Abstract
The brain renin-angiotensin system plays an important role in the regulation of arterial pressure in response to stress, in part due to activation of AT1 receptors in the hypothalamic median preoptic nucleus (MnPO) by endogenous angiotensin II (ANG II). N-methyl-d-aspartate (NMDA) receptors are also involved in the angiotensinergic signaling pathway through the MnPO. We investigated whether AT1 and NMDA receptors in the MnPO are responsible for variable hemodynamic response patterns to stress. Cocaine or startle with cold water evoked a pressor response in Sprague-Dawley rats due, in some rats [vascular responders (VR)], to a large increase in systemic vascular resistance (SVR) and, in other rats [mixed responders (MR)], to small increases in SVR and cardiac output (CO). Microinjection of the GABAA agonist muscimol into the MnPO to block synaptic transmission attenuated the cocaine- or stress-induced increase in SVR and the decrease in CO seen in VR without altering either response in MR. Likewise, administration of either an AT1 receptor antagonist, losartan, or an NMDA receptor antagonist, MK-801, attenuated the increase in SVR and the decrease in CO in VR in response to either cocaine (losartan and MK-801) or startle with cold water (losartan) without altering either response in MR. We propose that the MnPO is responsible for greater SVR responses in VR and that AT1 and NMDA receptors play an important role in greater SVR responses in VR. These data provide additional support for the critical role of the MnPO in cardiovascular responses to stress.
Keywords: AT1 receptor, cocaine, anteroventral third ventricle region, systemic vascular resistance, cardiovascular response variability
ang ii is a vasoactive peptide that elicits an increase in arterial pressure. Circulating ANG II acts both directly on the vasculature and on circumventricular organs, including the subfornical organ and the organum vasculosum lamina terminalis in the forebrain and the area postrema in the brain stem (15, 19, 30, 56). Circulating ANG II may modulate central responses to stress due to actions in these nuclei. Alternatively, the central renin-angiotensin system may be responsible for integrating physiological responses to stress (reviewed in Ref. 56). We reported that ANG II receptor antagonists or a converting enzyme inhibitor administered intracerebroventricularly prevented the greater increase in systemic vascular resistance in vascular responders in response to cocaine or to startle with cold water (40). In contrast, intracerebroventricular administration of ANG II enhanced the increase in systemic vascular resistance (40). These data suggest that AT1 receptors in the central nervous system (CNS) mediate and may be responsible for response variability although the site of action was not clear.
Numerous studies have shown that the integrity of the anteroventral third ventricular (AV3V) region in the hypothalamus, which contains the median preoptic nucleus (MnPO), is critical for the development of disparate forms of experimental hypertension (7, 17, 18). The MnPO contains several components of the renin-angiotensin system, including ANG II (10, 28) and AT1 receptors (10, 25). N-methyl-d-aspartate (NMDA) receptors, particularly the NR1 subunit, are also present within the MnPO (13). Others have reported that NMDA receptor activation in the MnPO is involved in the increase in vasopressin release from the posterior pituitary that occurs during hypovolemia (58), suggesting that NMDA receptors may be involved in cardiovascular homeostasis.
Although specific patterns of responses to stressors depend on the nature of the stressor, acute stressors elicit a pressor response due to differing patterns of vasoconstriction or cardiac stimulation. Brod (6) summarized several early studies identifying subsets of humans designated vascular and cardiac responders that differ in their hemodynamic responses to behavioral stress. Some individuals, designated vascular responders, increase their blood pressure due to a large increase in systemic vascular resistance, while others have an increase in cardiac output (6). Several investigators have suggested that vascular responders are at greater risk of developing hypertension and cardiac disease (6, 12, 26, 49).
In a similar manner, we identified a rat model that exhibited differences in the hemodynamic response patterns to stress (21). Using cocaine or behavioral stress to elicit a pressor response, we found wide variability in the cardiac output and systemic vascular resistance responses in conscious rats. Male Sprague-Dawley rats were designated vascular responders if cocaine induced a pressor response that was mediated by a large increase in systemic vascular resistance despite a decrease in cardiac output. In contrast, rats were named mixed responders if the pressor response to cocaine occurred due to small increases in systemic vascular resistance and cardiac output (21). We reported that the vascular responders were more prone to develop hypertension, cardiomyopathies and toxicity to cocaine (1, 20, 21, 32, 55). These data are strikingly similar to the predisposition of human vascular responders to be more prone to develop hypertension and heart disease (6, 12, 26, 49). Therefore, we proposed that the rat may be an appropriate model for studying the causes of varying predisposition to cardiovascular disease (21).
We hypothesized that the greater vasoconstrictor responses to stressors observed in vascular responders are dependent on synaptic transmission in the MnPO. To test this hypothesis, we microinjected muscimol, a GABAA agonist, into the MnPO and then challenged rats with either a pharmacological stressor (cocaine) or a behavioral stressor (startle with cold water) to examine hemodynamic responsiveness. Furthermore, we proposed that both AT1 and NMDA receptors mediate the greater vascular responsiveness. To examine the contributions of these receptors, we microinjected losartan, an AT1 receptor antagonist, or MK-801, an NMDA receptor antagonist, into the MnPO, and then presented either cocaine or startle with cold water to examine hemodynamic responses in vascular and mixed responders.
METHODS
Surgical preparation.
All surgical and experimental procedures were approved by the St. Louis University Institutional Animal Care and Use Committee and followed guidelines described in the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, 1996). Specific pathogen-free, male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) weighing 300–400 g were anesthetized with isoflurane (2–2.5% AErrane; Baxter Pharmaceuticals Products, Deerfield, IL), intubated for artificial respiration, and a miniaturized pulsed Doppler flow probe (Iowa Doppler Products, Iowa City, IA) was placed on the ascending aorta to measure aortic blood flow. This, in turn, was used to estimate changes in cardiac output. Animals were placed in a Kopf stereotaxic apparatus, and the skull was leveled between bregma and lambda. A stainless steel internal guide cannula (26 awg; Plastics One, Roanoke, VA) was stereotaxically inserted 1 mm above the MnPO (coordinates from bregma: anterior-posterior, −0.3 mm; medial-lateral, 0.0 mm; dorsal-ventral, −6.0 mm, according to Ref. 35) for CNS administration of drugs and fixed to the skull with jewelers screws embedded in dental cement. Guide cannulae were plugged with an obturator when not in use. Rats were monitored for at least 2 h after surgery and allowed to recover for at least 1 wk. Animals that lost >10% of their presurgical weight or exhibited limited locomotion following cannulation were euthanized with an overdose of pentobarbital (70 mg/kg ip). After a recovery period of 7–10 days, the femoral artery and vein were cannulated under isoflurane anesthesia for arterial pressure determination and intravenous drug infusion, respectively. The cannulae were tunneled subcutaneously to the suprascapular region and were plugged when not in use. All surgical instrumentation was performed using an aseptic technique.
