Abstract
As the U.S. population ages, the prevalence of geriatric conditions in patients with heart failure is increasing, although they currently fall outside the traditional heart failure disease model. In this review, we describe the co-occurrence of four common geriatric conditions (cognitive impairment, frailty, falls, and incontinence) in older adults with heart failure, their mechanisms of interaction, and their association with outcomes. We propose a new paradigm to meet the needs of the aging heart failure population that includes comprehensive assessment of geriatric conditions and tailoring of therapy and surveillance accordingly. Coordination among relevant disciplines such as cardiology and geriatrics may facilitate this transition. Further research is needed in order to understand how to optimize care for patients with specific impairments in order to improve outcomes.
Introduction
The vast majority of patients with heart failure are older adults; in the United States, approximately 80 % are ≥65 years of age [1, 2], and the number of patients aged 80 or older has nearly doubled over the last two decades [3]. Despite the aging of the heart failure population, geriatric conditions, defined as multifactorial non-disease specific conditions such as frailty, cognitive impairment, incontinence, dizziness, and falls [4], have historically received relatively little attention as they fall outside the traditional heart failure disease model that dominates research and clinical care [5–8].
However, there is emerging evidence demonstrating that geriatric conditions are common in older adults with heart failure and influence the heart failure disease process in multiple ways, including clinical presentation, disease progression, and outcomes including hospitalization and mortality [8, 9] (Fig. 1). The purpose of this review is to summarize the available literature on the prevalence of four common geriatric conditions (cognitive impairment, frailty, falls, incontinence) in heart failure, their relationship with the disease process, and directions for future research.
Figure 1.
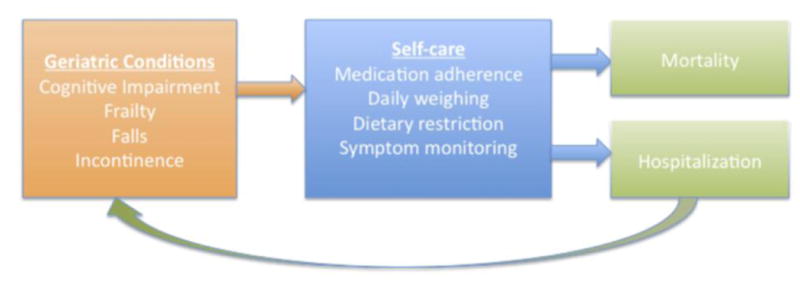
Theoretical relationship between geriatric conditions and outcomes in heart failure. In this model, which is simplified for illustration, geriatric conditions impair heart failure self-care, which subsequently may result in hospitalization and mortality. Hospitalization can further worsen impairments; for example, being confined to bed can exacerbate frailty, and delirium can precipitate falls.
Cognitive Impairment
Epidemiology
Cognitive impairment is relatively common in older adults with heart failure; most studies report a prevalence of at least 25 % [9–13], although some estimates are much higher. For example, a study by Cameron et al. of 93 consecutive patients (mean age 70 years, without known neurocognitive problems) hospitalized for heart failure in Australia found that mild cognitive impairment (identified by either the MMSE or MOCA) was present in 73 % of study participants [14] (Table 1). Most studies assessing prevalence of cognitive impairment in heart failure populations are small, and the variability in estimates is likely secondary to heterogeneous patient populations and differing definitions of cognitive impairment. For example, there is evidence that the prevalence of cognitive impairment is greater in heart failure patients who have recently been hospitalized [15], and in patients with advanced left ventricular systolic dysfunction [16].
Table 1. Prevalence of geriatric impairments in heart failure.
| Condition | Study | n (mean age) | Prevalence |
|---|---|---|---|
| Cognitive impairment | Cameron et al. [14] | 93 (70 years) | 73 % |
| Zuccalá et al. [11] | 1511 (82 years) | 35 % | |
| Frailty | Boxer et al. [33] | 60 (78 years) | 25 % |
| Newman et al. [34] | 299 (77 years) | 14% | |
| Falls | Tinetti et al. [52] | 336 (78 years) | 32 %a |
| Urinary incontinence | Van Der Wel et al. [66] | 269 (79 years) | 17.5 % |
| Palmer et al. [67] | 296 (62 years) | 45 % |
Fall rate per year; population not exclusively limited to heart failure
Numerous studies have shown that patients with heart failure are more likely to be cognitively impaired compared with patients without heart failure [10, 16, 17]. A pooled meta-analysis of 22 studies including 2,937 heart failure patients and 14,848 controls found that the odds ratio for cognitive impairment among patients with heart failure (relative to controls) was 1.62 [95 % confidence interval (CI): 1.48–1.79] [10]. Compared with other cardiovascular conditions, heart failure appears to confer a higher risk of cognitive impairment; for example, a study by Vogels et al. found that patients with heart failure were more likely to be cognitively impaired than age-matched patients with ischemic heart disease and preserved ejection fraction [17].
