Abstract
In the present study we examined the effects of normal aging in the hippocampus and cerebellum, as well as behaviors associated with these substrates. A total of 67 CB6F1 hybrid mice were tested at one of five ages (4, 8, 12, 18 or 25 months) on the context pre-exposure facilitation effect modification of fear conditioning (CPFE), rotorod, Barnes maze, acoustic startle, Morris water maze (MWM) and 500 ms trace eyeblink classical conditioning (EBCC). Behavioral tasks were chosen to increase the ability to detect age-related changes in learning, as trace EBCC is considered a more difficult paradigm (compared to delay EBCC) and the CPFE has been found to be more sensitive to hippocampus insults than standard contextual fear conditioning. To assess the effects of age on the brain, hippocampus volume was calculated and unbiased stereology was used to estimate the number of Purkinje neurons in the cerebellar cortex. A significant, age-related loss of Purkinje neurons was found—beginning at 12 months of age—and hippocampus volume remained stable over the adult life span. Age-related impairment was found, beginning at 12–18 months in the rotorod, and mice with fewer Purkinje neurons showed greater impairment in this task. CB6F1 mice retained auditory acuity across the life span and mice aged 25 months showed significant age-related impairment in the EBCC task; however, deficits were not associated with the loss of Purkinje neurons. Although the CPFE task is considered more sensitive to hippocampus insult, no age-related impairment was found. Spatial memory retention was impaired in the Barnes maze at 25 months, but no significant deficits were seen in the MWM. These results support the finding of differential aging in the hippocampus and cerebellum.
Keywords: aging, age sensitivity, cerebellum, hippocampus, learning, CB6F1
Brain memory systems and associated brain structures differ in the magnitude of age-related neuron loss. Aging in hippocampus-dependent learning and memory is associated with reduced functional capacity for new learning in pyramidal neurons in the perforant pathway (Lister & Barnes, 2009). However, during normal aging neuron number in this region is stable. Cerebellum-dependent learning and memory is associated with Purkinje neuron loss in cerebellar cortex and age-related impairment in morphology as well as function. Data over the adult life span in human (Woodruff-Pak & Finkbiner, 1995) and non-human mammals (Woodruff-Pak et al., 2010) suggest that cerebellum-essential tasks show age-related deficits at earlier ages than do hippocampus-essential tasks.
The goal of this study was to examine hippocampus- and cerebellum-dependent learning across the CB6F1mouse adult life span to expand on previous work regarding differential aging in these structures (Woodruff-Pak et al., 2010). In addition, the present study was designed to incorporate tasks that may be more sensitive to hippocampal function. Thus, we included hippocampus-dependent trace eyeblink classical conditioning (EBCC), as well as a modification of contextual fear conditioning that is more sensitive to hippocampus insult (Rudy, 2009). The Barnes maze, which is less stressful than the Morris water maze (MWM; Sternberg et al., 1992; Harrison et al., 2009), may be considered more sensitive to age-related disruptions of brain substrates (Kennard & Woodruff-Pak, 2011a). We chose CB6F1 mice because they maintain normal auditory acuity well into late life (Erway et al., 1993). This strain is maintained by the National Institute on Aging, but learning and memory has rarely been investigated, apart from radial arm maze in young adult mice (Roullet et al., 1993; Roullet & Lassalle, 1995). Learning ability across the life span has not yet been evaluated.
EBCC is one of the best-characterized learning and memory paradigms on a behavioral and neurobiological level. The circuitry required to support the formation of conditioned responses in EBCC has been almost entirely defined and parallels exist across a number of mammalian species (Thompson, 1986; Woodruff-Pak & Steinmetz, 2000a,b; Christian & Thompson 2003). Previously, we have reported age-related impairment in the delay EBCC paradigm in C57BL/6 (Woodruff-Pak, 2006) and CBA (Woodruff-Pak et al., 2010) mice, although deficits in C57BL/6 mice were confounded with age-related declines in hearing. Whereas cerebellar cortical circuitry is essential in delay eyeblink conditioning, it is appears to be bypassed in trace eyeblink conditioning when the stimulus-free trace period exceeds a critical interval (250 ms in mice; Tseng et al., 2004; Woodruff-Pak & Disterhoft, 2008) and additional regions such as the hippocampus and prefrontal cortex are recruited (Kim et al., 1995; Kronforst-Collins & Disterhoft, 1998; Weiss et al., 1999; Weible et al., 2000; Tseng et al., 2004). A common method to increase age sensitivity is to increase task difficulty (Gallagher et al., 1993; Frick et al., 1995; de Fiebre et al., 2006; Kennard & Woodruff-Pak, 2011a). The 500 ms trace paradigm is hippocampus-dependent, more difficult than the 500 ms delay paradigm (Beylin et al., 2001; Woodruff-Pak et al., 2007) and may reveal age-related deficits earlier in the life span. Acoustic startle was used to confirm that auditory acuity did not decrease with age.
As trace EBCC depends on both the hippocampus and the interpositus nucleus in the cerebellum, additional hippocampus- and cerebellum-dependent tasks were included. The rotorod test of motor learning that requires cerebellar cortex (Lalonde et al., 1995; Lalonde & Strazielle, 2003a) and is commonly used in aging studies. Deficits in this task have been seen around 12–13 months in C57BL/6 mice (Thouvarecq et al., 2001; Vogel et al., 2002). To date, no study has examined rotorod performance across the life span in the CB6F1 strain. Given that strain differences on this task exist (Logue et al., 1997a; Voikar et al., 2001), and that these differences can change across the life span (Ingram & Jucker, 1999), the present study adds to the literature concerned with the genetic basis of performance on the rotorod.
The MWM and Barnes maze are spatial learning tasks that depend on the hippocampus (McNaughton et al., 1989; Gerlai & Roder, 1996; Logue et al., 1997b; Moreau et al., 2008). In addition, there is a growing literature that suggests the cerebellum plays a role in spatial learning assessed by the MWM (D’Hooge & De Deyn, 2001; Lalonde & Strazielle, 2003a; 2003b; Woodruff-Pak et al., 2006); although to date this brain region has not been investigated in the Barnes maze. Age-related impairment is often reported with these tasks, generally between 15–25 months (Bach et al., 1999; van Praag et al., 2005; Harburger et al., 2007; Kennard & Woodruff-Pak, 2011b).
The context pre-exposure facilitation effect (CPFE) is a modification of the contextual fear conditioning paradigm which refers to enhanced conditioning due to pre-exposure to the context prior to shock administration (Rudy et al., 2004). This task that depends on the integrity of the hippocampus and importantly, lesions to or disruption of the hippocampus prior to training cause impairments in CPFE but not standard contextual fear conditioning (Rudy et al., 2002; Matus-Amat et al., 2004; Matus-Amat et al., 2007; Brown et al., 2011). This task was chosen due to the increased sensitivity to hippocampus insult—with the expectation that the accumulation of insults to the hippocampus across the life span would be more readily detected using CPFE than standard contextual fear conditioning. This task has not been incorporated in studies of the aging mouse (Kennard & Woodruff-Pak, 2011a), so another goal of the present study was to determine how normal aging affects CPFE conditioning ability.
In this study we tested CB6F1 mice of five ages (4, 8, 12, 18, or 25 months) on CPFE, rotorod, Barnes maze, acoustic startle, MWM and 500 ms trace EBCC. For a subset of mice we examined hippocampus volume and used unbiased stereology to estimate the total number of Purkinje neurons in the cerebellar cortex. Based on previous work in our lab, we expected to see an age-related loss of cerebellar cortical Purkinje neurons beginning around 18 months, in addition to stability in hippocampus volume (Woodruff-Pak, 2006; Woodruff-Pak et al., 2010). It was expected that the loss of Purkinje neurons would be correlated with tasks that depend upon the cerebellar cortex (rotorod). Impairment was expected in the behavioral tasks, although with the increased sensitivity afforded by the CPFE paradigm and the incorporation of the more difficult hippocampus-dependent trace EBCC paradigm, we hypothesized that deficits may be seen earlier in the life span in the present study.
2. Experimental Procedures
2.1. Subjects
This experiment included 67 male CB6F1 (BALB/c x C57BL/6) mice from the National Institute on Aging Aged Rodent Colony at Charles River Laboratory (Wilmington, MA) aged from 3.73 to 25.77 months at the time of behavioral testing. Mice were tested at one of five ages: 4 (n = 13; M = 4.38 mo, SD = 0.42 mo), 8 (n = 15; M = 8.57 mo, SD = 0.41 mo), 12 (n = 13; M = 12.95 mo, SD = 0.65 mo), 18 (n = 12; M = 18.94 mo, SD = 0.60 mo) or 25 (n = 14; M = 25.01 mo, SD = 0.70 mo) months. Mice were ordered at different ages (3–24 months) and were housed 2–4 per cage based on date of birth. Housing consisted of a polycarbonate cage with bedding, a toy and ad libitum access to food and water. Mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited animal facility at Temple University. The colony room was temperature- and humidity-controlled with a 12 hr light/dark schedule. This research was approved by Temple University’s Institutional Animal Care and Use Committee (IACUC).
2.2. Handling and Testing Procedure
Upon arrival, mice were kept undisturbed in the housing colony for at least three days before handling began. To acclimate mice to the experimenters, animals were taken from their cages and handled for several minutes each day until the experiment began. All mice were tested in the fixed order described in Table 1. Previous experimenters have used a fixed rather than random task order (Logue et al., 1997a; Owen et al., 1997). In addition, we have employed a fixed task order to examine learning across the mouse life span previously (Vogel et al., 2002). Mice were weighed during CPFE pre-handling and again before EBCC surgery. Prior to testing, mice were assigned an identification number and ear punched, which provided no information about the age of the mouse. A total of five groups of up to 20 mice were tested and each group contained a random selection of mice from different age groups. Experimenters were blind to age groups during behavioral testing.
Table 1.
Sequence of behavioral testing for all mice
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | |
|---|---|---|---|---|---|---|---|---|
| Day 1 | Ear Punch |
CPFE | Barnes Maze |
MWM | EBCC Surgery |
EBCC Training |
Perfusion | |
| Day 2 | ||||||||
| Day 3 | CPFE Pre- Handling |
|||||||
| Day 4 | Rotorod | Recovery from Surgery |
||||||
| Day 5 | Acoustic Startle |
CPFE: Context Preexposure Facilitation Effect; MWM: Morris Water Maze; EBCC: Eyeblink Classical Conditioning
2.3. Histology, Stereology and Volume Estimation
Mice were deeply anesthetized with isoflurane and perfused through the heart with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. One day before sectioning, each brain was refrigerated and stored in distilled water. Sections were embedded in gelatin and cut in the coronal plane through with a Vibratome 3000 Sectioning System (Vibratome Co., St. Louis, MO) at a thickness of 70 µm. Sections were mounted on glass slides and stained with thionin. Investigators carrying out the neuron counts and volume estimation were blind to mouse age-group membership.
2.3.1. Cerebellum
Stereological counts of Purkinje neurons in the cerebellum for eight brains in each of the five age groups were carried out. Each mouse cerebellum yielded between 25 and 50 sections. Methods for cerebellar Purkinje neuron counts were presented previously (Woodruff-Pak et al., 2010). An optical fractionator stereological design (West et al., 1991) was used to make unbiased estimates of total Purkinje neuron number (Stereo Investigator software, Version 10.21.1, MicroBrightField, Williston, VT). A three-dimensional optical dissector counting probe was applied to a systematic random sample of sites over the entire cerebellum. Every third section was probed. For each cerebellum there were approximately 11 (range: 7–16) sections outlined using the 4x objective. The outline of the section generated a path to select sampling sites using the designated step lengths (800 µm in the x direction and 900 µm in the y direction). This path completely covered the full extent of the outlined space. The mean number of sampling sites was 248.68 (SD = 61.22) for an entire cerebellum, and the range was 137–381. There were no significant age differences in the number of sampling sites. A 60x oil immersion objective was used for the neuron counts. The mean section thickness for all brains was 34.21 (SD = 10.17) µm. Some shrinkage of the tissue occurred due to processing, mounting and staining; however, there was not a significant difference in section thickness between age groups. A border guard of 5 µm was set at the top and the bottom of the section, and each Purkinje neuron with a visible nucleus was counted as the experimenter focused down through 10 µm of tissue in each 149 × 109 µm counting frame. The total number of Purkinje neurons in the entire cerebellum for each mouse was estimated when the number of Purkinje neurons counted in the known fraction of the cerebellar cortex was multiplied by the inverse of the sampling fraction.