Cocaine studies.
Two to three days later, arterial pressure, heart rate, and cardiac output were recorded in conscious, freely moving rats before and after administration of cocaine (5 mg/kg iv). Cocaine was administered 5–7 times (no more than twice daily, with a minimum of 3 h between doses, an interval previously shown to prevent tachyphylaxis [1]) and the responses over the first 60 s for these trials were averaged. Cocaine administration produced greater peak pressor responses than cold stress, so we used the average responses to cocaine during the first 60 s to define the phenotypic response of the animal (i.e., vascular responder vs. mixed responder). Those rats with an average increase in cardiac output were characterized as mixed responders, whereas those with a decrease were characterized as vascular responders (see Fig. 2). We previously showed that changes in cardiac output responses to four different stressors were correlated between individuals (23). In this study, 13 of 14 vascular responders to cocaine were also vascular responders to cold stress, and therefore responses to different stressors were similar in each animal.
Fig. 2.
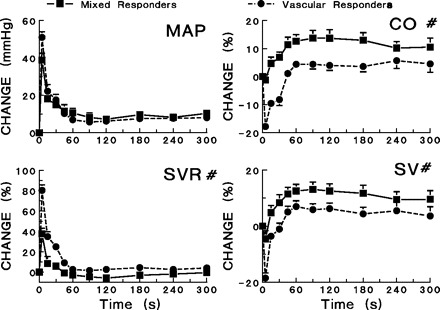
Responses to cocaine (5 mg/kg iv) in vascular and mixed responders. The mean arterial pressure (MAP), cardiac output (CO), systemic vascular resistance (SVR), and stroke volume (SV) following cocaine administration are depicted. #Significant differences exist between vascular and mixed responders in the changes in CO, SVR, and SV changes after cocaine administration (P < 0.05). No significant differences in heart rate changes after cocaine administration were observed between groups.
After recording response patterns to cocaine, we performed control experiments in rats instrumented with an MnPO guide cannula, where cocaine (5 mg/kg iv) was administered 10 min after a slow (1–2 min) intraparenchymal infusion of 100 nl of saline. A minimum of 3 h later, rats were given a 100-nl microinjection into the MnPO of one of three drugs: the GABAA agonist muscimol (80 pmol), the AT1 receptor antagonist losartan (43 pmol), or the NMDA receptor antagonist MK-801 (dizocilpine, 20 nmol). Microinjection of this dose of muscimol has been previously shown to produce increases in body temperature, heart rate, blood pressure, and plasma levels of ACTH, and locomotor activity when injected into the preoptic area (60) and reduced the increase in plasma ACTH after air jet stress when injected into either the paraventricular nucleus or the dorsomedial hypothalamic nucleus (46). Other investigators have reported that a lower dose of losartan (20 pmol) was effective in preventing ANG II inhibition of sexual behavior after injections in the medial amygdala (4). Finally, a similar dose of MK-801 injected into the central nucleus of the amygdala attenuated conditioned place aversion in rats following morphine withdrawal (51) and a similar dose of MK-801 injected into the AV3V was shown to prevent NMDA-induced increases in plasma vasopressin and arterial pressure without affecting resting arterial pressure (58). Microinjections were performed 10 min prior to cocaine (5 mg/kg iv) administration while hemodynamics were recorded. Control responses (saline microinjection 10 min before intravenous cocaine) were compared with cocaine responses following microinjection of drug into the MnPO. Rats were tested with each drug (muscimol, MK-801, and losartan) when possible, and only one drug was injected into the MnPO each day, so that on a given day responses in each animal to saline microinjection and cocaine and to one drug and cocaine were recorded. In cocaine studies, seven animals were tested with one drug, six animals were tested with two drugs on separate days, and 14 animals were tested with all three drugs (muscimol, losartan, and MK-801) on separate days prior to cocaine. A total of 10 animals were only tested with cocaine, and 14 animals were tested with both cocaine and with cold stress, with trials of each administered randomly, and only one type of stressor administered per day. Responses to each stressor were reproducible over the course of the experimental period in all animals included in the study.
Behavioral stress.
Hemodynamic responses were also recorded in conscious, freely moving rats suddenly exposed to ice-cold water (1 cm deep) for 1 min as previously described (23, see Fig. 3). The hemodynamic responses associated with the initial startle were analyzed separately from the sustained cold pressor response (15–60 s). At least four cold stress trials were conducted with a minimum of 30 min between trials.
Fig. 3.
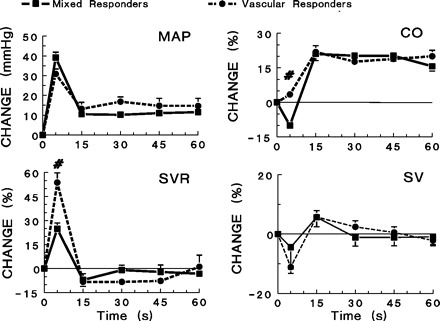
Responses to acute stress with ice-cold water added to a watertight cage rapidly to a depth of 1 cm. The initial response (phasic) was considered the startle response and was highly variable between animals, whereas the tonic response (15–60 s) evoked fairly consistent hemodynamic responses. Significant differences (P < 0.05) exist in the phasic increases in MAP and SVR between vascular and mixed responders. #Significant differences between vascular and mixed responders (P < 0.05).
As previously reported, hemodynamic response patterns to cocaine resembled those of startle with cold water (23). The response to startle was quantified as the average response in each rat during the initial peak pressor response (first 5–6 s) to the addition of cold water to the watertight enclosure. Those rats with a net increase in cardiac output were designated mixed responders, while those with a net decrease were named vascular responders to startle. On the second experimental day, we examined the effects of muscimol or losartan on the hemodynamic response to startle. In control experiments, rats were given a 100-nl microinjection of saline into the MnPO over 1 min. Ten minutes later, rats were startled by suddenly pouring cold water (1 cm deep) into the bottom of the cage. A minimum of 30 min later, rats were given a 100-nl microinjection of muscimol (80 pmol) or losartan (20 ng) into the MnPO over 1–2 min, and startled again with cold water after 10 min. Responses following saline microinjection into the MnPO were compared with startle responses following microinjection of drug into the MnPO. Rats were tested with each drug (muscimol and losartan) when possible, and only one drug was injected into the MnPO each day. In cold stress studies, 13 animals were tested with one drug and seven animals were tested with muscimol and losartan on separate days. A total of five animals were only tested with cold stress, while 14 animals were tested with both cocaine and cold stress. Responses to cold stress were reproducible over the course of the experimental period in all animals.