Assessment
The most widely utilized instrument to assess cognitive status in older adults with heart failure is the Folstein Mini Mental Status Examination (MMSE) [10, 18]. The MMSE consists of 11 items that assess domains of orientation, short-term memory, attention, and visual spatial skills, and is scored on a 30-point scale. A score of <25 is generally considered abnormal [19, 20], although there are a variety of cutpoints that adjust for age [21, 22] and education [21, 23]. More recently, the Montreal Cognitive Assessment (MOCA) has been developed as a brief screening tool to detect mild cognitive impairment [14, 24]. The MOCA contains cognitive domains including attention, memory, language, and conceptual thinking [24], with a total possible score of 30 and a score of <26 considered abnormal. Several studies have demonstrated that the MOCA has a higher sensitivity than the MMSE in detecting mild cognitive impairment [24–26]. A shorter alternative to the MMSE and MOCA is the Mini-Cog [27], which involves a composite of three-item recall and clock drawing, and can be administered in 3 min.
Mechanisms and Association with Outcomes
Two main pathophysiologial mechanisms for the association between heart failure and cognitive impairment have been postulated: intermittent cerebral hypoperfusion [28] and cerebral microemboli due to left ventricular thrombus formation [29]. As most studies have not utilized neuroimaging, the degree to which each mechanism contributes to cognitive impairment remains unclear.
Cognitive impairment may affect outcomes by impeding heart failure self-care, which is defined as an active process in which patients maintain health through adherence with medications, symptom monitoring, dietary compliance, and daily self-weighing [12, 14]. Cognitive impairment may interfere with any one of these necessary tasks; for example, doses of diuretics may be missed, or changes in symptoms (dyspnea, weight gain) may not be recognized until they are severe. The hospital to home transition may be especially difficult as therapeutic regimens and care plans are often changed [30]. Also, outpatients with heart failure and cognitive impairment may not remember to report ongoing problems at routine medical encounters [9].
Several studies have found that cognitive impairment is independently associated with mortality in patients with heart failure [8, 31]. In the largest study to date, Chaudhry et al. analyzed a sample of 62,330 Medicare beneficiaries ≥65 years of age with heart failure and found that the adjusted odds ratios (95 % CI) for death at 30 days and 5 years were 1.86 (1.73–2.01) and 2.01 (1.84–2.19), respectively [8]. The evidence base for cognitive impairment and hospital utilization in heart failure is limited. In a small study by McLennan et al. [31] of 200 inpatients admitted with heart failure, patients with cognitive impairment (n = 27, based on MMSE) were more likely than those without impairment (n =173) to experience an unplanned rehospitalization or death at 5 years, although rates were very high in both groups (100 vs. 94 %, relative risk 1.44, 95% CI 1.06–1.95). To our knowledge, definitive large-scale epidemiologic studies evaluating the relationship between cognitive impairment and hospital utilization are currently lacking.
Frailty
Epidemiology
Frailty is defined as an increased physiologic vulnerability to stressors [32]. Frailty becomes more common with advancing age, but even in the oldest old it is not ubiquitous; in the Cardiovascular Health Study, frailty was is present in 7 % of participants ≥65 years of age, and increased in prevalence to one-quarter of those ≥80 years of age [32]. Among patients with existing heart failure, estimates of the prevalence frailty are limited. A study by Boxer et al. of 60 heart failure patients (mean age 78 years) with ejection fraction <40 % found that frailty was present in 25 % of individuals [33]. Other studies focusing exclusively on frail patients have found that frailty and heart failure frequently co-occur [34, 35]. In the Cardiovascular Health Study, 14 % of patients identified as frail also had congestive heart failure, compared with 1.8 % among individuals who were not frail (odds ratio = 7.51, 95 % CI 4.7–12.1) [34]. A similar pattern was seen in women aged 65–79 enrolled in the Women's Health Initiative (WHI); frailty was present in 16 % of participants, and women with frailty were nearly six times as likely to have heart failure compared with women without frailty [35].
