Abstract
Objectives
To test the hypotheses that ischemia during stress testing has prognostic value and that it identifies those coronary artery disease (CAD) patients with left ventricular (LV) dysfunction who derive the greatest benefit from coronary artery bypass graft surgery (CABG) compared to medical therapy.
Background
The clinical significance of stress-induced ischemia in patients with CAD and moderately to severely reduced LV ejection fraction (EF) is largely unknown.
Methods
The Surgical Treatment of IsChemic Heart failure (STICH) trial randomized patients with CAD and EF ≤35% to CABG or medical therapy. In this study, we assessed the outcomes of those STICH patients who underwent either a radionuclide (RN) stress test or a dobutamine stress echocardiogram (DSE). A test was considered positive for ischemia by RN if the summed difference score (difference in tracer activity between stress and rest) was ≥4 or if ≥2 of 16 segments were ischemic during DSE. Clinical endpoints were assessed by intention-to-treat during a median follow-up of 56 months.
Results
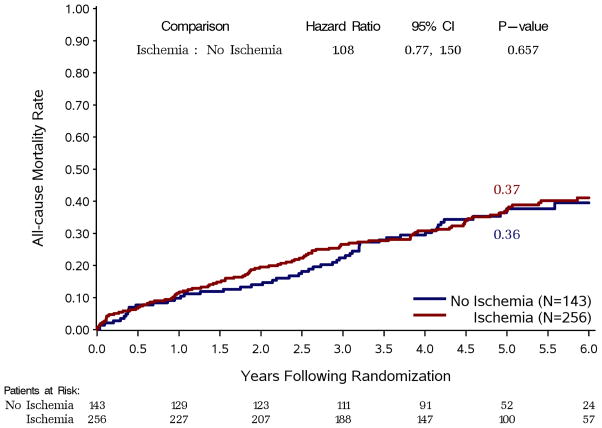
Of the 399 study patients (51 women, mean EF 26±8%), 197 were randomized to CABG and 202 to medical therapy. Myocardial ischemia was induced during stress testing in 256 patients (64% of the study population). Patients with and without ischemia were similar in age, multi-vessel CAD, previous myocardial infarction, LV EF, LV volumes, and treatment allocation (all p=NS). There was no difference between patients with vs. those without ischemia in all-cause mortality (hazard ratio: 1.08; 95% CI: 0.77–1.50; p=0.66), cardiovascular mortality, or all-cause mortality plus cardiovascular hospitalization. There was no interaction between ischemia and treatment for any clinical endpoint.
Conclusions
In CAD with severe LV dysfunction, inducible myocardial ischemia does not identify patients with worse prognosis or those with greater benefit from CABG over optimal medical therapy.
Clinical Trial ID: ClinicalTrials.gov number, NCT00023595
Keywords: coronary artery disease, left ventricular dysfunction, myocardial ischemia, heart failure, outcomes
It is widely accepted that, among patients with coronary artery disease (CAD), the presence of myocardial ischemia induced during stress testing is associated with worse prognosis and plays a role in the decision for myocardial revascularization (1–3). The evidence substantiating such a critical significance of stress-induced ischemia has emanated largely from studies in patients with normal or only mildly impaired left ventricular (LV) systolic function (4–6). In fact, until the recent publication of the Surgical Treatment of IsChemic Heart failure (STICH) trial (7), none of the contemporary studies addressing the impact of revascularization on outcome of CAD patients included those with moderately or severely reduced LV ejection fraction (EF) (8–10). Hence, the clinical relevance of identifying the presence of inducible ischemia in these patients is fundamentally unknown.
A growing number of patients with CAD present with heart failure associated with LV systolic dysfunction as a consequence of previous myocardial infarction(s) (11). In these patients, improvement in LV function with revascularization may be expected if there is a significant amount of hypocontractile but viable myocardium. This concept has been postulated based on the results of retrospective cohort studies and meta-analyses (12–15), but not proven in prospective trials. Indeed, the recent viability substudy of the STICH trial failed to show an interaction between myocardial viability and the effect of coronary bypass graft surgery (CABG) over optimal medical therapy on clinical outcomes (16).
It is conceivable that the salutary effects of revascularization are not mechanistically linked to the presence or extent of viable myocardium, but rather to the overall extent of jeopardized myocardium that might be identified by the presence of inducible ischemia on stress testing. However, there is no prospective randomized study to date demonstrating the significance of ischemia in patients with CAD and LV dysfunction. Hence, the present study was conducted in the STICH trial population to test the hypotheses that the presence of inducible myocardial ischemia identifies those patients with CAD and LV dysfunction with worse prognosis and those who derive the greatest benefit from CABG compared to medical therapy.