At the conclusion of all experiments, rats were anesthetized with isoflurane, and a 100-nl microinjection of Chicago Sky Blue dye was delivered into the MnPO. Ten minutes later, rats were given a lethal dose of pentobarbital sodium (70 mg/kg ip) and perfused with saline. Brains were removed, fixed in a solution containing 10% formalin and 10% sucrose, and cut in 40-μm coronal sections with a cryostat to verify cannula placement and lack of spread to cerebral ventricles, according to the atlas of Paxinos and Watson (35).
Drugs and chemicals.
Cocaine hydrochloride was obtained from the National Institute of Drug Abuse and dissolved in 0.9% sodium hydrochloride (American Pharmaceutical Partners, Los Angeles, CA) to a final concentration of 5 mg/ml. Losartan was donated by Merck Pharmaceuticals (Rahway, NJ). Isoflurane (AErrane, Baxter Pharmaceutical Products, Deerfield, IL) was purchased from Henry Schein and pentobarbital, muscimol, Chicago Sky Blue 6B, and MK-801 were purchased from Sigma (St. Louis, MO). Cefotaxime (5 mg/kg Claforan; Hoechst-Roussel Pharmaceuticals, Somerville, NJ) was used to prevent infection and buprenorphine (0.05 mg/kg; Hospira, Lake Forest, IL) was administered to alleviate pain following all surgeries.
Data analysis.
Data were recorded and analyzed using WINDAQ Pro+ software (Dataq Instruments, Akron, OH). Hemodynamic responses to cocaine were analyzed at the time of initial peak increase in arterial pressure, at 15-s intervals to 1 min, and then at 1 min intervals for a total of 10 min. For the cold-water stress paradigm, data were analyzed at the time of initial peak increase in arterial pressure (startle), and at 15-s intervals for 1 min (cold pressor test).
Data were analyzed using two-way repeated-measures analysis of variance (ANOVA) with Newman-Keuls correction to compare peak (startle) and 60-s average (cocaine) responses, and three-way ANOVA to compare data obtained at multiple time points (e.g., comparison of cocaine and cold stress data, or before and after drug data for 1 stressor). A P value of <0.05 was considered significant. Data are expressed as means ± SE.
RESULTS
Verification of cannula position and microinjection localization.
Accurate cannula placement and microinjection were verified in each rat at the conclusion of the experimental period. Rats were included in the final analysis if the cannula tract and localization of the dye spot were limited to the MnPO or tissue immediately adjacent to it (Fig. 1) and were excluded if the cannula tract or dye localization were lateral to the MnPO or not immediately adjacent. These criteria resulted in the exclusion of four animals from a total of 20 animals due to inaccurate cannula placement. We estimated the size and volume of injection for each animal based upon the extent of dye spread for animals included in the study. The average diameter of the dye spread was 0.45 mm ± 0.04 mm, and the volume of dye spheres averaged 0.49 mm3 ± 0.13 mm3. We analyzed responses to stress in animals with misplaced cannulae and found that drug effects were limited to the area including and immediately surrounding the MnPO. These effects are described in further detail below.
Fig. 1.
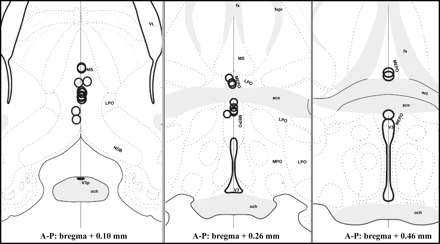
Location of microinjection sites are shown as circles indicating the locations and average spread of 100-nl microinjections of Chicago Sky Blue dye from animals that were included in the analysis. Some injection sites are overlapped due to their close proximity. Maps are based on templates from the Swanson atlas (47). aco, Anterior commissure; act, tract of anterior commissure; fx, fornix; LPO, lateral preoptic area; MPO, medial preoptic area; MS, medial septum; MEPO, median preoptic nucleus; NDB, diagonal band of Broca; och, optic chiasm; V3, 3rd ventricle; A-P, anterior-posterior; V3p, third ventricle, preoptic recess; VL, lateral ventricle; fxpr, precommissural fornix.
Resting hemodynamic values and control responses.
Mixed responders had an average resting arterial pressure of 119 ± 4 mmHg, a resting heart rate of 389 ± 8 beats/min, and a resting cardiac output of 10.5 ± 0.5 kHz shift. Vascular responders had an average resting arterial pressure of 115 ± 4 mmHg, a resting heart rate of 396 ± 11 b/min, and a resting cardiac output of 11.1 ± 0.4 kHz shift. Cocaine administration produced greater peak pressor responses than cold stress, so we used this stressor to characterize animals as vascular or mixed responders. Among animals participating in cocaine experiments, we found no difference in resting arterial pressure, heart rate, or cardiac output between vascular and mixed responders (Fig. 2). Additionally, we found no differences in hemodynamic responses in either mixed or vascular responders when we compared the hemodynamic measurements from the first and second cocaine trials each day (Table 1). Furthermore, we compared responses to cocaine and to cold stress in individual animals on consecutive days and found that microinjection of saline into the MnPO prior to cocaine (n = 5) or to cold stress (n = 6) on the 1st day had no effect on hemodynamic responses to saline microinjection prior to either stressor on subsequent days.
Table 1.
Comparison of hemodynamic responses to cocaine (5 mg/kg iv) in first vs. second daily trials
| Class | No. | Time of Trial | Δ MAP, mmHg | Δ CO, kHz | Δ SVR, % | Δ SV, % |
|---|---|---|---|---|---|---|
| VR | 28 | AM | 22±1 | −7.1±1.3 | 33.3±2.8 | −1.4±1.3 |
| VR | 28 | PM | 20±1 | −5.2±1.3 | 27.1±2.5 | −1.4±1.3 |
| MR | 7 | AM | 22±5 | 3.0±1.6 | 16.9±4.4 | 5.8±3.0 |
| MR | 7 | PM | 20±3 | 6.0±2.2 | 11.9±2.4 | 7.7±2.1 |
Values are means ± SE. No. is number of rats studied. AM, time of 1st trial (conducted in morning); CO, cardiac output; HR, heart rate; MAP, mean arterial pressure; MR, mixed responders; PM, time of 2nd trial (conducted in afternoon); SV, stroke volume; SVR, systemic vascular resistance; VR, vascular responders.
We wanted to determine whether different types of stressors produce similar hemodynamic response variability. Therefore, we performed experiments that examined responses in vascular and mixed responders to cold stress. Among animals participating in cold stress experiments, there were no differences in resting arterial pressure, heart rate, or cardiac output between vascular and mixed responders. The phasic (peak startle) response elicited a greater increase in systemic vascular resistance and an initial decrease in cardiac output in vascular responders compared with mixed responders (Fig. 3).