Assessment
The most widely used criteria to describe the frailty phenotype were defined by Fried et al. in 2001, using data from the Cardiovascular Health Study. In this framework, frailty is a condition in which three or more of the following are present: (1) unintentional weight loss (≥10 pounds within the past year), (2) self-reported exhaustion, (3) weakness (grip strength), (4) slow walking speed, and (5) low physical activity [32]. In the same study, the frailty phenotype was independently predictive of incident falls, worsening ADL disability, hospitalization, and death.
Gait speed is an easily reproducible singular measure that correlates well with the frailty phenotype [36] and has strong associations with survival [37, 38] as well as other patient-centered outcomes, including loss of independence [39], hospital readmission [40], and nursing home placement [41, 42]. Gait speed is typically measured as one's usual pace over a pre-specified distance (such as 5 m) [43]. While the relationship between gait speed and survival is continuous [37], several cutoffs for “slow gait” have been used to ease categorization of patients, ranging from 0.65 m/s (36) to 0.8 m/s [37].
Mechanisms and Association with Outcomes
The causal relationship between frailty and heart failure is complex, and each condition can exacerbate the other. In the Cardiovascular Health Study, patients who were frail were found to have reduced global left ventricular function and increased left ventricular mass compared with non-frail individuals [34]. The muscle weakness that is characteristic of frailty may impair adherence to daily self-care activities such as measurement of body weight, and may also worsen symptoms of dyspnea secondary to respiratory muscle weakness. In turn, worsening heart failure may lead to decreased activity and low nutritional intake, which can result in cardiac cachexia and worsened frailty [44]. Frail patients also appear more prone to developing iatrogenic heart failure due to excessive fluid administration compared with patients who are not frail [45].
Several investigations have demonstrated that frailty is independently associated with adverse outcomes in heart failure patients including hospitalization and mortality [9, 46, 47], although to date studies have been small. Cacciatore et al. studied 120 patients with heart failure and 1,139 controls (all ≥65 years of age) over a 12-year time period, and found that, while frailty was independently predictive of mortality in all subjects, the independent effect of frailty was greater in those with heart failure than without [46]: among the 18 heart failure patients with advanced frailty [defined according to the Frailty Staging System (FSS)], survival declined markedly after 5 years, and none were alive after 9 years of follow-up. A separate study of 59 outpatients with heart failure ≥60 years of age also found that baseline frailty status was independently associated with mortality at 4-year follow-up (hazard ratio 1.57, 95 % CI 1.05–2.33) [48].
Falls
Epidemiology and Assessment
Most literature on the epidemiology falls has assessed fall risk in the broader population of older adults, although more recent studies have focused on patients with heart failure [49, 50]. In general, more than one-third of patients ≥65 years of age experience a fall each year, and approximately 1 in 10 falls results in serious injury such as hip fracture or subdural hematoma [51, 52]. Among older adults, falls are independently associated with restricted mobility, a decline in the ability to perform independent activities such as dressing or bathing, and nursing home placement [51]. Clinicians are advised to screen for falls in older adults at annual follow-up, as information may not always be volunteered [53], and a multidisciplinary intervention may help to prevent future falls in patients at risk [54]
Heart failure has been associated with incident fall-related fracture in several studies [49, 50, 55]. Van Diepen et al. studied 2,041 consecutive patients who presented to emergency departments in Alberta, Canada with a new-onset diagnosis of heart failure, and found that within the first year after diagnosis the risk for any fracture or hip fracture (which is overwhelmingly due to falls) were elevated compared with a control population [adjusted odds ratios (95 % CI): all fracture 4.0 (2.9–5.3); hip fracture 6.3 (3.4–11.8)] [49].
Mechanisms and Association with outcomes
The relationship between heart failure and fracture has been postulated to be due to a mechanistic link between heart failure and osteoporosis shown in animal studies, specifically due to hyperaldosteronism and calcium wasting [56, 57]. The importance of aldosterone in the pathogenesis of fractures was further supported by evidence that use of the aldosteone antagonist spirnonolactone may have benefit in the prevention of fractures; in a case control study of Veterans, use of spironolactone was inversely associated with total fracture [odds ratio (95% CI) for fracture with spironolactone: 0.575 (0.346–0.955)] [55].
