Summary
Following transplantation of allogeneic tissues, CD4+ recipient T cells recognize donor antigens via two distinct mechanisms: direct allorecognition, in which T cells interact with intact MHC class II on donor bone marrow-derived passenger leukocytes, and indirect allorecognition, in which T cells recognize allogeneic peptide determinants bound to self-MHC class II molecules on recipient antigen presenting cells (APCs) [1]. Activation of pro-inflammatory T cells through each of these pathways elicits different types of alloresponses leading to acute and/or chronic rejection of allografts [2]. Therefore, suppression of both of these pathways is needed to achieve transplantation tolerance. In this study, different allogeneic dendritic cells (DCs) modified to lack expression of CD80/86 or over-express indoleamine 2,3-dioxygenase (IDO) were shown to inhibit either direct and/or indirect pro-inflammatory T cell alloresponses in vitro and in vivo. Most remarkably, suppression of both pathways was achieved through linked suppression via coexpression of self- and allo-MHC molecules on semi-allogeneic DCs. Finally, this approach accomplished long-term survival of allogeneic corneal allografts whose rejection relies on indirect activation of CD4+ T cells specific for minor histocompatibility antigens. This study further demonstrates the tolerogenic potential of genetically-modified DCs that could pave the way for the design of DC-based therapy in clinical transplantation.
Keywords: Dendritic cells, transplantation, tolerance, regulatory T cells, allorecognition pathways
Pro-inflammatory direct and indirect T cell alloresponses elicited after transplantation are both capable of causing acute rejection of fully allogeneic skin allografts [3]. Alternatively, early acute rejection of vascularized solid organ allotransplants is essentially mediated through direct allorecognition, while indirect allorecognition is commonly associated with chronic rejection of kidney and heart transplants (a slow process characterized by graft vasculopathy and tissue fibrosis) [4]. Therefore, abrogation of both types of inflammatory alloresponses will be required to achieve tolerance to allogeneic transplants—defined as indefinite graft survival without chronic dysfunction—in the absence of ongoing immunosuppression.
There is ample evidence showing that MHC class II-restricted antigen presentation by APCs incapable of delivering proper costimulatory signals results in CD4+ T cell unresponsiveness [5]. Actually, peripheral tolerance to autologous antigens is believed to be maintained by continuous presentation of self-determinants by resting dendritic cells in the absence of inflammatory signals, a phenomenon causing T cell anergy and activation of some regulatory T cells Tregs) [6–8]. Based upon this principle, many transplant tolerance protocols have been designed using donor cell administration in combination with antibodies blocking key costimulatory pathways such as CD28/B7 and CD40/CD40L [9]. For example, infusion of transplant recipients with donor bone marrow or spleen cells, along with anti-CD40L antibodies or CTLA4-Ig regularly achieves long-term survival of some organ allografts [10–12]. However, in most cases, these treatments do not prevent chronic rejection, suggesting that they fail to tolerize T cells activated in an indirect fashion. This viewpoint is also supported by studies showing that T cell costimulation blockade using anti-CD40L mAbs prolongs the survival of cardiac, but not skin allografts [9]. This may be explained by the observation that, in contrast to hearts, skin allografts induce potent indirect alloresponses, which are somewhat resistant to this antibody treatment (G. Benichou, unpublished data). Consequently, tolerization of T cells responding to alloantigens through the indirect allorecognition pathway has become a major challenge in the field of transplantation. In the present study, A. George’s group demonstrates that inflammatory CD4+ T cells activated though both direct and indirect allorecognition pathways can be rendered tolerant using either donor DCs lacking CD80/86 costimulatory receptor membrane expression, or DCs over-expressing IDO.
