Abstract
Alcoholic liver disease (ALD) remains a major cause of morbidity and mortality worldwide. For example, the Veterans Administration Cooperative Studies reported that patients with cirrhosis and superimposed alcoholic hepatitis had a 4-year mortality of >60%. The poor prognosis of ALD implies that preventing disease progression would be more effective than treating end-stage liver disease. An obvious avenue of prevention would be to remove the damaging agent; however, the infamously high rate of recidivism in alcoholics makes maintaining abstinence a difficult treatment goal to prevent ALD. Indeed, although the progression of ALD is well-characterized, there is no universally accepted therapy available to halt or reverse this process in humans. With better understanding of the mechanism(s) and risk factors that mediate the initiation and progression of ALD, rational targeted therapy can be developed to treat or prevent ALD. The purpose of this review is to summarize the established and proposed mechanisms by which chronic alcohol abuse damages the liver and to highlight key signaling events known or hypothesized to mediate these effects.
Keywords: alcohol metabolism, alcoholic liver disease, cytokines, inflammation, oxidative stress
Introduction: alcoholic liver disease
Alcoholic liver disease (ALD) affects millions of patients worldwide each year. In 2004, 3.8% of all global deaths were as a result of alcohol (Rehm et al., 2009). The progression of ALD is well-characterized and is actually a spectrum of liver diseases, ranging from steatosis, to inflammation and necrosis (steatohepatitis), to fibrosis and cirrhosis, and eventually hepatocellular carcinoma (HCC) in some cases. The risk of ALD increases in a dose- and time-dependent manner with consumption of alcohol (Mann et al., 2003). However, only a minor proportion of even heavy drinkers develop the severe form of the disease, suggesting that other environmental (e.g., cigarette smoking, obesity or HCV infection) or genetic (e.g., gender or polymorphisms in key genes) factors contribute to overall risk (Day, 2000). Clinical management of ALD focuses predominantly on maintaining abstinence in the patient, and on treating sequelae associated with acute alcoholic hepatitis or cirrhosis (Diehl, 2002). Short of a successful liver transplant, the effects of decompensation (e.g., hepatorenal syndrome) usually lead to the death of the patient (Powell and Klatskin, 1968). Even if compensated cirrhosis is maintained (i.e., ‘stable cirrhotics’), the overall risk of developing hepatocellular carcinoma, which has a very low survival rate is increased ~20-fold by pre-existing cirrhosis (Gogel et al., 2000). Although the progression of ALD is well-characterized, there is no universally accepted therapy available to halt or reverse this process in humans except abstinence. With better understanding of the mechanisms (e.g., signaling pathways) that mediate the initiation and progression of ALD, rational targeted therapy can be developed to treat or prevent ALD. The purpose of this review is to summarize the established and proposed mechanisms by which chronic alcohol abuse damages the liver (see Figure 1) and to highlight key signaling events known or hypothesized to mediate these effects.
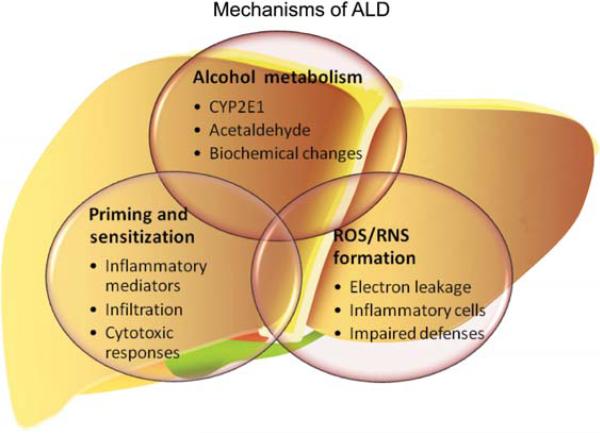
Figure 1. Proposed mechanism by which alcohol causes alcoholic liver disease.
Alcoholic liver disease is a chronic disease of the liver that encompasses fatty liver (steatosis) inflammation (hepatitis) and fibrosis/cirrhosis. Effects of alcohol exposure that are proposed to initiate and mediate the progression of the disease include alcohol metabolism, oxidative stress and priming and sensitization of the inflammatory response.
Ethanol metabolism and its role in ALD
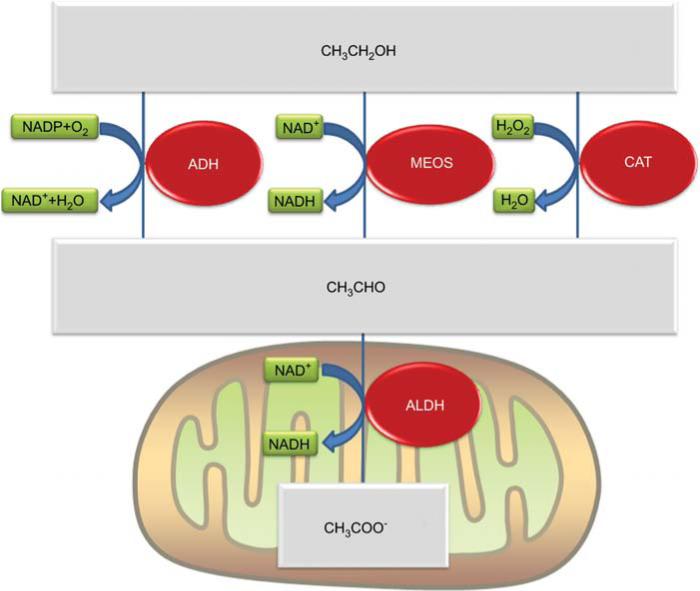
The liver is the main organ responsible for metabolizing ethanol. The major route of ethanol metabolism is the oxidation of ethanol to acetaldehyde. The dominant enzyme system involved in this process is alcohol dehydrogenases (ADH). However, cytochrome P450 systems, also called microsomal ethanol oxidizing system or ‘MEOS’ (mostly CYP2E1), and catalase also play crucial roles. ADH oxidizes ~80% of the ethanol, with MEOS mediating most of the remainder, and catalase oxidizing only a small component. Electrons from alcohol are transferred to NADP+ by ADH. By contrast, molecular oxygen (O2) is the recipient of the electrons from ethanol via the MEOS system. Lastly, hydrogen peroxide (H2O2) is reduced to water by catalase in the process of oxidizing ethanol (see Figure 2). The oxidation of ethanol to acetaldehyde is mediated by three distinct enzyme systems; aldehyde dehydrogenase (ALDH), however, is the only enzyme system that oxidizes acetaldehyde to acetate (Figure 2). Analogous to ADH, ALDH uses NAD+ as the electron acceptor for this reaction; however, ALDH is located in the mitochondria (Figure 2). Whereas the rapid removal of ethanol by the liver is clearly a protective mechanism, there are potentially toxic metabolic and biochemical processes of ethanol metabolism that could contribute to the development and progression of alcoholic liver disease, including induction of CYP2E1, production of toxic metabolites (e.g., acetaldehyde), and alteration of biochemical processes.
Figure 2. Oxidative metabolism of alcohol by the liver.
Alcohol (CH3CH2OH) is oxidized to acetaldehyde (CH3CHO) by three enzyme systems: the microsomal ethanol oxidizing system (MEOS), alcohol dehydrogenase (ADH) and catalase (CAT). Acetaldehyde, in turn, is metabolized to acetate (CH3COO−) by aldehyde dehydrogenase (ALDH) in the mitochondria. The metabolic and biochemical effects of alcohol metabolism might contribute to ADH.
CYP2E1
Whereas the relative role of the MEOS system to total alcohol metabolism is low a priori, CYP2E1 is robustly induced by alcohol and can contribute to a far greater amount of total alcohol metabolism in alcohol-dependent individuals (Lieber, 1997). This induction could be a protective effect at the organismal level, however, it might have side effects that contribute to hepatotoxicity. For example, CYP2E1 is localized in regions of the liver lobule that are damaged by alcohol. Furthermore, selective inhibitors of CYP2E1 partially inhibit liver injury caused by ethanol in animal models, supporting this hypothesis (Bardag-Gorce et al., 2000). Whereas these studies serve as proof-of-concept that CYP2E1 contributes to ALD, most CYP2E1 inhibitors, although selective for CYP2E1 among the P450s, often target other biomolecules that can confound the conclusions. More specific studies in cultured cells have historically been difficult, because cultured cells possess very low activities of CYP enzymes.
In recent years, the above-mentioned limitations of the research have been bypassed using new techniques. For example, experiments with HepG2 cells that overexpress CYP2E1 support the hypothesis that CYP2E1 is involved in hepatocyte damage owing to alcohol (Cederbaum et al., 2001). Furthermore, CYP2E1 knockout mice have also been employed (Kono et al., 1999). Interestingly, CYP2E1-deficient mice developed liver injury analogous to their wild-type counterparts (Kono et al., 1999). It has been suggested that other isoforms of CYP (e.g., CYP4A) could play a compensatory role in the onset of early alcohol-induced liver injury in the absence of CYP2E1 in the knockouts (Leclercq et al., 2001). Although it appears that CYP2E1 is not required for at least the initiation of alcohol-induced liver injury in mice, it does not preclude a role for CYP2E1 in later stages of disease progression in mice or in other species.
The potential mechanisms by which CYP2E1 contributes to ALD are still being explored; however, there are some plausible hypotheses. For example, CYP2E1 has been shown to contribute to oxidative stress caused by alcohol. This enzyme is relatively loosely coupled with cytochrome reductase; it can therefore leak electrons to oxygen to form O2•−, or catalyze lipid peroxidation (Ekstrom and Ingelman-Sundberg, 1989). Furthermore, CYP2E1 has also been shown to bioactivate several hepatotoxic agents (e.g., acetaminophen). Therefore, the induction of this enzyme by chronic abuse of ethanol can increase the risk of liver damage by other agents. Furthermore, CYP2E1 can be induced in the resident macrophages, the Kupffer cells and macrophages overexpressing CYP2E1 have a more robust response to stimulation in culture (Cao et al., 2005), which could contribute to the ‘priming’ effect of alcohol on these cells (see below).
Acetaldehyde and other toxic byproducts
Whereas acetaldehyde is oxidized to acetate by ALDH (see Figure 2), the kinetics of this reaction are relatively slow and therefore allow detectable increases in acetaldehyde in humans consuming alcohol. Acetaldehyde is toxic and several of the systemic effects of alcohol abuse (e.g., flushing, headaches and nausea) are mediated, at least in part, by direct or indirect effects of elevated acetaldehyde levels. Furthermore, it is proposed that acetaldehyde might also play a causal role in ALD (Lieber, 1988). Acetaldehyde can form adducts with reactive residues on proteins or small molecules (e.g., cysteines). These chemical modifications can alter and/or interfere with normal biologic processes, such as signal transduction, and/or be directly toxic to the cell. Modified biologic molecules could also stimulate the host immune response and cause an autoimmune-like disease. Antibodies against such oxidatively modified proteins have been reported in both humans and animal models of ALD (Klassen et al., 1995; Niemela, 2001). Furthermore, acetaldehyde promotes enhanced GSH utilization/turnover and significantly depletes GSH stores (Lieber, 1997), thus contributing to oxidative stress (see below).