2.3.2. Hippocampus
Despite the contributions of the unbiased stereology measure in the study of the hippocampus, in the present study we compared hippocampus volume (rather than stereological counts of pyramidal neurons) across the life span. Previously we have found no differences in pyramidal neurons in CBA mice aged 4–24 months (Woodruff-Pak et al., 2010). In addition, we have found that across several mouse strains (CBA, C57 and CB6F1) there is a statistically significant correlation between stereology and volume measures in the hippocampus, r(22) = 0.598, p < .01 (data not shown). Volumetric estimations were carried out in the hippocampus of the same animals used for stereology. Sectioning began at the posterior portion of the brain and continued until the hippocampus was not visible for five consecutive slices. Each mouse hippocampus yielded between 25 and 50 sections. Each tracing included the hippocampus in one hemisphere. Every third section was probed. For each hippocampus there were approximately 11 (range: 8–14) sections outlined using the 4x objective. A Cavalieri point grid with 150 µm spacing was placed over the tracings and points that fell within the tracing region were summed. The volume was estimated using the Cavalieri probe in Stereo Investigator.
2.4. CPFE
2.4.1. Apparatus
Med Associates Near Infrared Video Fear Conditioning System for Mouse (MED-VFC-NIR-M) as described in Anagnostaras and colleagues (2010) was used for fear conditioning in the present study. This system has demonstrated reliability in video coding of common dependent measures of fear (e.g., freezing; activity levels), thus allowing experimenters to avoid labor-intensive post hoc coding of fear behavior. Use of this system has the further advantage of avoiding potential biases in situations in which experimenters coding fear behavior are not blind to group or treatment conditions. Parameters used in the present study replicate those that yielded maximum reliability with experimenter coding of freezing in the Anagnostaras and colleagues (2010) studies.
Two identical conditioning chambers (32 cm wide, 25 cm high, 25 cm deep; part number VFC-008) located in the same room were used, with each chamber consisting of clear polycarbonate (top, front), white acrylic (back), and stainless steel (sides, shock grids, pan below grids). Each grid floor consisted of 36 rods, and each rod was 2 mm in diameter. Conditioning chambers were each stationed within separate white sound-attenuating boxes (63.5 cm wide, 35.5 cm high, 76 cm deep; NIR-022MD). An overhead LED-based light source (NIR-100) provided visible broad-spectrum white light (450–650 nm) and near infrared (NIR) light (940 nm). Background noise (65 db) was provided by internal ventilation fans. Video images of fear training were recorded at a frame rate of 30 frames per second using a video camera (VID-CAM-MONO-2A) secured to the inner door of the sound-attenuating box. Data collection was controlled by Med Associates software (Video Freeze) running on a Windows computer.
2.4.2. Procedure
Procedures were identical to those published previously (Brown et al., 2011). Prior to the start of training, all mice were given 2–3 days to acclimate to standard polycarbonate temporary housing or transport cages. Following transport from the housing colony, all mice were given 45–60 min to acclimate to the laboratory area in which behavioral training occurred. The same experimenter handled mice throughout all phases of training. Mice were trained using behavioral procedures identical to those used for rats as outlined by Rudy and colleagues (Matus-Amat et al., 2004). These mice were transported two at a time from their home cage to the conditioning chamber (approx 10 – 15 sec transport time) in a black ice bucket with the lid secured. All transport between the temporary housing cage and the conditioning context for these mice occurred in these buckets. During day 1 (Phase 1) of training, mice were given 5 min to explore the novel context (70% alcohol odor in black transport bucket and in conditioning chamber) and then transported back to their temporary housing cage for 40 sec before getting transported back to the conditioning chamber where they remained for 40 sec. This 40 sec alternating temporary housing cage/40 sec conditioning chamber exposure was repeated four times. The purpose of these multiple exposures was to establish features of the transport bucket as retrieval cues that could activate representation of the context (Rudy et al., 2002; Matus-Amat et al., 2004; Rudy et al., 2004; Rudy & O’Reilly, 2011) – there was no shock delivery during Phase 1 of training.
Twenty-four hours later (Phase 2) the mice were transported from temporary housing cages to the same conditioning context explored in Phase 1. Immediately after entry into the context, mice received a single, 2 sec, 0.75 mA foot shock through the grid floors. Mice were removed immediately after shock termination and transported to their home cage. Phase 3 training occurred twenty-four hours later, consisting of exposure to the same conditioning context experienced in Phases 1 and 2 for 6 min (no shock deliveries during Phase 3). The dependent measures recorded during this task were freezing (absence of movement apart from respiration) and general activity levels.
2.5. Rotorod
2.5.1. Apparatus
A four-lane motorized rotorod (San Diego Instruments, Inc., San Diego, CA) was used. Each rod was 3 cm in diameter and 11 cm long, and maintained at 46 cm above the foam-covered base. An electronically controlled motor maintained the rod speed. Photo beams detected falling in each lane.
2.5.2. Procedure
Training took place over two days. Each session consisted of eight trials, four at each speed (15 and 25 RPM). There was a five-minute inter-trial interval and 30 minutes between speed changes. On each day, mice were placed in the direction opposite of the rotation of the rod. To avoid falling, mice were required to move forward in a coordinated manner. The latency to fall from the rod for each trial was recorded as the dependent measure. Trials ended if the mouse did not fall after 60 seconds, at which point a latency score of 60 was given.
2.6 Barnes Maze
2.6.1. Apparatus
The maze consists of a platform 122 cm in diameter with 40 holes (5 cm diameter) spaced evenly around the perimeter (Med Associates, St. Albans, VT). Various pictures and paintings surrounding the maze served as spatial cues and bright lights were shined onto the surface of the maze. Underneath one hole was the escape box, which was filled with bedding and into which the mouse had to climb to end each trial. The location of the escape box was unique for each mouse and remained fixed throughout testing. The surface of the maze was cleaned with 5% white vinegar after each trial to remove odor cues and escape box bedding was changed between cages of animals. The maze surface, escape box, and metal start bowl were sanitized with 70% alcohol at the end of each day.
2.6.2. Procedure
Mice were acclimated to the maze and escape box with two practice trials before the first day of acquisition training (pre-training). For practice trials, each mouse was placed on a portion of the maze surface next to an escape hole that would not be used in training and visual cues were obstructed. The mouse could explore the small, confined area of the maze surface but eventually found the escape hole and climbed down into the escape box. If the mouse had not entered the escape box after 45 seconds it was gently guided into the hole. Once the mouse had entered the escape box it was allowed to rest for 30 seconds.
Testing took place over four consecutive days. Each day mice were given a total of three 4-minute trials with an inter-trial interval of 1 minute. The mouse was placed in a metal start bowl at the center of the maze for 15 seconds, which was then removed. Mice were motivated to escape by the flat, open, brightly illuminated surface. Once the mouse had entered the escape box, it was allowed to rest for 30 seconds. If the mouse had not entered the escape box after 4 minutes it was gently guided to the escape hole and allowed to enter. The latency to escape the platform, as well as the distance covered and the number of errors made (visiting the incorrect hole), were recorded by the SMART tracking software (Panlab, Barcelona, Spain).
Due to performance differences between mice and rats and the exploratory tendencies of mice, the traditional variables of latency, distance and errors can be biased and inaccurate (Koopmans et al., 2003). To address this problem, we calculated latency and distance, but only until the first encounter with the escape hole (Harrison et al., 2006; Patil et al., 2009). These variables (termed primary) are a better indication of whether the mouse learned the location of the escape hole. These measures can show learning that is not captured by total path length and total latency, which can be inflated and confounded by the exploratory nature of mice. Primary errors are not reported due to difficulties tracking mice around some of the escape holes, which prevented an accurate count of errors.
2.7 Acoustic Startle
2.7.1. Apparatus
A two-channel SR-Lab System (San Diego Instruments, San Diego, CA) was used to test mice for reflexive startle responses to acoustic stimuli. The system included two 35 × 33 × 38.5-cm sound-attenuating chambers that were ventilated and illuminated. The chambers contained a stabilimeter affixed to a clear Plexiglas cylinder (16 × 8.75 cm) mounted to a Plexiglas frame (12.5 × 20.5 × 0.6 cm). The cylinder and frame were elevated 2.75 cm above a 30 × 30 × 4 cm Plexiglas base by four screws stationed under each corner of the stabilimeter frame. A 6-cm speaker, placed 27 cm above the cylinder, delivered acoustic stimuli. Startle responses were transduced by a perizoelectric accelerometer mounted beneath the stabilimeter frame. Output signals were digitized, rectified, and recorded as consecutive 1-msec readings on a PC computer. Intensity of acoustic stimuli was verified by placing an audiometer (Radio Shack) in the Plexiglas cylinder, with the chamber door closed, and monitoring decibel levels through a viewing lens while running a test session.
2.7.2. Procedure
The chamber light and ventilation fan remained on throughout the session. A 75-dB white noise was presented through the overhead speaker to provide continuous diffuse background noise. Each session was controlled by San Diego Instruments software for Windows XP. There were a total of 80 trials. The inter-trial interval was random, ranging from 10 to 20 sec at 1-sec intervals, and all trials were presented in a pseudo-randomized order such that no two of the same trial-type were contiguous. Mice were tested in groups of two and each session lasted one-half hour.
Mice were placed in the cylinder for a 5-min acclimation period. Testing began immediately after acclimation. Startle stimuli were a 40-msec burst of white noise at one of ten startle intensity levels (80–125 dB, levels separated by 5 dB). Acoustic startle consisted of a total of 55 trials with an inter-trial interval of 15 ± 5 sec. Each startle intensity level was presented five times and sessions also included five trials with no stimulus. Peak amplitude of the startle response (Vmax) was used as the primary measure of startle response.
2.8. Morris Water Maze
2.8.1. Apparatus
The training apparatus was a circular pool, 100 cm diameter and 60 cm deep located in a laboratory room containing camera and computer equipment, a curtain to reduce the viewing area, and various visual cues. The interior of the pool was painted black. The water was maintained between 24 and 26°C, and the depth was 16 cm. The hidden platform was an 11 cm square clear Plexiglas platform positioned 1 cm below the surface of the water. The same platform was used for the visible platform, and it was marked by a black flag (10 by 7 cm) suspended 15 cm above it on a wooden stick.
2.8.2. Procedure
Training in the MWM took place over five days. On the walls of the test room were multiple cues such as posters and graphic prints. Camera equipment used to record the session was also visible to the mouse; however, computer equipment and experimenters were not. Each trial was initiated by placing the animal in the water at the edge of the pool in a quadrant either opposite or adjacent to the quadrant containing the platform. The start locations were varied among the three quadrants not containing the platform; with three different start locations being used in each block of four trials. The platform remained in the same location on every trial during the hidden platform task and varied across the four quadrants in the visible platform task. Each trial lasted 90 seconds or until the mouse located the platform. Mice that did not find the platform were guided to it, placed on it, and given a latency score of 90 seconds. Whether the platform was located or not, each mouse was required to spend 15 seconds on the platform at the end of each trial. Between blocks of four trials the mice were placed in individually heated, extra-absorbent paper towel lined plastic holding cages for at least 30 minutes. Two commonly reported MWM measures, time (latency to escape) and distance traveled to reach the platform, were recorded for each trial and averaged for each block of 4 trials during acquisition.
2.8.3. Hidden Platform Training
Each subject was given three blocks of four trials (12 trials/day/mouse) for three consecutive days of training. Mice were returned to the holding cage between blocks. On the fourth training day, the subjects were given a probe trial, in which the platform was removed from the pool. After swimming for the entire length of the trial (90 seconds) the mouse was removed from the pool and returned to its holding cage. All trials were videotaped and recorded using the SMART (Spontaneous Motor Activity Recording and Tracking) program manufactured by Panlab (Barcelona, Spain). The probe trial was analyzed to measure the amount of time spent in each quadrant and the number of crossings made over the platform location in the trained quadrant and the equivalent area in the untrained quadrants.
