Abstract
Objective
Our objective is to determine whether quetiapine was superior to placebo in increasing weight or reducing core symptoms of anorexia nervosa as assessed by the Yale–Brown–Cornell Eating Disorder Scale and the Eating Disorder Inventory-2.
Method
Participants were randomised to 8 weeks of quetiapine or placebo.
Results
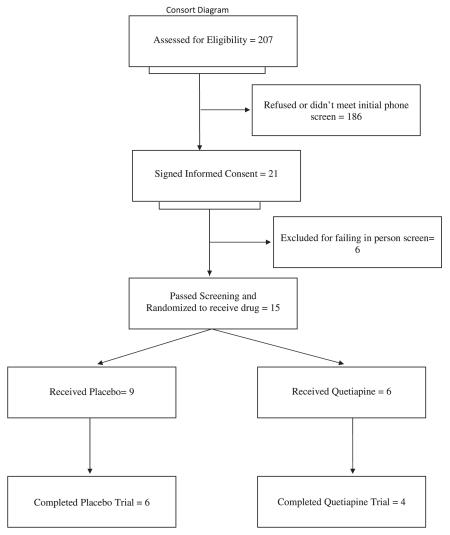
There are 21 participants who signed informed consent, 15 were randomised, 14 returned for at least one visit after receiving drug and 10 completed the study. There were no differences between drug and placebo in questionnaire scores, weight or measures of anxiety or depression.
Discussion
There was no difference between quetiapine and placebo on weight gain or core symptoms. Small effect sizes suggest that a higher number of participants would not increase significant differences between groups.
Keywords: anorexia nervosa, trial, pharmacotherapy, treatment
Introduction
Anorexia nervosa (AN) is a serious life-threatening illness with the highest premature mortality rate of any psychiatric disorder (Sullivan, 1995) that affects about 0.9% of women (Hudson, Hiripi, Pope, & Kessler, 2007). Treatment is difficult, expensive, and even when remission of symptoms occurs, relapse is common. Although there are guidelines for the management of AN (NICE, 2004; Yager et al., 2006), these are primarily consensus statements with few evidence-based treatments available. (Keys, Brozek, Henschel, Mickelsen, & Taylor, 1950; Nilsson, Gillberg, Gillberg, Gillberg, & Rastam, 1999).
There are no medications for AN, which are approved by the Food and Drug Administration. There has been considerable interest in whether the atypical antipsychotics are useful. For example, in a recent double-blind placebo-controlled study of olanzapine, participants gained to ideal body weight slightly more rapidly and had a greater reduction in obsessions than participants on placebo (Bissada, Tasca, Barber, & Bradwejn, 2008). However, a recent placebo-controlled study of adjunctive olanzapine in underweight adolescents with anorexia nervosa found that weight gain and improvements in eating attitudes, behaviours and psychological functioning were similar in both groups (Kafantaris et al., 2011; Norris et al., 2011). In terms of quetiapine, an open label study (Powers, Bannon, Eubanks, & McCormick, 2007) using a dose ranging from 150–300 mg/day was administered to 19 participants, 14 of whom completed the study; four of the five dropouts returned for a termination visit. Participants had reductions in core eating disorder symptoms as well as decreases in depression, anxiety and obsessionality. Mean weight gain from baseline to last observation carried forward (LOCF) was 1.6 lb.
Study aims and objectives
The primary outcome was to determine the effect of quetiapine compared with placebo in terms of reducing core eating disorder symptoms on the Yale–Brown–Cornell Eating Disorder Scale (YBC-EDS) and the Eating Disorder Inventory-2 (EDI-2). Secondary outcomes were to determine if quetiapine is superior to placebo in reducing anxiety, depression and obsessionality assessed with the State Trait Anxiety Inventory (STAI), Hamilton Depression Rating Scale (HAM D) and Yale–Brown Obsessive Compulsive Scale, respectively. In addition, another secondary goal was to determine if quetiapine is superior to placebo in terms of weight gain. Adverse events were also determined.
Methods
Inclusion and exclusion criteria
This was a dual site study conducted at the University of California at San Diego and the University of South Florida. It was a double-blind placebo-controlled 8-week trial of participants who met DSM-IV-TR criteria for AN. Inclusion criteria were as follows: (1) men and women 18–65 years of age who meet criteria for DSM-IV-TR AN (restricting or binge eating/purging types); (2) at least 15% below ideal body weight; (3) for participants who met all diagnostic criteria except amenorrhea, exceptions could be made by the investigator to permit entry into the study; (4) able to decide whether or not to sign informed consent; (5) able to take all tests and examinations; and (6) judged sufficiently medically and psychologically stable to participate as an outpatient. Exclusion criteria were as follows: (1) participants who met DSM-IV-TR criteria for schizophrenia or schizoaffective disorder; (2) any electrocardiogram abnormality considered clinically significant by the investigator; (3) laboratory abnormalities requiring acute medical intervention; (4) pregnant women or women who were trying to be pregnant; (5) lactating women; (6) serious suicide risk; (7) any medical condition precluding outpatient treatment; (8) history of severe allergies or allergy to quetiapine; and (9) use of psychotropic medications (except benzodiazepines) within 7 days preceding randomization.
