Abstract
The parathyroid hormone, PTH, is responsible for calcium and phosphate ion homeostasis in the body. The first thirty-four amino acids of the peptide maintains the biological activity of the hormone and is currently marketed for calcium imbalance disorders. Although several methods for the production of recombinant PTH(1–34) have been reported, most involve the use of cleavage conditions that result in a modified peptide or unfavorable side products. Herein, we detail the recombinant production of 15N-enriched human parathyroid hormone, 15N PTH(1–34), generated via a plasmid vector that gives reasonable yield, low cost protease cleavage (leaving the native N-terminal serine in its amino form) and purification by affinity and size exclusion chromatography. We characterize the product by multidimensional, heteronuclear NMR, circular dichroism and LC/MS.
INTRODUCTION
The human parathyroid hormone, PTH, and its paracrine acting counterpart, parathyroid hormone related peptide, PTHrP, are the main hormones for regulating calcium and phosphate ions homeostasis in the human body [1, 2]. PTH is produced as a 115 residue prepropeptide, which undergoes cleavage by multiple proteases to yield the final 84 residue hormone that acts upon parathyroid receptors 1 and 2 [3, 4]. Cleavage studies have demonstrated that the first 34 amino acids of PTH retains the biological activity of the hormone [5].
Efforts to produce recombinant PTH have been ongoing since the 1980’s [6–14], with the first report of full hormone production detailed in 1988 [6]. In general, recombinant production offers advantages over standard synthetic methodologies: fast cellular growth achieving high density using inexpensive substrates [15], facile isotope enrichment and post-translational modifications such as glycosylation. Several studies describing the recombinant production of PTH employing fusion tags subsequently cleaved using cyanogen bromide [11], enterokinase [16] and/or factor X [14] have been reported. All of these cleavage methods are either relatively harsh or utilize expensive reagents. A most promising production reported by Oldenburg et al. [11], showed the concatenation of PTH(1–34) cDNA in a vector that expressed robustly and allowed for the cleavage of PTH fragments by cyanogen bromide. While this method produced the desired peptide in high yield (>300 mg/L), both native methionine residues (M8 and M18) were mutated to Leu to prevent their conversion to homoserine upon CNBr cleavage. Additionally, to cleave each PTH peptide following isolation, a C-terminal methionine was cloned into the construct, resulting in a C-terminal homoserine residue post cleavage. In light of the critical roles of the N-terminal residue of PTH in receptor activation [5] and of the native methionine residues in receptor binding [17], it is desirable to develop an expression method that gives reasonable yields with the native sequence of PTH. Equally desirable would be to find a cleavage method that is mild and not requiring expensive reagents.
To address these issues, we report our efforts to generate 15N-labeled PTH(1–34) with the amino terminus as a free base in a constructed plasmid vector, pJCC04a—a modified pET32b vector containing a thioredoxin fusion tag upstream of a tobacco etch virus (TEV) protease cleavage site. We believe this production method to be more versatile and economical than currently reported methods particularly since TEV protease can be freely generated recombinantly and cleaves over a wide range of amino acid residues following glutamine in its recognition sequence.
MATERIALS AND METHODS
Design of pJCC04a plasmid vector
To generate the pJCC04a vector, the QuikChange site-directed mutagenesis kit (Stratagene) was used to insert a BamHI site downstream from the coding sequence for the N-terminal Thioredoxin (TrxA) and 6x-His purification tag in the pET32b vector (Novagen) using the following oligonucleotides (Integrated DNA Technologies):
5’-GCACCATCATCATCATCATTCTTCTGGATCCGTGCCACGCGGTTCTGGTATGAAAG-3’
5’-CTTTCATACCAGAACCGCGTGGCACGGATCCAGAAGAATGATGATGATGATGGTGC-3’
To introduce the coding sequence for a TEV protease cleavage recognition site and a multiple cloning site, a synthetic insert was designed using the following 5’-phosphorylated oligonucleotides (Integrated DNA Technologies):
5’-GATCTGAGAACCTGTACTTTCAGGGATCCATGGGCCTCGAGTGAGC-3’
5’-GGCCGCTCACTCGAGGCCCATGGATCCCTGAAAGTACAGGTTCTCA-3’
These oligonucleotides were mixed (50 µM each), heated to 95 °C for 5 min, slowly annealed by shifting the temperature to 70 °C over 30 min, then rapidly cooled to 25 °C using a thermocycler. The modified pET32b plasmid was digested with BamHI and NotI, and the synthetic insert was ligated using Quick T4 DNA ligase (New England BioLabs). The resulting vector was confirmed by DNA sequencing at the Dartmouth Core Sequencing facility.
Expression and Purification of 15N-PTH(1–34)
PTH(1–34) cDNA was incorporated into pJCC04a cloning site via site directed mutagenesis according to QuikChange Lightning protocol (Stratagene) using the following cDNA primer (Integrated DNA Technologies): 5‘-TCTGGATCTGAGAACCTGTACTTTCAGTCTGTGAGTGAAATACAGCTTATGCATAACCTGGGAAAACATCTGAACTCGATGGAGAGAGTAGAATGGCTGCGTAAGAAGCTGCAGGATGTGCACAATTTTTGAGCGGCCGCACTCGAG-3’. The underlined sequences are the complementary strand overhangs for pJCC04a vector.