2.8.4. Visible Platform Training
On the fifth day of training, all mice were given the visible platform task. Training was the same as in the hidden platform version except the location of the platform and the start position were varied across trials. The latency and distance to escape were recorded.
2.9. EBCC
2.9.1. Surgery
A randomly selected subset of mice at each age group received surgery to implant recording and stimulating electrodes for eyeblink classical conditioning. Anesthesia was induced in a chamber with O2 + 3% isoflurane at a flow rate of 1 L/min. The isoflurane was then reduced to 2.5% as the mouse was placed on a surgical platform and fitted with a nose cone for anesthesia maintenance throughout the procedure. Opthalmic ointment was applied to each eye to prevent drying, and mice were covered with gauze strips to maintain normal thermoregulation. After a small incision was made to expose the top of the skull and two screws were inserted into the skull, four Teflon-coated stainless steel wires (0.003-in bare, 0.0045-in coated; A–M Systems, Everett, WA, USA) soldered to a four-pin male header (Jameco Electronics, Belmont, CA, USA) were implanted intramuscularly in the orbicularis oculi of the left upper eyelid. Wires were stripped of Teflon and carefully placed such that only the muscle-embedded wire was bare. To ensure that the wires would not move or recede back into the periorbital cavity, wires were glued to the skull. The two wires most rostral were used to record differential electromyography (EMG) activity, and the two most caudal were used to deliver the eyeblink-eliciting stimulus. When all wires were placed, the four-pin headstage was cemented to the skull (with the glue adhering to the skull screws) and the incision was closed. Following surgery, mice were given Baytril antibiotic (85 mg/kg sc) to prevent infection and Ketofen (2 mg/kg sc) for analgesia. Mice were allowed a minimum of 5 days to recover from surgery.
2.9.2. Apparatus and Procedure
The conditioned eyeblink training apparatus consisted of four sound- and light-attenuating chambers (Med Associates, St. Albans, VT, USA). Each chamber contained an enclosure in which the mouse was placed, a copper Faraday cage covering the enclosure, a ventilation fan, and a wall-mounted speaker. A shielded four-conductor wire was attached to the mouse's headstage and was used to deliver a blink-eliciting stimulus to the orbicularis oculi and to record EMG activity. EMG activity was passed through a 300–5,000 Hz filter and amplified by 10 K. The signal was then integrated and digitized before being read into a system compatible with IBM (White Plains, NY, USA; described by Chen and Steinmetz, 1998) for processing.
Mice were run for 5 days in the 500 ms trace EBCC paradigm with each day consisting of one session of 90 paired CS–US and 10 CS alone trials. Each training session was controlled by a program written in C++ language (Chen and Steinmetz, 1998). The inter-trial interval was random, ranging from 15 to 30-s at 1-s intervals. Mice were trained in groups of four. Each session lasted approximately 1 h and mice were allowed to move about freely within the enclosure during testing. The ventilation fan generated a 70-dB background noise. Each daily session (5 sessions of acquisition total) consisted of 100 trials (presented in blocks of 10). Each 10-trial block consisted of nine paired trials and one CS-alone test trial. Trials included a 250-ms tone CS (85 dB 1 kHz) followed 500 ms after its onset (and 250 ms after its offset) by a 100-ms stimulation US (0.3–1.0 mA) to the orbicularis oculi muscles. The 250 ms period between CS offset and US onset represented a stimulus-free—trace” interval. The first day of training also included a baseline session without CS or US presentations.
2.9.3. Analysis
Each session was computer-scored with a macro written in Visual Basic, which analyzed each trial individually for responses. Response threshold was set to 1.5 units above the highest point of pre-CS activity. A startle (or—alpha”) response was scored if the response occurred in the first 60 ms after CS onset. A CR was scored if a response occurred after the 60 ms startle period and before the US onset (500 ms after CS onset) for paired CS–US trials. All data are reported from paired CS–US trials. Trials were excluded from analysis in cases where (a) excessive pre-CS activity (also termed—noise”) was present, or (b) activity that originated in the pre-CS period exceeded the response threshold after CS onset. Subjects were excluded from analyses if 30% or more of their trials (averaged across the 10 training sessions) were determined to be unusable. The primary dependent measures of interest were percentage of CRs and peak amplitude of CRs.
2.10. Statistical Analyses
All statistical analyses were carried out using the IBM SPSS Statistics (Version 19) software package. The main analyses used were the mixed analysis of variance (ANOVA) and one-way ANOVA to compare the effects of normal aging on dependent measures from behavioral tasks. Significance was set at p < .05. When appropriate, post-hoc analyses were conducted using the Tukey Honestly Significant Difference (HSD) test incorporating adjusted critical values. When reporting multiple comparisons and all p values fell within the same category (i.e. p < .05), these values were reported together (i.e. ps < .05). Before conducting analyses, the data were examined for statistical outliers (mean performance scores at least 1.5 times greater than the inter-quartile range). Based on performance in the visible platform portion of the MWM, and considering latency and primary latency in the Barnes maze, two mice were identified as outliers (4 month and 12 month) and excluded from analyses. No other outliers were identified. For EBCC, data are presented for a total of 45 mice that underwent successful surgery and did not meet exclusion criteria (4 mo n = 9, 8 mo n = 8, 12 mo n = 10, 18 mo n = 9, 25 mo n = 9).
3. Results
3.1. Cerebellum Stereology
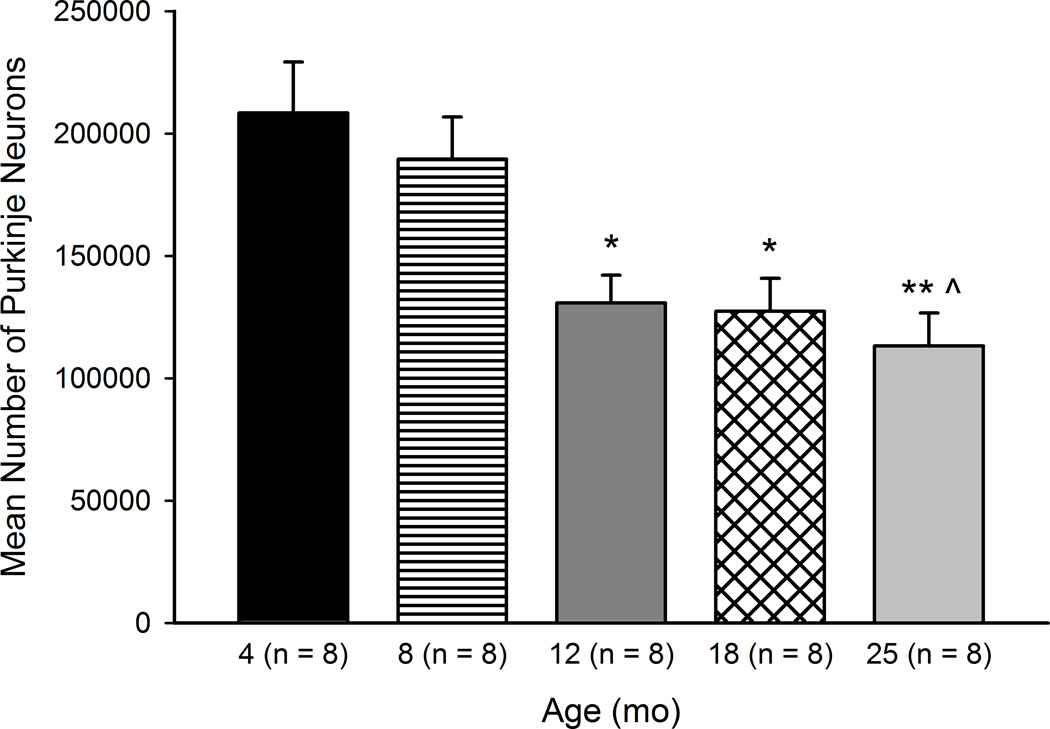
A one-way ANOVA was used to compare unbiased estimates of total Purkinje neuron number in cerebella of a subset of randomly selected mice aged 4–25 months (Figure 1). There was a statistically significant effect of Age, F(4, 35) = 7.28, p < .001. Post-hoc analyses indicated significantly fewer Purkinje neurons in the 25 month age group compared to the 4 (p < .01) and 8 (p < .05) month age groups. Mice aged 18 and 12 months had significantly fewer Purkinje neurons than 4 month-old mice (ps < .05). No significant differences were found between age groups in the number of sections analyzed, section thickness, or number of sampling sites (Table 2). The correlation between age and Purkinje neuron number was large and statistically significant (r(39) = −0.640, p < .001).
Figure 1.
Age-related deficits in cerebellar cortical Purkinje neurons. Total estimated number of Purkinje neurons in mice aged 4–25 months is shown. Significant loss of neurons was found in mice aged 12, 18, and 25 months. Values are mean ± SE. *p < .05 compared to 4 mo, ** p < .01 compared to 4 mo, ^ p < .05 compared to 8 mo.
Table 2.
Means (SD) of stereology parameters by age group
| Age Group |
n | Sections | Sampling Sites | Section Thickness |
Number of Cells Counted* |
Gundersen CE |
|---|---|---|---|---|---|---|
| 4 mo | 8 | 12.00 (2.07) | 251.13 (64.46) | 37.44 (8.72) | 446.25 (173.09) | .065 (.019) |
| 8 mo | 8 | 11.38 (1.51) | 232.88 (35.54) | 37.04 (5.98) | 387.00 (77.34) | .069 (.023) |
| 12 mo | 8 | 11.13 (2.10) | 248.25 (82.04) | 36.45 (15.48) | 303.63 (119.20) | .066 (.018) |
| 18 mo | 8 | 10.88 (2.10) | 248.38 (65.78) | 31.84 (4.86) | 297.00 (80.09) | .066 (.014) |
| 25 mo | 8 | 11.13 (1.25) | 262.75 (62.36) | 28.27 (11.32) | 317.50 (92.88) | .065 (.018) |
| Mean | 11.30 (1.79) | 248.68 (61.22) | 34.21 (10.17) | 350.28 (122.95) | .066 (.018) |
F(4, 35) = 2.55, p = .056
3.2. Hippocampus Volume
The volume of the hippocampus in each age group was compared in a subset of randomly selected mice using a one-way ANOVA. There were no significant age-related differences in hippocampus volume. In addition, no age-related differences were found for the number of slices analyzed or the number of points counted in each age group (Table 3). The Coefficient of Error (Gundersen CE, m = 1) for this analysis was low and within an acceptable range (0.055, SD = .019).
Table 3.
Means (SD) of Cavalieri volume parameters by age group
| Age Group |
n | Sections | Points | Gundersen CE |
|---|---|---|---|---|
| 4 mo | 5 | 10.00 (2.00) | 465.00 (89.14) | .060 (.014) |
| 8 mo | 6 | 11.33 (2.16) | 455.33 (119.22) | .065 (.029) |
| 12 mo | 6 | 10.67 (0.82) | 521.67 (87.45) | .055 (.014) |
| 18 mo | 5 | 10.20 (1.64) | 488.40 (80.59) | .044 (.005) |
| 25 mo | 6 | 11.50 (0.84) | 581.33 (76.35) | .048 (.018) |
| Mean | 10.79 (1.57) | 504.18 (97.56) | .055 (.019) |
3.3. Weight
Mouse weights were compared using a one-way ANOVA. There were significant differences in weight both at pre-handling for CPFE (F (4, 66) = 10.92, p < .001) and preparation for EBCC surgery four weeks later (F (4, 57) = 4.75, p < .01). Before CPFE testing, post-hoc tests determined that mice aged 4 months weighed significantly less than all age groups (ps < .01). In the subset of mice that received EBCC surgery, mice aged 4 months weighed significantly less than all age groups apart from 8 month-old mice (ps < .01).