Two-phase study
The study was approved by the institutional review board at both sites. After the participants completed the informed consent, there were two phases. The first phase was a screening phase and could last up to 14 days and included a 7 day washout period for participants on psychoactive drugs. The participants completed a comprehensive psychiatric and physiological examination including the Structured Clinical Interview for DSM Disorders (SCID) (First, Spitzer, Gibbon, & Williams, 2002) interview. Medical history was obtained, physical examination performed and laboratory assessment was completed including an electrocardiogram. The participants who completed the screening phase with no exclusion criteria detected began the second phase and were randomised to either placebo or quetiapine for 8 weeks. The participants were evaluated on Day 1 (day of randomization), Day 4, Day 10, Week 2 and then weekly through Week 8. At each session, weight and vital signs were obtained and assessment of symptoms and possible adverse events were completed. On Day 1, Weeks 2, 4, 6 and 8 or termination visit, the participants completed the HAM D (Hamilton, 1960), Yale–Brown Obsessive Compulsive Scale (Goodman, et al., 1989) and YBC-EDS (Mazure, Halmi, Sunday, Romano, & Einhorn, 1994). The Positive and Negative Syndrome Scale (PANSS) (Kay, Fiszbein, & Opler, 1987), EDI-2 (Garner,Olmstead, & Polivy, 1983) and STAI (Spielberger, 1983) were completed at Day 1 and Week 8, or early termination. The PANSS was administered for two reasons: first, quetiapine has been used primarily as an antipsychotic medication and, second, because previous pilot studies of neuroleptics for AN (including quetiapine) have found changes in scores on the PANSS.
Adverse events were classified as serious if they met the criteria defined by the Food and Drug Administration, which includes those events that result in hospitalisation, death or permanent disability.
Data analysis
Descriptive analyses were utilised for demographic and clinical data. Analyses were performed to determine if there were differences between the placebo and study drug groups when baseline and Week 8 (or early termination) measures were compared. Differences over time that occurred within the entire study group were also calculated. Both StatView (Sadock & Sadock, 1999) and spss (Statistical Package for Social Services, Chicago IL, 2001) were utilised to conduct analyses. p-values of .05 or less were considered statistically significant, and Bonferonni corrections were used to account for multiple comparisons.
To assess overall differences between the placebo and quetiapine groups’ means on body mass index (BMI), self-report and structured interview assessments, non-paired t-tests were determined using intent-to-treat analyses. Symptoms assessed by various questionnaires were compared between Day 1 and Week 8 or for participants who discontinued early but completed at least one follow-up visit after receiving study drug using the LOCF.
In addition to assessing differences that occurred between drug groups, paired t-tests were used to assess changes in core and secondary symptoms that occurred over time (Day 1 and Week 8 or LOCF) within all study participants, irrespective of whether they were on placebo or study drug.
Results
Participant enrollment
Two hundred seven participants were screened and 21 (10.4%) signed informed consent after explanation of the study. Of these 21, 15 were randomised and began the drug; the other six participants failed the in-person screening phase. Nine patients signed informed consent at the University of California at San Diego and 12 at the University of South Florida. Of the 15 participants who began the study, 14 completed at least one visit after beginning drug and 10 participants completed the trial (see Consort Diagram for details). Seven patients were on quetiapine; the mean dose of quetiapine was 177.7 (SD = 90.8). Three participants had serious adverse events; two participants were on drug (one was hospitalised for worsening weight loss, one was hospitalised after she was hit by a car while compulsively riding her bicycle), and one participant was on placebo (she was hospitalised for worsening manic symptoms). None of the serious adverse events were considered due to the study drug quetiapine.
Of the 186 participants who decided not to participate, the most common reason given was fear of weight gain that might occur with administration of the study drug. One participant who dropped out (who was on placebo) said she was fearful of the increased appetite she had after taking the first few doses of medication. Nine participants received the study drug quetiapine, and seven participants received placebo.
Demographic and clinical data
The mean age of the participants was 34 years (SD = 13.48), and of the 21 participants who signed informed consent, one was male. For the participants who were randomised, mean BMI (weight in kilogrammes divided by height in metres squared) was 15.9 kg/m2 on Day 1 (SD = 2.27). Half of the female participants had DSM-IV-TR AN, binge/purge type, and half had AN, restricting type. The one male participant had AN, restricting type. Of the female participants who began drug on Day 1, all but one had at least 3 months of amenorrhea.