Biochemical changes
The concentrations of ethanol in the systemic blood can reach high levels; for example a blood alcohol concentration (BAC) of 0.08% equates to an alcohol concentration of approximately 20 mm in the blood. The hepatic concentrations of ethanol are much higher than systemic owing to the first-pass effect of the liver on ethanol. High alcohol concentrations, coupled with the remarkable rate of metabolism by the liver, results in biochemical stress for liver cells. Indeed, whereas acetate from ethanol oxidation can enter the citric acid cycle after conversion to acetyl-CoA, the various metabolic and biochemical alterations caused by ethanol exposure result in a negative energy balance (see Lieber, 1997, for review). It is suggested that these biochemical changes caused by alcohol metabolism, at least in part, mediate and/or exacerbate ALD.
Steatosis, one of the earliest hepatic changes caused by alcohol, was originally thought to be a pathologically inert histological change. More recent work, however, showed that steatosis could play a crucial role not only in the initiation, but also in the progression of ALD (Yang et al., 1997; Day and James, 1998). Fatty livers are more sensitive to hepatotoxicity caused by agents such as endotoxin (Eastin et al., 1997; Yang et al., 1997). Furthermore, the degree of fat accumulation is predictive of the severity of later stages of ALD (i.e., fibrosis and cirrhosis) (Sorensen et al., 1984; Teli et al., 1995). As mentioned above, the oxidation of ethanol to acetaldehyde by ADH and subsequent oxidation to acetate by ALDH utilizes NAD+ as an electron acceptor (see Figure 2), causing a pronounced shift in the NADH:NAD+ ratio to a more reduced state. This increase in the reduced state of pyridine nucleotides is also proposed to be involved in the accumulation of lipids during alcohol ingestion. Specifically, the shift in the NADH:NAD+ ratio increases the rate of fatty acid synthesis and esterification, while simultaneously decreasing mitochondrial β-oxidation of free fatty acids. This change in the redox state can also impair normal carbohydrate metabolism, resulting in multiple effects, including a decreased supply of ATP to the cell (Lieber, 2000). Furthermore, it has been demonstrated that this shift in the pyridine nucleotide redox state can activate the sirtuin family of NAD+-dependent histone deacteylases (You et al., 2008), which can alter gene expression profiles and thereby indirectly affect the metabolic state and signal transduction of the liver. For example, SIRT1 has been shown to activate AMP-activated protein kinase (AMPK, see below) via deacetylation/activation of serine threonine liver kinase B1 (LKB1; Ruderman et al., 2010). However, a decrease in energy state or activation of AMPK also leads to activation of SIRT1, possibly by increasing NAD+ or the NADH:NAD+ ratio (Canto and Auwerx, 2009).
As mentioned above, the oxidation of ethanol by the MEOS consumes oxygen (see Figure 2). Furthermore, alcohol causes an acute hypermetabolic state in the liver, resulting in a doubled oxygen consumption rate (Yuki and Thurman, 1980). This increase in the oxygen consumption rate also increases the intralobular oxygen gradient in the liver (Ji et al., 1982) with subsequent pericentral hypoxia (Arteel et al., 1996, 1997). Hypoxia aggravates the metabolic stress on the liver by further increasing the pyridine nucleotide redox state. A decrease in cellular oxygen tension will also exacerbate the impaired mitochondrial electron flow caused by alcohol by reducing the delivery of O2 to the mitochondria in the steep intracellular oxygen gradient (De Groot et al., 1985). After alcohol levels decrease, the subsequent reoxygenation could increase pro-oxidant production via hypoxia/reoxygenation. This effect together with the impairment of free radical defenses caused by hypoxia (Shan et al., 1989) can add to the observed oxidative stress in the liver after ethanol exposure (Shan et al., 1989).
Oxidative stress
Reactive oxygen and nitrogen species (ROS and RNS, respectively) are products of normal cellular metabolism and have beneficial effects. For example, ROS have been shown to mediate a variety of cellular signaling pathways. However, owing to the potential of these molecules also to damage normal tissue, the balance between pro-oxidants and antioxidants is crucial for the survival and function of aerobic organisms. If the balance is tipped to favor overproduction of these species, oxidative stress can occur. Oxidative stress has been proposed to be crucially involved in ALD (Shaw et al., 1981; Arteel, 2003). In support of this hypothesis, numerous antioxidants have been shown to protect against the damaging effects of ethanol in vitro and in vivo models of ALD (Kono et al., 2000a, 2001a,b). Although important advances have been made in understanding the role of pro-oxidants in experimental alcohol-induced liver injury, this work has yet to translate into an accepted antioxidant therapy for ALD.
Types and sources of ROS and RNS in ALD
The study of ROS/RNS in vivo is complicated. Owing to their rapid reactions with biomolecules, they generally cannot be measured directly. Therefore, to assess these reactions the products of the reaction of pro-oxidants with endogenous (e.g., lipid peroxides) or exogenous (e.g., spin traps) targets are measured. This indirect detection makes it difficult to identify the parent oxidant. However, there are species with relatively clear data supporting their involvement in experimental ALD. These species are usually derived from superoxide (O2•−) or nitric oxide (NO•). There has been major debate over the identity of the major source(s) of oxidants during alcohol exposure in ALD. Based on experimental data, key pro-oxidant enzymes or enzyme systems have been consistently proposed to play a role in alcohol-induced liver injury localized in hepatocytes, inflammatory cells (e.g., Kupffer cells and neutrophils) and other nonparenchymal cells (e.g., stellate cells).
Types of ROS: a role for superoxide
Superoxide (O2•−) is believed to play a central role in alcohol-induced liver injury. Not only is O2•− readily produced by numerous processes in vivo, but many other oxidants found in vivo are also derived from O2•−. Indeed, exogenous SOD has protective/antioxidant effects in both in vitro and in situ models of alcohol exposure. Genetic overexpression of either cytosolic Cu/Zn-SOD or mitochondrial Mn-SOD in liver cells has been shown to prevent alcohol-induced liver injury in rats fed enteral alcohol (Wheeler et al., 2001a,b). The finding that both cytosolic and mitochondrial SOD isoforms were protective against alcohol-induced liver injury suggests at least two distinct pools of O2•− production are involved.
There is clear evidence that pro-oxidant formation is dependent on the production of O2•− during experimental ALD. However, the presence of this radical alone is not sufficient to explain the oxidative damage caused by alcohol. Indeed, O2•− is not a strong oxidant and does not oxidize many biologic molecules. A well-known radical that can be detected after alcohol exposure is the α-hydroxyethyl free radical. The formation of this radical depends on O2•− production (Wheeler et al., 2001a,b), however, O2•− is too weak an oxidant to directly react with ethanol to form this product (Knecht et al., 1993). Alternatively, O2•− can react via catalytic pathways in the cell to form more potent oxidants. For example, the reduction of O2•− by SOD forms H2O2 and H2O2 plus transition metals can lead to formation of hydroxyl radicals [OH•; the Fenton reaction (Fridovich, 1995)]. Superoxide could also react with NO• to form peroxynitrite (ONOO−), another strong oxidizing and nitrating species (Beckman et al., 1990; Beckman, 1996). Both of these products (OH• and ONOO−) are potent oxidants and capable of reacting with ethanol and form the α-hydroxyethyl radical. Therefore, although O2•− is not a potent pro-oxidant per se, it appears to be a key initiator of oxidative stress during alcohol exposure.
Types of RNS: a role for nitric oxide
Most RNS found in vivo are derived from NO•. Whether NO• production is protective or damaging in ALD is not clear (Hon et al., 2002). For example, hepatic vasoconstriction caused by acute ethanol or by cirrhosis is, at least in part, owing to low production of NO• (Oshita et al., 1994). Nitric oxide is also antiapoptotic in hepatocytes and is required for normal hepatic regeneration (Rai et al., 1998; Kim et al., 2000). Nitric oxide can also terminate lipid peroxidation chain reactions by attacking lipid peroxyl radicals (Rubbo et al., 2000). Studies in support of the hypothesis that NO• plays a protective role in ALD showed that the NOS-inhibitor N(G)-nitro-l-arginine methyl ester (l-NAME) exacerbated experimental ALD, and that arginine supplementation reversed liver injury caused by ethanol (Nanji et al., 1995, 2001). However, NO• also plays a potentially damaging role in ALD by producing strong reactive species (e.g., ONOO−). These NO•-derived RNS can cause nitration reactions (e.g., 3-nitrotyrosine formation) and nitrosation reactions (e.g., nitrosothiol formation), as well as oxidation reactions during alcohol exposure. Reactive intermediates formed during ONOO− degradation can also cause one-electron oxidation reactions with ethanol, leading to the formation of hydroxyethyl radicals (Gatti et al., 1998). Thus, NO• might play a dual role in ALD, mediating both protective effects and tissue damage by overproduction of RNS. These events in vivo are critically dependent on the cell type, NOS isoform and stage of disease.
Sources of ROS/RNS: hepatocytes
The most obvious pathologic changes to the liver during alcohol exposure occur in the hepatocytes. Moreover, the accumulation of indices of oxidative stress (e.g., lipid peroxides) are predominantly a hepatocellular event during alcohol administration. Therefore, hepatocellular oxidant production probably plays a key role in alcoholic liver injury. The proposed major sources of pro-oxidants in hepatocytes are the ethanol-inducible CYP2E1, mitochondria and NAD(P)H oxidase (NOX). In chronic liver disease, activation of NADPH oxidase isoforms leads to increased ROS generation and oxidative stress, resulting in inflammation and fibrogenesis. Activation of NADPH oxidase by hepatotoxic agents (e.g., bile salts) activate death pathways such as CD95-induced signaling to induce cell apoptosis (Reinehr et al., 2005b). Moreover, NOX isoforms found in hepatocytes participate in a variety of signal transduction cascades (Lambeth et al., 2000). Elevated pro-oxidant production not only increases the net amount of pro-oxidants in the hepatocyte, but also directly damages mitochondrial proteins and DNA, which can aggravate mitochondrial aging and activate apoptotic pathways (see Cunningham and Bailey, 2001, for review). In support of this hypothesis, overexpression of Mn-SOD in rats protects against alcohol-induced liver injury (Wheeler et al., 2001b). Furthermore, alcohol depletes mitochondrial GSH (Fernandez-Checa et al., 1997), resulting in increased hepatocellular apoptosis (Colell et al., 1998). Therefore, hepatocellular pro-oxidant production is a crucial step in the progression of ALD.
Sources of ROS/RNS: inflammatory cells
Another crucial component in the development of alcohol-induced liver injury is inflammation, involving both resident (e.g., Kupffer cells) and recruited (e.g., neutrophils and lymphocytes) inflammatory cells. Unlike parenchymal cells where ROS production is a ‘side effect’ of biochemical processes caused by electron leakage, inflammatory cells are actively generating high concentrations of ROS/RNS into their surrounding environment. Although the production of these species is key for host defense, they can also cause damage to normal tissue if inappropriately upregulated.
It is known that inappropriate activation of Kupffer cells plays a key role in the initiation of alcoholic liver injury (see Thurman, 1998, for review). The production of pro-oxidants is stimulated in activated Kupffer cells. The proposed major sources of pro-oxidants in these cells are NOX and the inducible form of NOS (iNOS or NOS2). Work from Thurman's group showed that the NAD(P)H oxidase inhibitor, diphenyliodonium (DPI), blocks alcohol-induced liver injury in rats exposed to enteral alcohol administration (Kono et al., 2001b). However, DPI is a flavoprotein inhibitor rather than a specific NAD(P)H oxidase inhibitor and could therefore have non-specific effects. Moreover, mice deficient in NAD(P)H oxidase (p47phox knockout mice), were also protected against experimental ALD (Kono et al., 2000b). Together, these results support the hypothesis that O2•− production from this enzyme plays a key role in the initiation of oxidative stress and experimental ALD.