After hospitalization for fracture, older adults with heart failure have worse outcomes than those without heart failure [50, 58]. A study of 1,116 patients in Olmsted County, MN, who underwent operative repair for hip fracture found that preoperative heart failure was associated with increased length of stay, more frequent discharge to a skilled nursing facility, and higher postoperative mortality (1 year mortality rate: heart failure 37.2 %, no heart failure 19.8 %, p < 0.001) [50]. A registry of patients in Denmark with fall-related fracture also demonstrated that the presence of heart failure was independently associated with long-term mortality [hazard ratio (95% CI) for men: 1.2 (1.1–1.3); for women: 1.3 (1.3–1.4)] [58]. These studies imply that heart failure alone may classify patients as “high-risk”, with increased need for postoperative surveillance to avoid complications such as iatrogenic heart failure exacerbations (i.e. secondary to postoperative blood transfusions).
The presence of falls may also complicate adherence to guideline-based therapy for heart failure, especially with beta blocker therapy [59, 60]. Specifically, age-related conduction disease may make prescribing or up-titrating of beta blockers difficult, as they may exacerbate bradycardia or hypotension [59, 60] that may theoretically contribute to falls [61]. An observational study of 1,030 patients ≥70 years of age with heart failure starting the beta blocker carvedilol found that 20 % did not tolerate the drug at 6 months, with symptomatic hypotension and bradycardia cited as common reasons for discontinuation [60]. A smaller study of 51 heart failure patients aged 70–89 initiating bisoprolol found that 31 % did not tolerate the medication (most frequently due to hypotension and fatigue) [62]. However, a direct causal relationship between beta blockers and falls has not been established; two meta-analyses have failed to find a significant association between beta blocker use and falls in patients ≥60 years of age [63, 64], although most studies have been observational in design with limited ability to adjust for confounders, including poor candidacy for beta blocker therapy due to preexisting bradycardia.
Urinary Incontinence
Assessment and Prevalence
Urinary incontinence is a common problem in older adults with prevalence estimates ranging from 17 to 55% women and 11 to 34% in men [65]. Prevalence estimates in heart failure also vary: a Dutch study of 269 heart failure patients ≥65 years of age found that urinary incontinence was present in 17.5 % of subjects overall [66], and 42.9 % in patients ≥85 years of age. Another study of U.S. heart failure patients (mean age 62 years) found that urinary incontinence was present in 45 % of respondents [67].
Mechanisms and Association with Outcomes
The clearest mechanistic link between urinary incontinence and heart failure relates to diuretic therapy, and it is likely that incontinence adversely influences heart failure outcomes by diminishing adherence with these medications. Although data are limited, a small study of incontinent older adults in England found that of 21 patients prescribed diuretics, 20 did not take them as prescribed in an attempt to control their incontinence [68]. Withdrawal of diuretic therapy, even in stable heart failure patients, has been shown to lead to recurrence of heart failure symptoms [69] and may account for preventable reshospitalization. Urinary incontinence is known to also adversely influence outcomes such as quality of life [70] and the development of pressure ulcers [71], although whether these effects are more pronounced in patients with heart failure is unknown.
Conclusion
To meet the needs of the aging HF population, a new clinical paradigm is needed—one that starts with the comprehensive assessment of geriatric conditions and then tailors therapy and surveillance accordingly. The disease-oriented focus of medical care that has dominated heart failure practice does not adequately address the needs of older adults who have critical vulnerabilities in cognition and physical capacity. An acute heart failure exacerbation may be easily treated with intravenous diuretics, but 25 % of patients are readmitted to the hospital within 1 month, and 50 % within 6 months, often for non-cardiac reasons [72]. We need a new paradigm to care for this population, one that incorporates the routine assessment and management of geriatric conditions, and which considers the attainment of individually tailored patient outcomes such as functional status and symptom alleviation. These conditions should be considered together given their co-occurrence and potential for interaction; for example, cognitive impairment is included in some frailty scores and may improve the ability to risk-stratify patients for adverse outcomes [73, 74].