E. coli strain BL21 Star (DE3) (Invitrogen), transformed with the above pJCC04a plasmid containing the PTH(1–34) cDNA, was grown in Terrific Broth (TB) at 37 °C with shaking at 220 rpm until the optical density of the culture at 600 nm was approximately 4. The culture was then centrifuged slowly at 1500 rpm for 20 min, and the supernatant discarded. The residual pellet, for 15N enriched growth, was resuspended in minimal media (48 mM Na2HPO4, 22 mM KH2PO4, 9 mM NaCl, 5 mM MgSO4, 0.2 mM CaCl2, 0.25× trace metals solution [29], 21 µM Na2MoO4.2H2O, 0.25× BME Vitamins (Sigma, 100× stock), 1 µg/ml Thiamine, 10 g/l dextrose (Fisher Scientific), 3 g/l 15N NH4Cl (Cambridge Isotope Laboratories), pH 8.0). The resulting suspension was grown at 37 °C for 1 hr before inducing peptide overexpression with 2.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) in the presence of 1 mM MgSO4 and 1 ml ultra-pure glycerol. The cells were then grown at 20 °C for 16–18 hrs and then centrifuged at 4000 rpm for 30 min. Pelleted cells were subsequently resuspended into 50 ml lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole, pH 8.0) containing 1 tablet of EDTA-free protease inhibitor cocktail (Roche), 2 µl benzonase (250 U/µl, Sigma Aldrich), 2 mM MgCl2 and 1 ml ultrapure glycerol. The resulting suspension was lysed by French press at 15,000 psi.
8M urea was added to the cell lysate and stirred at room temperature for 18–20 hrs before pelleting at 40,000 rpm for 40 min. On an Amersham Biosciences AKTAxpress fast protein liquid chromatography (FPLC) instrument, a 5ml Histrap™ HP nickel affinity column (GE Healthcare) was equilibrated with loading buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM Imidazole, pH 8.0) and the supernatant from the urea prep was directly loaded onto it, followed by a 10 column volume wash with loading buffer. A gradual gradient elution (buffer: 50 mM NaH2PO4, 300 mM NaCl, 500 mM Imidazole, pH 8.0) was used to elute TrxA-15N PTH(1–34) fusion protein off the nickel affinity column. Protein elution was monitored by UV light at 280nm. The fractions obtained were assessed by SDS-PAGE, with the cleanest fractions pooled and subsequently subjected to 16 hrs overnight TEV protease digestion via dialysis into 50 mM Tris-HCl, 150 mM NaCl, 1 mM DTT and 10 % glycerol at 4 °C (1:10 ratio of protease to protein). The resulting mixture was loaded on a 5 ml HisTrap™ affinity column to remove the TEV protease and thioredoxin proteins. SDS-PAGE analysis was conducted on a 16 % Tricine peptide gel (Invitrogen). Fractions containing the peptide alone, and with trace TrxA, were pooled, concentrated and loaded onto a GE© HiLoad Superdex75 16/60 size exclusion column. dH2O, pH 3.1 was used to separate the peptide from the TrxA. The purest fractions of 15N PTH(1–34) were pooled, and the concentration determined by UV absorbance at 280 nm before storage at −80 °C.
All UV readings for peptide concentration were carried out on a Beckman Coulter DU800 spectrophotometer. Before further analysis, peptide solutions were lyophilized and reconstituted in the appropriate buffer and, as needed, further concentrated (3.5 kDal MWCO centrifugal concentrator; Vivaspin, Sartorius Stedim Biosciences). Unlabeled, synthetically derived PTH(1–34) was purchased from the Tufts University Core Facility.
Circular Dichroism Analysis
Circular dichroism experiments were conducted on a Jasco J-815 Spectrometer (cell path length 0.1 cm) on a 52 µM sample of 15N PTH(1–34) peptide in dH2O, pH 3.1. The absorbance was converted to residue molar ellipticity (θ) using standard conversion equation [18] and the percent helicity was calculated as previously described [19].
Liquid Chromatography and Mass Spectrometry (LC/MS)
LC/MS analysis was run on a Shimadzu LCMS-8030 Tandem Quadrupole LC/MS/MS instrument with a detection limit of 2000 m/z. Methanol/dH2O (0.1% trifluoroacetic acid, TFA) were used as the eluting solvents. A gradient elution was employed with a flow rate of 8.4 ml/min with monitoring at wavelengths of 190–800 nm. Ionization was obtained by electrospray (ESI) with a DL temperature of 250 °C and a heat block temperature of 400 °C. The column oven was set to 40–90 °C. Both positive and negative ions were monitored. A Savitzky-Golay processing method was employed to smooth the data [20].
NMR characterization
All NMR experiments were acquired on a Bruker 700 MHz spectrometer equipped with a cryoprobe and pulse field gradients. Data acquisition and processing were performed with Topspin software (version 3.2; Bruker) running on a Linux workstation. All experiments were run at 298 K. One-dimensional 1H NMR, with a total of 128 scans, were acquired for each experiment with labeled (85 µM) and unlabeled (1 mM) PTH(1–34) peptides. Spectra were acquired in 1× phosphate buffered saline (PBS), 5% D2O at pH 6.8. 1H, 15N HSQC experiments were conducted using recombinantly produced, 15N-labeled PTH(1–34) at 30 µM in H2O, pH 6.8, 5% D2O. A total of 32 scans were accumulated for each experiment, with 1024 and 256 points in the F2 and F1 dimensions, respectively.