In infected tissues, microbial antigen capture by DCs in infected tissues and their presentation in secondary lymphoid organs is clearly the most efficient mechanism to trigger a pro-inflammatory adaptive immune response [13]. This response initiates the destruction of microbes and the generation of memory T cells, conferring long-term protective immunity. This type of antigen presentation occurs during acute infections associated with potent innate immune responses and inflammatory cytokine secretion, causing DC maturation and expression of certain key costimulation receptors such as B71/2 and CD40. Likewise, alloantigen presentation by donor DCs is known to induce powerful pro-inflammatory T cell immune responses leading to acute allograft rejection [14]. Based upon these principles, many studies have focused on the elimination of donor DCs in an effort to induce transplant tolerance. However, while elimination of donor passenger leukocytes brought about some prolongation of graft survival by reducing direct alloreactivity, it neither achieved tolerance nor suppressed indirect alloresponses [15]. On the other hand, there is increasing evidence to suggest that inactivation of pro-inflammatory T cells against antigens also relies on antigen presentation by DCs. Indeed, antigens displayed by immature DCs in the absence of innate inflammatory signals may preferentially activate Tregs and T cell anergy [6–8]. This mechanism is essential to maintainance of peripheral tolerance to autoantigens [6–8]. Likewise, there are several lines of data suggesting that donor DCs are necessary for tolerance induction to allografts [16–18]. Therefore, allogeneic DCs are required for both rejection and tolerance of allografts.
In the CBK to CBA single MHC class I-disparate model (Model A), CBK “CTLA-4-DCs” (CBA Kb transgenic) lacking membrane expression of CD80/86 induced both in vivo and in vitro expansion/activation of Tregs. These Tregs were capable of suppressing the indirect alloresponse of CD4+ pro-inflammatory CBA T cells recognizing Kb peptides presented by Ak/Ek recipient MHC class II molecules. T cell suppression was presumably mediated via IL-10 and TGF-β cytokine release, and resulted in anergy confined to donor-specific CD4+ T cells. In the BALB/c to C3H full MHC-disparate model (Model B), BALB/c “CTLA-4-DC” (H-2d) were cultured with C3H (H-2k) T cells or administered to C3H mice. In both cases, C3H CD4+ T cells became anergic to BALB/c but not to third-party allogeneic cells, and allospecific tolerance could be transferred in vitro and in vivo with C3H CD4+ T cells from cultures or mice exposed to BALB/c CTLA-4-DCs.
The results obtained in model A presumably reflect the mechanism by which Tregs—activated through the presentation of peptides presented on a nominal antigen—suppress effector T cells (Teff) recognizing the same or other determinants presented by the same MHC class II molecules. The process by which Tregs mediate suppression in model B is more intriguing. The results suggest that such induced Tregs recognize alloantigens in the same fashion as pro-inflammatory CD4+ T cells. Indeed, the fact that donor BALB/c DCs and recipient C3H Tregs do not share any MHC class II molecules hints that Tregs are activated in a direct fashion, a conclusion which challenges the traditional view that Treg activation occurs exclusively through indirect antigen presentation. Alternatively, one can speculate that Tregs recognize determinants presented by self-MHC class II molecules present on partially activated effector T cells and not on donor DCs—i.e., in an indirect fashion. This question could be resolved by using effector T cells lacking MHC class II expression.
Next, A. Khan and colleagues showed that Tregs generated in CBA mice via administration of CBK CTLA-4-DCs inhibit T cell responses to (B6 x CBA) F1 semi-allogeneic stimulators expressing allogeneic MHC class I (Lb and Db) and class II alleles (Ab) that are not present in CBK mice. These data demonstrate that both indirect alloresponses to Lb/Db and direct alloresponses to Ab of CBA T cells were abrogated though linked suppression, as depicted in Figure 1. This conclusion was confirmed by lack of suppression to (BALB/c x B6) F1 allogeneic stimulators, which express Kb, but not the proper self-MHC class II allele. These observations further illustrate how Tregs activated indirectly can suppress direct alloresponses by effector T cells. It is not clear whether the effector phase of suppression requires Treg-Teff cell contact. Experiments using transwell plates in which Teff, Tregs, and DCs are cultured in separate compartments could be conducted to answer this question.
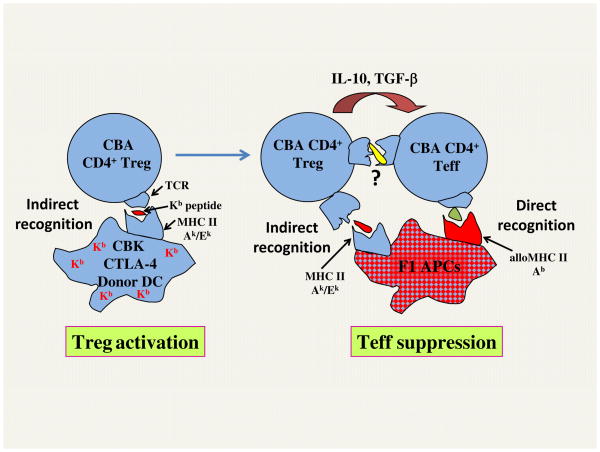
Figure 1. Potential mechanism involved in linked suppression.