Although many geriatric conditions are not reversible, there are multiple potential interventions for patients with heart failure who are identified as having geriatric conditions. For example, in patients with incontinence, once daily dosing of diuretics may help to improve adherence and reduce inconvenience. In patients with cognitive impairment, medication regimens may also be simplified so that once-daily beta blockers or ACE inhibitors are prescribed, instead of shorter-acting formulations. Heart failure discharge education, which is a core quality measure [75], can be simplified, provided in written and pictorial formats, and targeted to include caregivers for patients who are cognitively impaired [76]. For patients identified with frailty, the targeting of resource-intensive disease management programs may help to prevent hospital readmission [47].
Despite the importance of geriatric conditions in heart failure, screening for them remains outside routine clinical heart failure care. Underscoring the relative inattention to geriatric conditions, even guidelines developed specifically for older patients with heart failure—such as the Assessing Care of Vulnerable Elders (ACOVE) quality indicators for heart failure care [77]—do not mention geriatric conditions. There are several potential reasons for the lack of screening, including clinicians' limited time during patient encounters, unfamiliarity with the prevalence of these conditions in heart failure patients, or focusing on disease-specific outcomes rather than broader health states. Coordination among relevant disciplines such as cardiology and geriatrics may help in realigning care to improve identification and reduce fragmented management decisions. Future research is also necessary to further understand whether large-scale interventions for geriatric conditions in heart failure, such as rehabilitation for frailty, home assessment for fall risk, and individualized discharge education for cognitive impairment, may improve meaningful outcomes such as hospitalization, mortality, and quality of life.
Acknowledgments
Funding Sources: Dr. Dodson is supported by a training grant in Geriatric Clinical Epidemiology from the National Institute on Aging (T32 AG019134) and a Clinical Research Loan Repayment Award from the National Heart, Lung, and Blood Institute.
Dr. Chaudhry is supported by a Beeson Career Development Award from the National Institute on Aging (K23 AG030986).
Footnotes
Disclosure: No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of outstanding importance
- 1.Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: The Framingham Study. J Am Coll Cardiol. 1993;4:A6–A13. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. Heart Disease and Stroke Statistics—2011 Update. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in Comorbidity, Disability, and Polypharmacy in Heart Failure. Am J Med. 2011;124:136–143. doi: 10.1016/j.amjmed.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the health and retirement study. Ann Intern Med. 2007;147:156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116:179–185. doi: 10.1016/j.amjmed.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 7.Lang CC, Mancini DM. Non-cardiac comorbidities in chronic heart failure. Heart. 2007;93:665–671. doi: 10.1136/hrt.2005.068296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Chaudhry SI, Wang Y, Gill TM, Krumholz HM. Geriatric conditions and subsequent mortality in older patients with heart failure. J Am Coll Cardiol. 2010;55:309–316. doi: 10.1016/j.jacc.2009.07.066. Describes independent association of cognitive impairment with short- and long-term mortality in a large cohort of older adults with heart failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lien CTC, Gillespie ND, Struthers AD, McMurdo MET. Heart failure in frail elderly patients: diagnostic difficulties, co-morbidities, polypharmacy and treatment dilemmas. Eur J Heart Fail. 2002;4:91–98. doi: 10.1016/s1388-9842(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 10.Vogels RLC, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Zuccalà G, Marzetti E, Cesari M, Lo Monaco MR, Antonica L, et al. Correlates of cognitive impairment among patients with heart failure: results of a multicenter survey. Am J Med. 2005;118:496–502. doi: 10.1016/j.amjmed.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Dickson VV, Tkacs N, Riegel B. Cognitive influences on self-care decision making in persons with heart failure. Am Heart J. 2007;154:424–431. doi: 10.1016/j.ahj.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 13.Debette S, Bauters C, Leys D, Lamblin N, Pasquier F, et al. Prevalence and determinants of cognitive impairment in chronic heart failure patients. Congest Heart Fail. 2007;13:205–208. doi: 10.1111/j.1527-5299.2007.06612.x. [DOI] [PubMed] [Google Scholar]