RESULTS
pJCC04a Plasmid Vector Design
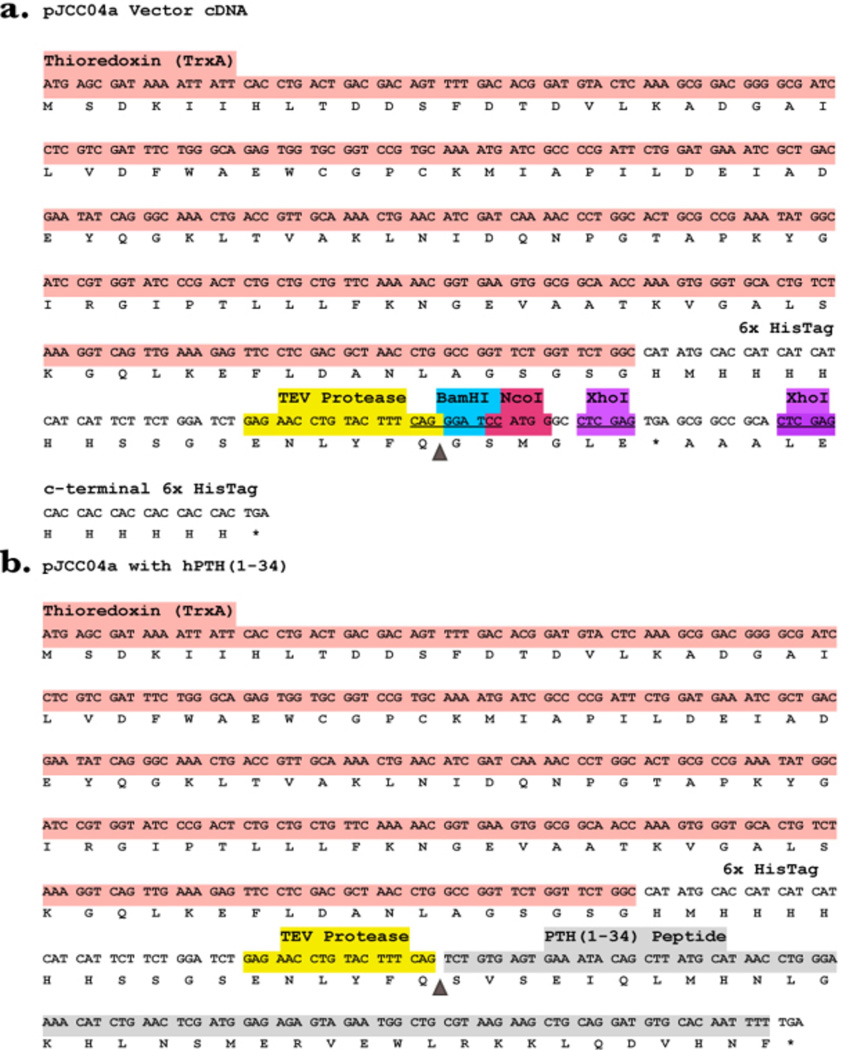
The pJCC04a plasmid is modified from pET32b and contains the ampicillin resistance gene and a thioredoxin tag. Our modifications introduced the coding sequence for a TEV protease cleavage recognition site downstream from the 6xHis tag sequence, as well as a multiple cloning site containing the sequences for BamHI, NcoI, and XhoI restriction endonucleases. The full recombinant vector plasmid (native and with PTH insert) is shown in Figure 2.
Figure 2.
(a) Color coded cDNA and amino acid sequence of pJCC04a — a modified pET32b vector. (b) cDNA and amino acid sequence of pJCC04a with PTH(1–34) insert. The triangle in both panels shows the TEV protease scissile bond.