3.4. CPFE Fear Conditioning
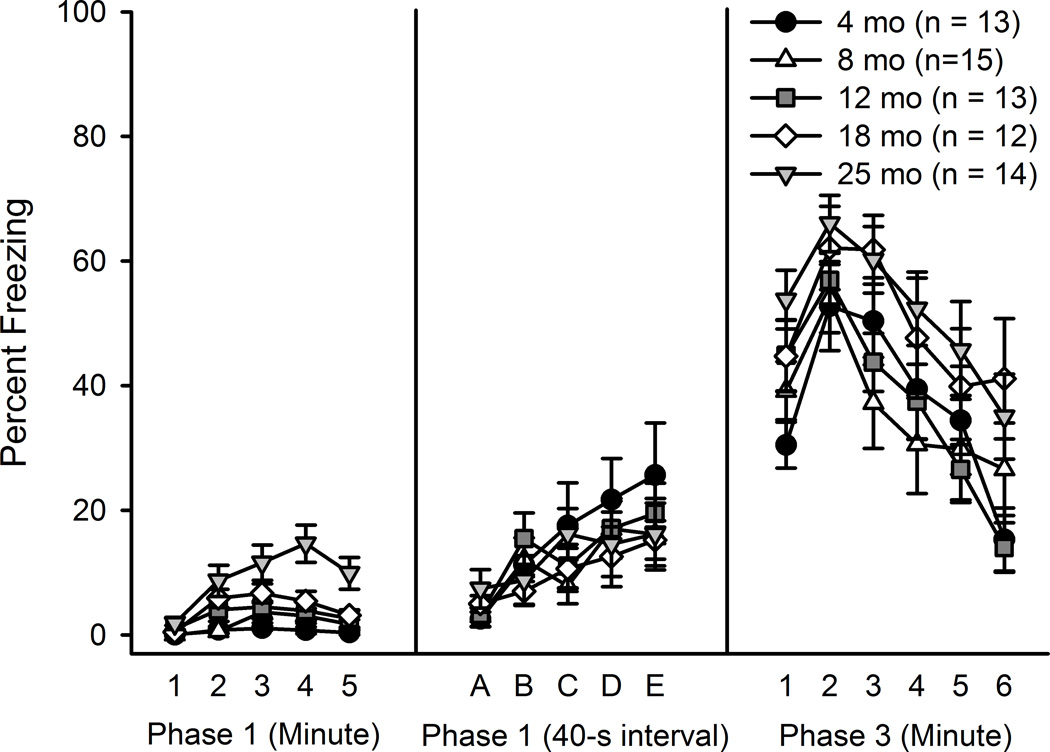
Freezing was assessed during the initial five-minute exposure to the conditioning chamber in the absence of a shock (Phase 1) using a 5 (Age) × 5 (Minute) mixed ANOVA. In all mice the amount of freezing was minimal; however, freezing tended to increase across the five minutes, F(4, 248) = 18.92, p < .001, with the largest amount of freezing (about 5.5%, Figure 2) in minutes 3–4. Post hoc tests determined that mice aged 25 months froze significantly more than all other ages (ps < .05) during this period. A 5 (Age) × 5 (Exposure) mixed ANOVA revealed that freezing also increased significantly across the repeated 40 sec exposure to the conditioning chamber, F(4, 248) = 14.11, p < .001, though no age-related differences in freezing were found. Significant differences in reactivity to the foot shock (Phase 2) were found, F(4, 61) = 6.97 p < .001, as mice aged 12, 18 and 25 months displayed higher activity levels than 4 month-old mice (ps < .01) during the 2 sec shock administration, as determined by a one-way ANOVA (data not shown).
Figure 2.
CPFE fear conditioning across the life span. Percentage of time freezing during pre-exposure (Phase 1) and context test (Phase 3) in mice aged 4–25 months is shown. 25 month-old mice froze significantly more during the initial five minute pre-exposure, but no differences were found during the six minute context test.
A 5 (Age) × 6 (Minute) mixed ANOVA on Phase 3 (context test) freezing revealed a significant effect of Minute, F(5, 310) = 31.85, p< .001 (Figure 2). The Age × Minute interaction was not significant and the effect of Age approached significance (p = .08). Freezing levels were largest in the first three minutes of the context test and tended to decrease in minutes 4–6. Mice aged 18 and 25 months tended to freeze more than younger mice, although this difference was not statistically significant.
3.5. Rotorod
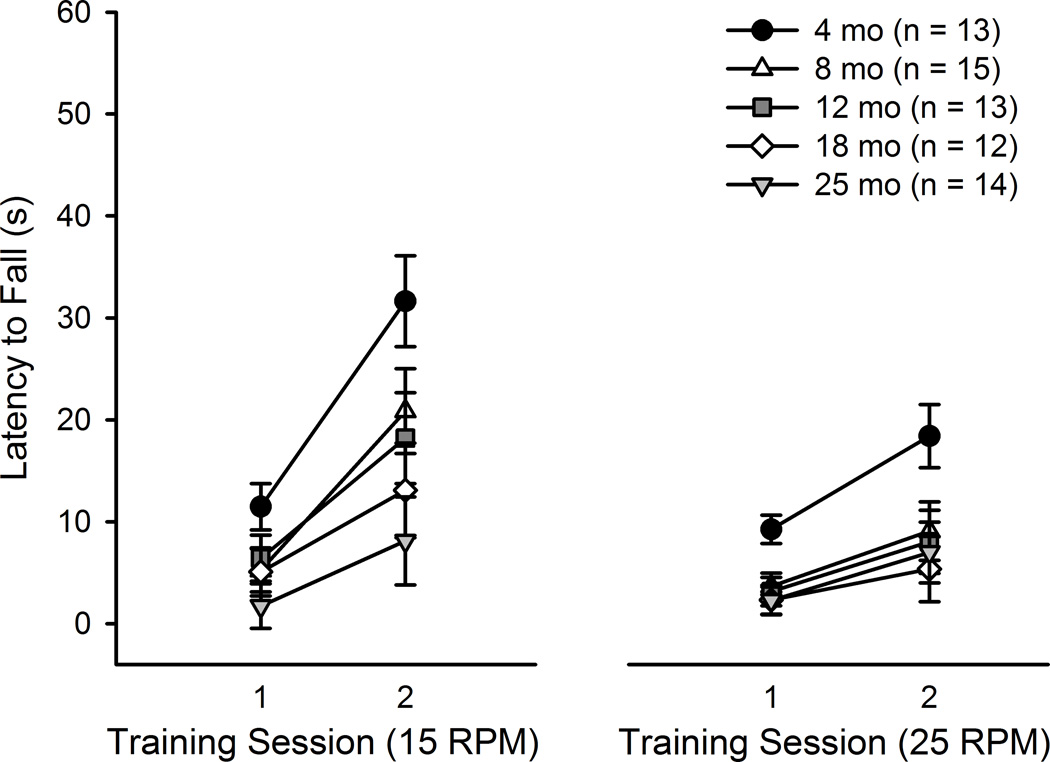
At each rotation speed, latency to fall was analyzed with 5 (Age) × 2 (Training Session) mixed ANOVAs. At the 15 RPM speed, significant effects of Training Session (F(1, 62) = 45.90, p < .001) and Age (F(4, 62) = 4.67, p < .01) were found, but the interaction was not significant (Figure 3). All mice showed evidence of learning, as the latency to fall from the rotating beam increased across Training Sessions. Post-hoc tests determined that mice aged 18 (p < .05) and 25 (p < .01) months displayed significant impairment relative to mice aged 4 months. Some researchers have reported that weight can be a potential confound in the rotorod, as maintaining balance on the rotating beam is more difficult for heavier mice (Ingram, 1983). To examine this potential confound, the data were re-analyzed with weight measured during pre-handling for CPFE (one week before rotorod testing) as a covariate. In this analysis, the effect of Weight was significant (F(1, 61) = 10.30, p < .01), and the effect of Age was no longer significant.
Figure 3.
Age-related deficits in motor learning on the rotorod. Latency to fall from the rotating beam in mice aged 4–25 months is shown. Main effects of Age were found at both rotation speeds (15 and 25 RPM). Post-hoc tests determined that mice aged 18 (p < .05) and 25 (p < .01) months displayed significant impairment relative to mice aged 4 months at 15 RPM. At 25 RPM, mice aged 12 (p = .05), 18 (p < .05), and 25 months (p < .05) displayed significant impairment compared to mice aged 4 months.
Similar results were found at the 25 RPM speed. Training Session (F(1, 62) = 27.14, p < .001) and Age (F(4, 62) = 3.68, p < .01) effects were significant, whereas the interaction was not (Figure 3). Latency to fall increased across Training Sessions, and mice aged 12 (p = .05), 18 (p < .05), and 25 months (p < .05) displayed significant impairment compared to mice aged 4 months. Weight was also a significant covariate at the 25 RPM speed (F(1, 61) = 8.65, p < .01).
Latency to fall values on Day 2 at 15 RPM were significantly correlated with total estimated Purkinje neuron number, r(39) = 0.325, p < .05). Correlations between neuron number and other days and training speeds were not significant. A significant correlation was found when comparing the slope of performance on the rotorod with total Purkinje neuron number. Mice with fewer Purkinje neurons tended to have a smaller performance slope at 15 RPM (r (39) = 0.317, p < .05). The correlation between slope and Purkinje neuron number was not significant at 25 RPM.
3.6. Barnes Maze
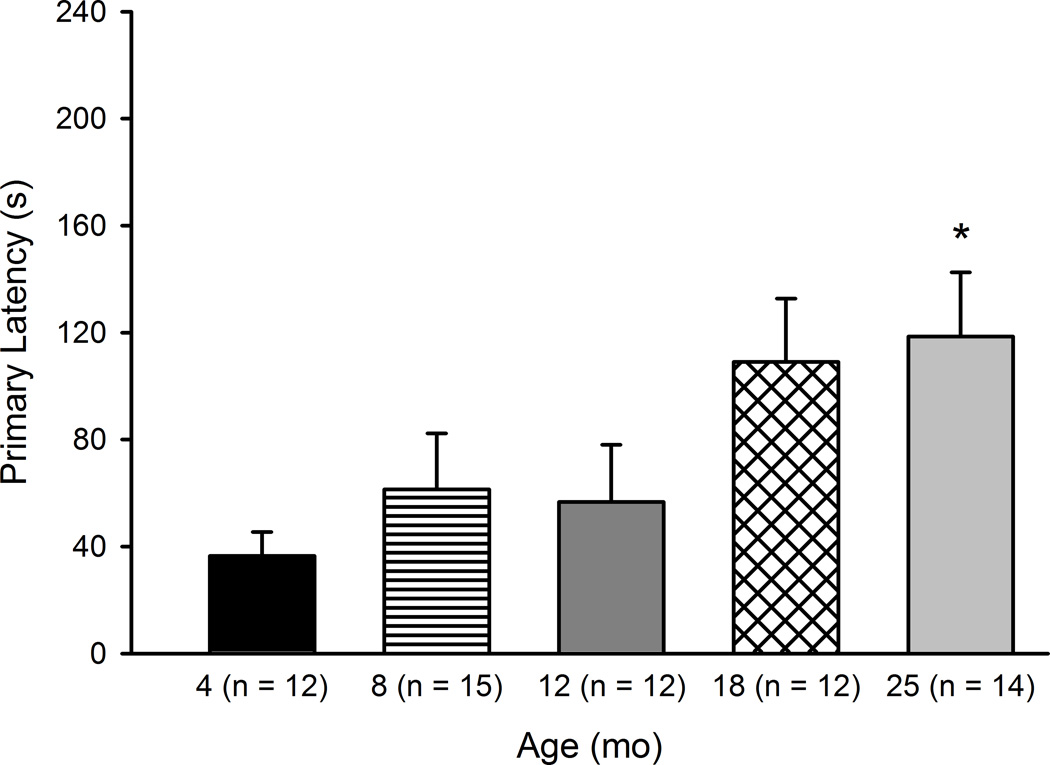
Primary latency and primary distance were evaluated with 5 (Age) × 4 (Training Day) Mixed Model ANOVAs. For primary latency, a main effect of Training Day was found, F(3, 180) = 89.52, p < .001, and the effect of Age was not significant (data not shown). Post-hoc tests determine that primary latency decreased significantly across the four Training Days, which was indicative of learning (ps < .01). A measure of learning we planned to examine was the first trial of the second day of training—to examine learning at a 24-hour retention interval. A one-way ANOVA comparing primary latency in the five age groups revealed a significant effect of Age at Day 2, Trial 1, F(4, 60) = 2.85, p < .05. Post-hoc tests determined that 25 month mice had significantly larger primary latencies (p < .05) compared to 4 month mice (Figure 4). Primary distance decreased significantly across all four Training Days (F(3, 180) = 80.80, p < .001) but the effect of Age and the Age × Training Day interaction were not significant. A significant correlation was found for Purkinje neuron number and Barnes maze primary latency, r(39) = −0.433, p < .01, though correlations between neuron number and the other measures did not achieve statistical significance.