Previous work has also shown that iNOS knockout mice are protected against oxidative stress caused by alcohol and nitration of tyrosine residues was prevented as well (McKim et al., 2003). Similar results were demonstrated using the highly specific iNOS inhibitor, 1400W, in wild-type mice. The finding that injury and oxidative stress are prevented in both iNOS knockout mice (McKim et al., 2003), and in NAD(P)H oxidase-deficient mice (Kono et al., 2000b), demonstrates that the damaging oxidant in experimental ALD is dependent on the production of O2•− and NO•. This in turn gives weight to the idea that ONOO− might be a key player in the onset of alcoholic liver damage. These results also support the hypothesis that NO• production might play dual roles in ALD, with iNOS activity in macrophages a critical early step in the initiation and the progression of liver injury by increasing RNS production. Disadvantages of ‘specific’ NOS inhibitors in vivo are significant cross-reactions with other NOS isoforms (Boer et al., 2000), particularly when these isoforms serve different functions in the disease state, as in ALD (Wiest and Groszmann, 2002).
Sources of ROS/RNS: other nonparenchymal cells
Recent studies have shown that oxidative stress could play a role not only in infiltrating inflammatory cells, Kupffer cells or hepatocytes, but also in the transformation of stellate cells into myofibroblasts, the critical matrix-depositioning cell in the fibrotic liver (Poli, 2000). It is unclear at this time how much this cell type contributes to oxidative stress in the liver, or whether pro-oxidant production in this cell acts mainly as an autocrine/paracrine signaling event. Because activation of this cell type is crucial in the progression to severe ALD, more research in this area is required.
Oxidative stress independent of ROS/RNS production in ALD
Oxidative stress can be mediated by an increase in ROS/RNS production, by a decrease in the antioxidant defense, or an increase in the reactivity of ROS/RNS (Sies, 1986). Alcohol causes modifications to the cell that might also favor oxidative stress via these mechanisms. For example, alcoholics have lower antioxidant levels owing to the nutritional deficiencies (Lieber, 2003). Hypoxia caused by alcohol exposure can impair antioxidant defenses (Jones, 1985; Tribble and Jones, 1990). Free iron is mobilized by alcohol (Shaw et al., 1988), which can also lead to an increase in transition-metal catalysis to potent oxidants (e.g., the Fenton reaction). Another example is that alcohol exposure inhibits the 26S proteosome in hepatocytes (Bardag-Gorce et al., 2000), which is responsible for degrading proteins damaged by ROS/RNS. The inhibition of this complex results in an accumulation of proteins damaged by ROS/RNS (Donohue, 2002). Finally, there is a mass of proteins and systems involved in the ‘antioxidant network’. This family does not directly block pro-oxidants, but serves as an additional reductant and maintains the catalytic activity of antioxidant proteins or small molecules. These reactions are energy-dependent to maintain such cycles. Biochemical stress (see above) caused by alcohol exposure can therefore indirectly impair cellular antioxidant defenses.
Oxidative stress and signaling
Chemical modification/damage to biologic molecules, which can alter and/or interfere with normal processes within the cell, is a major mechanism by which ROS/RNS can cause cellular injury. It is also known that pro-oxidants can mediate and/or amplify their signal by modifying signaling cascades within the cell. Many reviews have focused on the role of signaling cascades in damage owing to oxidative stress (Kamata and Hirata, 1999; Allen and Tresini, 2000; Forman and Torres, 2001; Droge, 2002). Signaling cascades that are oxidant sensitive include small molecules [e.g., intracellular Ca2+ (Ermak and Davies, 2002)], stress-activated protein kinases [e.g., SAPK, JNK, ERK1/2, and p38 (Suzuki et al., 1997)], transcription factors [e.g., AP-1, HIF-1 and NF-κB (D'Angio and Finkelstein, 2000)], and modulators of apoptosis signaling [e.g., caspases, Bad and Bcl-2 (Hoek and Pastorino, 2002)]. Ethanol has been shown to alter the signal of many of these pathways in vitro and/or in experimental ALD (see Mandrekar and Szabo, 2009; Szabo and Bala, 2010, for review); however, whether or not these effects are mediated by oxidative stress per se is often unclear.
The role of inflammation in ALD
Priming and sensitization in ALD
The concept of priming and sensitization is important in alcoholic liver damage. As noted, the natural history of ALD is characterized by chronic inflammation in the liver, involving an increase in proinflammatory cytokines and a decrease in anti-inflammatory cytokines. Activation of the inflammatory response is, at least in part, owing to increased levels of activators of this response, such as lipopolysaccharides (LPS; Spencer et al., 1983; Bigatello et al., 1987). However, elevated levels of LPS found in alcoholics and in experimental ALD are relatively low compared with those found in experimental endotoxemia and human sepsis. Furthermore, liver injury caused by alcohol cannot be mimicked by chronic low-dose LPS in the absence of ethanol (Deaciuc et al., 1999). However, inflammatory cells seem to be primed to activation by chronic alcohol administration. For example, peripheral blood monocytes from patients with alcoholic hepatitis spontaneously produce proinflammatory mediators (e.g., TNFα). In response to LPS, these monocytes produce more proinflammatory mediators than their control counterparts (McClain and Cohen, 1989).
Hepatocytes also appear to be sensitized to inflammatory stimuli by alcohol administration. For example, although TNFα is proproliferative in hepatocytes isolated from naive animals, it is proapoptotic in cells isolated from ethanol-treated animals (Colell et al., 1998), via cellular ‘death domain’ pathways (Liu et al., 2002). Other cell types such as stellate cells also appear to be primed/sensitized owing to alcohol administration (Reeves et al., 2000; Kim et al., 2001). The concept of priming and sensitization also means that there could be a series of sequential events in the progression of liver damage owing to alcohol exposure. Specifically, the priming of inflammatory cells by ethanol leads to a more robust cell-killing response that is increased in sensitized hepatocytes, explaining why blocking the activation of Kupffer cells (Adachi et al., 1994) or employing knockouts with an impaired Kupffer cell response (Kono et al., 2000b) prevent hepatocyte damage.
It is known that ALD is associated with an increase in apoptotic cell death. Hepatocyte death via apoptosis is associated with alcohol consumption in animals and humans and the number of apoptotic cells detected in the liver correlates with the development of ethanol-induced liver injury (Deaciuc et al., 1999). Studies have shown that hepatocyte apoptosis owing to ethanol can be linked to pathways involving oxidative stress mechanisms via the induction enzymes such as CYP2E1, the effects of cytokines (e.g., TNFα), or the involvement of death receptors (e.g., Fas/CD95; see McVicker et al., 2007 for review). The extrinsic death receptor pathway via TNFα and Fas involves the downstream activation of ‘initiator caspases’ (e.g., caspase 8), which then activate ‘executioner caspases’ (e.g., caspase 3). Intertwined in these pathways are proteins that control the intrinsic or extrinsic routes of apoptosis (e.g., BCL-2 and Bax). Under normal conditions, the highly regulated apoptotic system is counterbalanced by cytoprotective signals that maintain tissue homeostasis. Alcohol consumption however, might increase expression of proapoptotic death factors and result in subsequent apoptotic cell death (see McVicker et al., 2007, for review). The expression of membrane-bound Fas, soluble Fas and Fas ligand have been increased with pathological conditions associated with alcoholic hepatitis (Taieb et al., 1998). This increased expression of Fas ligand in hepatocytes owing to ethanol has been also shown to induce apoptosis of adjacent cells by interacting with their Fas receptors (Taieb et al., 1998). Furthermore, it has been demonstrated that oxidative stress contributes to Fas receptor activation (Reinehr et al., 2005a). Whether this is true for the enhancement of ethanol-induced Fas receptor activation has yet to be determined.
As mentioned above, the enhanced production of TNFα is considered a key factor in the priming effect of ethanol on macrophage activation. This effect has largely been studied in the context of stimulating macrophages with LPS. Ethanol causes changes in several components along the signaling cascade from the LPS receptor partners (CD14/TLR4) to expression of TNFα protein, all leading to enhanced production (see Figure 3). For example, the expression of CD14 on the surface of Kupffer cells is upregulated by oxidant-dependent activation of the transcription factor AP-1 by alcohol (see Figure 3; Wheeler and Thurman, 2003). Two transcription factors that are critical in TNFα expression are NF-κB and EGR-1. Studies have shown that ethanol increases NF-κB activation via both ROS-dependent (Hill et al., 1999) and ROS-independent pathways (Roman et al., 1999). Ethanol also increases EGR-1 transcriptional activity via enhancing ERK1/2 signaling (see Nagy, 2004a, for review). Therefore oxidative stress is hypothesized to be key factor in the priming effect of ethanol. Furthermore, alcohol itself enhances LPS-stimulated ROS production by macrophages via MyD88-independent TLR4 signaling (Hritz et al., 2008), which implies that alcohol induces a feed-forward mechanism to further enhance the above-mentioned pathways and subsequently the expression of TNFα (see Figure 3). It is important to note that there are great similarities between obesity related fatty liver and alcoholic steatohepatitis. These include increased gut permeability with subsequent endotoxemia and TLR4 activation, with both priming and sensitization. However, the TLR4 signaling pathway for hepatotoxicity diverge, with alcohol being MyD88 independent and obesity being MyD88 dependent.
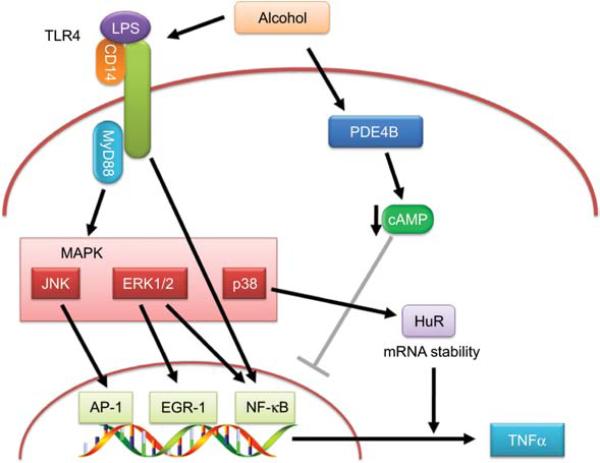
Figure 3. Enhancement of cell signaling caused by alcohol.
In LPS-exposed macrophages, toll-like receptor TLR4 is activated, resulting in MyD88-dependent or MyD88-independent activation of MAP kinases and their downstream signaling molecules such as AP-1, EGR-1, NF-κB and HuR. These are inducers of TNFα transcription and all pathways are enhanced by alcohol exposure. Alcohol also downregulates cAMP via inducing PDE4B. This decrease in cAMP results in a disinhibition of NF-κB, which further enhances TNFα transcription.
In addition to alcohol-induced oxidative stress, other alcohol-mediated mechanisms also probably contribute to the enhanced production of TNFα protein by macrophages after alcohol exposure (see Figure 3). For example, alcohol activates phosphodiesterase 4B (PDE4B), which is a cAMP-specific PDE isozyme (Gobejishvili et al., 2008). The subsequent decrease in cAMP is proposed to enhance TNFα production via disinhibition of NF-κB activity (see Figure 3; Gobejishvili et al., 2006). Ethanol-induced TNFα expression is not only regulated at the level of transcription but also by increased mRNA stability. Increased LPS stimulation of p38 mitogen-activated protein kinase contributes to this stabilization of TNFα mRNA in macrophages (see Figure 3). Moreover, studies have shown that at least one mRNA-binding protein, HuR, is also involved in stabilization of TNFα mRNA stability after chronic exposure to ethanol (see Nagy, 2004b, for review). The net effect is an increase in TNFα protein release in alcohol-exposed macrophages after stimulation (see Figure 3).