Treg activation: CBA (H-2k) mice were injected with CTLA-4-DCs from CBK mice (CBA mice expressing MHC class I, Kb transgene). Presentation of Kb determinants by self-MHC class II Ak/Ek (indirect presentation), in the absence of proper costimulation, leads to activation/expansion of some CD4+ regulatory T cells (Tregs). Teff suppression: Upon adoptive transfer, Tregs recognizing Ak/Ek-Kb peptide complexes presented on (B6 x BALB/c) F1 donor cells (indirect pathway) suppress the alloresponse of CBA CD4+ effector T cells (Teff) recognizing donor MHC class II Ab molecules (direct pathway) presented on the same F1 APCs (three cell model). Teff suppression is mediated via IL-10 and TGFβ and potentially some cognate interactions between Tregs and Teff (T-T interaction).
Although IDO-DCs suppressed both direct and indirect alloresponses by CD4+ T cells, they failed to induce T cell anergy and regulatory T cell immunity. This is in apparent contradiction with the general view that DCs expressing IDO inhibit inflammatory reactions via cell cycle arrest, apoptosis and anergy of TH1/CT1 cells [19]. The authors speculate that this discrepancy may be explained by the fact that high IDO levels result in non-specific T cell hyporesponsiveness and death rather than anergy. Additionally, we surmise that IDO-DC injection does not cause substantial inflammation and subsequent γIFN production, a cytokine presumably required for TGF-β production by activated DCs [20]. In this scenario, kynurenines and other metabolites of tryptophan degradation generated through IDO activity may lead to T-cell apoptosis but not anergy and generation of Tregs, in the absence of TGF-β [21]. This might explain why, apparently, “artificially” increased IDO expression in DCs mitigates some effector T cell functions in a non-specific manner but not antigen-dependent induction of the T cell response.
Lastly, A. Khan et al. investigated the effects of modified DCs on the rejection of corneal allografts in mice. The alloresponse to and rejection of allogeneic transplants are exclusively mediated via indirect alloreactivity by CD4+ T cells directed toward minor histocompatibility antigens [22, 23]. Indeed, lack of corneal APCs expressing allo-MHC class II+ and low expression of allo-MHC class I result in an absence of CD4+ T cell direct alloreactivity [24]. On the other hand, some CD8+ T cells secreting γIFN are activated though the direct allorecognition of donor MHC class I antigens, but fail to display cytotoxic functions and do not influence the rejection process [22]. In the C3H to BALB/c model, injection of CTLA-4-DCs resulted in a modest but significant prolongation of graft survival (18d vs. 12d), while IDO-DCs had no effect. In this setting, it is conceivable that Tregs recognizing allo-MHC class II directly do not suppress the CD4+ T cell indirect alloresponse and thereby fail to induce tolerance of corneal allografts. This hypothesis is supported by the observation that administration of CBA recipients with semi-allogeneic (CBA x BALB/c) F1 CTLA-4-DCs, which apparently generate Tregs blocking both direct and indirect alloresponses by CD4+ T cells, resulted in indefinite survival of corneal allografts (>100d). It would have been interesting to test the effects of CTLA-4-DCs on the rejection of high-risk corneal transplants, which are performed on an inflamed eye bed, induce direct CD4+ alloresponses, and represent a major problem in clinical settings [25, 26].
In summary, the article from A. George’s group demonstrates that allogeneic DCs can be modified in a fashion that achieves donor-specific (CTLA-4-DCs) or non-specific (IDO-DCs) tolerance of T cells responding to alloantigens via both direct and indirect allorecognition pathways. It is clear that the injection of transplant recipient DCs presenting defined alloantigens—while lacking proper costimulatory receptors—is expected to achieve selective suppression of donor-specific inflammatory T cells. In comparison, the administration of donor APCs along with antibodies blocking the same costimulation pathways can potentially suppress other T cells. In the present study, injection of mice with modified allogeneic DCs achieved long-term survival of corneal allografts (which are otherwise rejected by CD4+ T cells activated indirectly). Whether delivery of tolerogenic DCs would be effective at preventing rejection of more immunogenic transplants such as heart and skin allografts remains to be investigated. In addition, the ability of modified DCs to achieve tolerance in allo-sensitized mice should be explored, given the documented contribution of donor-reactive memory T cells to transplant tolerance resistance in primates [27]. Finally, since the tolerogenic DCs designed in this study can suppress indirect alloreactivity, it would be interesting to determine whether administration of these cells could prevent or suppress alloantibody production and chronic rejection of vascularized organ transplants in recipients treated with short-term immunosuppressive therapy using calcineurin inhibitors and/or a lymphocyte costimulation blockade.