Figure 4.
Barnes maze retention across the life span: Primary latency Day 2, Trial 1. Latency to the first visit to the escape hole during Trial 1 of Day 2 in mice aged 4–25 months is shown. Mice aged 25 months took significantly longer to first visit the escape hole than mice aged 4 months. Values are mean ± SE. *p < .05 compared to 4 mo.
3.7. Acoustic Startle
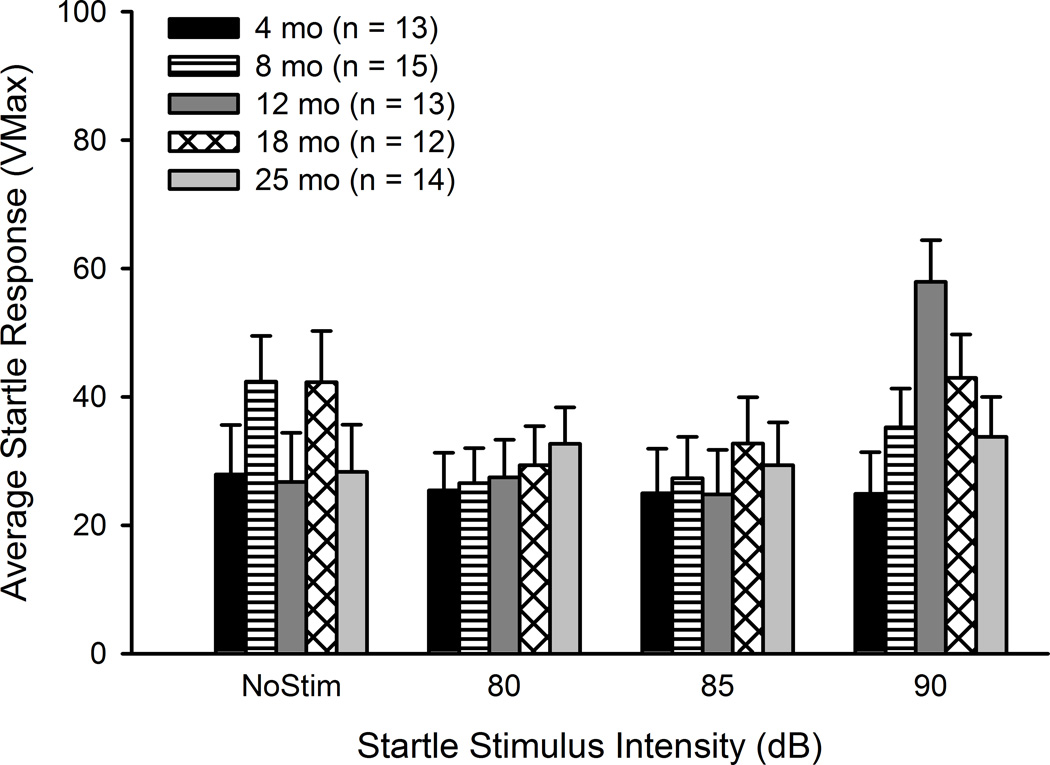
Peak amplitude of the startle response (Vmax) was compared in a 5 (Age) × 11 (Stimulus Intensity) mixed ANOVA. The effect of Age was significant (F(4, 62) = 10.02, p < .001), as mice aged 25 months tended to have smaller startle amplitude than younger mice. Older mice displayed some auditory deficits at the highest intensities tested; however, the effect of Age was not significant when comparing stimuli at the 80–90 dB range. Thus, at the stimulus intensity range used in EBCC testing there were no age-related impairments in auditory acuity (Figure 5).
Figure 5.
Auditory acuity is maintained across the adult life span in CB6F1 mice. Average startle intensity (Vmax) to stimuli ranging from 80–90 dB in mice aged 4–25 months is shown. Mice of all ages had similar startle responses, which confirmed that older mice retained hearing ability.
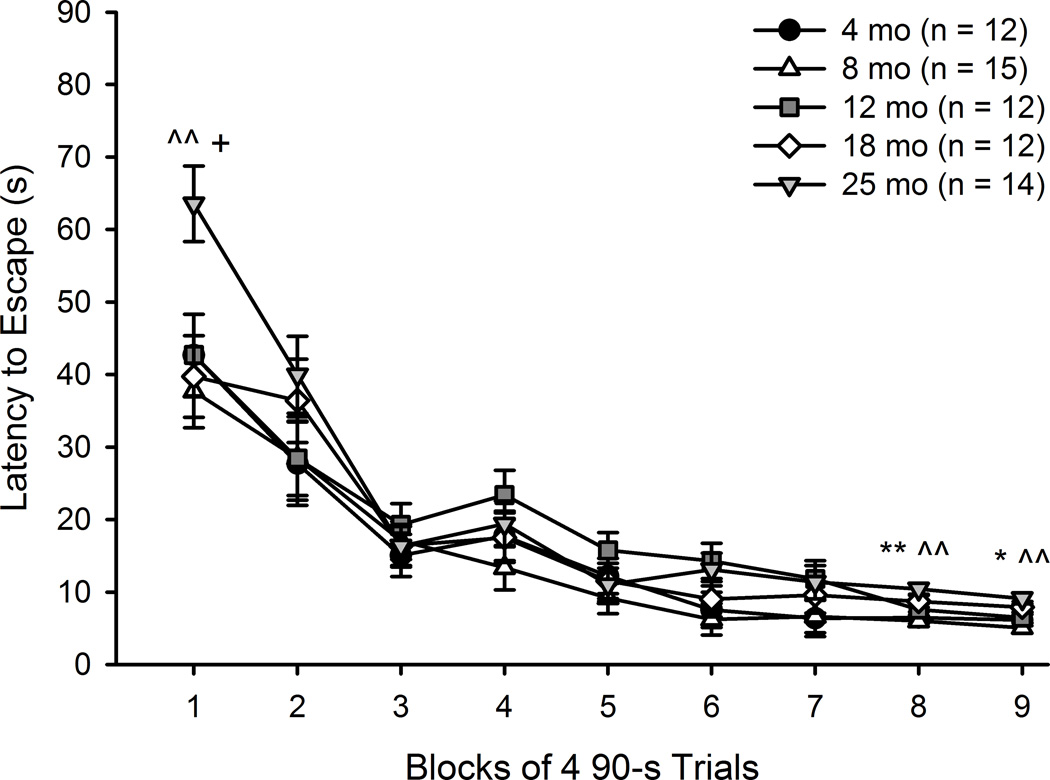
3.8. Morris Water Maze
Acquisition data (latency, distance and swim speed) were analyzed using 5 (Age) × 9 (Training Session) mixed ANOVAs. For latency to escape, significant main effects of Training Session (F(8, 480) = 101.64, p < .001) and Age (F(4, 60) = 2.47, p = .05) were found. The Age x Training Session interaction was also significant, F(32, 480) = 1.84, p < .01. All mice showed evidence of learning, as latency to escape decreased across Training Sessions (Figure 6).
Figure 6.
MWM acquisition across the life span: Latency. Latency to escape the MWM in mice aged 4–25 months is shown. Learning is expressed by all groups, as latency decreases across Training Sessions. Overall, mice aged 25 months took significantly longer to find the platform than did 8 month-old mice (p < .05). The Age x Training Session interaction revealed differences in performance between 25 month-old mice and younger mice at Training Sessions 1, 8 and 9. Values are mean ± SE. *p < .05 compared to 4 mo, ** p < .01 compared to 4 mo, ^ p < .05 compared to 8 mo, ^^ p < .01 compared to 8 mo, + p < .05 compared to 18 mo.
Post-hoc tests determined that 25 month mice took significantly longer to find the hidden platform than did 8 month-old mice (p < .05). To investigate the Training Session x Age interaction, post-hoc tests determined that 25 month mice were significantly slower than 8 (p < .01) and 18 (p < .05) month mice in Training Session 1. In Training Session 8, 25 month mice were significantly slower than 4 and 8 month mice (ps < .01). Finally, in Training Session 9, 25 month mice were significantly slower than both 4 (p = .05) and 8 (p < .01) month mice.
Significant age-related differences were found for the swim speed (F(4, 60) = 5.91, p < .001) but not distance traveled measures. Post-hoc tests determined that mice aged 25 months swam significantly slower than mice aged 4 and 8 months (ps < .01).
For platform crossings during the retention probe test a 5 (Age) × 4 (Quadrant) mixed ANOVA revealed a significant main effect of Quadrant, F(3, 180) = 208.73, p < .001, and an Age x Quadrant interaction, F(12, 180) = 2.24, p < .05. There was no effect of Age. As performance in the trained quadrant is the most indicative of successful retention of spatial memory, we planned to examine age differences in platform crossings in only the trained quadrant. A one-way ANOVA revealed no significant effect of Age. The second probe dependent variable, swim time, revealed a main effect of Quadrant, F(3, 180) = 60.73, p < .001, but no other effects were significant. Mice spent significantly more time swimming in the trained quadrant compared to the other three quadrants (ps < .001).
Since the probe test was administered on Day 4, a point where most all mice demonstrated learning of the platform location, the latency to escape and distance traveled were analyzed for Trial 1 of Day 2. This trial represented the first attempt to find the platform after a 24-hour retention interval. No significant age differences were found for latency or distance traveled in this trial.
For visible platform training, a 5 (Age) × 3 (Training Session) mixed ANOVA revealed a significant main effect of Training Session, F(2, 120) = 78.19, p < .001, but no other significant effects. Latency to escape decreased significantly across the three Training Sessions but all age groups performed similarly (data not shown). Analyses comparing distance and swim speed during visible platform training paralleled the latency results. No age-related differences were found for distance and though the effect of Age was significant in swim speed (F(4, 60), = 2.61, p < .05), this effect was driven by the swim speed difference between 12 and 25 month mice. The oldest mice were not significantly impaired in swim speed compared to the youngest (4 and 8 month) mice.