As noted, the sensitization effect of alcohol is described as an increase in sensitivity of parenchymal cells to cytotoxic killing. TNFα-induced signaling via TNFR1 appears to play a key role. In normal hepatocytes, TNFα is a mitogenic stimulus. However, alcohol is proposed to cause changes in intracellular signaling that switch this mitogenic response to a cytoxic response. Although this pathway is not completely elucidated, the sustained activation of JNK (which is an apoptotic signal) coupled with the inhibition of NF-κB activation in parenchymal cells are proposed to mediate the sensitization effect of ethanol (see Han et al., 2009, for review).
Chronic alcohol feeding has been shown to sensitize to the hepatotoxicity induced by gut-derived endotoxin and TNFα in animal models (Szabo and Bala, 2010), and patients with alcoholic hepatitis (AH) have increased basal and endotoxin stimulated monocyte TNFα production (McClain and Cohen, 1989). Moreover, mice that were given anti-TNFα antibodies or mice deficient in TNFR1 were protected against the development of experimental ALD (Iimuro et al., 1997; Yin et al., 1999). However, recent human studies have shown no therapeutic efficacy for anti-TNFα antibodies or TNFα soluble receptors in AH (Naveau et al., 2004; Boetticher et al., 2008). Thus, it appears that completely blocking TNFα is not a viable therapeutic option in ALD, possibly because of the necessary role for a basal level of TNFα in liver regeneration (Fausto, 2006). Another strategy is to attenuate TNFα production/activity with agents such as pentoxyfilline, a broad phosphodiesterase inhibitor. As noted previously, in animal models of ALD highly specific PDE4 inhibition is very effective at attenuating LPS-stimulated TNFα production. Importantly, human trials support a role for pentoxyfilline and phosphodiesterase inhibition in the treatment of alcoholic hepatitis, with the most recent randomized trial showing superiority to corticosteroids (De et al., 2009).
Do cytokines mediate steatosis caused by alcohol?
As mentioned above, alcohol-induced steatosis has historically been thought to be the direct result of alcohol metabolism. However, whereas alcohol metabolism is indeed likely to contribute to alcohol-induced steatosis, this process alone does not fully explain the phenomenon. For example, experimental alcohol-induced steatosis can be blunted by using pharmacologic agents but alcohol metabolism per se is not affected (Kono et al., 2001a,b). Similarly, many knockout strains [e.g., NADPH oxidase, iNOS and CD14 (Kono et al., 2000b; Yin et al., 2001a; McKim et al., 2003)] are protected against experimental alcohol-induced steatosis without changes in alcohol metabolism. Therefore, these results support the hypothesis that alcohol metabolism is not the only cause of alcoholic fatty liver.
A possible alternative mechanism by which alcohol can cause steatosis is via the production of cytokines such as TNFα. Indeed, many of the treatments/knockouts discussed above all block the increase in cytokine production caused by alcohol. In support of the role of cytokines in alcohol-induced steatosis, TNFR1 knockout mice were also almost completely protected from alcohol-induced fatty liver (Yin et al., 1999, 2001b). Furthermore, TNFα and other cytokines have been shown to influence lipid metabolism in both liver and the periphery (see Pessayre et al., 2002, for review). TNFα increases free fatty acid release from adipocytes in the periphery (Hardardottir et al., 1992), augments lipogenesis in hepatocytes (Feingold and Grunfeld, 1987), and blocks β-oxidation of fatty acids (Nachiappan et al., 1994). Alcohol-induced cytokines might also impair transport and secretion of triglycerides as VLDL (Navasa et al., 1998). The net effect of alcohol exposure is that cytokines could increase the supply of fatty acids to the liver while simultaneously impairing the ability of the hepatocytes to metabolize and secrete them. However, the specific mechanism(s) by which cytokines might mediate these effects have not been completely elucidated.
Steatosis and signaling
It has been hypothesized that cytokines could alter hepatic fat metabolism by increasing the expression and DNA binding activity of the transcription factor, sterol regulatory element binding protein 1 (SREBP-1; You and Crabb, 2004). SREBP-1 activation could directly cause steatosis by upregulating the expression of lipogenic enzymes (e.g., fatty acid synthetase) and indirectly by impairing β-oxidation via increasing the production of malonyl-CoA by acetyl CoA carboxylase (ACC). Malonyl CoA is an allosteric inhibitor of carnitine palmitoyl-transferase-1 (CPT-1), which is a crucial player in the β-oxidation of fatty acids (see Rawson, 2003, for review). In the cell, the effects of SREBP-1 on lipid metabolism appear to be almost exactly opposed to the effects of AMPK (Zhou et al., 2001). It has therefore been proposed that inducers of AMPK might protect against alcohol-induced steatosis by counteracting the effects of SREBP-1.
Selected emerging concepts in ALD
There are still crucial gaps in our understanding of ALD but the major concepts discussed above are well-established. More recent findings expand our understanding of ALD; these include the potential roles of S-adenosylmethionine (SAM), zinc and fibrin metabolism in the initiation and progression of ALD.
SAM and altered methionine metabolism
SAM is a precursor for syntheses of polyamines, choline, and is the major methylating agent for a host of molecules via specific methyltransferases (see Figure 4; Avila et al., 2005). Furthermore, SAM is a precursor for glutathione, a major endogenous antioxidant that prevents cellular injury by scavenging free radicals. Under normal conditions, most of the SAM produced is used in transmethylation reactions in which SAM is converted to SAH by transferring the methyl group to diverse biological acceptors (Finkelstein, 1990). SAH hydrolase then catalyzes the conversion of SAH to homocysteine and adenosine in a reversible reaction (Finkelstein, 1990). The conversion of homocysteine to methio-nine is an essential reaction to conserve methionine, detoxify homocysteine, and generate SAM (see Figure 4).
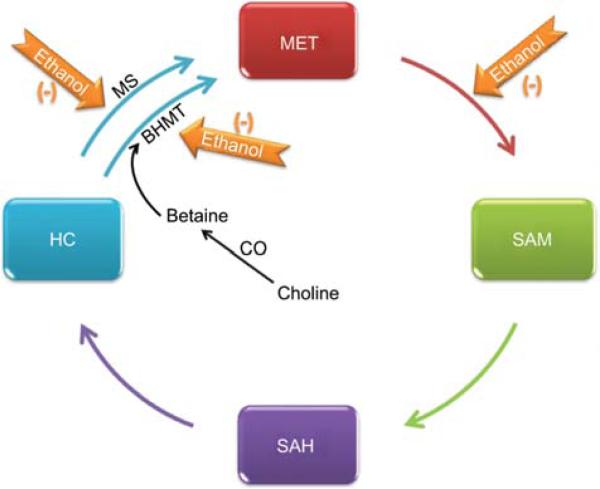
Figure 4. Effect of alcohol on the transmethylation pathway.
The transmethylation pathway is the critical supplier of methyl groups to the cell. Under normal conditions, most of the S-adenosylmethionine (SAM) generated is used in transmethylation reactions, in which SAM is converted to S-adenosyl homocysteine (SAH) by transferring the methyl group to diverse biological acceptors. SAH is then converted to homocysteine (HC) and adenosine in a reversible reaction catalyzed by SAH hydrolase. The conversion of homocysteine to methionine is an essential reaction to conserve methionine (MET), detoxify homocysteine and produce SAM. Ethanol directly or indirectly impairs the formation of SAM from MET by methionine adenosyl transferases (MAT). Alcohol also impairs the remethylation of HC to MET by both folate-dependent methionine synthase (MS) and by betaine homocysteine methyltransferase (BHMT).
It has been known for over 50 years that alcohol consumption leads to deficiencies of components involved in the transulfuration/methylation pathways (Best et al., 1949). However, the knowledge of the contribution of methyl donor deficiency in human alcoholic liver disease has recently increased. Specifically, alcohol consumption causes a hepatic deficiency in SAM, and this appears to be key to the development of animal model and human ALD (Lu et al., 2002). Indeed, hepatic-specific methionine adenosyl transferase (MAT) is highly sensitive to oxidative stress, and it is probable that decreased hepatic MAT activity in ALD is owing in part to oxidation of the active site. Furthermore, homocysteine accumulates after alcohol exposure in both humans and rats (Hultberg et al., 1993; Cravo and Camilo, 2000; Barak et al., 2001). The mRNA expression of the two enzymes responsible for remethylation of homocysteine, methylfolate-homocysteine methyltransferase (methionine synthase; MS) and betaine-homocysteine methyltransferase (BHMT) is also decreased (Avila et al., 2000). Levels of precursors needed for remethylation of homocysteine (e.g., folate) might be depleted in alcoholics (Cravo et al., 1996). Taken together, the increase in homocysteine observed in alcoholics can at least be explained in part by impaired ability of the liver to remethylate this compound (see Figure 4). It has been shown that patients with compensated alcoholic cirrhosis who were randomized to receive SAM (1200 mg/day orally) for 2 years had decreased liver mortality/liver transplantation (16% vs. 30%) compared with the placebo-treated group (Mato et al., 2002). Betaine supplementation was also shown to prevent alcohol-induced liver damage in mice (Ji and Kaplowitz, 2003). These studies suggest that altered methionine metabolism owing to alcohol intake contributes to ALD.
SAM metabolism and signaling
Depletion of SAM can change the methylation status of DNA, RNA, biogenic amines, phospholipids, histones, and other proteins, which probably contributes to liver injury. Altered DNA methylation might also be a post-translational modification that can affect transcription of key genes involved in the inflammatory response to alcohol. SAM also decreases TNFα expression in animal models of liver injury and in peripheral blood monocytes or macrophage cell lines in vitro (Song et al., 2005). This effect is most probably mediated by blunting LPS-induced NF-κB transcriptional activation of TNFα expression (Veal et al., 2004). Furthermore, SAM increases IL-10 concentrations in animal models or macrophage cell lines (Hevia et al., 2004; Song et al., 2005). Therefore, SAM deficiency can contribute to the priming effect of alcohol on inflammatory cells, with both an increase in proinflammatory TNFα and a decrease in anti-inflammatory IL-10. Other members of the transmethylation pathway (e.g., SAH, homocysteine) could also contribute to the sensitizing effect of ethanol via altering signal transduction responses (Song et al., 2007). Indeed, Song and co-workers showed that elevated SAH sensitized hepatocytes in a dose-dependent fashion to TNF hepatotoxicity. Moreover, a mitochondrial SAM transporter has been identified, and cytosolic SAM is exchanged for mitochondrial SAH. Inhibiting the SAM transporter or increasing cytosolic SAH have both been shown to sensitize to LPS/TNF hepatotoxicity. Thus, altered methionine/SAM metabolism plays a crucial role in both sensitization and priming to hepatotoxicity.
