence factors of Neisseria meningitidis that determine meningococcal interaction with human dendritic cells. Infect Immun. 2002;70:2454–2462. doi: 10.1128/IAI.70.5.2454-2462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban CF, Lourido S, Zychlinsky A. How do microbes evade neutrophil killing? Cell Microbiol. 2006;8:1687–1696. doi: 10.1111/j.1462-5822.2006.00792.x. [DOI] [PubMed] [Google Scholar]
- van Deuren M, Brandtzaeg P, van der Meer JW. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev. 2000;13:144–166. doi: 10.1128/cmr.13.1.144-166.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veale DR, Goldner M, Penn CW, Ward J, Smith H. The intracellular survival and growth of gonococci in human phagocytes. J Gen Microbiol. 1979;113:383–393. doi: 10.1099/00221287-113-2-383. [DOI] [PubMed] [Google Scholar]
- Virji M, Makepeace K, Ferguson DJ, Watt SM. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- Vogel U, Frosch M. Mechanisms of neisserial serum resistance. Mol Microbiol. 1999;32:1133–1139. doi: 10.1046/j.1365-2958.1999.01469.x. [DOI] [PubMed] [Google Scholar]
- Weichselbaum A. Ueber die Aetiologie der akuten Meningitis Cerebrospinalis. Fortschrift Medizin. 1887;5:573–587. [Google Scholar]
- Yazdankhah SP, Caugant DA. Neisseria meningitidis: an overview of the carriage state. J Med Microbiol. 2004;53:821–832. doi: 10.1099/jmm.0.45529-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.