All 21 participants who signed informed consent participated in a structured interview and completed the SCID. Five of the 21 participants had AN and no other DSM-IV-TR Axis I diagnosis. For the remaining 16 participants, the number of SCID diagnoses ranged from one additional diagnosis to six additional diagnoses, but the majority of the participants had at least three additional Axis I diagnoses. The most common diagnoses were major depressive disorders, either single or recurrent episode, which occurred in 11 participants. The next most common diagnoses were obsessive-compulsive disorder (eight participants) and specific phobias (eight participants). Social phobia was present in seven participants, six participants had generalised anxiety disorder, four participants had panic disorder (two with agoraphobia and two without agoraphobia), three had posttraumatic stress disorder, three had a past history of alcohol abuse and one had Bipolar I disorder.
Quetiapine versus placebo: core eating disorder symptoms
The difference in hours occupied by preoccupations and by rituals on the YBC-EDS from Day 1 to LOCF was analysed by the unpaired groups of quetiapine versus placebo, and no statistical differences were found (t = 2.8, p = .15, partial η2 = .15, power = .29) for hours of preoccupations and (t = .56, p = .40, partial η2 = .06, power = .13) for hours of rituals. The differences in scores on the EDI-2 from Day 1 to LOCF were analysed using the unpaired groups of quetiapine versus placebo, and no statistically significant differences were found for the total scores on the EDI-2 or any of the subscales (EDI Total Scale; t = .13, p = .72, η2 = .01, power = .06).
The difference between the two different groups on BMI was analysed using unpaired groups of quetiapine versus placebo (Day 1 compared with LOCF), and no statistical differences were found (t = .01, p = .93, partial η2 = .00, power = .05).
Quetiapine versus placebo: secondary measures
Differences in individual participants’ scores between Day 1 and LOCF time points on the STAI were compared between the quetiapine and placebo groups. No statistically significant differences on either state or trait scales were found between quetiapine and placebo groups (trait: t = .52, p = .48 partial η2 = .04, power = .10; state: t = .09, p = .77, partial η2 = .01, power = .06). Similarly, there were no differences in the HAM D between quetiapine and placebo when the differences in scores between Day 1 and LOCF were analysed (t = .05, p = .83, partial η2 = .01, power = .06). Finally, the differences in scores on the PANSS from Day 1 to Week 8 were studied comparing the quetiapine group with the placebo group, and no statistically significant differences were found in the Positive Scale, Negative Scale or General Scale scores (Positive Scale: t = .78, p = .40, partial η2 = .05, power = .13; Negative Scale: t = 3.0, p = .11, partial η2 = .18, power = .36; General Scale: t = .02, p = .90, partial η2 = .00, power = .05).
Comparison of changes for entire group
When the entire group was analysed, comparing findings from Day 1 to LOCF, statistically significant findings were noted. There were no statistically significant differences in either preoccupations or rituals on the YBC-EDS, but there were statistically significant differences in EDI-2 including the total scores (t = 2.6. p = .02) and the subscales of Bulimia (B), Ineffectiveness (I), Interpersonal Distrust (ID), Interoceptive Awareness (IA) and Maturity Fears (MF) scales. Although there was an increase in BMI when Day 1 was compared with LOCF (15.9 on Day 1 and 16.5 at LOCF), this did not reach statistical significance (t = .78, p = .45). Significance was also seen on a secondary measure that compared Day 1 and LOCF on the HAM D (t = 3.2, p < .01). Analyses on the STAI-Y Trait approached significance (STAI-Y Trait: t = 23.3, p = .01).
Discussion
There was no difference in outcome for any of the measures between the group of participants who received quetiapine and the group who received placebo. In fact, quetiapine appeared to have little direct effect on improving core eating disorder symptoms and secondary measures when compared with placebo.
Recruiting participants for this study was difficult. Both sites have very experienced treatment teams, and there were many potential participants who contacted the research teams who met the diagnostic criteria to participate in the study, but few participants were willing to participate. Many potential participants indicated they did not want to take medication, especially once that they thought it might result in weight gain, even though the primary reason for considering research or treatment was their apparent semistarvation. This paradox is well-known and is one of the main difficulties in determining if there are medications that might be useful in the treatment of AN.
Despite difficulties in recruitment, the finding of multiple comorbid Axis I diagnoses in the majority of participants who signed the informed consent is in line with findings from other studies (Preti et al., 2009). One-third of the participants met formal criteria for obsessive–compulsive disorder. Depressive and anxiety-related disorders were the most common comorbidities. These findings suggest that the participants who were treated are very similar to those who present for treatment at specialised treatment centres. Most of the patients recruited for the study had been ill for many years and had various treatments that had not resulted in enduring recovery. Longer duration of illness is likely associated with an increase in DSM-IV psychiatric disorders. This study also used the SCID, which typically detects more psychiatric disorders (particularly anxiety disorders) than the typical clinical interview.