Acknowledgments
The authors of this commentary are supported by the United States National Institute of Health (NIH grants R03AI09423 and R21AI100278). We would also like to thank Ms. Kate Capetta for her assistance with the preparation of this manuscript.
References
- 1.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 2.Illigens BM, Yamada A, Fedoseyeva EV, Anosova N, Boisgerault F, Valujskikh A, Heeger PS, Sayegh MH, Boehm B, Benichou G. The relative contribution of direct and indirect antigen recognition pathways to the alloresponse and graft rejection depends upon the nature of the transplant. Hum Immunol. 2002;63:912–925. doi: 10.1016/s0198-8859(02)00449-4. [DOI] [PubMed] [Google Scholar]
- 3.Boisgerault F, Liu Y, Anosova N, Dana R, Benichou G. Differential roles of direct and indirect allorecognition pathways in the rejection of skin and corneal transplants. Transplantation. 2009;87:16–23. doi: 10.1097/TP.0b013e318191b38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Cook HT, Lechler RI. Loss of direct and maintenance of indirect alloresponses in renal allograft recipients: implications for the pathogenesis of chronic allograft nephropathy. Journal of Immunology. 2001;167:7199–7206. doi: 10.4049/jimmunol.167.12.7199. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 6.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 7.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 8.Huang FP, MacPherson GG. Continuing education of the immune system--dendritic cells, immune regulation and tolerance. Curr Mol Med. 2001;1:457–468. doi: 10.2174/1566524013363573. [DOI] [PubMed] [Google Scholar]
- 9.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: evidence for anergy and deletion but not regulatory cells. Blood. 2004;103:4336–4343. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 11.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bushell A, Karim M, Kingsley CI, Wood KJ. Pretransplant blood transfusion without additional immunotherapy generates CD25+CD4+ regulatory T cells: a potential explanation for the blood-transfusion effect. Transplantation. 2003;76:449–455. doi: 10.1097/01.TP.0000083043.84630.99. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Larsen CP, Steinman RM, Witmer-Pack M, Hankins DF, Morris PJ, Austyn JM. Migration and maturation of Langerhans cells in skin transplants and explants. J Exp Med. 1990;172:1483–1493. doi: 10.1084/jem.172.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 17.McCurry KR, Colvin BL, Zahorchak AF, Thomson AW. Regulatory dendritic cell therapy in organ transplantation. Transpl Int. 2006;19:525–538. doi: 10.1111/j.1432-2277.2006.00306.x. [DOI] [PubMed] [Google Scholar]
- 18.Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23:252–263. doi: 10.1016/j.smim.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 20.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 21.Belladonna ML, Orabona C, Grohmann U, Puccetti P. TGF-beta and kynurenines as the key to infectious tolerance. Trends Mol Med. 2009;15:41–49. doi: 10.1016/j.molmed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Boisgerault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. Journal of Immunology. 2001;167:1891–1899. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 23.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice--evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54:694–704. doi: 10.1097/00007890-199210000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Streilein JW, Toews GB, Bergstresser PR. Corneal allografts fail to express Ia antigens. Nature. 1979;282:326–327. doi: 10.1038/282326a0. [DOI] [PubMed] [Google Scholar]
- 25.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol. 2004;173:4464–4469. doi: 10.4049/jimmunol.173.7.4464. [DOI] [PubMed] [Google Scholar]
- 26.Qian Y, Hamrah P, Boisgerault F, Yamagami S, Vora S, Benichou G, Dana MR. Mechanisms of immunotherapeutic intervention by anti-CD154 (CD40L) antibody in high-risk corneal transplantation. J Interferon Cytokine Res. 2002;22:1217–1225. doi: 10.1089/10799900260475740. [DOI] [PubMed] [Google Scholar]
- 27.Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, Sachs DH, Allan J, Madsen JC, Kawai T, Cosimi AB, Benichou G. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3:86–90. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]