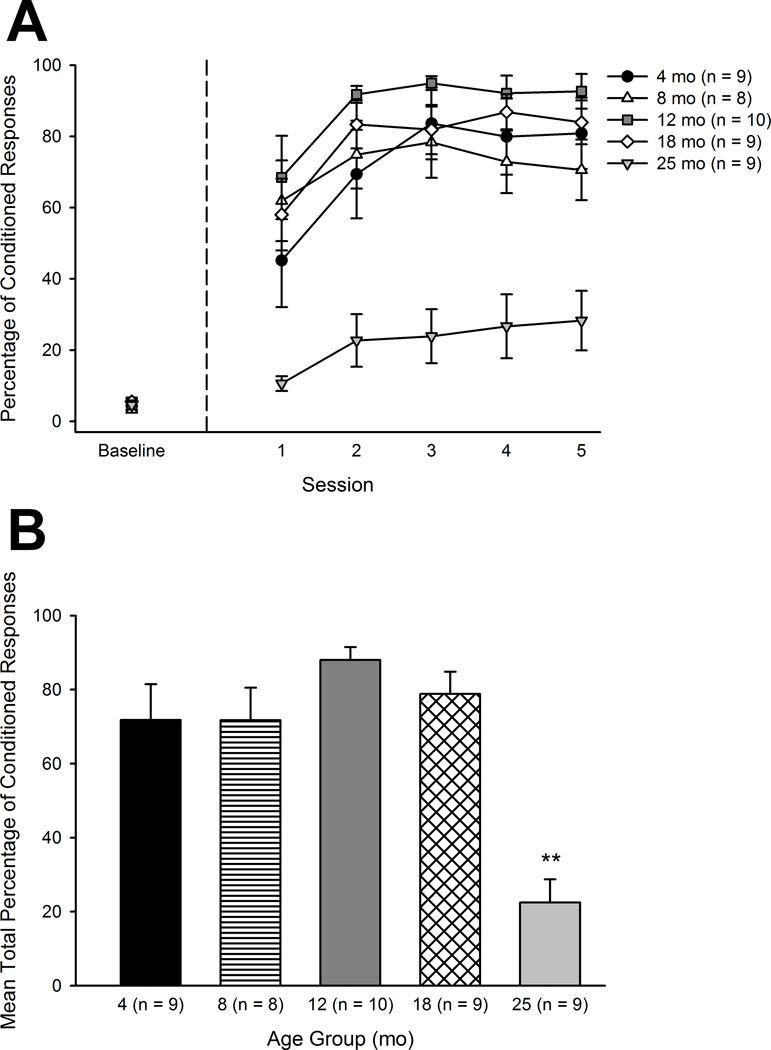
3.9. EBCC
Total percentage of conditioned responses (CRs) was examined using a 5 (Age) × 5 (Session) mixed ANOVA. Significant effects of Age (F(4, 40) = 13.58, p < .001) and Session (F(4, 160) = 18.10, p < .001) were found, but the interaction was not significant (Figure 7A). All mice showed evidence of learning, as CR percentage increased across Sessions. Post hoc tests determined that mice aged 25 months displayed significantly fewer CRs than all other age groups (ps < .001). A one-way ANOVA was used to compare mean total percentage of CRs across the five age groups and mice aged 25 months were found to be significantly impaired on this measure as well (F(4, 40) = 13.58, p < .001; Figure 7B). The correlation between total number of CRs and Purkinje neuron number was not significant (r(27) = .33, p = .09). Correlations within each age group were not significant. Overall, mean total percentage of CRs was significantly correlated with mean performance on the rotorod at 15 but not 25 RPM (r(45) = .35, p < .05).
Figure 7.
Age-related impairment in 500 ms trace EBCC. A. The percentage of conditioned responses across baseline and five training sessions in mice aged 4–25 months is shown. Overall, mice aged 25 months produced significantly fewer conditioned responses (ps < .001). B. The mean total percentage of conditioned responses during training in mice aged 4–25 months is shown. Mice aged 25 months were significantly impaired on this measure (p < .001) as well.
Amplitude of the CR was also assessed using a 5 (Age) × 5 (Session) mixed ANOVA. Significant effects of Age (F(4, 40) = 5.33, p < .01), Session (F(4, 160) = 11.47, p < .001), and the Age × Session interaction (F(16, 160) = 2.54, p < .01) were found. CR amplitude increased across Sessions and mice aged 12 months displayed larger CR amplitudes than 8 (p < .05) and 25 (p < .01) month-old mice, according to post hoc tests. The elevated CR amplitudes in the 12 month-old group may have been due to noise or artifacts within the EMG recording.
4. Discussion
In this study we examined the effects of normal aging on brain and behavior in CB6F1 hybrid mice. Similar to previous reports from our lab (Woodruff-Pak et al., 2010), we found evidence of differential aging in the hippocampus and cerebellum. There were no significant changes in hippocampus volume with age and no age-related impairment in the CPFE fear conditioning task. Some impairment was found in the MWM, although swim speed differences complicate interpretation. Deficits in the Barnes maze were found in retention but not acquisition. Cerebellar cortical Purkinje neuron numbers decreased significantly across the adult life span of the CB6F1 mouse, beginning at 12 months. In addition, age-related impairment was reported in the rotorod task for which cerebellar cortex is required and trace EBCC for which the cerebellar interpositus nucleus as well as the hippocampus are essential. These results extend previous findings to include a more sensitive hippocampus-dependent task (Rudy et al., 2002; Kennard & Woodruff-Pak, 2011a), as well as trace eyeblink conditioning across the life span in a strain of mouse that maintains hearing ability into old age.
Beginning in middle age, the delay EBCC paradigm produces age-related deficits in many different mammalian species (Harrison & Buchwald 1983; Woodruff-Pak & Thompson, 1988; Solomon et al., 1989; Woodruff-Pak & Jaeger, 1998; Vogel et al., 2002). We previously attempted to replicate these results in the C57BL/6 mouse (a commonly used strain) and data were confounded by age-related hearing loss beginning at 12 months in this strain (Woodruff-Pak, 2006). In the CBA strain, which maintains hearing ability, EBCC deficits were evident at 24 months (Woodruff-Pak et al., 2010). The present study has extended these conclusions to the hybrid CB6F1 strain tested on the 500 ms trace EBCC paradigm. Impairment in CB6F1 mice aged 25 months was not due to deficits in auditory acuity, as confirmed by the acoustic startle response. Aged mice showed no significant decreases in CR amplitude. Significant age-related impairment at 25 months in the CB6F1 strain is in line with previous studies incorporating the 500 ms trace EBCC paradigm using either a whisker (22 mo; Galvez et al., 2011) or tone (12 & 22 mo; Kishimoto et al., 2001a) CS.
Though no significant age-related impairment in auditory acuity was reported, the lack of differences in response to the no stimulus condition compared to conditions with 80–90 dB startle bursts was unexpected. These results were likely due to the intensity of the white noise background sound used (75 dB) being too similar to startle bursts. Increased responses were seen in all age groups as the startle burst intensity increased up to 125 dB (data not shown). Given that previous reports have found that auditory acuity is maintained in older age in the CB6F1 strain (Erway et al., 1993) and our findings that only 25 month-old mice were impaired in EBCC (despite younger mice showing similar startle responses to the no stimulus and 80–90 dB conditions), it seems unlikely that EBCC deficits were due to the loss of hearing ability.
Taking into consideration the age-related stability on the sensitive hippocampus-dependent CPFE task, the deficit in trace eyeblink conditioning may be cerebellum-dependent rather than hippocampus-dependent. The interpositus nucleus ipsilateral to the conditioned eye is essential in trace eyeblink conditioning (Pakaprot et al., 2009; Woodruff-Pak et al., 1985). An investigation using transmission electron microscopy was carried out on cerebellar tissue from guinea pigs trained in delay, trace, or unpaired eyeblink conditioning (Hu et al., 2012). Results demonstrated that changes of synaptic ultrastructure in the interpositus nucleus were associated with response magnitude and timing. The investigators concluded that trace eyeblink conditioning induces structural plasticity in the interpositus nucleus, which may play a crucial role in acquiring and executing conditioned eyeblink responses.
Purkinje cells project directly to the interpositus nucleus, and the documented loss of Purkinje cells in CB6F1 mice likely reduced interpositus neuron numbers. Although studies of the consequences of Purkinje cell loss in normal aging have not been carried out, studies of ethanol effects on the cerebellum include impaired delay and trace eyeblink classical conditioning and loss of Purkinje and interpositus neurons (Goodlett & Ellers, 1997; Green, 2004; Green et al., 2006).
Our hypothesis regarding brain measures was confirmed. We found no evidence of total volume loss in the hippocampus, although MRI may have provided a more sensitive measure of volume that would account for tissue shrinkage. Given the previous findings of stability in pyramidal neuron number in the CA field of the hippocampus (West, 1993; West et al., 1994; Rasmussen et al., 1996; Woodruff-Pak et al., 2010), as well as other research suggesting more localized loss of synapses (Nicolle et al., 1999; Smith et al., 1999) or decreases in neurogenesis (Bondolfi et al., 2004; van Praag et al., 2005) with age, our results are to be expected. More refined and specific hippocampus analyses may have yielded evidence of aging; however, the focus in this paper was to compare anatomical (rather than functional) changes in the hippocampus and cerebellum.
The phenomenon of age-related loss of Purkinje neurons has been reported in a number species, including mice, rats and humans (Larsen et al., 2000; Anderson et al., 2003; Woodruff-Pak, 2006; Woodruff-Pak et al., 2010). Previous work in our lab suggested that in mice Purkinje neuron loss begins around 12 months and is statistically significant at 18 months. Neuron loss was significant at 12 months in the present study, though this result may be due to increased statistical power resulting from more mice per age group (8/group compared to 6/group). In humans, we have reported an age-related loss of cerebellar volume that was associated with impairment in delay EBCC (Woodruff-Pak et al., 2001). It has been reported that a statistically significant correlation exists between cerebellar volume and Purkinje neuron number in human autopsy tissue (Nairn et al., 1989). This finding, combined with the large size of Purkinje neurons, suggests that these cells contribute significantly to cerebellar volume.
The relationship between age-related changes in learning ability and brain measures partially confirmed our hypothesis, as a shorter latency to fall from the rotorod beam was associated with fewer Purkinje neurons. Whereas we replicated the loss of Purkinje neurons across the life span in a third mouse strain (in addition to C57BL/6 and CBA), neuron number did not correlate with age-related impairment in the trace EBCC task. We have previously reported a correlation between EBCC learning ability and Purkinje neuron number in hearing-intact C57BL/6 mice (Woodruff-Pak, 2006); however, this effect was not replicated in the present study. One potential explanation for this difference comes from the literature suggesting that the cerebellar cortex is not required for trace EBCC. Studies using knockout and mutant mice (Kishimoto et al., 2001b; 2001c; Woodruff-Pak et al., 2006; Brown et al., 2010) have shown that trace EBCC can be accomplished in the presence of an altered or dysfunctional cerebellar cortex. Given that the cerebellar cortex is not an essential substrate for trace EBCC, a correlation between the loss of Purkinje neurons and age-related impairment on this task would not be expected.
An inverse relationship exists between Purkinje neuron number and performance in spatial learning tasks (Goodlett et al., 1992; Lalonde & Strazielle, 2003a; Woodruff-Pak et al., 2006). The role of the cerebellum in spatial learning appears to be primarily during acquisition (Petrosini et al., 1998; Wrenn & Wiley, 2001; Lalonde & Strazielle, 2003b), as the utilization of a previous spatial map does not appear to be cerebellum-dependent. In the present study, reduced Purkinje neuron number was associated with worse performance on the Barnes maze; however, no relationship was found in the MWM. The role of the cerebellar cortex in Barnes maze performance has yet to be fully described. Our data suggest that acquisition of spatial memory in this task is associated with Purkinje neuron number. Why the Barnes maze would be sensitive to the age-related loss of Purkinje neurons and not the MWM is unclear. The Barnes maze may be more sensitive to cerebellar dysfunction, or the present results may be due to differences between land and water mazes (Whishaw, 1995; Whishaw & Tomie, 1996). In addition, an aged cerebellum is not the same as a cerebellum that has been lesioned or altered due to genetic manipulation. Knockout and mutant mice lose Purkinje neurons early in development and, if tested as adults, may have undergone compensatory changes (Freeman et al., 1995). Cerebellar lesions can also lead to motor impairment that may obscure any deficits in spatial learning (Gandhi et al., 2000).
The CPFE fear conditioning task revealed no behavioral effects of aging in the hippocampus and no significant impairment was reported in the MWM. Age-related impairment was found in retention, but not acquisition assessed by the Barnes maze. Our volume assessment suggested no significant decline of the principal neurons of the CA field of the hippocampus, a finding that has been replicated across a number of species (West, 1993; West et al., 1994; Rasmussen et al., 1996; Woodruff-Pak et al., 2010). Alterations in synapses or changes in neurogenesis are the most likely contributors to aging in the hippocampus (Geinisman et al., 1992; Bondolfi et al., 2004) and these changes were not detected by either our behavioral tasks or anatomical volumetric measure. As standard contextual fear conditioning can be accomplished with an absent or disrupted hippocampus (Logue et al., 1997b; Frankland et al., 1998; Gerlai, 1998), the CPFE paradigm was developed as a more sensitive hippocampus-dependent task (Rudy, 2009). We hypothesized that with the increased sensitivity afforded by the CPFE paradigm, age-related deficits in the hippocampus would be more readily detected. Our results did not confirm this hypothesis, as the oldest mice tested showed no impairment in the CPFE task. In addition, the oldest mice tended to freeze the most, which is generally indicative of a stronger fear memory. A likely explanation for this pattern of elevated freezing is that the oldest mice may have altered anxiety responses. Based on the reactivity data during the shock presentation, older mice (12–25 mo) showed increased sensitivity relative to 4 month-old mice. Mice aged 25 months froze significantly more during pre-exposure (before any shocks had been administered). We have previously found elevated (though numerically very minimal) freezing during pre-exposure in C57BL/6 mice tested using the same CPFE procedures (Brown et al., 2011) that does not appear to occur when rats are the training subjects. Thus, a potential limitation of the CPFE task in mice is that the extra handling and transport during pre-exposure to the conditioning chamber can lead to an increased freezing response. This response, combined with findings of increased stress reactivity in older mice (Pardon et al., 2000; Pietrelli et al., 2012) could explain the lack of age-related impairment in the CPFE task in the present study.