- 14.Cameron J, Worrall-Carter L, Page K, Riegel B, Lo SK, et al. Does cognitive impairment predict poor self-care in patients with heart failure? Eur J Heart Fail. 2010;12:508–515. doi: 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 15.Harkness K, Demers C, Heckman GA, McKelvie RS. Screening for cognitive deficits using the Montreal Cognitive Assessment Tool in outpatients >=65 Years of age with heart failure. Am J Cardiol. 2011;107:1203–1207. doi: 10.1016/j.amjcard.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Zuccalà G, Cattel C, Manes-Gravina E, Di Niro MG, Cocchi A, et al. Left ventricular dysfunction: a clue to cognitive impairment in older patients with heart failure. Journal of Neurology, Neurosurgery & Psychiatry. 1997;63:509–512. doi: 10.1136/jnnp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogels RLC, Oosterman JM, Van Harten B, Scheltens P, Van Der Flier WM, et al. Profile of cognitive impairment in chronic heart failure. J Am Geriatr Soc. 2007;55:1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 18.Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002-July 2007) J Cardiovasc Nurs. 2008;23:239–249. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- 19.Young J, Meagher D, MacLullich A. Cognitive assessment of older people. BMJ. 2011;343:d5042. doi: 10.1136/bmj.d5042. [DOI] [PubMed] [Google Scholar]
- 20.Mungas D. In-office mental status testing: a practical guide. Geriatrics. 1991;46:54–66. [PubMed] [Google Scholar]
- 21.Ylikoski R, Erkinjuntti T, Sulkava R, Juva K, Tilvis R, et al. Correction for age, education and other demographic variables in the use of the Mini Mental State Examination in Finland. Acta Neurol Scand. 1992;85:391–396. doi: 10.1111/j.1600-0404.1992.tb06034.x. [DOI] [PubMed] [Google Scholar]
- 22.Kahle-Wrobleski K, Corrada MM, Li B, Kawas CH. Sensitivity and specificity of the Mini-Mental State Examination for identifying dementia in the oldest-old: The 90+ Study. J Am Geriatr Soc. 2007;55:284–289. doi: 10.1111/j.1532-5415.2007.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. J Am Med Assoc. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith T, Gildeh N, Holmes C. The Montreal Cognitive Assessment: validity and utility in a memory clinic setting. Can J Psychiatry. 2007;52:329–332. doi: 10.1177/070674370705200508. [DOI] [PubMed] [Google Scholar]
- 26.Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24:197–201. doi: 10.1002/gps.2101. [DOI] [PubMed] [Google Scholar]
- 27.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The Mini-Cog: a cognitive ‘vital signs’ measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 28.Pullicino PM, Hart J. Cognitive impairment in congestive heart failure? Neurology. 2001;57:1945–1946. doi: 10.1212/wnl.57.11.1945. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt R, Fazekas F, Offenbacher H, Dusleag J, Lechner H. Brain magnetic resonance imaging and neuropsychologic evaluation of patients with idiopathic dilated cardiomyopathy. Stroke. 1991;22:195–199. doi: 10.1161/01.str.22.2.195. [DOI] [PubMed] [Google Scholar]
- 30.Ekman I, Fagerberg B, Skoog I. The clinical implications of cognitive impairment in elderly patients with chronic heart failure. J Cardiovasc Nurs. 2001;16:47–55. doi: 10.1097/00005082-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 31.McLennan SN, Pearson SA, Cameron J, Stewart S. Prognostic importance of cognitive impairment in chronic heart failure patients: does specialist management make a difference? Eur J Heart Fail. 2006;8:494–501. doi: 10.1016/j.ejheart.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, et al. Frailty in older adults: evidence for a phenotype. J Gerontol Ser A: Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 33.Boxer RS, Wang Z, Walsh SJ, Hager D, Kenny AM. The utility of the 6-Minute Walk Test as a measure of frailty in older adults with heart failure. Am J Geriatr Cardiol. 2008;17:7–12. doi: 10.1111/j.1076-7460.2007.06457.x. [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol Ser A: Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 35.Fugate Woods N, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 36.Purser JL, Kuchibhatla MN, Fillenbaum GG, Harding T, Peterson ED, et al. Identifying frailty in hospitalized older adults with significant coronary artery disease. J Am Geriatr Soc. 2006;54:1674–1681. doi: 10.1111/j.1532-5415.