METHODS
Study Population
STICH is a prospective, multicenter, randomized trial sponsored by the National Heart, Lung, and Blood Institute (NHLBI) that recruited 2,136 patients with CAD and LV EF ≤35% between 2002 and 2007. The trial addressed two primary hypotheses: 1) that CABG combined with optimal medical therapy improves survival compared to optimal medical therapy alone (surgical revascularization hypothesis), and 2) that surgical ventricular reconstruction added to CABG improves survival free of cardiovascular hospitalization compared to CABG alone in patients with significant anterior wall akinesis (surgical ventricular reconstruction hypothesis). The trial design and the results of the two primary hypotheses have been reported (7,17,18). Only the 1,212 patients in the surgical revascularization hypothesis were considered for this study. The inclusion and exclusion criteria and the requirements for insuring high-quality surgical revascularization have been described previously (17). The NHLBI and the ethics committee at each recruiting institution approved the study protocol. All patients provided written informed consent. A “risk at randomization” (RAR) score was calculated for each patient with an equation derived using multiple variables with known predictive power (19).
Although non-invasive tests were initially mandated as part of the STICH trial protocol, this requirement was discontinued to facilitate patient enrollment. Hence, only a proportion of patients included in STICH could be considered for inclusion into this study. Specifically, those patients who had either a radionuclide (RN) stress test or a dobutamine stress echocardiogram (DSE) within 90 days of randomization and prior to the initiation of therapy allocated by randomization were selected.
Stress Testing
For RN stress testing, several protocols were allowed, including exercise, dobutamine, or vasodilator stress with adenosine or dipyridamole. Technetium-99m sestamibi or tetrofosmin or thallium-201 was injected one minute prior to the end of stress. Patients exercised to the development of fatigue, chest pain, or ST segment deviation, as is customary in clinical practice. For adenosine stress, the protocol used a 6-minute adenosine infusion (0.14 mg/kg/min) with radiotracer injection 3 minutes into the infusion. For dipyridamole stress, the tracer was injected 3 minutes after the 4-minute infusion (0.14 mg/kg/min). For DSE, conventional parasternal and apical images were obtained at rest and during the infusion of incremental doses of dobutamine. A DSE test was considered suitable to assess presence or absence of inducible ischemia (and hence included into this study), if it met at least one of the following criteria: 1) achievement of a dobutamine dose ≥30 μg/kg/min; 2) achievement of ≥85% of age-predicted maximum heart rate; 3) presence of myocardial ischemia (as defined below).
RN and DSE images were reviewed at core laboratories, independently funded by the NHLBI, by investigators blinded to treatment assignment and all individual patient characteristics. Criteria for presence or absence of myocardial ischemia were defined prospectively and separately for each method without knowledge of baseline characteristics, results of other tests, or follow-up outcomes.
For RN studies, a semi-quantitative visual assessment of myocardial perfusion was performed using a 17-segment model of the LV (20). In each segment, tracer activity was assessed using a 5-point scale, where 0 = normal and 4 = absent uptake of tracer. The scores for each of the 17 segments were summed to give the summed stress and summed rest scores. When dedicated viability imaging was performed along with the stress study, the “viability” images were used for the rest score. The summed difference score (SDS) was obtained by subtracting the stress from the rest score and thus reflects the extent of the ischemic defect. A RN test was considered as positive for myocardial ischemia when the SDS was ≥4. As a reflection of the overall magnitude of ischemia, combining both ischemic extent and severity of ischemia, the SDS was also analyzed as a continuous variable expressed as the percent of the maximum possible SDS (5,21,22).
For DSE analysis, the LV was divided into 16 segments and systolic wall thickening was assessed separately for each segment (23). A segment was considered to display inducible myocardial ischemia when systolic wall thickening during the infusion of dobutamine worsened when compared to that seen at baseline or during the preceding dose (24). A patient was considered to have a DSE positive for ischemia when an ischemic response was observed in ≥2 LV segments (25). Myocardial ischemia was also analyzed as a continuous variable expressed as the number (and percent) of ischemic segments.
In patients who had both RN and DSE tests, the presence of an ischemic response on either test was considered sufficient for the demonstration of ischemia.
Follow-up and Outcomes
After enrollment, patients were followed every 4 months for the first year and every 6 months thereafter. The primary outcome was death from any cause. Secondary end points included death from cardiovascular causes and a composite of death from any cause or hospitalization for cardiovascular causes. Definitions of the trial end points have been previously reported (7). All death causes were adjudicated by an independent clinical events committee. Median follow-up was 56 months.
Statistical Analyses
Baseline patient characteristics are descriptively summarized using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Because stress test information was not available in every patient, we first examined the baseline characteristics and clinical outcomes of patients for whom a stress-test was available compared to the patients without a stress test. The distributions of continuous or ordinal variables were compared between groups using the Wilcoxon rank-sum test, and categorical variables were compared using the chi-square test or Fisher’s Exact Test. The primary and secondary endpoints were compared between patients in the stress-test cohort and the patients without a stress test. Event-rate estimates in each group and for each endpoint were calculated using the Kaplan-Meier method (26) and statistically compared using the log-rank test (27). Relative risks, expressed as hazard ratios with associated 95% confidence intervals, were derived using the Cox regression model (28). The log-rank test and Cox model were also used to examine the randomized treatment comparisons with respect to the primary and secondary endpoints among the patients in the stress-test cohort and in the excluded patients to assess comparability of treatment comparisons relative to the overall trial results.