Stress-related effects of aging may have contributed to the present results. The battery of procedures used over the course of seven weeks of testing may have differentially affected the oldest mice and contributed to the age-related impairment seen, particularly in the final task implemented (EBCC). Although if stress had compounded the effects of aging, then it would have been expected that deficits seen in earlier tests (i.e. 18 month mice in the rotorod) would have carried through to EBCC where only 25 month mice were impaired. The effects of stress cannot be entirely ruled out, though, as we did not measure corticosterone and our hippocampus measure could not capture the deleterious effects of stress (i.e. dendritic atrophy; Conrad et al., 1999). The age-related impairment seen in EBCC in particular seems unlikely to be driven by stress, as evidence generally suggests that both acute and chronic stressors can facilitate associative learning (Beylin & Shors, 2003; Sandi & Pinelo-Nava, 2007).
Age-related impairment in the rotorod was found beginning at 18 (15 RPM) or 12 months (25 RPM) and the latency to fall from the beam was positively and significantly correlated with Purkinje neuron number. Mice with fewer neurons spent less time on the rotating beam. It should be noted that weight was a significant covariate at both rotation speeds tested, so impairment at older ages cannot be completely attributed to age. There is some disagreement in the literature regarding what the rotorod measures—balance, coordination, motor skills, motor learning, or some combination of these abilities (Thouvarecq et al., 2001; Serradj & Jamon, 2007). Weight could affect some of these abilities, but with the current apparatus and analysis it is not possible to determine the cause of the decreased latency to fall from the beam in the older mice in the present study. Other tasks that are independent of the weight confound, such as the horizontal beam, would strengthen conclusions regarding age, motor learning and the cerebellar cortex in the present study. Some evidence suggesting that impairment in the older mice was due to learning deficits comes from the significant and positive correlation between scores on the rotorod and EBCC. The ability to learn to blink to a CS is not affected by weight, and mice that tended to show poorer learning ability in EBCC also were poorer performers on the rotorod.
Impairment on the rotorod task is somewhat in line with previous research in C57BL/6 mice that suggests deficits begin around 12–13 months (Thouvarecq et al., 2001; Vogel et al., 2002). In the present study, impairment was generally restricted to older ages (18 and 25 mo). Age-related impairment in the trace EBCC task began later than expected (25 mo) as well. No significant impairment was found in the MWM and only the oldest mice (25 mo) were impaired in retention tested on the Barnes maze. As the trace EBCC task is generally considered more difficult task (Beylin et al., 2001; Woodruff-Pak et al., 2007), we had hypothesized that deficits would be evident earlier than previously reported in the delay EBCC task (24 months in hearing-intact CBA mice; Woodruff-Pak et al., 2010). One potential explanation for the current results is the use of a hybrid mouse strain. Heterosis, or hybrid vigor, is the phenomenon whereby the offspring of inbred parents display an enhanced phenotype (Shull, 1914; Hannon et al., 2010). Behavioral superiority of hybrid strains in tasks of learning and memory, including the MWM, is commonly reported (Owen et al., 1997). We have noticed that the CB6F1 mice tested show superior MWM acquisition ability than C57BL/6 mice of comparable ages (unpublished data), which suggests that age-related deficits may be obscured by strong hybrid performance.
The exact mechanism for the age-related loss of Purkinje neurons is not known. These cells are particularly vulnerable, due to high metabolic activity. Purkinje neurons fire at a high rate during both sleep and wake cycles and require a constant replenishment of oxygen and glucose (Woodruff-Pak et al., 2001). They are vulnerable to hypoxia (Welsh et al., 2002) and toxins (Goodlett & Eilers, 1997; Breton et al., 1998; Cavanagh et al., 1998; Pierce et al., 1999; Green, 2004). The cerebellum in particular is sensitive to the effects of oxidative stress (Wang & Michaelis, 2010). Thus, it appears that a lifetime of insults, combined with age-related increase in oxidative stress (Sykora et al., 2013), may contribute to the age-related loss of Purkinje neurons.
The analysis of hippocampus- and cerebellum-dependent learning across the adult life span of the CB6F1 mouse adds to the literature regarding differential effects of aging in these substrates. We have replicated and extended results of earlier aging in anatomical measures of the cerebellum relative to the hippocampus to a new strain of mouse. Age-related impairment in this strain was found at 25 months in the trace EBCC task, although learning deficits were not associated with the age-related loss of Purkinje neurons in the cerebellar cortex. No evidence of impairment was found in the hippocampus-dependent CPFE fear conditioning and MWM tasks, nor did hippocampus volume change across the adult life span. Spatial learning impairment (at 25 mo) was only seen in the retention test on the Barnes maze. Aged mice (12–25 mo) were impaired in the cerebellum-dependent rotorod task, although a potential weight confound was reported. In this study we incorporated measures to improve detection of age-related impairment in learning and memory, such as the more difficult trace EBCC paradigm and the hippocampus-sensitive CPFE task. Significant age-related deficits were reported, although the robust learning ability of CB6F1 hybrid mice may have obscured the detection of impairment at earlier ages. Taken together, these results (from tasks considered more age sensitive) support the finding of differential rates of aging in the hippocampus and cerebellum.
Highlights.
We compared age effects on hippocampus- and cerebellum-dependent learning in CB6F1 mice
Tasks were chosen to increase the ability to detect age-related changes in learning
Rotorod impairment began at 12 mo and correlated with Purkinje neuron loss
Trace EBCC deficits began at 25 mo, but CPFE fear conditioning was not impaired
Results support the finding of differential aging in the hippocampus and cerebellum
Acknowledgments
The authors are grateful to David Comalli, Christopher de Solis and Jennifer Gooch for assisting in behavioral testing and care of mice and to Daniel Han for assistance with hippocampus stereology. This research was supported by grants from the National Institute on Aging, 1 R01 AG021925 and 1 R01 AG023742 to DSW-P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest.
Author Contributions: Research design, data analysis and manuscript writing: JAK, KLB, DSW-P
References
- Anagnostaras SG, Wood SC, Shuman T, Cai DJ, LeDuc AD, Zum KR, et al. Automated assessment of Pavlovian conditioned freezing and shock reactivity in mice using the VideoFreeze system. Front Behav Neurosci. 2010;4:1–11. doi: 10.3389/fnbeh.2010.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Gunderson H, Pakkenberg B. Aging of the human cerebellum: A stereological study. J Comp Neurol. 2003;466:356–365. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, et al. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96(9):5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: Temporal discontinuity or task difficulty? Neurobiol Learn Mem. 2001;76(3):447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm Behav. 2003;43:124–131. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L, Ermini F, Long JM, Ingram DK, Jucker M. Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol Aging. 2004;25(3):333–340. doi: 10.1016/S0197-4580(03)00083-6. [DOI] [PubMed] [Google Scholar]
- Breton P, Bizot JC, Buee J, De La Manche I. Brain neurotoxicity of penitrem a: electrophysiological, behavioral and histopathological study. Toxicon. 1998;36(4):645–655. doi: 10.1016/s0041-0101(97)00084-6. [DOI] [PubMed] [Google Scholar]
- Brown KL, Agelan A, Woodruff-Pak DS. Unimpaired trace eyeblink classical conditioning in Purkinje cell degeneration (pcd) mutant mice. Neurobiol Learn Mem. 2010;93:303–311. doi: 10.1016/j.nlm.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KL, Kennard JA, Sherer DJ, Comalli DM, Woodruff-Pak DS. The context preexposure facilitation effect in mice: A dose-response analysis of pretraining scopolamine administration. Behav Brain Res. 2011;225:290–296. doi: 10.1016/j.bbr.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JB, Holton JL, Nolan CC, Ray DE, Naik JT, Mantle PG. The effects of the tremorgenic mycotoxin penitrem a on the rat cerebellum. Vet Pathol. 1998;35:53–63. doi: 10.1177/030098589803500105. [DOI] [PubMed] [Google Scholar]
- Chen G, Steinmetz JE. A general-purpose computer system for behavioral conditioning and neural recording experiments. Behav Res Methods Instrum Comput. 1998;30:384–391. [Google Scholar]
- Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: Acquisition and retention. Learn Mem. 2003;10(6):427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by Tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- de Fiebre NC, Sumien N, Forster MJ, de Fiebre CM. Spatial learning and psychomotor performance of C57BL/6 mice: age sensitivity and reliability of individual differences. AGE. 2006;28(3):235–253. doi: 10.1007/s11357-006-9027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in hybrid strains of mice: I. Inbred and F1. Hearing Res. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-h. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Cestari V, Filipkowski RK, McDonald RJ, Silva AJ. The dorsal hippocampus is essential for context discrimination but not for contextual conditioning. Behav Neurosci. 1998;112(4):863–874. doi: 10.1037//0735-7044.112.4.863. [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working-memory deficits assessed in the water maze. Neurobiol Aging. 1995;16(2):149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107(4):618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Galvez R, Cua S, Disterhoft JF. Age-related deficits in a forebrain-dependent task, trace-eyeblink conditioning. Neurobiol Aging. 2011;32(10):1915–1922. doi: 10.1016/j.neurobiolaging.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by 2 afferent systems in the rat dentate gyrus revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2(4):437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Contextual learning and cue association in fear conditioning in mice: a strain comparison and a lesion study. Behav Brain Res. 1998;95(2):191–203. doi: 10.1016/s0166-4328(97)00144-7. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Roder J. Spatial and nonspatial learning in mice: Effects of S100 beta overexpression and age. Neurobiol Learn Mem. 1996;66(2):143–154. doi: 10.1006/nlme.1996.0055. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: A stereological study of temporal windows of vulnerability. Alcohol Clin Exp Res. 1997;21(4):738–744. [PubMed] [Google Scholar]
- Goodlett CR, Hamre KM, West JR. Dissociation of spatial navigation and visual guidance performance in Purkinje cell degeneration (pcd) mutant mice. Behav Brain Res. 1992;47(2):129–141. doi: 10.1016/s0166-4328(05)80119-6. [DOI] [PubMed] [Google Scholar]
- Green JT. The effects of ethanol on the developing cerebellum and eyeblink classical conditioning. Cerebellum. 2004;3(3):178–187. doi: 10.1080/14734220410017338. [DOI] [PubMed] [Google Scholar]
- Green JT, Arenos JD, Dillon CJ. The effects of moderate neonatal ethanol exposure on eyeblink conditioning and deep cerebellar nuclei neuron numbers in the rat. Alcohol. 2006;39(3):135–150. doi: 10.1016/j.alcohol.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Hannon RM, Meek TH, Acosta W, Maciel RC, Schutz H, Garland T., Jr. Sex-specific heterosis in line crosses of mice selectively bread for high locomotor activity. Behav Genet. 2010;41:615–624. doi: 10.1007/s10519-010-9432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger LL, Lambert TJ, Frick KM. Age-dependent effects of environmental enrichment on spatial reference memory in male mice. Behav Brain Res. 2007;185(1):43–48. doi: 10.1016/j.bbr.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J, Buchwald J. Eyeblink conditioning deficits in the old cat. Neurobiol Aging. 1983;4(1):45–51. doi: 10.1016/0197-4580(83)90053-2. [DOI] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198(1):247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem. 2006;13(6):809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Chen H, Yang L, Tao ZF, Yan J, Zhang YH, Zhu ZR, Sun WZ, Huang W, Huang WQ, Sui JF. Changes of synaptic ultrastructure in the guinea pig interpositus nuclei associate with response magnitude and timing after trace eyeblink conditioning. Behav Brain Res 2012. 2012;226(2):529–537. doi: 10.1016/j.bbr.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Toward the behavioral assessment of biological aging in the laboratory mouse: Concepts, terminology and objectives. Exp Aging Res. 1983;9(4):225–238. doi: 10.1080/03610738308258457. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Jucker M. Developing mouse models of aging: a consideration of strain differences in age-related behavioral and neural parameters. Neurobiol Aging. 1999;20(2):137–145. doi: 10.1016/s0197-4580(99)00033-0. [DOI] [PubMed] [Google Scholar]