Peptide Expression and Purification
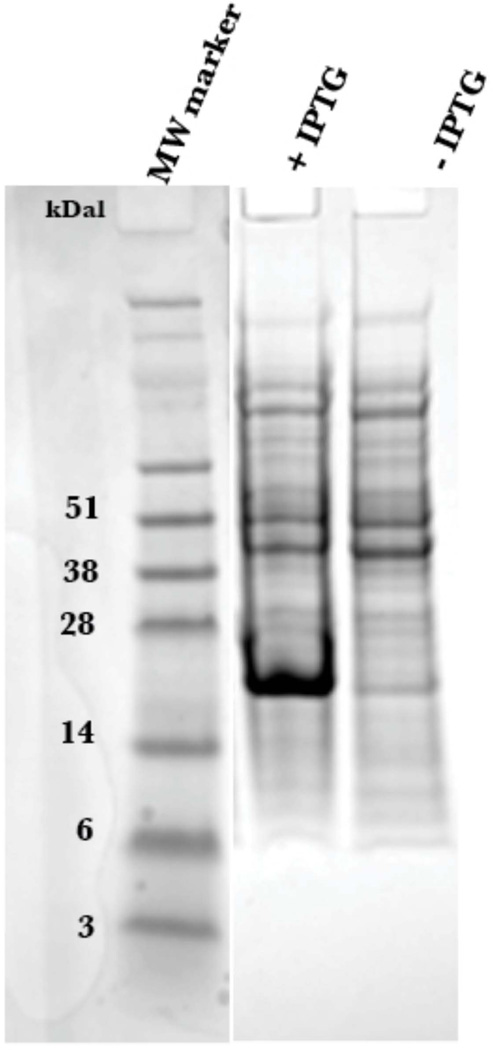
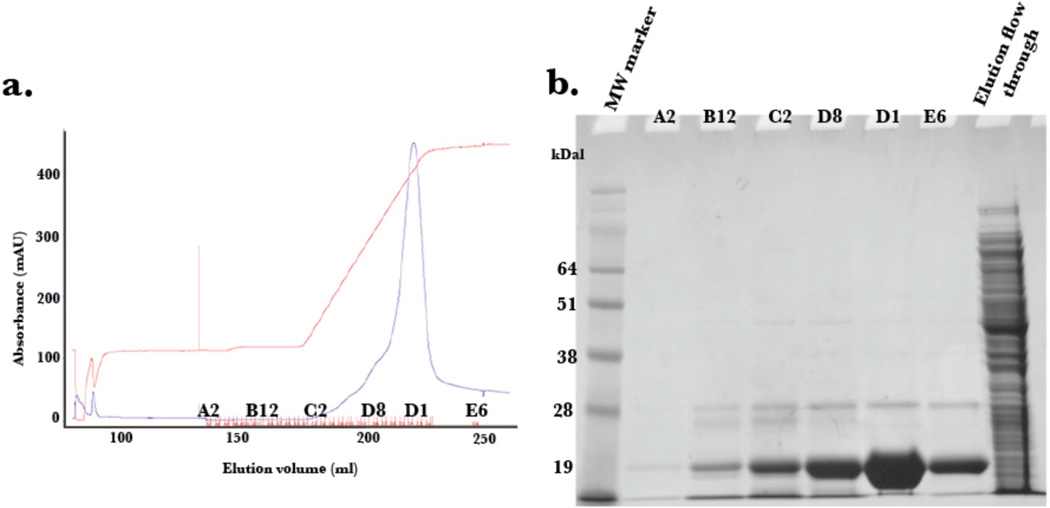
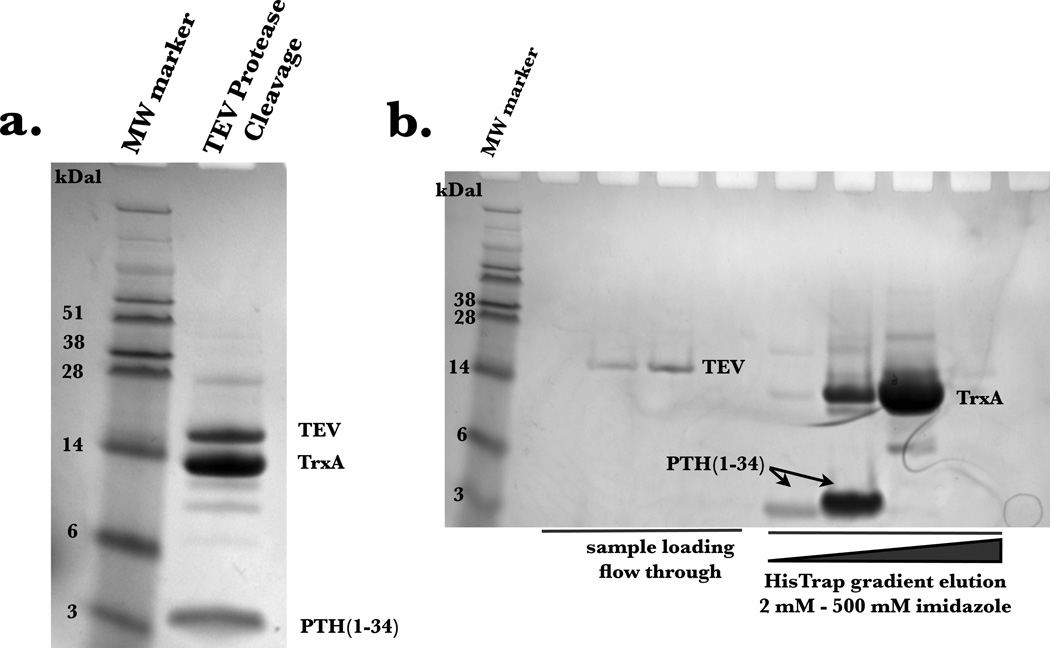
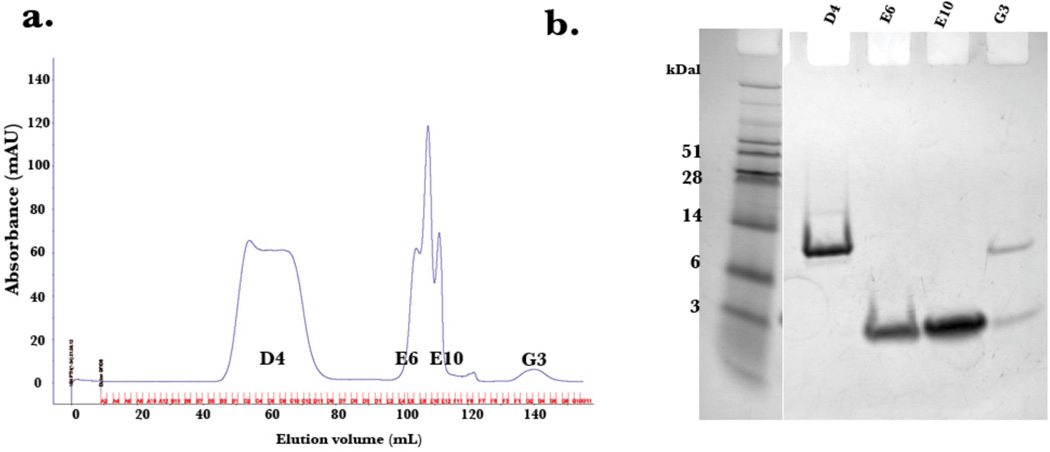
The expression of PTH(1–34) yielded approximately 20 mg per liter of minimal media, with greater than 95% purity as determined by LC/MS. The pJCC04a vector was induced for protein production using IPTG (Figure 3). The thioredoxin fusion tag on the plasmid vector allowed for robust expression, although, as previously reported [21], the generated fusion protein, TrxA-15N PTH(1–34), was insoluble following cell lysis. To recover the protein, the cell pellet was resuspended using the urea solubilizing preparation described in Materials and Methods. The fusion protein, washed with excess loading buffer to remove residual urea, was purified to homogeneity using a gradient elution on Ni2+ affinity column (Figure 4). The incorporation of the TEV protease cleavage site allowed for the generation of untagged peptide following TEV digestion, and is visible on SDS-PAGE analysis (Figure 5a). Attempts to isolate the peptide from thioredoxin and TEV post digestion by Ni2+ affinity column resulted in most of the protein eluting during the imidazole gradient (Figure 5b). This is likely due to non-specific interactions of the peptide with the Ni2+ column. Nonetheless, the peptide (~4 kDa) was readily separated from residual thioredoxin protein (15 kDa) by size exclusion chromatography (Figure 6).