Similar analyses to those described above were also performed to compare the baseline characteristics, the primary and secondary endpoints, and the CABG versus medical treatment comparisons in the patients with versus those without myocardial ischemia during stress test (treating ischemia as a dichotomous variable). In addition, we addressed the question of whether there was a differential effect of CABG in patients with vs. those without demonstrated ischemia. This assessment was performed by testing for the presence of a treatment by ischemia interaction using the Cox model. Finally, ischemia was examined as a continuous variable with the Cox model to assess the relationship of the amount of ischemia with the primary and secondary clinical endpoints.
The various treatment comparisons described above were performed with the treatment groups defined according to the randomized treatment assignments (intention-to-treat). As previously reported (7), because of treatment crossovers, some patients did not receive the treatment to which they were randomized. Supplementary analyses were performed based on the treatment the patients actually received (“as treated”), and excluding crossover patients (“per-protocol”).
RESULTS
Study Population
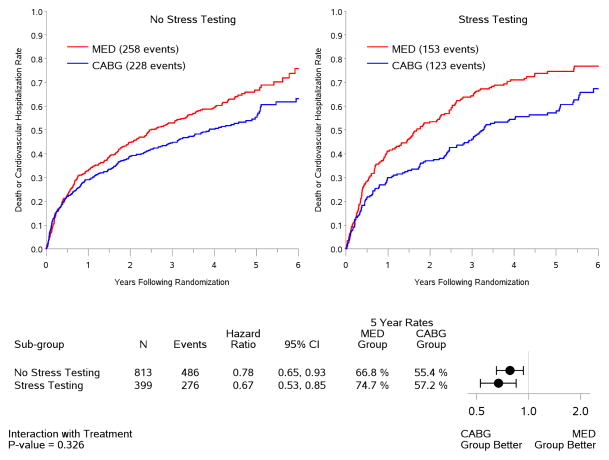
A total of 399 patients (or 33% of those enrolled into the STICH revascularization hypothesis trial) fulfilled the inclusion criteria and were included in this study. There were 348 men and 51 women. Mean age was 61±10 years and mean EF was 26±8%. Of the 399 study patients, 219 had a RN test and 205 had a DSE. Both tests were available for analysis in 25 patients. Table 1 shows a comparison of key baseline characteristics between the 399 study patients and the 813 enrolled in STICH who did not meet the inclusion criteria for this study. There was a greater proportion of white patients among those included in this study, due to a higher rate of ischemia tests performed in European countries where white race is more prevalent. The RAR score was similar in the two groups. There were less advanced heart failure presentations and a trend toward less advanced angina in the patients included in this study. Of note, patients with ischemia testing had lower LV EF and larger LV volumes, and a higher rate of ICD use during the study. These findings notwithstanding, there were no statistically significant differences between the patients included and those excluded from this analysis in terms of all-cause mortality (p=0.36) or cardiovascular mortality (p=0.32). Similarly, there was no significant difference in the treatment effect of CABG plus medical therapy versus medical therapy alone among the patients included in this study compared to those excluded due to the lack of ischemia data (Figure 1).
Table 1.
Comparison of Key Baseline Variables in STICH Patients With and Without Ischemia Testing
| Variable | Patients With Ischemia Testing (n= 399) | Patients Without Ischemia Testing (n= 813) | P value |
|---|---|---|---|
| Age (years) (mean±SD) | 61±10 | 60±9 | 0.07 |
| Female (n (%)) | 51 (13%) | 97 (12%) | 0.67 |
| White race (n (%)) | 331 (83%) | 496 (61%) | <0.0001 |
| Body mass index (mean±SD) | 27±4 | 27±5 | 0.31 |
| History of previous myocardial infarction (n (%)) | 299 (75%) | 635 (78%) | 0.22 |
| Previous CABG (n (%)) | 11 (3%) | 25 (3%) | 0.76 |
| Previous PCI (n (%)) | 59 (15%) | 97 (12%) | 0.16 |
| Advanced angina*(n (%)) | 13 (3%) | 45 (6%) | 0.08 |
| Advanced heart failure† (n (%)) | 127 (32%) | 320 (39%) | 0.01 |
| Multi-vessel disease‡ (n (%)) | 289 (72%) | 615 (76%) | 0.21 |
| LV EF (%) (mean±SD) | 26±8 | 29±8 | <0.0001 |
| ESVI§ (ml/m2) (mean±SD) | 92±38 | 82±35 | <0.0001 |
| EDVI¶ (ml/m2) (mean±SD) | 123±41 | 114±38 | 0.001 |
| ICD# use n (%)) | 86 (22%) | 117 (14%) | 0.002 |
| Risk at randomization score (mean±SD) | 13±9 | 13±9 | 0.66 |
Canadian Cardiac Society Class III or IV
New York Heart Association Functional Class III or IV
Presence of ≥75% stenosis in two or three coronary arteries
End-systolic volume index
End-diastolic volume index
Intra-cardiac defibrillator use at any point during the study
Figure 1. Kaplan-Meier rate estimates of all-cause mortality (panel A), cardiovascular mortality (panel B), and all-cause mortality or cardiovascular hospitalization (panel C).