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- 37*.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, et al. Gait Speed and Survival in Older Adults. J Am Med Assoc. 2011;305:50–58. doi: 10.1001/jama.2010.1923. Comprehensive meta-analysis of gait speed and its association with mortality in older adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan R, et al. Gait Velocity as a Single Predictor of Adverse Events in Healthy Seniors Aged 75 Years and Older. J Gerontol Ser A: Biol Sci Med Sci. 2005;60:1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 39.Gill TM, Williams CS, Tinetti ME. Assessing risk for the onset of functional dependence among older adults: the role of physical performance. J Am Geriatr Soc. 1995;43:603–609. doi: 10.1111/j.1532-5415.1995.tb07192.x. [DOI] [PubMed] [Google Scholar]
- 40.Cesari M, Kritchevsky SB, Penninx BWHJ, Nicklas BJ, Simonsick EM, et al. Prognostic value of usual gait speed in well-functioning older people: results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 41.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, et al. A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 42.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–2216. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alfilalo J. Frailty in patients with cardiovascular disease: why, when, and how to measure. Curr Cardiovasc Risk Rep. 2011;5:467–472. doi: 10.1007/s12170-011-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahmed N, Mandel R, Fain MJ. Frailty: an emerging geriatric syndrome. 2007;120:748–753. doi: 10.1016/j.amjmed.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Rich MW, Shah AS, Vinson JM, Freedland KE, Kuru T, et al. Iatrogenic congestive heart failure in older adults: clinical course and prognosis. J Am Geriatr Soc. 1996;44:638–643. doi: 10.1111/j.1532-5415.1996.tb01824.x. [DOI] [PubMed] [Google Scholar]
- 46.Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 47.Pulignano G, Del Sindaco D, Di Lenarda A, Tarantini L, Cioffi G, et al. Usefulness of frailty profile for targeting older heart failure patients in disease management programs: a cost-effectiveness, pilot study. J Cardiovasc Med. 2010;11:739–742. doi: 10.2459/JCM.0b013e328339d981. [DOI] [PubMed] [Google Scholar]
- 48.Boxer R, Kleppinger A, Ahmad A, Annis K, Hager D, et al. The 6-Minute walk is associated with frailty and predicts mortality in older adults with heart failure. Congest Heart Fail. 2010;16:208–213. doi: 10.1111/j.1751-7133.2010.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Diepen S, Majumdar SR, Bakal JA, McAlister FA, Ezekowitz JA. Heart failure is a risk factor for orthopedic fracture. Circulation. 2008;118:1946–1952. doi: 10.1161/CIRCULATIONAHA.108.784009. [DOI] [PubMed] [Google Scholar]
- 50.Cullen MW, Gullerud RE, Larson DR, Melton LJ, Huddleston JM. Impact of heart failure on hip fracture outcomes: a population-based study. J Hospital Med. 2011;6:507–512. doi: 10.1002/jhm.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tinetti ME. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 52.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 53.American Geriatrics Society, British Geriatrics Society, American Academy of Orthopaedic Surgeons Panel on Falls Prevention. Guideline for the Prevention of Falls in Older Persons. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- 54.Tinetti ME, Baker DI, McAvay G, Claus EB, Garrett P, et al. A multifactorial intervention to reduce the risk of falling among elderly people living in the community. N Engl J Med. 1994;331:821–827. doi: 10.1056/NEJM199409293311301. [DOI] [PubMed] [Google Scholar]
- 55.Carbone LD, Cross JD, Raza SH, Bush AJ, Sepanski RJ, et al. Fracture risk in men with congestive heart failure: risk reduction with spironolactone. J Am Coll Cardiol. 2008;52:135–138. doi: 10.1016/j.jacc.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 56.Chhokar VS, Sun Y, Bhattacharya SK, Ahokas RA, Myers LK, et al. Hyperparathyroidism and the calcium paradox of aldosteronism. Circulation. 2005;111:871–878. doi: 10.1161/01.CIR.0000155621.10213.06. [DOI] [PubMed] [Google Scholar]
- 57.Law PH, Sun Y, Bhattacharya SK, Chhokar VS, Weber KT. Diuretics and bone loss in rats with aldosteronism. J Am Coll Cardiol. 2005;46:142–146. doi: 10.1016/j.jacc.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 58.Kannegaard PN, van der Mark S, Eiken P, Abrahamsen B. Excess mortality in men compared with women following a hip fracture: national analysis of comedications, comorbidity and survival. Age Ageing. 2010;39:203–209. doi: 10.1093/ageing/afp221. [DOI] [PubMed] [Google Scholar]