Zinc metabolism
Zinc is an essential trace element, and zinc deficiency is a common occurrence in ALD (Kang and Zhou, 2005). Selected complications of zinc deficiency of relevance to ALD include skin lesions, anorexia, depressed wound healing, hypogonadism, altered immune function, impaired night vision and depressed mental function with possible encephalopathy (Kang and Zhou, 2005). The mechanisms for altered zinc metabolism in ALD are multifactorial and include impaired intake, impaired absorption and increased urinary zinc excretion. Stress/inflammation caused by a variety of factors including LPS/TNFα also cause an internal redistribution of zinc, with loss of zinc from tissues (deficiency) and targeting to other tissues/organs such as the liver. Moreover, zinc deficiency can occur through an oxidative stress setting in which thiol oxidation of zinc-finger transcription factors causes zinc loss from these proteins leading to loss of DNA-binding activity (Zhou et al., 2007). Zinc participates in cellular function through hundreds of zinc proteins including zinc metalloenzymes and crucial zinc transcription factors (Kang and Zhou, 2005). Zinc deficiency has been associated with multiple forms of clinical and experimental liver injury, and it sensitizes to experimental LPS-induced hepatotoxicity (Kang and Zhou, 2005; Shea-Budgell et al., 2006). In ALD, alcohol intake and oxidative stress can cause disruption of tight junctions in the intestine, which leads to translocation of bacterial products, such as endotoxin (Zhong et al., 2010). Endotoxin causes TLR4 activation and TNFα production with subsequent oxidative stress and liver injury. Endotoxin and TNFα also play a crucial role in liver fibrosis as well as liver injury. Disruption of tight-junction proteins occurs not only in the intestine but also in the lung and probably at the blood-brain barrier related to oxidative stress and thiol oxidation, thus potentially predisposing to lung injury and hepatic encephalopathy (Joshi et al., 2009). Zinc treatment in experimental animals with ALD has been shown to attenuate the increased gut permeability, endotoxemia, decrease TNFα production, oxidative stress, and decrease liver injury (Kang and Zhou, 2005). Thus, zinc supplementation targets most postulated mechanisms for the development of ALD. It is well documented that zinc supplementation corrects the manifestations of zinc deficiency in human ALD, and a human pilot trial suggests it might stabilize or cause regression of hepatic fibrosis (Takahashi et al., 2007).
Fibrin metabolism
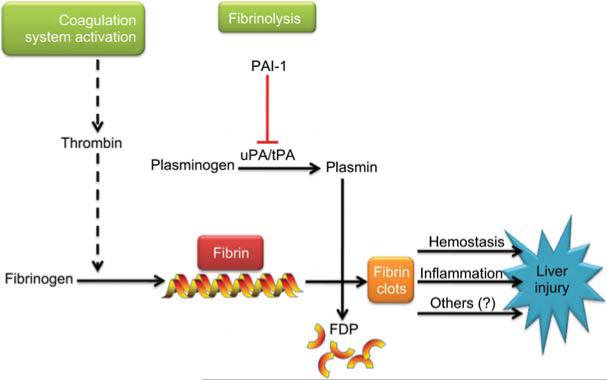
The liver is a critical organ in the regulation of blood coagulation. Hepatocytes synthesize and release numerous coagulation factors into the blood, including fibrinogen and prothrombin, as well as anticoagulant factors such as protein C (Mammen, 1992; Monroe and Hoffman, 2009). Under normal physiological conditions, thrombin, the main effector protease of the coagulation cascade, catalyzes the conversion of fibrinogen to fibrin, which then mediates clot formation (see Figure 5). Homeostasis of the coagulation cascade is crucial for normal organ/organism function; too little activity can lead to edema and clotting dysfunction, and too much activity can lead to hypercoagulation and hemostasis owing to increased fibrin extracellular matrix (ECM) accumulation (Northup et al., 2008). In addition to fibrin deposition by coagulation, the level of fibrin ECM is also regulated by degradation of the existing matrix (fibrinolysis; Kolev and Machovich, 2003). Specifically, inhibition of fibrinolysis can cause ECM to accumulate, even in the absence of enhanced deposition by the thrombin cascade (see Figure 5). A major inhibitor of fibrinolysis is plasminogen activator inhibitor 1 (PAI-1), via blocking the activation of plasmin by plasminogen activators (uPA and tPA; Arteel, 2008).
Figure 5. Fibrin metabolism.
Cross-linked fibrin deposition is initiated by activation of the coagulation cascade through thrombin. PAI-1 inhibits the activity of the plasminogen activators uPA and tPA, blocking the activation of plasmin, thereby blunting fibrinolysis of fibrin matrices to fibrin degradation products (FDP).
Thrombin-mediated signaling
Thrombin is an acute phase protein that is rapidly activated under conditions of stress in the body. The activation of this protease initiates the coagulation cascade that ultimately causes fibrin to accumulate in the extracellular matrices. Thrombin also has a host of direct actions on the cells (Coughlin, 2000). Most of the cellular effects of thrombin are mediated via proteinase-activated receptor 1 (PAR1). PAR1 receptors are present on hepatic stellate cells which, when activated, deposit fibrous matrix (Fibbi et al., 1999). Increased thrombin generation could thus directly promote hepatic fibrogenesis. Indeed, thrombin inhibition decreased experimental liver fibrosis caused by CCl4 in rats (Duplantier et al., 2004). Furthermore, PAR1 antagonists also ameliorate experimental hepatic fibrosis (Fiorucci et al., 2004). In addition to direct activation by PAR1, thrombin might indirectly activate hepatic stellate cells via stimulating cytokine secretion by macrophages and platelets, which also express PAR1 (Marra et al., 1995). Thrombin signaling could also affect endothelial cells and angiogenesis (Zania et al., 2008), which in principle could also contribute to the pathologic changes in liver caused by alcohol. However, the potential effect of thrombin under these conditions is not well studied.
PAI-1-mediated signaling
Under normal conditions, PAI-1 is only expressed in adipocytes and endothelial cells; however, PAI-1 can be upregulated to high levels in multiple cell types under conditions of injury and/or inflammation, most probably via the MAP kinases pathways (Kruithof, 1988; Fearns and Loskutoff, 1997). PAI-1 is the major inhibitor of both tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA). It therefore plays a key regulatory role in fibrinolysis by inhibiting the activation of plasminogen. Specifically, high PAI-1 levels can contribute to fibrin ECM accumulation by blocking degradation (see Figure 5).
A common problem in patients with cirrhosis is hyperfibrinolysis (high PA/PAI-1 ratio). It has been shown that these patients have more severe consequences and poor outcome of the disease (Violi et al., 1992; Hu et al., 2001). In contrast to end-stage liver disease, PAI-1 is known to be upregulated with alcohol consumption (Marques-Vidal et al., 1995; Mukamal et al., 2001), and its level is an index of severity during disease development (Tran-Thang et al., 1989). However, whether increased PAI-1 expression is a cause or an effect in the development of alcoholic liver disease remains unclear.
It was recently shown that acute ethanol rapidly and robustly induced PAI-1 expression in the mouse liver, and that steatosis under these conditions was prevented by genetic (PAI-1−/− mice) or pharmacologic inhibition of PAI-1 expression (Bergheim et al., 2006b). The mechanisms by which PAI-1 blunts steatosis were most probable via increasing HGF receptor (cMET) activation, leading to enhanced VLDL synthesis and export from the hepatocyte. Steatosis owing to chronic enteral alcohol exposure was also blunted by preventing the induction of PAI-1 expression (Bergheim et al., 2006b). Furthermore, PAI-1 has been shown to contribute to hepatic inflammation caused by alcohol (Bergheim et al., 2006b; Beier et al., 2009), as well as experimental hepatic fibrosis (Bergheim et al., 2006a). The latter effect is most probable via inhibiting ECM degradation during fibrogenesis; the former effect is still being elucidated, but is probably mediated via a fibrin matrix and/or fibrin signaling (see below).
Role of fibrin metabolism in ALD
Hepatic injury in models of alcohol- or drug-induced liver disease involve dysregulation of the coagulation cascade/fibrinolysis, favoring the formation of fibrin clots in the hepatic sinusoids (Beier et al., 2008, 2009). This accumulation might be mediated by de novo fibrin deposition via the coagulation cascade, or also by a decrease in fibrinolysis (e.g., by PAI-1, see above). The fibrin clots disrupt the flow of blood within the hepatic parenchyma (i.e., hemostasis), causing microregional hypoxia and subsequent hepatocellular death (Pearson et al., 1996; Ganey et al., 2004). For example, previous studies have shown that ethanol enhances LPS-induced liver damage via mechanisms involving excess fibrin accumulation. Exaggerated fibrin accumulation induced by ethanol was not associated with an enhancement of LPS-induced coagulation, per se, as thrombin-antithrombin levels were similar in the LPS and ethanol/LPS groups.
One potential mechanism by which fibrinogen could alter signal transduction cascades is via enhanced hemostasis. Specifically, fibrin clots disrupt the flow of blood within the hepatic parenchyma (i.e., hemostasis), the subsequent micro-regional hypoxia and hepatocellular death might directly (e.g., HIF1α) and indirectly cause changes in cell signaling (Pearson et al., 1996; Ganey et al., 2004). Fibrin(ogen) ECM not only serves as physical structure, but also binds/interacts with several biomolecules that can directly or indirectly alter responses. One family of receptors for which fibrin(ogen) is a ligand are the integrins. Integrins are receptors that mediate attachment between a cell and the tissues surrounding it, which might be other cells or ECM. Integrins transfer information from the ECM to the cell, allowing rapid and flexible responses to changes in the environment. Integrins play a myriad of roles within the body, including proliferation/angiogenesis, as well as inflammation and apoptosis (Hodivala-Dilke et al., 2003; Zhou et al., 2009). Fibrin(ogen) is a known ligand for several integrins, including integrin αIIbβ3, and integrin αMβ2 and integrin αvβ3. These integrins are found on several non-parenchymal cells in the liver. Therefore, fibrin(ogen) ECM has the potential to alter intracellular signaling in liver via a myriad of mechanisms. Modulators of integrin function are in human clinical trials for nonhepatic diseases, and might be ultimately used in hepatic problems such as ALD.
Summary and conclusions
As summarized in this review, there are multiple signaling pathways that are proposed in the mechanisms by which alcohol causes liver disease. These proposed mechanisms are not mutually exclusive and probably work in tandem to cause initiation and progression of ALD (see Figure 1). Whereas there is no universally-accepted therapy to treat ALD or halt its progression, there are many potential therapies being tested that target at least one of these potential mechanisms (see Bergheim et al., 2005, for review). Likewise, owing to the multiple mechanisms by which alcohol can damage the liver, it is unlikely that one therapy will be sufficient to treat ALD. Effective therapy might probably be a mix of lifestyle modification, dietary care and pharmacologic intervention.
Another avenue in which improvements need to be made is in modeling the disease. Specifically, most research to date focuses on early stages of ALD and often involves concomitant administration of the potential therapy with ethanol, or in the case of transgenic/knockout mice, using strains in which the expression of the protein of interest is a life-long event. Such research has given us useful information and is the foundation on where we now stand. However, the relevance of this research to the clinical situation in which alcoholics are seeking therapy to reverse existing ALD is unclear. Further translational research and research involving multiple modulators/targets are required.
Acknowledgments
Supported, in part, by grants from National Institute on Alcohol Abuse and Alcoholism (R37 AA010762, P01 AA017103, R01 AA015970, RC2 AA019388, R01 AA018869). J.I.B. was supported by a postdoctoral (T32) fellowship from National Institute of Environmental Health Science (ES011564).