- Kennard JA, Woodruff-Pak DS. Age sensitivity of behavioral tests and brain substrates of normal aging in mice. Front Aging Neurosci. 2011a;3(9):1–22. doi: 10.3389/fnagi.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennard JA, Woodruff-Pak DS. Aging and exercise effects on motor learning and spatial memory. Ageing Res. 2011b;3(e2):4–11. [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109(2):195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Hirono M, Sugiyama T, Kawahara S, Nakao K, Kishio M, et al. Impaired delay but normal trace eyeblink conditioning in PLCβ4 mutant mice. Neuroreport. 2001b;12:2919–2922. doi: 10.1097/00001756-200109170-00033. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Kawahara S, Suzuki M, Mori H, Mishina M, Kirino Y. Classical conditioning in glutamate receptor subunit delta 2 mutant mice is impaired in the delay paradigm but not in the trace paradigm. Eur J Neurosci. 2001c;13:1249–1253. doi: 10.1046/j.0953-816x.2001.01488.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Suzuki M, Kawahara S, Kirino Y. Age-dependent impairment of delay and trace eyeblink conditioning in mice. Neuroreport. 2001a;12(15):3349–3352. doi: 10.1097/00001756-200110290-00040. [DOI] [PubMed] [Google Scholar]
- Koopmans G, Blokland A, van Nieuwenhuijzen P, Prickaerts J. Assessment of spatial learning abilities of mice in a new circular maze. Physiol Behav. 2003;79(4–5):683–693. doi: 10.1016/s0031-9384(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Kronforst-Collins MA, Disterhoft JF. Lesions of the caudal area of rabbit medial prefrontal cortex impair trace eyeblink conditioning. Neurobiol Learn Mem. 1998;69(2):147–162. doi: 10.1006/nlme.1997.3818. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Bensoula AN, Filali M. Rotorod sensorimotor learning in cerebellar mutant mice. Neurosci Res. 1995;22(4):423–426. doi: 10.1016/0168-0102(95)00916-h. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Motor coordination, exploration, and spatial learning in a natural mouse mutation (nervous) with Purkinje cell degeneration. Behav Genet. 2003a;33(1):59–66. doi: 10.1023/a:1021003600900. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. The effects of cerebellar damage on maze learning in animals. Cerebellum. 2003b;2(4):300–309. doi: 10.1080/14734220310017456. [DOI] [PubMed] [Google Scholar]
- Larsen J, Skalicky M, Viidick A. Does long-term physical exercise counteract age-related Purkinje cell loss? A stereological study of rat cerebellum. J Comp Neurol. 2000;428:213–222. [PubMed] [Google Scholar]
- Lister JP, Barnes CA. Neurobiological changes in the hippocampus during normative aging. Arch Neurol. 2009;66(7):829–833. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- Logue SE, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: Implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997a;80(4):1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Logue SE, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997b;111(1):104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav Neurosci. 2007;121(4):721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Meltzer J, Sutherland RJ. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp Brain Res. 1989;76(3):485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- Moreau PH, Cosquer B, Jeltsch H, Cassel JC, Mathis C. Neuroanatomical and behavioral effects of a novel version of the cholinergic immunotoxin mu p75-saporin in mice. Hippocampus. 2008;18(6):610–622. doi: 10.1002/hipo.20422. [DOI] [PubMed] [Google Scholar]
- Nairn JG, Bedi KS, Mayhew TM, Campbell LF. On the number of Purkine cells in the human cerebellum: Unbiased estimates obtained by using the—fractionator.”. J Comp Neurol. 1989;290:527–532. doi: 10.1002/cne.902900407. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Gallagher M, McKinney M. Analyses of hippocampal circuitry in aging. Neurobiol Aging. 1999;20(3):359–360. doi: 10.1016/s0197-4580(99)00054-8. [DOI] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F-1 hybrids: Implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80(4):1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Pakaprot N, Kim S, Thompson RF. The role of the cerebellar interpositus nucleus in short and long term memory for trace eyeblink conditioning. Behav Neurosci. 2009;123(1):54–61. doi: 10.1037/a0014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon M, Perez-Diaz F, Joubert C, Cohen-Salmon C. Age-dependent effects of a chronic ultramild stress procedure on open-field behavior in B6D2F1 female mice. Physiol Behav. 2000;70(1–2):7–13. doi: 10.1016/s0031-9384(00)00216-x. [DOI] [PubMed] [Google Scholar]
- Patil SS, Sunyer B, Hoger H, Lubec G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav Brain Res. 2009;198(1):58–68. doi: 10.1016/j.bbr.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Petrosini L, Leggio MG, Molinari M. The cerebellum in the spatial problem solving: A co-star or guest star? Prog Neurobiol. 1998;56:191–210. doi: 10.1016/s0301-0082(98)00036-7. [DOI] [PubMed] [Google Scholar]
- Pietrelli A, Lopez-Costa J, Goni R, Brusco A, Basso N. Aerobic exercise prevents age-dependent cognitive decline and reduces anxiety-related behaviors in middle aged and old rats. Neuroscience. 2012;202:252–266. doi: 10.1016/j.neuroscience.2011.11.054. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: No loss of principal hippocampal and subicular neurons. Neurobiol Aging. 1996;17(1):143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Roullet P, Lassalle JM. Radial maze learning using exclusively distant visual cues reveals learners and nonlearners among inbred mouse strains. Physiol Behav. 1995;58(6):1189–1195. doi: 10.1016/0031-9384(95)02066-7. [DOI] [PubMed] [Google Scholar]
- Roullet P, Lassalle JM, Jegat R. A study of behavioral and sensorial bases of radial maze learning in mice. Behav Neural Biol. 1993;59:173–179. doi: 10.1016/0163-1047(93)90926-9. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O’Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neurosci Biobehav Rev. 2004;8:675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn Aff Behav Neurosci. 2001;1:66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learn Mem. 2009;16(10):573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Pinelo-Nava MT. Stress and memory: Behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:1–20. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serradj N, Jamon M. Age-related changes in the motricity of the inbred mice strains 129/sv and C57BL/6j. Behav Brain Res. 2007;177(1):80–89. doi: 10.1016/j.bbr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Shull AF. Duplicate genes for capsule form in Bursa bursa-pastoris. Mol Gen Genet. 1914;12(1):97–149. [Google Scholar]
- Smith TD, Calhoun HE, Rapp PR. Circuit and morphological specificity of synaptic change in the aged hippocampal formation. Neurobiol Aging. 1999;20(3):357–358. doi: 10.1016/s0197-4580(99)00073-1. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Pomerleau D, Bennett L, James J, Morse DL. Acquisition of the classically-conditioned eyeblink response in humans over the life-span. Psychol Aging. 1989;4(1):34–41. doi: 10.1037//0882-7974.4.1.34. [DOI] [PubMed] [Google Scholar]
- Sternberg EM, Glowa JR, Smith MA, Calogero AE, Listwak SJ, Aksentijevich S, et al. Corticotropin releasing hormone related behavioral and neuroendocrine responses to stress in Lewis and Fischer rats. Brain Res. 1992;570(1–2):54–60. doi: 10.1016/0006-8993(92)90563-o. [DOI] [PubMed] [Google Scholar]
- Sykora P, Willson DM, III, Bohr VA. Base excision repair in the mammalian brain: Implications for age related neurodegeneration. Mech Ageing Dev. 2013 doi: 10.1016/j.mad.2013.04.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF. The neurobiology of learning and memory. Science. 1986;233(4767):941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Thouvarecq R, Protais P, Jouen F, Caston J. Influence of cholinergic system on motor learning during aging in mice. Behav Brain Res. 2001;118(2):209–218. doi: 10.1016/s0166-4328(00)00330-2. [DOI] [PubMed] [Google Scholar]
- Tseng W, Guan R, Disterhoft JF, Weiss C. Trace eyeblink conditioning is hippocampally dependent in mice. Hippocampus. 2004;14(1):58–65. doi: 10.1002/hipo.10157. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao CM, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel RW, Ewers M, Ross C, Gould TJ, Woodruff-Pak DS. Age-related impairment in the 250-millisecond delay eyeblink classical conditioning procedure in C57BL/6 mice. Learn Mem. 2002;9(5):321–336. doi: 10.1101/lm.50902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72(1–2):271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2(12):1–13. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weible AP, McEchron MD, Disterhoft JF. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav Neurosci. 2000;114(6):1058–1067. doi: 10.1037//0735-7044.114.6.1058. [DOI] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav Brain Res. 1999;99(2):123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O’Hearn E, et al. Why do Purkinje cells die so easily after global brain ischemia? Adolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol. 2002;89:331–359. [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;2002;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14(4):287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal aging and Alzheimer’s disease. Lancet. 1994;344(8925):769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ. A comparison of rats and mice in a swimming pool place task and matching to place task: Some surprising differences. Physiol Behav. 1995;58(4):687–693. doi: 10.1016/0031-9384(95)00110-5. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Of mice and mazes: Similarities between mice and rats on dry land but not water mazes. Physiol Behav. 1996;60(5):1191–1197. doi: 10.1016/s0031-9384(96)00176-x. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS. Stereological estimation of Purkinje neuron number in C57BL/6 mice and its relation to associative learning. Neuroscience. 2006;141(1):233–243. doi: 10.1016/j.neuroscience.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Disterhoft JF. Where is the trace in trace conditioning? Trends Neurosci. 2008;31:105–112. doi: 10.1016/j.tins.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Finkbiner Larger nondeclarative than declarative deficits in learning and memory in human aging. Psychol Aging. 1995;10(3):416–426. doi: 10.1037//0882-7974.10.3.416. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Green JT, Levin SI, Meisler MH. Inactivation of sodium channel Scn8A (Na(v)1.6) in Purkinje neurons impairs learning in Morris water maze and delay but not trace eyeblink classical conditioning. Behav Neurosci. 2006;120(2):229–240. doi: 10.1037/0735-7044.120.2.229. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Jaeger ME. Predictors of eyeblink classical conditioning over the adult age span. Psychol Aging. 1998;13(2):193–205. doi: 10.1037//0882-7974.13.2.193. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res. 1985;348:249–260. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink classical conditioning: Applications in humans. Vol. 1. Boston: Kluwer Academic Publishers; 2000a. [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink classical conditioning: Animal models. Vol. 2. Boston: Kluwer Academic Publishers; 2000b. [Google Scholar]
- Woodruff-Pak DS, Thompson RF. Classical conditioning of the eyeblink response in the delay paradigm in adults aged 18–83 years. Psychol Aging. 1988;3(3):219–229. doi: 10.1037//0882-7974.3.3.219. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KPT, et al. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci USA. 2010;107(4):1624–1629. doi: 10.1073/pnas.0914207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Green JT, Levin SI, Meisler MH. Inactivation of sodium channel Scn8A (Na(v)1.6) in Purkinje neurons impairs learning in Morris water maze and delay but not trace eyeblink classical conditioning. Behav Neurosci. 2006;120(2):229–240. doi: 10.1037/0735-7044.120.2.229. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Seta SE, Roker LA, Lehr MA. Effects of paradigm and inter-stimulus interval on age differences in eyeblink classical conditioning in rabbits. Learn Mem. 2007;14(4):287–294. doi: 10.1101/lm.504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Vogel RW, Ewers M, Coffey J, Boyko OB, Lemieux SK. MRI-assessed volume of cerebellum correlates with associative learning. Neurobiol Learn Mem. 2001;76(3):342–357. doi: 10.1006/nlme.2001.4026. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Wiley RG. Lack of effect of moderate purkinje cell loss on working memory. Neuroscience. 2001;107(3):433–445. doi: 10.1016/s0306-4522(01)00326-8. [DOI] [PubMed] [Google Scholar]