Effect of muscimol on hemodynamic responses to stress in vascular and mixed responders.
To test whether synaptic transmission through the MnPO was necessary to produce hemodynamic response variability, muscimol, a GABAA agonist, was administered to six mixed and seven vascular responders (Fig. 4) prior to cocaine dosing. Muscimol pretreatment did not affect resting hemodynamic values in either group (Table 2). Delivery of muscimol (80 pmol) into the MnPO did not significantly change hemodynamic responses following cocaine administration in mixed responders. In vascular responders, microinjection of muscimol into the MnPO prevented the cocaine-induced decrease in cardiac output (P < 0.05, Fig. 4). The cardiac output responses to cocaine in vascular and mixed responders after administration of muscimol were no longer different. Muscimol attenuated the increase in systemic vascular resistance in vascular responders (P < 0.01) without changing the systemic vascular resistance in mixed responders (Fig. 4). To ensure that these effects of muscimol were limited to the MnPO, we analyzed hemodynamic data for four rats (2 mixed and 2 vascular responders) whose cannulae were located >1 mm rostral or lateral to the MnPO in the rostral medial septum or the ventral diagonal band. Muscimol microinjection in these animals did not result in changes in the hemodynamic responses to cocaine observed in either vascular or mixed responders. Therefore, only vascular responders with cannulae in or immediately adjacent to the MnPO exhibited an alteration in response to cocaine following muscimol pretreatment.
Fig. 4.
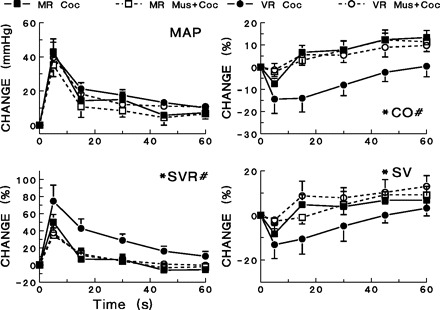
Effects of microinjection of muscimol (80 pmol) into the median preoptic nucleus (MnPO) on hemodynamic responses to cocaine [coc: (co → coc); 5 mg/kg iv]. Hemodynamic responses are shown for vascular responders and mixed responders following cocaine administration alone and for cocaine administration following muscimol (Mus) pretreatment. #Significant differences (P < 0.05) between vascular and mixed responders exist in the changes seen in CO and SVR. *Muscimol pretreatment produced significant differences (P < 0.05) among vascular responders in the changes seen in CO, SVR, and stroke volume (SV) after cocaine administration. Muscimol did not produce significant differences in heart rate changes.
Table 2.
Effects of drug treatment alone immediately before cocaine administration
| Stimulus | Class | No. | Mean AP, mmHg | Δ MAP, mmHg | HR, beats/min | Δ HR, beats/min | CO, kHz | Δ CO, kHz | Δ SVR, % | Δ SV, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Muscimol | MR | 6 | 115±4 | −1.0±1.5 | 417±9 | −4±8 | −2.7±0.1 | −4.1±2 | 3.6±2.8 | −2.9±3.6 |
| VR | 7 | 118±7 | −0.4±0.5 | 409±12 | 7±4 | −2.9±0.1 | 0.1±2 | −0.3±2.3 | −1.5±2.3 | |
| Losartan | MR | 5 | 120±8 | −0.2±3.1 | 428±16 | −14±5 | −3.1±0.1 | −3.5±1.9 | 3.6±2.8 | −0.2±2.6 |
| VR | 7 | 122±3 | −0.4±2.3 | 425±12 | −4±7 | −3.0±0.2 | −6.7±2.4* | 6.9±2.9* | −5.5±3.4 | |
| MK-801 | MR | 7 | 123±7 | −0.5±1.0 | 377±21 | −11±9 | −2.5±0.2 | 1.6±1.4 | −2.2±2.3 | 5.5±2.9 |
| VR | 6 | 118±4 | 0.8±3.2 | 394±28 | −4±3 | −2.8±0.1 | −1.4±2.1 | 2.4±2.1 | −0.6±2.5 |
Values are means ± SE. No. is number of rats studied.
Significant difference with treatment alone (P < 0.05).
We also microinjected muscimol in six mixed and nine vascular responders prior to cold water startle (Fig. 5) to test whether the MnPO mediated hemodynamic responses to a behavioral stressor. The hemodynamic response profile following startle did not significantly change after muscimol administration in mixed responders compared with control experiments (Fig. 5). However, in vascular responders, muscimol microinjection into the MnPO reduced the initial large increase in systemic vascular resistance (P < 0.01).
Fig. 5.
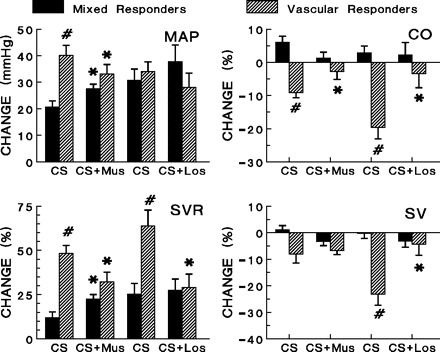
Effects of microinjection of muscimol (80 pmol) or losartan (20 ng) into the MnPO on initial hemodynamic responses to cold stress (startle). Hemodynamic responses are shown for vascular responders and mixed responders following cold stress alone and for cold stress following muscimol pretreatment. #Significant differences (P < 0.05) between vascular and mixed responders exist in the control MAP, CO, SVR, and SV responses to startle. *Muscimol pretreatment produced significant differences (P < 0.05) among vascular responders in MAP, CO, and SVR after cold stress; and losartan pretreatment produced significant differences (P < 0.05) among vascular responders in CO, SVR, and SV after cold stress. Neither drug produced significant differences in heart rate changes.
Effect of losartan on hemodynamic responses to stress in vascular and mixed responders.
The contribution of MnPO AT1 receptors to response variability was examined by microinjection of losartan to five mixed and seven vascular responders (Fig. 6) prior to cocaine dosing. Losartan pretreatment produced an increase in SVR and a decrease in cardiac output in vascular responders but did not affect resting values in mixed responders (Table 2). Microinjection of losartan into the MnPO did not affect hemodynamic responses to cocaine (5 mg/kg iv) in mixed responders. In vascular responders, losartan microinjection blocked the decrease in cardiac output typically seen after cocaine administration (P < 0.01, Fig. 6). In addition, the cardiac output responses in mixed and vascular responders after losartan administration were no longer different. Losartan also attenuated the increase in systemic vascular resistance in vascular responders (P < 0.01) without significantly changing the systemic vascular resistance response in mixed responders so that the two groups were no longer different (Fig. 6). An example of the effect of losartan pretreatment on the decrease in cardiac output after cocaine in vascular responders is depicted in Fig. 7.