- 59.Flather MD, Shibata MC, Coats AJS, Van Veldhuisen DJ, Parkhomenko A, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 60.Krum H, Hill J, Fruhwald F, Sharpe C, Abraham G, et al. Tolerability of beta-blockers in elderly patients with chronic heart failure: the COLA II study. Eur J Heart Fail. 2006;8:302–307. doi: 10.1016/j.ejheart.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Sztramko R, Chau V, Wong R. Adverse drug events and associated factors in heart failure therapy among the very elderly. Can Geriatr J. 2011;14:79–92. doi: 10.5770/cgj.v14i4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baxter AJ, Spensley A, Hildreth A, Karimova G, O'Connell JE, et al. Beta Blockers in older persons with heart failure: tolerability and impact on quality of life. Heart. 2002;88:611–614. doi: 10.1136/heart.88.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II cardiac and analgesic drugs. 1999;47:40–50. doi: 10.1111/j.1532-5415.1999.tb01899.x. [DOI] [PubMed] [Google Scholar]
- 64.Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–1960. doi: 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 65.Thom D. Variation in estimates of urinary incontinence prevalence in the community: effects of differences in definition, population characteristics, and study type. J Am Geriatr Soc. 1998;46:473–80. doi: 10.1111/j.1532-5415.1998.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 66.van der Wel MC, Jansen RWMM, Bakx JC, Bor HHJ, OldeRikkert MGM, et al. Non-cardiovascular co-morbidity in elderly patients with heart failure outnumbers cardiovascular co-morbidity. Eur J Heart Fail. 2007;9:709–715. doi: 10.1016/j.ejheart.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Palmer MH, Hardin SR, Behrend C, Collins SK, Madigan CK, et al. Urinary Incontinence and Overactive Bladder in Patients With Heart Failure. J Urol. 2009;182:196–202. doi: 10.1016/j.juro.2009.02.115. [DOI] [PubMed] [Google Scholar]
- 68.McKeever MP. An investigation of recognized incontinence within a health authority. J Adv Nurs. 1990;15:1197–1207. doi: 10.1111/j.1365-2648.1990.tb01713.x. [DOI] [PubMed] [Google Scholar]
- 69.Walma Edmond, van Dooren Colette, Prins Ad, van der Does Emiel, Hoes Arno. Withdrawal of long term diuretic medication in elderly patients: a double blind randomised trial. BMJ. 1997;315:464–468. doi: 10.1136/bmj.315.7106.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Temml C, Haidinger G, Schmidbauer J, Schatzl G, Madersbacher S. Urinary incontinence in both sexes: Prevalence rates and impact on quality of life and sexual life. Neurourol Urodyn. 2000;19:259–271. doi: 10.1002/(sici)1520-6777(2000)19:3<259::aid-nau7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 71.Baumgarten M, Margolis DJ, Localio AR, Kagan SH, Lowe RA, et al. Pressure ulcers among elderly patients early in the hospital stay. J Gerontol Ser A: Biol Sci Med Sci. 2006;61:749–754. doi: 10.1093/gerona/61.7.749. [DOI] [PubMed] [Google Scholar]
- 72*.Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, et al. Recent national trends in readmission rates after heart failure hospitalization. Circulation: Heart Failure. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. Demonstrates that over time, 30-day readmission rates for older adults hospitalized for heart failure have not changed significantly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ávila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: The Three-City Study. J Am Geriatr Soc. 2009;57:453–61. doi: 10.1111/j.1532-5415.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 74.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–6. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonow RO, Bennett S, Casey DE, Ganiats TG, Hlatky MA, et al. ACC/AHA Clinical Performance Measures for Adults With Chronic Heart Failure. Circulation. 2005;112:1853–1887. doi: 10.1161/CIRCULATIONAHA.105.170072. [DOI] [PubMed] [Google Scholar]
- 76.Strömberg A. The crucial role of patient education in heart failure. Eur J Heart Fail. 2005;7:363–369. doi: 10.1016/j.ejheart.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Heidenreich PA, Fonarow GC. Quality Indicators for the Care of Heart Failure in Vulnerable Elders. J Am Geriatr Soc. 2007;55:S340–S346. doi: 10.1111/j.1532-5415.2007.01341.x. [DOI] [PubMed] [Google Scholar]