In terms of limitations, small effect sizes raise the possibility that differences between groups might not be found with a larger sample size. Still, given the difficulty in recruiting adult AN participants and the few controlled trials of atypicals in this field, presentation of this data is warranted. It is difficult to show differences in weight in an 8-week trial. The brief period was chosen as we wanted to test dose and acceptance of this drug in addition to determining whether there were any positive effects on symptoms. Our clinical experience is that some individuals with AN have good responses to atypicals, particularly in terms of a reduction in anxiety. Despite these negative results for this drug, we think further studies of this class of medications are important to pursue.
REFERENCES
- Bissada H, Tasca G, Barber A, Bradwejn J. Olanzapine in the treatment of low body weight and obsessive thinking in women with anorexia nervosa: A randomized, double-blind, placebo-controlled trial. American Journal of Psychiatry. 2008;165:1281–1288. doi: 10.1176/appi.ajp.2008.07121900. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. Biometrics Research, New York State Psychiatric Institute; (SCID-I/P) New York: Nov, 2002. [Google Scholar]
- Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia and bulimia nervosa. International Journal of Eating Disorders. 1983;2:15–34. [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J, Hiripi E, Pope H, Kessler R. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafantaris V, Leigh E, Hertz S, Berest A, Schebendach J, Sterling W, et al. A placebo-controlled pilot study of adjunctive olanzapine for adolescents with anorexia nervosa. Journal of Child and Adolescent Psychopharmacology. 2011;21:207–212. doi: 10.1089/cap.2010.0139. [DOI] [PubMed] [Google Scholar]
- Kaplan A, Saddock BJ. Comprehensive textbook of psychiatry. Lippincott Williams & Wilkins; Philadelpia, PA: 1999. [Google Scholar]
- Kay SF, Fiszbein A, Opler L. The Positive and Negative Syndrome Scale (PANSS) of schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Keys A, Brozek J, Henschel A, Mickelsen O, Taylor H. The biology of human starvation. The University of Minnesota Press; Minneapolis, MN: 1950. [Google Scholar]
- Mazure CM, Halmi KA, Sunday SR, Romano SJ, Einhorn AM. The Yale-Brown-Cornell Eating Disorder Scale: development, use, reliability and validity. Journal of Psychiatric Research. 1994;28:425–445. doi: 10.1016/0022-3956(94)90002-7. [DOI] [PubMed] [Google Scholar]
- NICE . Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders (Clinical Guideline 9) National Collaborating Centre for Medical Health; London: 2004. [Google Scholar]
- Nilsson EW, Gillberg C, Gillberg IC, Gillberg C, Rastam M. Ten-year follow-up of adolescent-onset anorexia nervosa: Personality disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1389–1395. doi: 10.1097/00004583-199911000-00013. [DOI] [PubMed] [Google Scholar]
- Norris M, Spettigue W, Buchholz A, Henderson K, Gomez R, Maras D, et al. Olanzapine use for the adjunctive treatment of adolescents with anorexia nervosa. Journal of Child and Adolescent Psychopharmacology. 2011;21:213–220. doi: 10.1089/cap.2010.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers P, Bannon Y, Eubanks R, McCormick T. Quetiapine in anorexia nervosa patients: An open label outpatient pilot study. International Journal of Eating Disorders. 2007;40:21–26. doi: 10.1002/eat.20325. [DOI] [PubMed] [Google Scholar]
- Preti A, Girolamo G, Vilagut G, Alonso J, Graaf R, Bruffaerts R, et al. The epidemiology of eating disorders in six European countries: Results of the ESEMeD-WMH project. Journal of Psychiatric Research. 2009;43:1125–1132. doi: 10.1016/j.jpsychires.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Sadock B, Sadock V. Kaplan and Sadock’s comprehensive textbook of psychiatry. 7th ed. Lippincott Williams & Wilkins; Chicago, IL: 1999. [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc.; Palo Alto, CA: 1983. [Google Scholar]
- SPSS . SPSS for Windows. Chicago: 2001. Rel. 11.0.1. edn. [Google Scholar]
- Sullivan PF. Mortality in anorexia nervosa. The American Journal of Psychiatry. 1995;152:1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- Yager J, Devlin MH, Halmi KA, Herzog D, Mitchelolo J, Powes P, et al. American Psychiatric Association Practice Guideline for the Treatment of Psychiatric Disorders Compendium. 3rd ed. American Psychiatric Association; Arlington, VA: 2006. Practice guidelines for the treatment of patients with eating disorders; pp. 1097–1222. [Google Scholar]