Fig. 6.
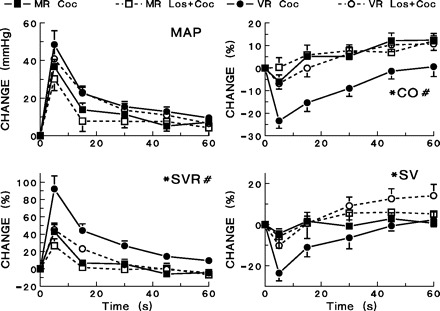
Effects of microinjection of losartan (20 ng) into the MnPO on hemodynamic responses to cocaine (5 mg/kg iv). Hemodynamic responses are shown for vascular responders and mixed responders following cocaine administration alone and for cocaine administration following losartan (Los) pretreatment. #Significant differences (P < 0.05) between vascular and mixed responders exist in the changes seen in MAP, CO, and SVR. *Losartan pretreatment produced significant differences (P < 0.05) among vascular responders in the changes seen in CO, SVR, and SV after cocaine administration. Losartan did not produce any differences in heart rate changes.
Fig. 7.
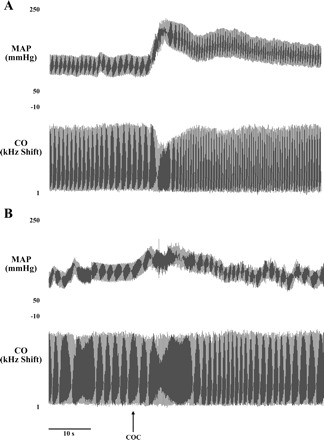
Tracings from a representative vascular responder depicting effects of MnPO microinjection of saline (A) or losartan (B) on arterial pressure (mmHg) and CO (kHz shift) changes following cocaine administration. Cocaine was administered (5 mg/kg iv) at the time point indicated by the arrow; microinjection of saline or losartan occurred 10 min prior to cocaine injection. Time scale (10 s) is indicated by bar.
To test whether AT1 receptors in the MnPO mediate hemodynamic response variability during startle, we administered losartan to five mixed and eight vascular responders prior to cold water stress (Fig. 5). Prior to losartan microinjection, changes in systemic vascular resistance after cold water startle differed between mixed and vascular responders (P < 0.05, Table 3). After losartan microinjection, the hemodynamic responses to cold water stress did not change significantly in mixed responders compared with responses in control trials. In vascular responders, losartan significantly attenuated the increase in systemic vascular resistance (P < 0.01, Fig. 5).
Table 3.
Effects of drug treatment alone immediately before cold stress
| Stimulus | Class | No. | Mean AP, mmHg | Δ MAP, mmHg | HR, beats/min | Δ HR, beats/min | CO, kHz | Δ CO, kHz | Δ SVR, % | Δ SV, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Muscimol | MR | 6 | 115±6 | 0.17±3.2 | 423±15 | −4±7 | −3.0±0.2 | −1.4±5.4 | 3.5±6.0 | −4.3±6.0 |
| VR | 9 | 119±5 | 1.04±1.0 | 405±11 | 1±4 | −3.0±0.1 | −3.0±1.2 | 4.1±1.7 | −3.3±1.4 | |
| Losartan | MR | 5 | 117±4 | −2.2±1.4 | 403±25 | 10±11 | −2.7±0.2 | 5.3±5.6 | −6.0±4.7 | 2.9±6.3 |
| VR | 7 | 119±6 | 0.24±1.2 | 404±24 | 2±8 | −2.9±0.2 | 1.2±2.2* | −0.5±2.3* | 0.97±3.1* |
Values are means ± SE. No. is number of rats studied.
Significant change with treatment alone (P < 0.05); #significant difference between vascular and mixed responders (P < 0.05).
Effect of MK-801 on hemodynamic responses to cocaine in vascular and mixed responders.
To examine the contribution of NMDA receptors within the MnPO to hemodynamic response variability, MK-801 (dizocilpine) was administered to seven mixed and six vascular responders (Fig. 8) prior to cocaine dosing. MK-801 pretreatment did not significantly affect resting hemodynamic values in either group (Table 1). In mixed responders, microinjection of MK-801 into the MnPO followed by cocaine administration (5 mg/kg iv) did not significantly change recorded hemodynamic parameters compared with control experiments. Microinjection of MK-801 into the MnPO preceding cocaine administration blocked the decrease in cardiac output in vascular responders (P < 0.05, Fig. 8). Thus, the cardiac output responses to cocaine seen in vascular and mixed responders after administration of MK-801 were no longer different. MK-801 also produced a reduction in the large increase in systemic vascular resistance in vascular (P < 0.05) but not in mixed responders (Fig. 8).
Fig. 8.
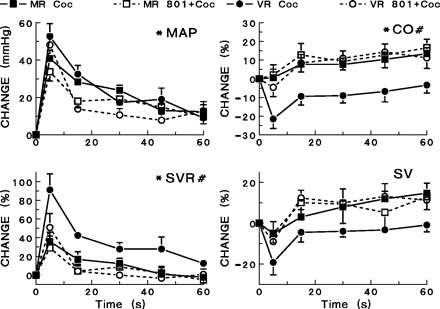
Effects of microinjection of MK-801 (20 nmol) into the MnPO on hemodynamic responses to cocaine (5 mg/kg iv). Hemodynamic responses are shown for vascular responders and mixed responders following cocaine administration alone and for cocaine administration following MK-801 pretreatment. #Significant differences (P < 0.05) between vascular and mixed responders exist in the changes seen in CO and SVR. *MK-801 pretreatment produced significant differences (P < 0.05) among vascular responders in the changes seen in MAP, CO, and SVR.
DISCUSSION
The present study identifies the MnPO as a critical synaptic site mediating the acute hemodynamic responses to stress, particularly in vascular responders, and represents the first evidence that vasoconstrictor responses to cocaine and to startle depend on synaptic connections in the MnPO. We also demonstrated that ANG II receptors in this tissue, and presumably ANG II, are responsible for greater vascular responsiveness in vascular responders. Furthermore, neurons in the MnPO containing NMDA receptors also play a role in mediating greater vasoconstrictor responses in vascular responders. Although the significance of these results in relation to the predisposition to cardiovascular disease has yet to be determined, the data suggest that the MnPO and surrounding tissue is important in mediating greater vasoconstrictor responses to acute stress in conscious rats.
Synaptic transmission in the MnPO is critical for vascular response.