The 813 STICH patients with no evaluable stress testing and hence excluded from this study are shown on the left panels. The 399 patients with stress testing included in this study are shown on the right panels. Analysis based on intention-to-treat. MED= medical therapy; CABG= coronary artery bypass graft surgery.
Inducible Myocardial Ischemia
Of the 399 study patients, 256 (64%) had demonstrable myocardial ischemia during stress testing and 143 (36%) did not. Notably, the prevalence of a positive ischemic response was similar among patients with a RN test (135 of 219, or 62%) and those with a DSE test (129 of 205, or 63%). Table 2 shows a comparison of key characteristics between patients with versus those without myocardial ischemia. None of the baseline characteristics were significantly different between the two groups, although there was a trend for a higher RAR score index in patients with ischemia.
Table 2.
Comparison of Key Baseline Variables between Patients With and Without Inducible Myocardial Ischemia on Stress Testing
| Variable | Patients With Ischemia (n= 256) | Patients Without Ischemia (n= 143) | P value |
|---|---|---|---|
| Age (years) (mean±SD) | 61±10 | 60±9 | 0.27 |
| Female (n (%)) | 29 (11%) | 22 (15%) | 0.24 |
| White race (n (%)) | 212 (83%) | 119 (83%) | 0.95 |
| Body mass index (mean±SD) | 27±4 | 28±5 | 0.09 |
| History of previous myocardial infarction (n (%)) | 187 (73%) | 112 (78%) | 0.24 |
| Previous CABG (n) | 9 (4%) | 2 (1%) | 0.34 |
| Previous PCI (n (%)) | 42 (16%) | 17 (12%) | 0.22 |
| Advanced angina* (n (%)) | 9 (4%) | 4 (3%) | 0.78 |
| Advanced heart failure† (n (%)) | 81 (32%) | 46 (32%) | 0.91 |
| Multi-vessel disease‡ (n (%)) | 192 (75%) | 97 (68%) | 0.12 |
| LV EF (%) (mean±SD) | 26±8 | 26±8 | 0.38 |
| ESVI§ (ml/m2) (mean±SD) | 91±37 | 94±39 | 0.55 |
| EDVI¶ (ml/m2) (mean±SD) | 121±41 | 125±41 | 0.31 |
| ICD# use n (%)) | 57 (22%) | 29 (20%) | 0.64 |
| Risk at randomization score (mean±SD) | 14±9 | 12±9 | 0.07 |
| Percent of ischemic myocardium (mean±SD) | 18±11 | 2±2 | <0.0001 |
Canadian Cardiac Society Class III or IV
New York Heart Association Functional Class III or IV
Presence of ≥75% stenosis in two or three coronary arteries
End-systolic volume index
End-diastolic volume index
Intra-cardiac defibrillator use at any point during the study
When ischemia was analyzed as a continuous variable, the percent ischemic myocardium was 12.2±11.5% for the 399 patients included in the study and, as expected, was higher among those with an ischemic response (Table 2). The amount of ischemic myocardium was ≥ 10% in 199 patients (or 50% of the study group) and ≥20% in 75 patients (or 19% of the study group).
Effect of Ischemia on Events during Follow-Up
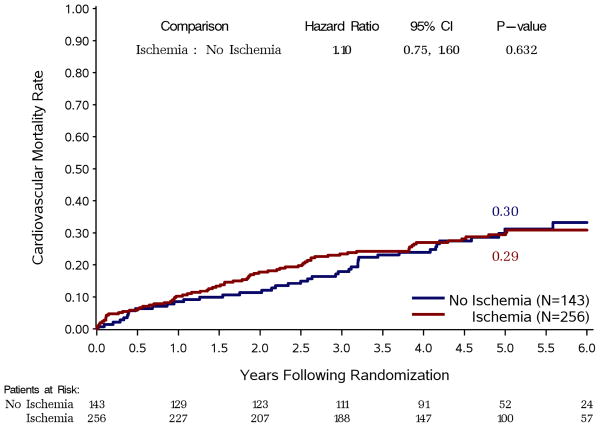
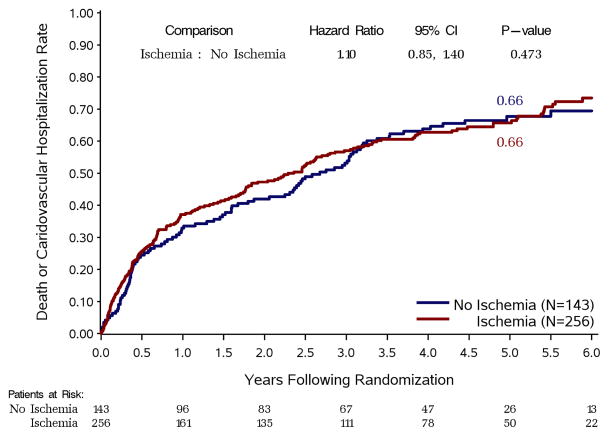
When myocardial ischemia was analyzed as a dichotomous variable, there was no difference in all-cause mortality between patients with and without ischemia (Figure 2). Similarly, there were no differences in outcomes between these two groups for the secondary endpoints of cardiovascular mortality (Figure 3, panel A) or death plus cardiovascular hospitalization (Figure 3, panel B).