Figure 3.
SDS-PAGE of whole cell E. coli lysates with and without IPTG for 15N PTH(1–34)-TrxA fusion protein (MW: ~20 kDa). Coomassie blue was used for gel staining.
Figure 4.
(a) Elution profile of 15N PTH(1–34)-TrxA fusion protein off the Ni2+ HisTrap affinity chromatography column with accompanying SDS PAGE (b).
Figure 5.
(a) SDS PAGE analysis of solution resulting from overnight TEV protease cleavage. 15N PTH(1–34) peptide is evident at 4 kDa. (b) SDS PAGE analysis following HisTrap affinity chromatography to isolate 15N PTH(1–34) peptide. A gradient elution was used to separate the peptide from thioredoxin.
Figure 6.
Elution profile of size exclusion chromatography (a) with corresponding SDS PAGE gel (b) depicting the separation of thioredoxin (fraction D4) and 15N PTH(1–34) peptide (fractions E6–E10).
Peptide Composition and Structure
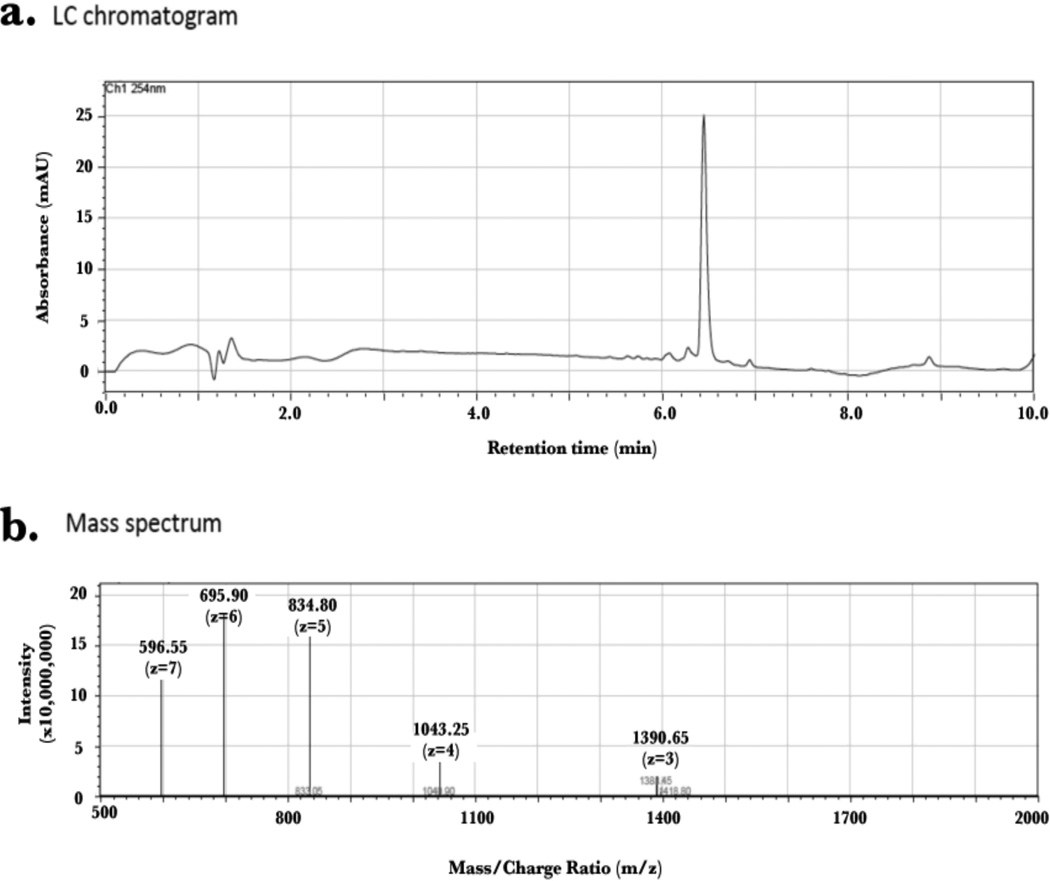
An aliquot of generated 15N PTH(1–34) peptide was lyophilized and subjected to LC/MS for analysis (Figure 7). This showed a peptide composition of 98% purity eluting at 6.5 min over a gradient elution of methanol/water (0.1 % TFA). The molecular weight of the 15N-labelled peptide (assuming 100% 15N incorporation) is 4127.33 g/mol. An estimated mass of 4171.95 m/z was obtained indicating that most residues were labeled. Peptide fragments of 1390.65 m/z, 1043.25 m/z, 834.80 m/z, 695.90 m/z and 596.55 m/z were obtained correlating to z (ion charge) values of 3, 4, 5, 6 and 7, respectively.