GABA-containing neurons and nerve terminals exist within the MnPO (31), and GABAA receptor activation has been shown by others to attenuate norepinephrine release in the MnPO (41). Unger et al. (50) reported that microdialysis of GABA or GABA analogs into the MnPO inhibited pressor and drinking responses to centrally administered ANG II. Thus, there appears to be a role for GABA in ANG II-related signaling in the MnPO.
There is some evidence to suggest that the MnPO is activated in response to acute behavioral stress. Whyte and Johnson (54) recently reported that AV3V ablation reduced pressor responses to acute heat stress. Others have determined that c-fos expression in the MnPO increases after water deprivation or heat stress (35), placement in a novel environment, restraint stress, and immobilization stress (5). Numerous ablation studies have also demonstrated the importance of AV3V tissues in both short- and long-term blood pressure regulation. AV3V lesions have been found to attenuate acute pressor responses to systemically injected ANG II (14), hypertonic saline (36) and to cholinergic activation of the subfornical organ (11), demonstrating that the AV3V modulates responses to a variety of pressor stimuli, possibly in response to input from the subfornical organ. Whalen et al. (53) reported that systemic injection of the excitatory amino acid analogs kainic acid and NMDA elicited an acute pressor response that was attenuated in rats that had undergone AV3V ablation. They argued that the AV3V plays a critical role in the increased sympathetic activity that elicits the pressor response. Ablation of the AV3V also attenuates the development of hypertension in several experimental and genetic models, including Goldblatt 2-kidney, 1-clip (7, 18), Grollman 1-kidney, 1-clip (8, 9), and DOCA-salt (8, 16) and in the Dahl salt-sensitive rat strain (17). Therefore, the MnPO, part of the AV3V, is involved in short- and long-term regulation of arterial pressure.
In the present study, microinjection of muscimol into the MnPO blocked the decrease in cardiac output and greater increase in SVR observed in vascular responders in response to cocaine or to startle. In fact, the hemodynamic response profiles of vascular and mixed responders after receiving muscimol were no longer different. Despite the changes in hemodynamic response profile in vascular responders, the pressor response to cocaine or to cold stress was not prevented. Our data suggest that synaptic transmission through the MnPO is critical for producing greater vasoconstrictor responses to stress in vascular responders without affecting the pressor response.
AT1 receptors in the MnPO mediate vascular response to stress.
The MnPO contains AT1 receptors (25), but it is not clear whether ANG II activating AT1 receptors on MnPO neurons directly affects fluid balance and cardiovascular responsiveness or whether these neurons participate indirectly by receiving angiotensinergic neural inputs from other nuclei and relaying those inputs to other areas of the brain. The MnPO and several other forebrain nuclei are linked in an angiotensinergic signaling pathway. For example, previous studies have determined that the subfornical organ, which responds to peripheral ANG II and contains AT1 receptors (25, 27), sends projections through the MnPO to the paraventricular nucleus and the supraoptic nucleus (29, 33, 42, 48). The MnPO itself sends a major projection to the paraventricular nucleus, which in turn projects to the rostral ventrolateral medulla and the intermediolateral cell column to contribute to modulation of sympathetic nerve activity and regulation of arterial pressure (44, 37). Angiotensinergic signaling through the MnPO may influence sympathetic outflow via such a pathway. We have evidence that vascular responders have greater increases in renal sympathetic nerve activity following cocaine administration (2, and Stonely TB, Purcell RM, Wall AE, Shields KA, McIntyre DC, Knuepfer MM, unpublished data) that is correlated to the larger increases in systemic vascular resistance typically seen in these animals.
In the present study, microinjection of losartan into the MnPO selectively attenuated the hemodynamic response profile of vascular responders in response to both cocaine and to startle with cold water. Both mixed responders and vascular responders exhibited similar pressor responses to each type of stressor. Losartan pretreatment attenuated the increase in systemic vascular resistance following either cocaine or startle with cold water only in vascular responders, producing a hemodynamic response profile similar to mixed responders. These data support the premise that the greater increase in systemic vascular resistance of vascular responders, but not the pressor response, is due to angiotensinergic conduction through the MnPO. Furthermore, losartan microinjection into the MnPO prior to cocaine administration affected vascular responders selectively, producing a decrease in SVR and an increase in cardiac output. This suggests that there may be tonic angiotensinergic activity in the MnPO that contributes to vascular tone, although this effect is marginal, since it was not seen in the group tested with startle to cold water.
NMDA receptors in the MnPO mediate vascular response to stress.
NMDA receptors are a class of ionotropic receptors that bind excitatory neurotransmitters, such as glutamate and aspartate within the CNS (3). In the MnPO, there is a particularly dense concentration of the NR1 subunit (13). It has been shown that NMDA receptors within the MnPO are involved in vasopressin secretion following blood loss (58, 59). In the present study, MK-801 microinjections into the MnPO blocked the decrease in cardiac output in vascular responders following cocaine administration, preventing the differences in vascular and mixed responder groups. Furthermore, MK-801 pretreatment reduced the large increase in systemic vascular resistance in vascular responders in response to cocaine without affecting the vasoconstrictor or pressor responses in mixed responders. Thus, NMDA receptors in the MnPO are critical for the hemodynamic response profile characteristic of vascular responders but are not responsible for the cocaine-induced increase in arterial pressure.
In some rats, the microinjection site appeared to be immediately rostral to the MnPO, yet drug administration was effective in attenuating vasoconstrictor responses. In contrast, those injections that were >0.5 mm from the MnPO (lateral or rostral) had little effect on responses to cocaine or cold water. Considering that the dye spread was typically 0.5–0.7 mm diameter, it appeared as though the critical tissue was in the MnPO. Alternatively, some neurons from the MnPO may extend into the medial septum or the medial septal neurons may have a similar function. Considering the resolution of these techniques, we cannot differentiate between these possibilities.
We propose that the vascular response is dependent on excessive stimulation of AT1 receptors, NMDA receptors, or a combination of both within the MnPO. For example, if activation of the AT1 receptor is required for subsequent downstream activation of the NMDA receptor, then blocking either receptor in the pathway may similarly attenuate the vascular response. Several studies suggest that NMDA and AT1 receptors in forebrain structures interact to influence cardiovascular homeostasis. Xu et al. (57) reported that intracerebroventricular microinjection of MK-801 reduced ANG II-evoked c-fos expression in several hypothalamic nuclei involved in cardiovascular regulation, including the MnPO and the paraventricular nucleus, and also attenuated dipsogenesis induced by intracerebroventricular infusion of ANG II. Electrophysiological studies by Latchford and Ferguson (24) showed that glutamate participates in ANG II-mediated excitation of magnocellular neurons in the paraventricular nucleus. Thus, it is possible that a similar functional relationship exists between AT1 and NMDA receptors in the MnPO, which could explain the ability of both losartan and MK-801 to attenuate the vascular response in the present study.