Figure 2. Kaplan-Meier estimates of all-cause mortality rates.
Study patients are divided according to the presence or absence of ischemia on stress testing, regardless of treatment allocation.
Figure 3. Kaplan-Meier rate estimates of cardiovascular mortality (panel A) and all-cause mortality plus cardiovascular hospitalization (panel B).
Study patients are divided according to the presence or absence of ischemia on stress testing, regardless of treatment allocation.
The impact of ischemia on clinical outcomes was also analyzed treating ischemia as a continuous variable. No relationship was observed between the amount of ischemic myocardium and the probability of an adverse outcome for all-cause mortality (p= 0.28), cardiovascular mortality (p=0.07) or death plus cardiovascular hospitalization (p=0.79). These findings were similar when the data were analyzed separately for patients with RN tests or with DSE tests (data not shown).
Interaction between Ischemia and Treatment
Of the 399 patients included in the study, 197 were randomized to CABG and 202 to medical therapy. The number of patients with an ischemic response was similar in the two treatment groups: 129/197 (or 66%) in the CABG group and 127/202 (or 63%) in the medical therapy group (p=0.59).
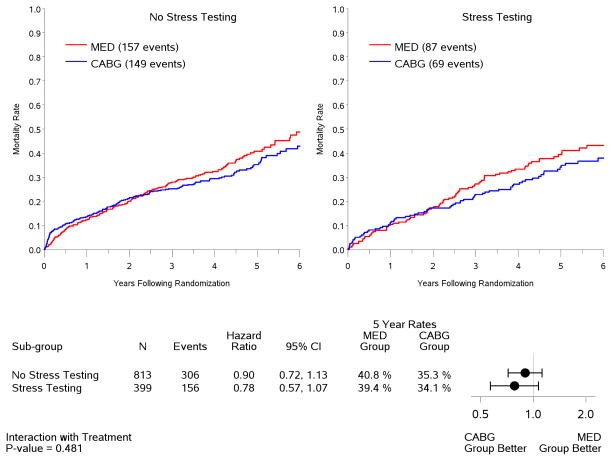
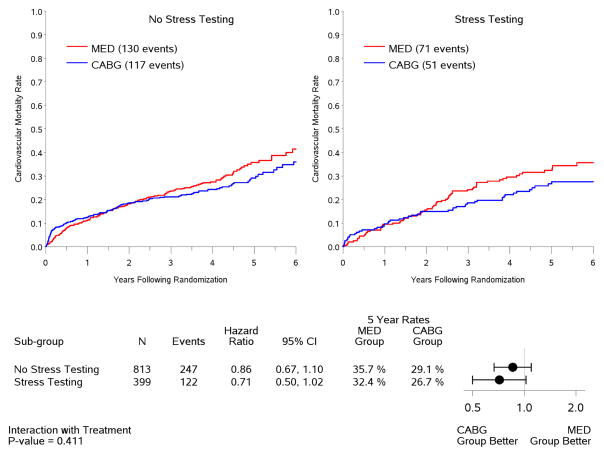
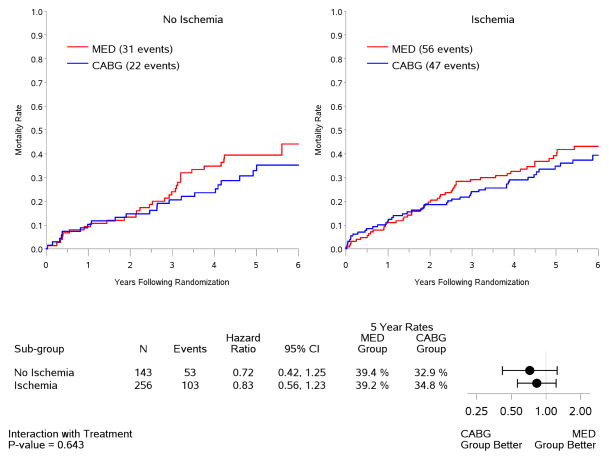
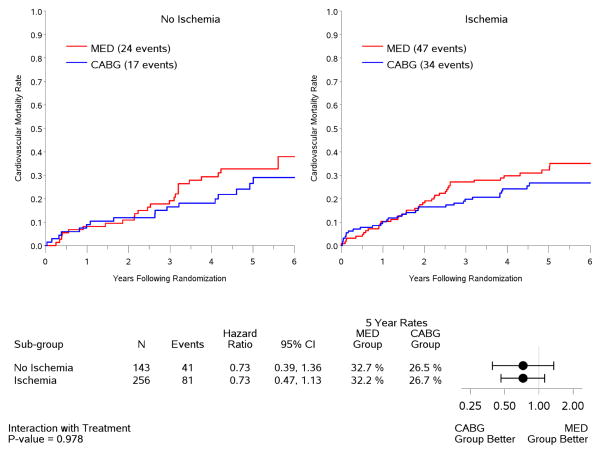
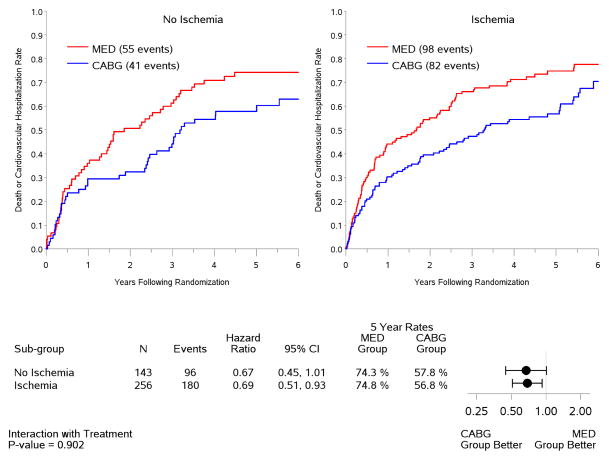
In the intention-to-treat analysis, there was a trend toward decreased all-cause mortality (p=0.13) and cardiovascular mortality (p=0.07), and a significant benefit in terms of death or cardiovascular hospitalization (p=0.001) for CABG compared to medical therapy, similar to the results observed for the entire population of 1,212 patients included in the STICH trial surgical revascularization hypothesis (7). However, no interaction was observed between the treatment effects of CABG over medical therapy and the presence or absence of myocardial ischemia for all-cause mortality (Figure 4) or either of the secondary endpoints (Figure 5). When myocardial ischemia was assessed as a continuous variable, there was no significant interaction between the extent of ischemia and the treatment effect of CABG on all-cause mortality (p=0.73,) cardiovascular mortality (p=0.79), or death plus cardiovascular hospitalization (p=0.89). Similar findings were observed when patients were grouped according to treatment received (i.e., “as treated” analysis) and when patients who crossed over from the randomized allocated treatment arm were excluded (i.e., “per protocol” analysis) (Table 3).