Figure 7.
LC/MS analysis of 15N PTH(1–34) fragments showing greater than 95% purity by LC (a) and m/z peaks correlating to z-values of 3–7 (b). Estimated molecular weight of peptide is 4171.95 m/z. Max m/z detectable is 2000.
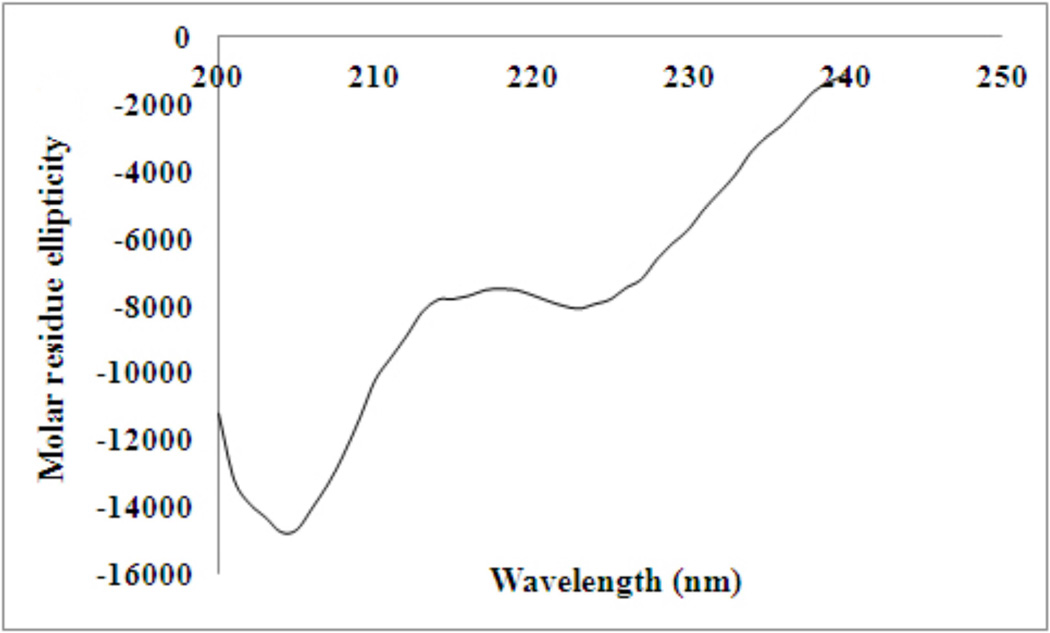
The secondary structure of 15N PTH(1–34) was assessed by circular dichroism spectroscopy (Figure 8) which revealed the hormone is primarily random coil with 28 % helical content, in accord with previous observations [22–24].
Figure 8.
Circular dichroism spectrum of 15N PTH(1–34) at 52 µM in dH2O, pH 3.1.