Perspectives and Significance
This study identifies the MnPO as a critical site that mediates differences in hemodynamic response patterns to stress between individuals, despite similar pressor responses. Our findings represent the first evidence that AT1 and NMDA receptors in the MnPO mediate the greater vascular responsiveness to cocaine and cold stress in vascular responders. Furthermore, these studies highlight the importance of specific central angiotensinergic and glutamatergic signaling pathways to the production of variable hemodynamic response patterns. The significance of our findings relates to the studies, described above, that suggest that vascular responders are more prone to developing hypertension and heart disease, while mixed responders have lower risks for these diseases (1, 20, 21, 32, 55). The results of the present study may also have clinical implications because a subset of humans has also been identified as vascular responders (6) and human vascular responders are more prone to develop hypertension and heart disease (12, 26). If the vascular response to acute stressors can be prevented, as we have reported for losartan and MK-801 administration into the MnPO, we may also attenuate the increased risks these individuals have for developing hypertension and its attendant sequelae.
GRANTS
This work was supported by grants from the US Public Health Service Grants DA-05180 and DA-13256 and a grant (to M. M. Knuepfer) from the American Heart Association, Heartland Affiliate. J. A. Schwartz was supported by a predoctoral fellowship from the American Heart Association, Heartland Affiliate.
Acknowledgments
The authors thank Qi Gan, Lance L. Lomax, Laura A. Willingham, and Megan R. Espenschied for technical assistance during these studies.
We also thank Merck Pharmaceuticals for the generous gift of losartan.
Some data have been published in abstract form (38, 39, 43).
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Branch CA, Knuepfer M. Causes of differential cardiovascular sensitivity to cocaine. I: studies in conscious rats. J Pharmacol Exp Ther 269: 674–683, 1994a [PubMed] [Google Scholar]
- 2.Branch CA, Knuepfer MM. Causes of differential cardiovascular sensitivity to cocaine. II: sympathetic, metabolic, and cardiac effects. J Pharmacol Exp Ther 271: 1103–1113, 1994b [PubMed] [Google Scholar]
- 3.Brann DW, Mahesh VB. Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation. Front Neuroendocrinol 15: 3–49, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Breigeiron MK, Morris M, Lucion AB, Sanvitto GL. Effects of angiotensin II microinjected into medial amygdala on male sexual behavior in rats. Horm Behav 41: 267–274, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Briski K, Gillen E. Differential distribution of Fos expression within the male rat preoptic area and hypothalamus in response to physical vs. psychological stress. Brain Res Bull 55: 401–8, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Brod J. Haemodynamic basis of acute pressor reactions and hypertension. Br Heart J 25: 227–245, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brody MJ, Fink GD, Buggy J, Haywood JR, Gordon FJ, Johnson AK. The role of the anteroventral third ventricle (AV3V) region in experimental hypertension. Circ Res 43, Suppl 1: I2–I13, 1978 [DOI] [PubMed] [Google Scholar]
- 8.Brody MJ, Johnson AK. Role of the anteroventral third ventricle region in fluid and electrolyte balance, arterial pressure regulation and hypertension. In: Frontiers in Neuroendocrinology, edited by L. Martini and W. F. Ganong. New York: Raven, 1980, pp. 249–292.
- 9.Buggy J, Fink GD, Johnson AK, Brody MJ. Prevention of the development of renal hypertension by anteroventral third ventricular tissue lesions. Circ Res 40, Suppl 1: 110, 1977 [PubMed] [Google Scholar]
- 10.Bunnemann B, Iwai N, Metzger R, Fuxe K, Inagami T, Ganten D. The distribution of angiotensin II AT1 receptor subtype mRNA in the rat brain. Neurosci Lett 142: 155–158, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Colombari DS, Saad WA, Camargo LA, Renzi A, de Luca LA Jr, Colombari E, Menani JV. AV3V lesion impairs responses induced by cholinergic activation of SFO in rats. Am J Physiol Regul Integr Comp Physiol 263: R1277–R1283, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Eliot RS. Stress and the heart: mechanisms, measurement, and management. Postgrad Med 92: 237–248, 1992 [DOI] [PubMed] [Google Scholar]
- 13.Eyigor O, Centers A, Jennes L. Distribution of ionotropic glutamate receptor subunit mRNAs in the rat hypothalamus. J Comp Neurol 434: 101–124, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Fink GD, Haywood JR, Bryan WJ, Packwood W, Brody MJ. Central site for pressor action of blood-borne angiotensin in the rat. Am J Physiol Regul Integr Comp Physiol 239: R358–R361, 1980 [DOI] [PubMed] [Google Scholar]
- 15.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev 78: 583–686, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Gavras H, Brunner HR, Laragh JH, Vaughan ED, Koss M, Cote LJ, Gavras I. Malignant hypertension resulting from deoxycorticosterone acetate and salt excess: role of renin and sodium in vascular changes. Circ Res 36: 300–309, 1975 [DOI] [PubMed] [Google Scholar]
- 17.Goto A, Ganguli M, Tobian L, Johnson MA, Iwai J. Effect of an anteroventral third ventricle lesion on NaCl hypertension in Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol 243: H614–H618, 1982 [DOI] [PubMed] [Google Scholar]
- 18.Haywood JR, Fink GD, Buggy J, Boutelle S, Johnson AK, Brody MJ. Prevention of two-kidney, one-clip renal hypertension in rat by ablation of AV3V tissue. Am J Physiol Heart Circ Physiol 245: H683–H689, 1983 [DOI] [PubMed] [Google Scholar]
- 19.Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 288: H680–H685, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Knuepfer MM, Branch CA, Gan Q, Fischer VW. Cocaine-induced myocardial ultrastructural alterations and cardiac output responses in rats. Exp Mol Pathol 59: 155–168, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Knuepfer MM, Mueller PJ. Review of evidence for a novel model of cocaine-induced cardiovascular toxicity. Pharm Biochem and Behav 63: 489–500, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Knuepfer MM. Cardiovascular disorders associated with cocaine use: myths and truths. Pharmacol Ther 97: 181–222, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Knuepfer MM, Purcell RM, Gan Q, Le KM. Hemodynamic response patterns to acute behavioral stressors resemble those to cocaine. Am J Physiol Regul Integr Comp Physiol 281: R1778–R1786, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Latchford KJ, Ferguson AV. ANG II-induced excitation of paraventricular nucleus magnocellular neurons: a role for glutamate interneurons. Am J Physiol Regul Integr Comp Physiol 286: R894–R902, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Neuroscience 82: 827–841, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Light KC, Dolan CA, Davis MR, Sherwood A. Cardiovascular responses to an active coping challenge as predictors of blood pressure patterns 10 to 15 years later. Psychosom Med 54: 217–230, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Lippoldt A, Bunnemann B, Iwai N, Metzger R, Inagami T, Fuxe K, Ganten D. Cellular localization of angiotensin type 1 receptor and angiotensinogen mRNAs in the subfornical organ of the rat brain. Neurosci Lett 150: 153–158, 1993 [DOI] [PubMed] [Google Scholar]
- 28.McKinley MJ, Allen AM, Mathai ML, May C, McAllen RM, Oldfield BJ, Weisinger RS. Brain angiotensin and body fluid homeostasis. Jpn J Physiol 51: 281–289, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Miselis RR. Subfornical organ efferents to neural systems for control of body water. Science 205: 1022–1025, 1979 [DOI] [PubMed] [Google Scholar]
- 30.Morris MJ, Wilson WL, Starbuck EM, Fitts DA. Forebrain circumventricular organs mediate salt appetite induced by intravenous angiotensin II in rats. Brain Res 949: 42–50, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunohistochemistry. In: Handbook of Chemical Neuroanatomy, edited by Bjorklund A and Hokfelt T. New York: Elsevier, 1985, vol. 4. p. 436–608.