Figure 4. Kaplan-Meier estimates of all-cause mortality rates according to treatment among patients with (right panel) or without (left panel) ischemia.
Analysis based on intention-to-treat. MED= medical therapy; CABG= coronary artery bypass graft surgery.
Figure 5. Kaplan-Meier rate estimates of cardiovascular mortality (panel A) and all-cause mortality plus cardiovascular hospitalization (panel B) according to treatment among patients with (right panels) or without (left panels) ischemia.
Analysis based on intention-to-treat. MED= medical therapy; CABG= coronary artery bypass graft surgery.
Table 3.
P values for the statistical assessment of an interaction between treatment (CABG v. MED) and presence or absence of myocardial ischemia for each of the pre-determined clinical endpoints, according to the treatment received by each patient (“as treated”) and after excluding patients who did not receive the treatment assigned by randomization (“per protocol”).
| Clinical endpoint | “As Treated” Analysis | “Per Protocol” Analysis |
|---|---|---|
| All-Cause Mortality | 0.28 | 0.41 |
| Cardiovascular Mortality | 0.55 | 0.70 |
| Death or Cardiovascular Hospitalization | 0.58 | 0.69 |
DISCUSSION
Despite the accepted significance of ischemia on stress testing, the evidence supporting its role in the treatment decisions for CAD patients with LV dysfunction is inadequate, emanating from studies conducted in patients with normal or slightly reduced LV systolic function (4), from retrospective assessment of registries (21), or from prospective trials with limited assessment of ischemia (5,8). Further, a major ongoing effort to determine the best management strategy for patients with stable CAD and at least moderate ischemia– the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial -- will exclude patients with EF <35% (ClinicalTrials.gov number, NCT01471522).
Present Study Findings
This study results indicate that the presence of inducible ischemia on stress testing in patients with CAD and severe LV dysfunction (mean EF: 26%) is not associated with worse prognosis nor does it identify those with greater therapeutic benefit from surgical revascularization. Thus, despite the trend for decreased overall and cardiovascular mortality for CABG compared to medical therapy, no interaction was found between the treatment effect of surgical revascularization and the presence of ischemia. In essence, these results suggest that the therapeutic effect of CABG is not limited to patients with inducible myocardial ischemia on stress testing. Similar findings were observed in separate analyses performed according to the treatment actually received (“as treated” analysis) and excluding patients not receiving the treatment allocated by randomization (“per protocol” analysis).