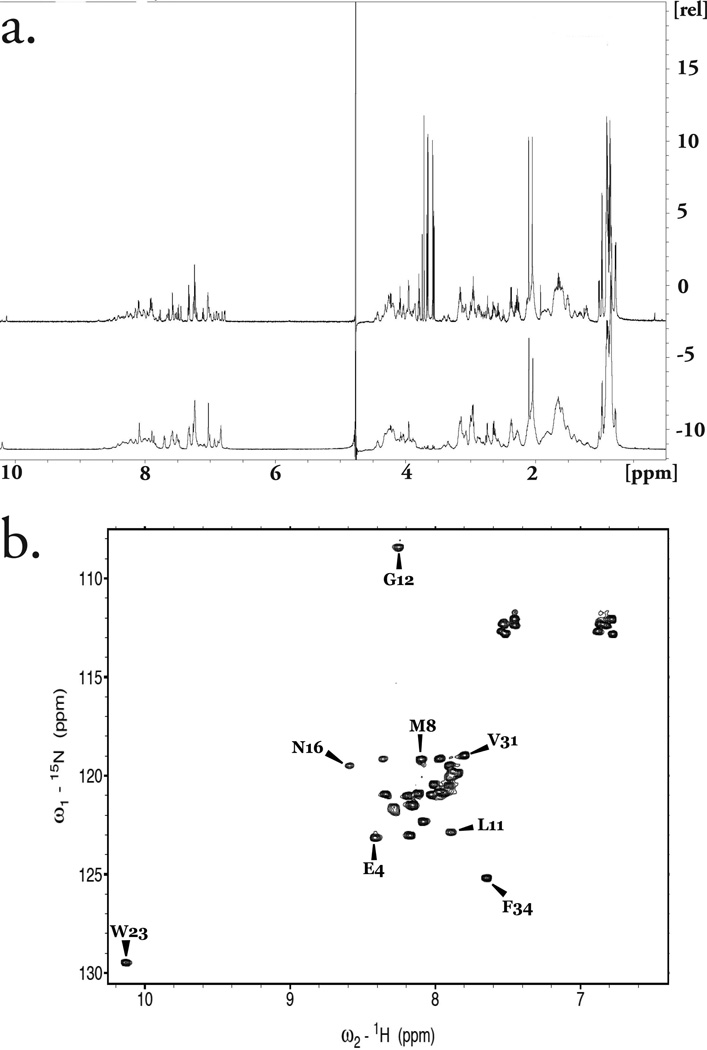
Comparison of the 1D 1H spectrum of this sample with synthetically derived, unlabeled PTH(1–34) revealed an identical number of peaks (Fig. 9a) that were readily superimposable. Further characterization was conducted by 2D HSQC 1H, 15N NMR. At pH 6.8 in H2O, strong peaks for PTH(1–34) were observed (Figure 6b). The sequence assignment could be easily deduced from those reported in similar conditions [25].
Figure 9.
(a) Overlay of 1D 1H NMR of recombinant 15N PTH(1–34) in 1× PBS, pH 6.8 (85 µM; top) with synthetic sample in same buffer (1mM; bottom) showing peak to peak comparison. The intense peaks observed between 3.5 – 3.8 ppm for the recombinant peptide corresponds to trace polypropylene obtained from the membranes used during centrifugal concentration. (b) 2D HSQC NMR spectroscopy showing well defined and sharp peaks for 24 amino acids of 15N PTH(1–34) in 1× PBS, pH 6.8, with some residues not visible at this pH.
DISCUSSION
The production method described here yields the biologically-active, N-terminal fragment of PTH utilizing proteolytic cleavage by a recombinantly expressed and relatively common TEV protease. We utilize pJCC04a—a pET32b vector plasmid containing a thioredoxin fusion protein which is reported to facilitate expression of short peptides otherwise not expressed [26], a TEV protease cleavage recognition site, and multi-endonuclease sites for BamHI, NcoI, and XhoI (Figure 2a). A similar technique was recently reported for the production of the third intracellular loop of the vasopressin receptor [27].
In our case, the desired PTH(1–34) peptide cDNA was readily inserted downstream of these modifications using long primer site directed mutagenesis (Figure 2b). Our routine production and analysis show that we are able to attain reasonable yields (approx. 20 mg peptide per 1L minimal media) of 15N PTH(1–34) peptide with high purity, as confirmed by LC/MS. It is commonly thought that PTH is mostly random coil with transient helical character [22] at the N- and C-termini. Our analysis by CD corroborates this finding, revealing a small absorption at 222 nm corresponding to 28 % helical character. The 1H NMR spectrum of our recombinant peptide is identical to that of synthetically derived peptide (Figure 9a). Recombinant production allows for the facile incorporation of isotopic labels necessary for biophysical characterization by multidimensional NMR—a useful tool in assessing binding interactions with the class B G-protein coupled receptor responsible for the cellular responses of the hormone.
The recombinant production method described in this report is particularly suited for generating peptides hormones, such as PTH, whose biological activity is dependent on having its N-terminal residue as a free amine. In the case of a peptide containing multiple cysteines, we have found that purification in the presence of a reducing agent improves yields. The TEV protease cleanly and specifically cleaves after Gln of the following recognition sequence: ENLYFQX, where X can be any amino acid other than Pro [28]. Indeed, this versatility is one of the advantages of using TEV as a protease. Given these benefits, we believe this vector and production methodology represents a generally applicable tool for the generation of recombinant peptides.