References
- Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460. [PubMed] [Google Scholar]
- Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778–790. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- Arteel GE. New role of plasminogen activator inhibitor-1 in alcohol-induced liver injury. J. Gastroenterol. Hepatol. 2008;23(Suppl. 1):S54–S59. doi: 10.1111/j.1440-1746.2007.05285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteel GE, Raleigh JA, Bradford BU, Thurman RG. Acute alcohol produces hypoxia directly in rat liver tissue in vivo: role of Kupffer cells. Am. J. Physiol. 1996;271:G494–G500. doi: 10.1152/ajpgi.1996.271.3.G494. [DOI] [PubMed] [Google Scholar]
- Arteel GE, Iimuro Y, Yin M, Raleigh JA, Thurman RG. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology. 1997;25:920–926. doi: 10.1002/hep.510250422. [DOI] [PubMed] [Google Scholar]
- Avila MA, Berasain C, Torres L, Martin-Duce A, Corrales FJ, Yang H, Prieto J, Lu SC, Caballeria J, Rodes J, et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J. Hepatol. 2000;33:907–914. doi: 10.1016/s0168-8278(00)80122-1. [DOI] [PubMed] [Google Scholar]
- Avila MA, Berasain C, Prieto J, Mato JM, Garcia-Trevijano ER, Corrales FJ. Influence of impaired liver methio-nine metabolism on the development of vascular disease and inflammation. Curr. Med. Chem. Cardiovasc. Hematol. Agents. 2005;3:267–281. doi: 10.2174/1568016054368197. [DOI] [PubMed] [Google Scholar]
- Barak AJ, Beckenhauer HC, Kharbanda KK, Tuma DJ. Chronic ethanol consumption increases homocysteine accumulation in hepatocytes. Alcohol. 2001;25:77–81. doi: 10.1016/s0741-8329(01)00168-9. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, Yuan QX, Li J, French BA, Fang C, Ingelman-Sundberg M, French SW. The effect of ethanol-induced cytochrome p4502E1 on the inhibition of proteasome activity by alcohol. Biochem. Biophys. Res. Commun. 2000;279:23–29. doi: 10.1006/bbrc.2000.3889. [DOI] [PubMed] [Google Scholar]
- Beckman JS. In: The physiological and pathophysiological chemistry of nitric oxide. In: Nitric Oxide: Principles and Actions. Lancaster J, editor. Academic Press; San Diego, CA, USA: 1996. pp. 1–82. [Google Scholar]
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JI, Guo L, von Montfort C, Kaiser JP, Joshi-Barve S, Arteel GE. New role of resistin in lipopolysaccha-ride-induced liver damage in mice. J. Pharmacol. Exp. Ther. 2008;325:801–808. doi: 10.1124/jpet.108.136721.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JI, Luyendyk JP, Guo L, von Montfort C, Staunton DE, Arteel GE. Fibrin accumulation plays a critical role in the sensitization to lipopolysaccharide-induced liver injury caused by ethanol in mice. Hepatology. 2009;49:1545–1553. doi: 10.1002/hep.22847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergheim I, McClain CJ, Arteel GE. Treatment of alcoholic liver disease. Dig. Dis. 2005;23:275–284. doi: 10.1159/000090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergheim I, Guo L, Davis MA, Duveau I, Arteel GE. Critical role of plasminogen activator inhibitor-1 in cholestatic liver injury and fibrosis. J. Pharmacol. Exp. Ther. 2006a;316:592–600. doi: 10.1124/jpet.105.095042. [DOI] [PubMed] [Google Scholar]
- Bergheim I, Guo L, Davis MA, Lambert JC, Beier JI, Duveau I, Luyendyk JP, Roth RA, Arteel GE. Metformin prevents alcohol-induced liver injury in the mouse: critical role of plasminogen activator inhibitor-1. Gastroenterology. 2006b;130:2099–2112. doi: 10.1053/j.gastro.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best CH, Hartroft WS, Lucas CC, Ridout JH. Liver damage produced by feeding alcohol or sugar and its prevention by choline. Br. Med. J. 1949;2:1001–1006. doi: 10.1136/bmj.2.4635.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigatello LM, Broitman SA, Fattori L, Di Paoli M, Pontello M, Bevilacqua G, Nespoli A. Endotoxemia, encephalopathy, and mortality in cirrhotic patients. Am. J. Gastroenterol. 1987;82:11–15. [PubMed] [Google Scholar]
- Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol. Pharmacol. 2000;58:1026–1034. [PubMed] [Google Scholar]
- Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–1960. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Mak KM, Lieber CS. Cytochrome P4502E1 primes macrophages to increase TNF-α production in response to lipopolysaccharide. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G95–G107. doi: 10.1152/ajpgi.00383.2004. [DOI] [PubMed] [Google Scholar]
- Cederbaum AI, Wu D, Mari M, Bai J. CYP2E1-dependent toxicity and oxidative stress in HepG2 cells. Free Radic. Biol. Med. 2001;31:1539–1543. doi: 10.1016/s0891-5849(01)00743-2. [DOI] [PubMed] [Google Scholar]
- Colell A, Garcia-Ruiz C, Miranda M, Ardite E, Mari M, Morales A, Corrales F, Kaplowitz N, Fernandez-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Cravo ML, Camilo ME. Hyperhomocysteinemia in chronic alcoholism: relations to folic acid and vitamins B6 and B12 status. Nutrition. 2000;16:296–302. doi: 10.1016/s0899-9007(99)00297-x. [DOI] [PubMed] [Google Scholar]
- Cravo ML, Gloria LM, Selhub J, Nadeau MR, Camilo ME, Resende MP, Cardoso JN, Leitao CN, Mira FC. Hyperhomocysteinemia in chronic alcoholism: correlation with folate, vitamin B-12, and vitamin B-6 status. Am. J. Clin. Nutr. 1996;63:220–224. doi: 10.1093/ajcn/63.2.220. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Bailey SM. Ethanol consumption and liver mitochondria function. Biol. Signals Recept. 2001;10:271–282. doi: 10.1159/000046892. [DOI] [PubMed] [Google Scholar]
- D'Angio CT, Finkelstein JN. Oxygen regulation of gene expression: a study in opposites. Mol. Genet. Metab. 2000;71:371–380. doi: 10.1006/mgme.2000.3074. [DOI] [PubMed] [Google Scholar]
- Day CP. Who gets alcoholic liver disease: nature or nurture? J. R. Coll. Physicians Lond. 2000;34:557–562. [PMC free article] [PubMed] [Google Scholar]
- Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463–1466. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- De BK, Gangopadhyay S, Dutta D, Baksi SD, Pani A, Ghosh P. Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial. World J. Gastroenterol. 2009;15:1613–1619. doi: 10.3748/wjg.15.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot H, Noll T, Sies H. Oxygen dependence and subcellular partitioning of hepatic menadione-mediated oxygen uptake. Arch. Biochem. Biophys. 1985;243:556–562. doi: 10.1016/0003-9861(85)90532-6. [DOI] [PubMed] [Google Scholar]
- Deaciuc IV, Fortunato F, D'Souza NB, Hill DB, Schmidt J, Lee EY, McClain CJ. Modulation of caspase-3 activity and Fas ligand mRNA expression in rat liver cells in vivo by alcohol and lipopolysaccharide. Alcohol Clin. Exp. Res. 1999;23:349–356. [PubMed] [Google Scholar]
- Diehl AM. Liver disease in alcohol abusers: clinical perspective. Alcohol. 2002;27:7–11. doi: 10.1016/s0741-8329(02)00204-5. [DOI] [PubMed] [Google Scholar]
- Donohue TM., Jr. The ubiquitin-proteasome system and its role in ethanol-induced disorders. Addict. Biol. 2002;7:15–28. doi: 10.1080/135562101200100562. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Duplantier JG, Dubuisson L, Senant N, Freyburger G, Lauren-deau I, Herbert JM, Desmouliere A, Rosenbaum J. A role for thrombin in liver fibrosis. Gut. 2004;53:1682–1687. doi: 10.1136/gut.2003.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastin CE, McClain CJ, Lee K, Bagby GJ, Chawla RK. Choline deficiency augments and antibody to tumor necrosis factor-α attenuates endotoxin-induced hepatic injury. Alcohol. Clin. Exp. Res. 1997;21:1037–1041. [PubMed] [Google Scholar]
- Ekstrom G, Ingelman-Sundberg M. Rat liver micro-somal NADPH-supported oxidase activity and lipid peroxidation dependent on ethanol-inducible cytochrome P-450 (P-450IIE1). Biochem. Pharmacol. 1989;38:1313–1319. doi: 10.1016/0006-2952(89)90338-9. [DOI] [PubMed] [Google Scholar]
- Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol. Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Fausto N. Involvement of the innate immune system in liver regeneration and injury. J. Hepatol. 2006;45:347–349. doi: 10.1016/j.jhep.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Fearns C, Loskutoff DJ. Induction of plasminogen activator inhibitor 1 gene expression in murine liver by lipopolysaccharide. Cellular localization and role of endogenous tumor necrosis factor-α. Am. J. Pathol. 1997;150:579–590. [PMC free article] [PubMed] [Google Scholar]
- Feingold KR, Grunfeld C. Tumor necrosis factor-α stimulates hepatic lipogenesis in the rat in vivo. J. Clin. Invest. 1987;80:184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A, Miranda M, Mari M, Ardite E, Morales A. GSH transport in mitochondria: defense against TNF-induced oxidative stress and alcohol-induced defect. Am. J. Physiol. 1997;273:G7–G17. doi: 10.1152/ajpgi.1997.273.1.G7. [DOI] [PubMed] [Google Scholar]
- Fibbi G, Pucci M, Grappone C, Pellegrini G, Salzano R, Casini A, Milani S, Del Rosso M. Functions of the fibrinolytic system in human Ito cells and its control by basic fibroblast and platelet-derived growth factor. Hepatology. 1999;29:868–878. doi: 10.1002/hep.510290343. [DOI] [PubMed] [Google Scholar]
- Finkelstein JD. Methionine metabolism in mammals. J. Nutr. Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Distrutti E, Severino B, Fiorentina R, Baldoni M, Caliendo G, Santagada V, Morelli A, Cirino G. PAR1 antagonism protects against experimental liver fibrosis. Role of proteinase receptors in stellate cell activation. Hepatology. 2004;39:365–375. doi: 10.1002/hep.20054. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Torres M. Redox signaling in macrophages. Mol. Aspects Med. 2001;22:189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Ganey PE, Luyendyk JP, Maddox JF, Roth RA. Adverse hepatic drug reactions: inflammatory episodes as consequence and contributor. Chem. Biol. Interact. 2004;150:35–51. doi: 10.1016/j.cbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Gatti RM, Alvarez B, Vasquez-Vivar J, Radi R, Augusto O. Formation of spin trap adducts during the decomposition of peroxynitrite. Arch. Biochem. Biophys. 1998;349:36–46. doi: 10.1006/abbi.1997.0451. [DOI] [PubMed] [Google Scholar]
- Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kB activity and TNF expression: relevance to alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:G681–G688. doi: 10.1152/ajpgi.00098.2006. [DOI] [PubMed] [Google Scholar]