Prior registry data have suggested a benefit of revascularization only when moderate-to-severe ischemia was present (22). However, in this study, the extent of myocardial ischemia was not associated with either worse prognosis or a beneficial effect of CABG. The number of patients with moderate-to-severe of ischemia was too small to provide a meaningful analysis of this subset. Nonetheless, and in support of these findings, when patients with extensive myocardial scarring were examined in the prior registry, the presence of ischemia no longer identified survival benefit from revascularization (22).
Limitations
The performance of a stress test was not mandated as part of the STICH trial protocol design and was left to the discretion of the recruiting investigators. This may have led to bias such that patients with severe ischemia were less likely to be included in the trial, thus limiting the number of patients in this cohort and reducing the statistical power to determine a treatment by ischemia interaction. Patients included in this study had more advanced forms of the disease, as expressed – for example – by lower EF and larger LV volumes. However, the outcomes of the 399 patients included in this study (regardless of treatment allocation) were similar to those of the other 813 STICH patients excluded from this analysis because of the lack of a stress test. In addition, the treatment effect of CABG over medical therapy was similar in the STICH patients included in this study and those excluded. Moreover, the randomized treatment assignment in the patients included in this study was similar to that of the entire STICH population with approximately half of the patients allocated to CABG. Finally, among the study patients, treatment allocation was also similar in patients with and without inducible ischemia. This indicates that there were likely no clinically meaningful biases to associate either the performance of a stress test or its results to the likelihood of randomization to surgical revascularization. Nevertheless, the power of the surgical revascularization hypothesis was calculated for the entire STICH trial population (7); therefore, no definitive conclusions regarding the treatment effect of CABG in patients with ischemic cardiomyopathy may be derived from the results of this sub-study.
We must also acknowledge that patients included in the study did not have serial testing to ascertain that CABG (or medical therapy) eliminated the presence of inducible ischemia. Hence, reduction of ischemia as a predictor of outcomes could not be analyzed as part of this study. Further, because we did not measure the presence or extent of scar, we cannot determine the additive value of the myocardial scarring information relative to that provided by the presence of ischemia. Finally, although the concept of complete revascularization was part of the STICH trial protocol (18), we did not have the ability to ascertain that the grafted coronary arteries corresponded to the location of myocardial ischemia in each individual study patient.
Potential Explanations and Implications
A number of potential explanations may account for the findings of this study and provide a framework for their interpretation. First, in the natural history process of coronary atherosclerosis, once severe LV dysfunction and remodeling have developed, the occurrence of inducible ischemia may not play a major role and may therefore become a less significant prognostic marker than in patients with milder forms of the disease. Second, the benefit of surgical revascularization may be related to the prevention of events that are not mechanistically linked to the induction of ischemia on stress testing. In this regard, a recent analysis on the mode of death in the STICH trial showed that CABG reduced the rate of sudden cardiac death and fatal myocardial infarction (29). In conjunction with those observations, the findings of this study suggest that the benefit of CABG is related to the protection of jeopardized myocardium when there is a subsequent arterial occlusion that might precipitate lethal arrhythmias, which is not causally associated to the lesion(s) responsible for ischemia during stress testing. Third, medical therapy may have also reduced myocardial ischemia, hence limiting the therapeutic value of surgical revascularization. Finally, the accuracy of imaging stress testing may be diminished in patients with severe LV dysfunction and remodeling, thus rendering the test less useful for its intended purpose.
The findings of this study have important clinical implications. First, these observations do not argue against a beneficial effect of CABG in patients with inducible ischemia. Instead, they indicate that the presence of ischemia does not select a group with greater benefit from revascularization. Based on these results, the demonstration of myocardial ischemia should not be viewed as a requisite for the indication of surgical revascularization in these patients (30). Hence, if indicated based on the patient’s clinical presentation, CABG should not be withheld because ischemia is not demonstrated on non-invasive studies. Nevertheless, when making clinical decisions, the physician must integrate all available information including location of ischemia, the possibility of imaging artifacts affecting the accuracy of the test, and feasibility of regional revascularization in order to formulate the best therapeutic choice for each individual patient.
Viability and Ischemia
We have previously reported that the assessment of myocardial viability in these patients does not identify those with greater therapeutic benefit of CABG (16). It must be noted that viability and inducible ischemia are distinct concepts and phenomena – all myocardial segments showing inducible ischemia must be viable, but not all viable segments are ischemic on stress testing. Further, not all segments with inducible ischemia are dysfunctional at rest. Hence, the findings of this study complement those previous observations and provide the basis for a more thorough understanding of the role of non-invasive imaging in the evaluation of patients with CAD and severe LV dysfunction. In this regard, the present study findings are in agreement with previous retrospective reports showing that, even among patients in whom viability information was predictive of long-term prognosis, the presence of inducible ischemia was not associated with benefit from revascularization (31).