- 32.Muller JR, Le KM, Haines W, Gan Q, Knuepfer MM. Hemodynamic response pattern predicts susceptibility to stress-induced elevation in arterial pressure in the Sprague-Dawley rat. Am J Physiol Regul Integr Comp Physiol 281: R31–R37, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Oldfield BJ, Miselis RR, McKinley MJ. Median preoptic nucleus projections to vasopressin-containing neurons of the supraoptic nucleus in sheep. A light and electron microscopic study. Brain Res 542: 193–200, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Patronas P, Horowitz M, Simon E, Gerstberger R. Differential stimulation of c-fos expression in hypothalamic nuclei of the rat brain during short-term heat acclimation and mild dehydration. Brain Res 798: 127–139, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (2nd ed.). San Diego, CA: Academic, 1986
- 36.Pedrino GR, Sera CTN, Cravo SL, Colombari DS. Anteroventral third ventricle lesions impair cardiovascular responses to intravenous hypertonic saline infusion. Auton Neurosci 117: 9–16, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100: 549–556, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Reilly NS, Wombles K, Bloodgood TA, Knuepfer MM. The median preoptic area (mnPOA) is critical for hemodynamic responses to cocaine and stress (Abstract). FASEB J 17: A1292, 2003 [Google Scholar]
- 39.Reilly NS, Lomax LL, Knuepfer MM. AT1 receptors in the median preoptic area (mnPOA) are responsible for regulating hemodynamic responses to cocaine and to behavioral stress (Abstract). FASEB J 18: A675-A676, 2004 [Google Scholar]
- 40.Rowe KD, Schwartz JA, Lomax LL, Knuepfer MM. Central angiotensin receptors mediate hemodynamic response variability to stress. Am J Physiol Regul Integr Comp Physiol 291: R719–R727, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Sakamaki K, Nomura M, Hatakenaka S, Miyakubo H, Tanaka J. GABAergic modulation of noradrenaline release in the median preoptic nucleus area in the rat. Neurosci Lett 342: 77–80, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Saper CB, Levisohn D. Afferent connections of the median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res 288: 21–31, 1983 [DOI] [PubMed] [Google Scholar]
- 43.Schwartz JA, Reilly NS, Knuepfer MM. NMDA receptors in the median preoptic area mediate hemodynamic response variability following stress (Abstract). FASEB J 20: A238, 2006 [Google Scholar]
- 44.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res 742: 1773–1179, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Swanson LW. Brain Maps: Structure of the Rat Brain (3rd ed.). Los Angeles, CA: Academic, 2003
- 48.Tanaka J, Saito H, Kaba H. Subfornical organ and hypothalamic paraventricular nucleus connections with median preoptic nucleus neurons: an electrophysiological study in the rat. Exp Brain Res 68: 579–585, 1987 [DOI] [PubMed] [Google Scholar]
- 49.Turner JR, Sherwood A, Light KC. Individuals Differences in Cardiovascular Response to Stress. New York: Plenum, 1992
- 50.Unger T, Bles F, Ganten D, Lang RE, Rettig R, Schwab NA. Gabaergic stimulation inhibits central actions of angiotensin II: pressor responses, drinking and release of vasopressin. Eur J Pharmacol 90: 1–9, 1983 [DOI] [PubMed] [Google Scholar]
- 51.Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M. Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol 88: 399–406, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Whalen EJ, Beltz TG, Lewis SJ, Johnson AK. AV3V lesions attenuate the cardiovascular responses produced by blood-borne excitatory amino acid analogs. Am J Physiol Heart Circ Physiol 276: H1409–H1415, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Whalen EJ, Beltz TG, Lewis SJ, Johnson AK. Periventricular anteroventral third ventricle lesions diminish the pressor response produced by systemic injection of the N-methyl-d-aspartate antagonist MK-801. Brain Res 836: 210–212, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Whyte DG, Johnson AK. Thermoregulatory role of periventricular tissue surrounding the anteroventral third ventricle (AV3V) during acute heat stress in the rat. Clin Exp Pharmacol Physiol 32: 457–461, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Williams JB, Keenan SM, Gan Q, Knuepfer MM. Hemodynamic response profile predicts susceptibility to cocaine-induced toxicity. Eur J Pharmacol 464: 189–196, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Wright JW, Harding JW. Brain angiotensin receptor subtypes in the control of physiological and behavioral responses. Neurosci Biobehav Rev 18: 1, 21–53, 1994 [DOI] [PubMed] [Google Scholar]
- 57.Xu Z, Lane JM, Zhu B, Herbert J. Dizocilpine maleate, an N-methyl-d-aspartate antagonist, inhibits dipsogenic responses and c-Fos expression induced by intracerebral infusion of angiotensin II. Neuroscience 78: 203–214, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi K, Watanabe K. Anteroventral third ventricular N-methyl-d-aspartate receptors, but not metabotropic glutamate receptors are involved in hemorrhagic AVP secretion. Brain Res Bull 66: 59–69, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi K, Watanabe K. Contribution of N-methyl-d-aspartate receptors in the anteroventral third ventricular region to vasopressin secretion, but not to cardiovascular responses provoked by hyperosmolality and prostaglandin E2 in conscious rats. Brain Res Bull 58: 301–309, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Zaretsky DV, Hunt JL, Zaretskaia MV, DiMicco JA. Microinjection of prostaglandin E2 and muscimol into the preoptic area in conscious rats: comparison of effects on plasma adrenocorticotrophic hormone (ACTH), body temperature, locomotor activity, and cardiovascular function. Neurosci Lett 397: 291–296, 2006 [DOI] [PubMed] [Google Scholar]