- Gobejishvili L, Barve S, Joshi-Barve S, McClain C. Enhanced PDE4B expression augments LPS-inducible TNF expression in ethanol-primed monocytes: relevance to alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G718–G724. doi: 10.1152/ajpgi.90232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogel BM, Goldstein RM, Kuhn JA, McCarty TM, Donahoe A, Glastad K. Diagnostic evaluation of hepatocellular carcinoma in a cirrhotic liver. Oncology. 2000;14:15–20. [PubMed] [Google Scholar]
- Han D, Ybanez MD, Ahmadi S, Yeh K, Kaplowitz N. Redox regulation of tumor necrosis factor signaling. Antioxid. Redox. Signal. 2009;11:2245–2263. doi: 10.1089/ars.2009.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardardottir I, Doerrler W, Feingold KR, Grunfeld C. Cytokines stimulate lipolysis and decrease lipoprotein lipase activity in cultured fat cells by a prostaglandin independent mechanism. Biochem. Biophys. Res. Commun. 1992;186:237–243. doi: 10.1016/s0006-291x(05)80798-3. [DOI] [PubMed] [Google Scholar]
- Hevia H, Varela-Rey M, Corrales FJ, Berasain C, Martinez-Chantar ML, Latasa MU, Lu SC, Mato JM, Garcia-Trevijano ER, Avila MA. 5'-Methylthioadenosine modulates the inflammatory response to endotoxin in mice and in rat hepatocytes. Hepatology. 2004;39:1088–1098. doi: 10.1002/hep.20154. [DOI] [PubMed] [Google Scholar]
- Hill DB, Devalaraja R, Joshi-Barve S, Barve S, McClain CJ. Antioxidants attenuate nuclear factor-kB activation and tumor necrosis factor-α production in alcoholic hepatitis patient monocytes and rat Kupffer cells, in vitro. Clin. Biochem. 1999;32:563–570. doi: 10.1016/s0009-9120(99)00056-9. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, Reynolds AR, Reynolds LE. Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res. 2003;314:131–144. doi: 10.1007/s00441-003-0774-5. [DOI] [PubMed] [Google Scholar]
- Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Hon WM, Lee KH, Khoo HE. Nitric oxide in liver diseases: friend, foe, or just passerby? Ann. N.Y. Acad. Sci. 2002;962:275–295. doi: 10.1111/j.1749-6632.2002.tb04074.x. [DOI] [PubMed] [Google Scholar]
- Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu KQ, Yu AS, Tiyyagura L, Redeker AG, Reynolds TB. Hyperfibrinolytic activity in hospitalized cirrhotic patients in a referral liver unit. Am. J. Gastroenterol. 2001;96:1581–1586. doi: 10.1111/j.1572-0241.2001.03781.x. [DOI] [PubMed] [Google Scholar]
- Hultberg B, Berglund M, Andersson A, Frank A. Elevated plasma homocysteine in alcoholics. Alcohol Clin. Exp. Res. 1993;17:687–689. doi: 10.1111/j.1530-0277.1993.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor-α attenuate hepatic necrosis and inflammation due to chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–1537. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- Ji S, Lemasters JJ, Thurman RG. Intralobular hepatic pyridine nucleotide fluorescence: evaluation of the hypothesis that chronic treatment with ethanol produces pericentral hypoxia. Proc. Natl. Acad. Sci. USA. 1982;80:5415–5419. doi: 10.1073/pnas.79.17.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. The role of oxygen concentration in oxidative stress: hypoxic and hyperoxic models. In: Sies H, editor. Oxidative Stress. Academic Press; London, UK: 1985. pp. 151–195. [Google Scholar]
- Joshi PC, Mehta A, Jabber WS, Fan X, Guidot DM. Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am. J. Respir. Cell Mol. Biol. 2009;41:207–216. doi: 10.1165/rcmb.2008-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Zhou Z. Zinc prevention and treatment of alcoholic liver disease. Mol. Aspects Med. 2005;26:391–404. doi: 10.1016/j.mam.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Kim YM, Kim TH, Chung HT, Talanian RV, Yin XM, Billiar TR. Nitric oxide prevents tumor necrosis factor α-induced rat hepatocyte apoptosis by the interruption of mitochondrial apoptotic signaling through S-nitrosylation of caspase-8. Hepatology. 2000;32:770–778. doi: 10.1053/jhep.2000.18291. [DOI] [PubMed] [Google Scholar]
- Kim KY, Rhim T, Choi I, Kim SS. N-Acetylcysteine induces cell cycle arrest in hepatic stellate cells through its reducing activity. J. Biol. Chem. 2001;276:40591–40598. doi: 10.1074/jbc.M100975200. [DOI] [PubMed] [Google Scholar]
- Klassen LW, Tuma D, Sorrell MF. Immune mechanisms of alcohol-induced liver disease. Hepatology. 1995;22:355–357. [PubMed] [Google Scholar]
- Knecht KT, Thurman RG, Mason RP. Role of superoxide and trace transition metals in the production of α-hydroxyethyl radical from ethanol by microsomes from alcohol dehydrogenase-deficient deer mice. Arch. Biochem. Biophys. 1993;303:339–348. doi: 10.1006/abbi.1993.1293. [DOI] [PubMed] [Google Scholar]
- Kolev K, Machovich R. Molecular and cellular modulation of fibrinolysis. Thromb. Haemost. 2003;89:610–621. [PubMed] [Google Scholar]
- Kono H, Bradford BU, Yin M, Sulik KK, Koop DR, Peters JM, Gonzalez FJ, McDonald T, Dikalova A, Kadiiska MB, et al. CYP2E1 is not involved in early alcohol-induced liver injury. Am. J. Physiol. 1999;277:G1259–G1267. doi: 10.1152/ajpgi.1999.277.6.G1259. [DOI] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Connor H, Mason RP, Thurman RG. Allopurinol prevents early alcohol-induced liver injury in rats. J. Pharmacol. Exp. Ther. 2000a;293:296–303. [PubMed] [Google Scholar]
- Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J. Clin. Invest. 2000b;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Arteel GE, Rusyn I, Sies H, Thurman RG. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic. Biol. Med. 2001a;30:403–411. doi: 10.1016/s0891-5849(00)00490-1. [DOI] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, Mason RP, Thurman RG. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am. J. Physiol. Gastroin-test. Liver Physiol. 2001b;280:G1005–G1012. doi: 10.1152/ajpgi.2001.280.5.G1005. [DOI] [PubMed] [Google Scholar]
- Kruithof EK. Plasminogen activator inhibitors – a review. Enzyme. 1988;40:113–121. doi: 10.1159/000469153. [DOI] [PubMed] [Google Scholar]
- Lambeth JD, Cheng G, Arnold RS, Edens WA. Novel homologs of gp91phox. Trends Biochem. Sci. 2000;25:459–461. doi: 10.1016/s0968-0004(00)01658-3. [DOI] [PubMed] [Google Scholar]
- Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. The CYP2E1 knockout delivers another punch: first ASH, now NASH. Hepatology. 2001;33:311–312. doi: 10.1053/jhep.2001.0330311. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Metabolic effects of acetaldehyde. Biochem. Soc. Trans. 1988;16:241–247. doi: 10.1042/bst0160241. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Ethanol metabolism, cirrhosis and alcoholism. Clin. Chim. Acta. 1997;257:59–84. doi: 10.1016/s0009-8981(96)06434-0. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Alcohol: its metabolism and interaction with nutrients. Annu. Rev. Nutr. 2000;20:395–430. doi: 10.1146/annurev.nutr.20.1.395. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res. Health. 2003;27:220–231. [PMC free article] [PubMed] [Google Scholar]
- Liu H, Jones BE, Bradham C, Czaja MJ. Increased cytochrome P-450 2E1 expression sensitizes hepatocytes to c-Jun-mediated cell death from TNF-α. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G257–G266. doi: 10.1152/ajpgi.00304.2001. [DOI] [PubMed] [Google Scholar]
- Lu SC, Tsukamoto H, Mato JM. Role of abnormal methionine metabolism in alcoholic liver injury. Alcohol. 2002;27:155–162. doi: 10.1016/s0741-8329(02)00226-4. [DOI] [PubMed] [Google Scholar]
- Mammen EF. Coagulation abnormalities in liver disease. Hematol. Oncol. Clin. North Am. 1992;6:1247–1257. [PubMed] [Google Scholar]
- Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res. Health. 2003;27:209–219. [PMC free article] [PubMed] [Google Scholar]
- Marques-Vidal P, Cambou JP, Nicaud V, Luc G, Evans A, Arveiler D, Bingham A, Cambien F. Cardiovascular risk factors and alcohol consumption in France and Northern Ireland. Atherosclerosis. 1995;115:225–232. doi: 10.1016/0021-9150(94)05517-m. [DOI] [PubMed] [Google Scholar]
- Marra F, Grandaliano G, Valente AJ, Abboud HE. Thrombin stimulates proliferation of liver fat-storing cells and expression of monocyte chemotactic protein-1: potential role in liver injury. Hepatology. 1995;22:780–787. [PubMed] [Google Scholar]
- Mato JM, Corrales FJ, Lu SC, Avila MA. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- McKim SE, Gabele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, Mason RP, Doll MA, Hein DW, et al. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834–1844. doi: 10.1053/j.gastro.2003.08.030. [DOI] [PubMed] [Google Scholar]
- McVicker BL, Tuma DJ, Casey CA. Effect of ethanol on pro-apoptotic mechanisms in polarized hepatic cells. World J. Gastroenterol. 2007;13:4960–4966. doi: 10.3748/wjg.v13.i37.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe DM, Hoffman M. The coagulation cascade in cirrhosis. Clin. Liver Dis. 2009;13:1–9. doi: 10.1016/j.cld.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Jadhav PP, D'Agostino RB, Massaro JM, Mittleman MA, Lipinska I, Sutherland PA, Matheney T, Levy D, Wilson PW, et al. Alcohol consumption and hemostatic factors: analysis of the Framingham Offspring cohort. Circulation. 2001;104:1367–1373. doi: 10.1161/hc3701.096067. [DOI] [PubMed] [Google Scholar]
- Nachiappan V, Curtiss D, Corkey BE, Kilpatrick L. Cytokines inhibit fatty acid oxidation in isolated rat hepatocytes: synergy among TNF, IL-6, and IL-1. Shock. 1994;1:123–129. doi: 10.1097/00024382-199402000-00007. [DOI] [PubMed] [Google Scholar]
- Nagy LE. Molecular aspects of alcohol metabolism: transcription factors involved in early ethanol-induced liver injury. Annu. Rev. Nutr. 2004a;24:55–78. doi: 10.1146/annurev.nutr.24.012003.132258. [DOI] [PubMed] [Google Scholar]
- Nagy LE. Stabilization of tumor necrosis factor-α mRNA in macrophages in response to chronic ethanol exposure. Alcohol. 2004b;33:229–233. doi: 10.1016/j.alcohol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Greenberg SS, Tahan SR, Fogt F, Loscalzo J, Sadrzadeh SM, Xie J, Stamler JS. Nitric oxide production in experimental alcoholic liver disease in the rat: role in protection from injury. Gastroenterology. 1995;109:899–907. doi: 10.1016/0016-5085(95)90400-x. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Jokelainen K, Lau GK, Rahemtulla A, Tipoe GL, Polavarapu R, Lalani E. Arginine reverses ethanol-induced inflammatory and fibrotic changes in liver despite continued ethanol administration. J. Pharmacol. Exp. Ther. 2001;299:832–839. [PubMed] [Google Scholar]
- Navasa M, Gordon DA, Hariharan N, Jamil H, Shigenaga JK, Moser A, Fiers W, Pollock A, Grunfeld C, Fein-gold KR. Regulation of microsomal triglyceride transfer protein mRNA expression by endotoxin and cytokines. J. Lipid Res. 1998;39:1220–1230. [PubMed] [Google Scholar]
- Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broet P, Emilie D. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390–1397. doi: 10.1002/hep.20206. [DOI] [PubMed] [Google Scholar]
- Niemela O. Distribution of ethanol-induced protein adducts in vivo: relationship to tissue injury. Free Radic. Biol. Med. 2001;31:1533–1538. doi: 10.1016/s0891-5849(01)00744-4. [DOI] [PubMed] [Google Scholar]
- Northup PG, Sundaram V, Fallon MB, Reddy KR, Balogun RA, Sanyal AJ, Anstee QM, Hoffman MR, Ikura Y, Caldwell SH. Hypercoagulation and thrombophilia in liver disease. J. Thromb. Haemost. 2008;6:2–9. doi: 10.1111/j.1538-7836.2007.02772.x. [DOI] [PubMed] [Google Scholar]
- Oshita M, Takei Y, Kawano S, Hijioka T, Masuda E, Goto M, Nishimura Y, Nagai H, Iio S, Tsuji S, et al. Endogenous nitric oxide attenuates ethanol-induced perturbation of hepatic circulation in the isolated perfused rat liver. Hepatology. 1994;20:961–965. doi: 10.1002/hep.1840200427. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Schultze AE, Schwartz KA, Scott MA, Davis JM, Roth RA. The thrombin inhibitor, hirudin, attenuates lipopolysaccharide-induced liver injury in the rat. J. Pharmacol. Exp. Ther. 1996;278:378–383. [PubMed] [Google Scholar]
- Pessayre D, Mansouri A, Fromenty B. Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G193–G199. doi: 10.1152/ajpgi.00426.2001. [DOI] [PubMed] [Google Scholar]
- Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol. Aspects Med. 2000;21:49–98. doi: 10.1016/s0098-2997(00)00004-2. [DOI] [PubMed] [Google Scholar]
- Powell WJ, Jr., Klatskin G. Duration of survival in patients with Laennec's cirrhosis. Influence of alcohol withdrawal, and possible effects of recent changes in general management of the disease. Am. J. Med. 1968;44:406–420. doi: 10.1016/0002-9343(68)90111-3. [DOI] [PubMed] [Google Scholar]
- Rai RM, Lee FY, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthase deficient mice. Proc. Natl. Acad. Sci. USA. 1998;95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RB. The SREBP pathway – insights from Insigs and insects. Nat. Rev. Mol. Cell Biol. 2003;4:631–640. doi: 10.1038/nrm1174. [DOI] [PubMed] [Google Scholar]
- Reeves HL, Dack CL, Peak M, Burt AD, Day CP. Stress-activated protein kinases in the activation of rat hepatic stellate cells in culture. J. Hepatol. 2000;32:465–472. doi: 10.1016/s0168-8278(00)80398-0. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Becker S, Eberle A, Grether-Beck S, Häussinger D. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J. Biol. Chem. 2005a;280:27179–27194. doi: 10.1074/jbc.M414361200. [DOI] [PubMed] [Google Scholar]
- Reinehr R, Becker S, Keitel V, Eberle A, Grether-Beck S, Häussinger D. Bile salt-induced apoptosis involves NADPH oxidase isoform activation. Gastroenterology. 2005b;129:2009–2031. doi: 10.1053/j.gastro.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Roman J, Colell A, Blasco C, Caballeria J, Pares A, Rodes J, Fernandez-Checa JC. Differential role of ethanol and acetaldehyde in the induction of oxidative stress in HEP G2 cells: effect on transcription factors AP-1 and NF-kB. Hepatology. 1999;30:1473–1480. doi: 10.1002/hep.510300623. [DOI] [PubMed] [Google Scholar]
- Rubbo H, Radi R, Anselmi D, Kirk M, Barnes S, Butler J, Eiserich JP, Freeman BA. Nitric oxide reaction with lipid peroxyl radicals spares alpha-tocopherol during lipid peroxidation. Greater oxidant protection from the pair nitric oxide/α-tocopherol than α-tocopherol/ascorbate. J. Biol. Chem. 2000;275:10812–10818. doi: 10.1074/jbc.275.15.10812. [DOI] [PubMed] [Google Scholar]
- Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan X, Aw TY, Shapira R, Jones DP. Oxygen dependence of glutathione synthesis in hepatocytes. Toxicol. Appl. Pharmacol. 1989;101:261–270. doi: 10.1016/0041-008x(89)90275-5. [DOI] [PubMed] [Google Scholar]
- Shaw S, Jayatilleke E, Ross WA, Gordon ER, Lieber S. Ethanol-induced lipid peroxidation: potentiation by long-term alcohol feeding and attenuation by methionine. J. Lab. Clin. Med. 1981;98:417–424. [PubMed] [Google Scholar]
- Shaw S, Jayatilleke E, Lieber CS. Lipid peroxidation as a mechanism of alcoholic liver injury: role of iron mobilization and microsomal induction. Alcohol. 1988;5:135–140. doi: 10.1016/0741-8329(88)90010-9. [DOI] [PubMed] [Google Scholar]
- Shea-Budgell M, Dojka M, Nimmo M, Lee D, Xu Z. Marginal zinc deficiency increased the susceptibility to acute lipopolysaccharide-induced liver injury in rats. Exp. Biol. Med. 2006;231:553–558. doi: 10.1177/153537020623100509. [DOI] [PubMed] [Google Scholar]
- Sies H. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. 1986;25:1058–1071. [Google Scholar]
- Song Z, Uriarte S, Sahoo R, Chen T, Barve S, Hill D, McClain C. S-adenosylmethionine (SAMe) modulates interleukin-10 and interleukin-6, but not TNF, production via the adenosine A2 receptor. Biochim. Biophys. Acta. 2005;1743:205–213. doi: 10.1016/j.bbamcr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Song Z, Zhou Z, Song M, Uriarte S, Chen T, Deaciuc I, McClain CJ. Alcohol-induced S-adenosylhomocysteine accumulation in the liver sensitizes to TNF hepatotoxicity: possible involvement of mitochondrial S-adenosylmethionine transport. Biochem. Pharmacol. 2007;74:521–531. doi: 10.1016/j.bcp.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen TI, Orholm M, Bentsen KD, Hoybye G, Eghoje K, Christoffersen P. Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet. 1984;2:241–244. doi: 10.1016/s0140-6736(84)90295-2. [DOI] [PubMed] [Google Scholar]
- Spencer S, Rubin KP, Lieber CS. Depressed hepatic glutathione and increased diene conjugates in alcoholic liver disease: evidence of lipid peroxidation. Dig. Dis. Sci. 1983;28:585–589. doi: 10.1007/BF01299917. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J. Gastroenterol. 2010;16:1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taieb J, Mathurin P, Poynard T, Gougerot-Pocidalo MA, Chollet-Martin S. Raised plasma soluble Fas and Fasligand in alcoholic liver disease. Lancet. 1998;351:1930–1931. doi: 10.1016/S0140-6736(05)78614-1. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Saito H, Higashimoto M, Hibi T. Possible inhibitory effect of oral zinc supplementation on hepatic fibrosis through downregulation of TIMP-1: a pilot study. Hepatol. Res. 2007;37:405–409. doi: 10.1111/j.1872-034X.2007.00065.x. [DOI] [PubMed] [Google Scholar]
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- Thurman RG. Mechanisms of hepatic toxicity. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am. J. Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- Tran-Thang C, Fasel-Felley J, Pralong G, Hofstetter JR, Bach-mann F, Kruithof EK. Plasminogen activators and plasminogen activator inhibitors in liver deficiencies caused by chronic alcoholism or infectious hepatitis. Thromb. Haemost. 1989;62:651–653. [PubMed] [Google Scholar]
- Tribble DL, Jones DP. Oxygen dependence of oxidative stress: rate of NADPH supply for maintaining the GSH pool during hypoxia. Biochem. Pharmacol. 1990;39:729–736. doi: 10.1016/0006-2952(90)90152-b. [DOI] [PubMed] [Google Scholar]
- Veal N, Hsieh CL, Xiong S, Mato JM, Lu S, Tsukamoto H. Inhibition of lipopolysaccharide-stimulated TNF-α promoter activity by S-adenosylmethionine and 5'-methylthioadenosine. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G352–G362. doi: 10.1152/ajpgi.00316.2003. [DOI] [PubMed] [Google Scholar]
- Violi F, Ferro D, Basili S, Quintarelli C, Saliola M, Alessandri C, Cordova C, Balsano F. Hyperfibrinolysis increases the risk of gastrointestinal hemorrhage in patients with advanced cirrhosis. Hepatology. 1992;15:672–676. doi: 10.1002/hep.1840150420. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Thurman RG. Up-regulation of CD14 in liver caused by acute ethanol involves oxidant-dependent AP-1 pathway. J. Biol. Chem. 2003;278:8435–8441. doi: 10.1074/jbc.M212076200. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Kono H, Yin M, Rusyn I, Froh M, Connor HD, Mason RP, Samulski RJ, Thurman RG. Delivery of Cu/Zn-superoxide dismutase gene with adenovirus reduces early alcohol-induced liver injury in rats. Gastroenterology. 2001a;120:1241–1250. doi: 10.1053/gast.2001.23253. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Nakagami M, Bradford BU, Uesugi T, Mason RP, Connor HD, Dikalova A, Kadiiska M, Thurman RG. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J. Biol. Chem. 2001b;276:36664–36672. doi: 10.1074/jbc.M105352200. [DOI] [PubMed] [Google Scholar]
- Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002;35:478–491. doi: 10.1053/jhep.2002.31432. [DOI] [PubMed] [Google Scholar]
- Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc. Natl. Acad. Sci. USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor α in alcohol-induced liver injury. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in cd14-deficient mice. J. Immunol. 2001a;166:4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- Yin M, Gabele E, Wheeler MD, Connor H, Bradford BU, Dikalova A, Rusyn I, Mason R, Thurman RG. Alcohol-induced free radicals in mice: direct toxicants or signaling molecules? Hepatology. 2001b;34:935–942. doi: 10.1053/jhep.2001.28888. [DOI] [PubMed] [Google Scholar]
- You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am. J. Physiol Gastrointest. Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- You M, Cao Q, Liang X, Ajmo JM, Ness GC. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J. Nutr. 2008;138:497–501. doi: 10.1093/jn/138.3.497. [DOI] [PubMed] [Google Scholar]
- Yuki T, Thurman RG. The swift increase in alcohol metabolism: time course for the increase in hepatic oxygen uptake and the involvement of glycolysis. Biochem. J. 1980;186:119–126. doi: 10.1042/bj1860119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zania P, Papaconstantinou M, Flordellis CS, Maragoudakis ME, Tsopanoglou NE. Thrombin mediates mito-genesis and survival of human endothelial cells through distinct mechanisms. Am. J. Physiol Cell Physiol. 2008;294:C1215–C1226. doi: 10.1152/ajpcell.00452.2007. [DOI] [PubMed] [Google Scholar]
- Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G625–G633. doi: 10.1152/ajpgi.00350.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Kang X, Jiang Y, Song Z, Feng W, McClain CJ, Kang YJ. Preservation of hepatocyte nuclear factor-4α is associated with zinc protection against TNF-α hepatotoxicity in mice. Exp. Biol. Med. (Maywood) 2007;232:622–628. [PubMed] [Google Scholar]
- Zhou HF, Chan HW, Wickline SA, Lanza GM, Pham CT. αvβ3-targeted nanotherapy suppresses inflammatory arthritis in mice. FASEB J. 2009;23:2978–2985. doi: 10.1096/fj.09-129874. [DOI] [PMC free article] [PubMed] [Google Scholar]