Acknowledgments
This work was supported by the National Institutes of Health (ClinicalTrials.gov number, NCT00023595)
ABBREVIATIONS
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- DSE
dobutamine stress echocardiogram
- EF
ejection fraction
- LV
left ventricle or left ventricular
- NHLBI
National Heart, Lung, and Blood Institute
- RAR
risk at randomization
- RN
radionuclide
- SDS
summed difference score
- STICH
Surgical Treatment of IsChemic Heart failure
Footnotes
There are no relationships with industry in reference to this manuscript
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2012;60:2564–603. doi: 10.1016/j.jacc.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Fox K, Garcia MAA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary. The task force on the management of stable angina pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–81. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2012;59:857–81. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DA, Ryan TJ, McCabe CH, et al. The role of exercise testing in identifying patients with improved survival after coronary artery bypass surgery. J Am Coll Cardio. 1986;8:741–8. doi: 10.1016/s0735-1097(86)80412-0. [DOI] [PubMed] [Google Scholar]
- 5.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–91. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 6.Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–24. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 7.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–16. doi: 10.1056/NEJMoa1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 9.BARI 2D Study Group. Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hueb W, Lopes N, Gersh BJ, et al. Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2010;122:949–57. doi: 10.1161/CIRCULATIONAHA.109.911669. [DOI] [PubMed] [Google Scholar]
- 11.Gheorghiade M, Sopko G, De Luca L, et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation. 2006;114:1202–13. doi: 10.1161/CIRCULATIONAHA.106.623199. [DOI] [PubMed] [Google Scholar]
- 12.Afridi I, Grayburn PA, Panza JA, Oh JK, Zoghbi WA, Marwick TH. Myocardial viability during dobutamine echocardiography predicts survival in patients with coronary artery disease and severe left ventricular systolic dysfunction. J Am Coll Cardiol. 1998;32:921–6. doi: 10.1016/s0735-1097(98)00321-0. [DOI] [PubMed] [Google Scholar]
- 13.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151–8. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 14.Bax JJ, Poldermans D, Elhendy A, Boersma E, Rahimtoola SH. Sensitivity, specificity, and predictive accuracies of various noninvasive techniques for detecting hibernating myocardium. Curr Probl Cardiol. 2001;26:147–86. doi: 10.1067/mcd.2001.109973. [DOI] [PubMed] [Google Scholar]
- 15.Pasquet A, Robert A, D’Hondt AM, Dion R, Melin JA, Vanoverschelde JL. Prognostic value of myocardial ischemia and viability in patients with chronic left ventricular ischemic dysfunction. Circulation. 1999;100:141–8. doi: 10.1161/01.cir.100.2.141. [DOI] [PubMed] [Google Scholar]
- 16.Bonow RO, Maurer G, Lee KL, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. N Engl J Med. 2011;364:1617–25. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones RH, Velazquez EJ, Michler RE, et al. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–17. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velazquez EJ, Lee KL, O’Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–7. doi: 10.1016/j.jtcvs.2007.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones RH, White H, Velazquez EJ, et al. STICH (Surgical Treatment for Ischemic Heart Failure) trial enrollment. J Am Coll Cardiol. 2010;56:490–8. doi: 10.1016/j.jacc.2009.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardica Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 21.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–7. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 22.Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32:1012–24. doi: 10.1093/eurheartj/ehq500. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardogr. 2007;20:1021–41. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Sicari R, Nihoyannopoulos P, Evangelista A, et al. Stress echocardiography expert consensus statement. Eur Heart J. 2009;30:278–89. doi: 10.1093/eurheartj/ehn492. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–81. [Google Scholar]
- 27.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2. New York: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 28.Cox DR. Regression models and life-tables (with discussion) J Royal Statist Soc B. 1972;34:187–220. [Google Scholar]
- 29.Carson PE, Miller A, O’Connor CM, Pina IL, Selzman C, Sueta C, Wertheimer J, She L, Greene D, Lee KL. Surgical treatment for ischemic heart failure (STICH) trial: mode of death results (abstract) Eur Heart J. 2012;33(Suppl 1):497–8. doi: 10.1016/j.jchf.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velazquez EJ. Myocardial imaging should not exclude patients with ischemic heart failure from coronary revascularization. Circ Cardiovasc Imaging. 2012;5:271–9. doi: 10.1161/CIRCIMAGING.111.964650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizzello V, Poldermans D, Schinkel AFL, et al. Long term prognostic value of myocardial viability and ischaemia during dobutamine stress echocardiography in patients with ischaemic cardiomyopathy undergoing coronary revascularization. Heart. 2006;92:239–44. doi: 10.1136/hrt.2004.055798. [DOI] [PMC free article] [PubMed] [Google Scholar]