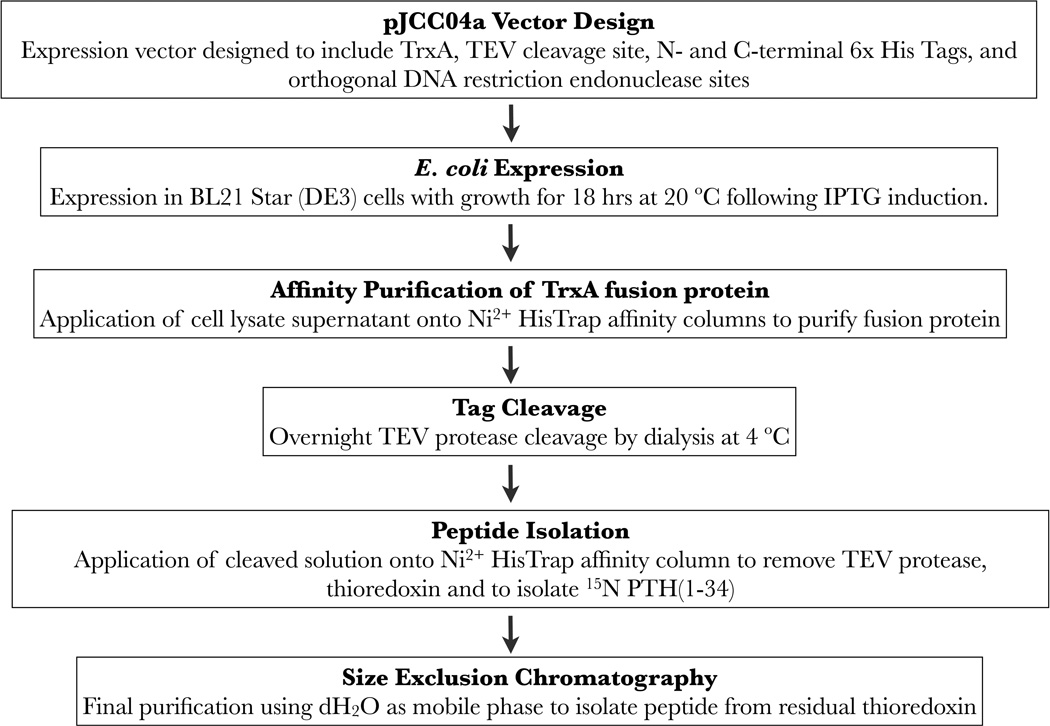
Figure 1.
A scheme for the expression and purification of 15N PTH(1–34).
ACKNOWLEDGMENTS
We thank Dr. Chamila Rupasinghe for his assistance with the LC/MS studies. This work was funded by NIH grants (S10 RR025084 and R01 GM54082) to DFM.
REFERENCES
- 1.Ramasamy L. Recent advances in physiological calcium homeostasis. Clin. Chem. Lab. Med. 2006;44:237–273. doi: 10.1515/CCLM.2006.046. [DOI] [PubMed] [Google Scholar]
- 2.Aslan D, Andersen MD, Gede LB, de Franca T, Jorgensen SR, Schwarz P, Jorgensen NR. Mechanisms for the bone anabolic effect of parathyroid hormone treatment in humans. Scand. J. Clin. Lab Invest. 2012;72:14–22. doi: 10.3109/00365513.2011.624631. [DOI] [PubMed] [Google Scholar]
- 3.Habener JF, Rosenblatt M, Potts JT., Jr Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action and metabolism. Physiol. Rev. 1984;64:985–1053. doi: 10.1152/physrev.1984.64.3.985. [DOI] [PubMed] [Google Scholar]
- 4.Habener JF, Potts JT., Jr Subcellular distributions of parathyroid hormone, hormonal precursors, and parathyroid secretory protein. Endocrinology. 1979;104:265–275. doi: 10.1210/endo-104-1-265. [DOI] [PubMed] [Google Scholar]
- 5.Gardella TJ, Jüppner H. Interaction of PTH and PTHrP with their receptors. Rev. Endocr. Metab. Disord. 2000;1:317–329. doi: 10.1023/a:1026522619828. [DOI] [PubMed] [Google Scholar]
- 6.Rabbani SA, Yasuda T, Bennett HP, Sung WL, Zahab DM, Tam CS, Goltzman D, Hendy GN. Recombinant human parathyroid hormone synthesized in Escherichia coli. Purification and characterization. J. Biol. Chem. 1988;263:1307–1313. [PubMed] [Google Scholar]
- 7.Harder MP, Sanders EA, Wingender E, Deckwer WD. Studies on the production of human parathyroid hormone by recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 1993;39:329–334. doi: 10.1007/BF00192087. [DOI] [PubMed] [Google Scholar]
- 8.Wingender E, Bercz G, Blocker H, Frank R, Mayer H. Expression of human parathyroid hormone in Escherichia coli. J. Biol. Chem. 1989;264:4367–4373. [PubMed] [Google Scholar]
- 9.Hogset A, Blingsmo OR, Gautvik VT, Saether O, Jacobsen PB, Gordeladze JO, Alestrom P, Gautvik KM. Expression of human parathyroid hormone in Escherichia coli. Biochem. Biophys. Res. Commun. 1990;166:50–60. doi: 10.1016/0006-291x(90)91910-k. [DOI] [PubMed] [Google Scholar]
- 10.Gram H, Ramage P, Memmert K, Gamse R, Kocher HP. A novel approach for high level production of a recombinant human parathyroid hormone fragment in Escherichia coli. Biotechnology. 1994;10:1017–1023. doi: 10.1038/nbt1094-1017. [DOI] [PubMed] [Google Scholar]
- 11.Oldenburg KR, D’Orfani AL, Selick HE. A Method for the High-Level Expression of a Parathyroid Hormone Analog in Escherichia coli. Prot. Express. Purif. 1994;5:278–284. doi: 10.1006/prep.1994.1042. [DOI] [PubMed] [Google Scholar]
- 12.Rokkones E, Kareem BN, Olstad OK, Hogset A, Schenstrom K, Hansson L, Gautvik KM. Expression of human parathyroid hormone in mammalian cells, Escherichia coli and Saccharomyces cerevisiae. J. Biotechnol. 1994;33:293–306. doi: 10.1016/0168-1656(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Yabuta M, Ohsuye K. High-level production of recombinant human parathyroid hormone 1–34. Appl. Environ. Microbiol. 1998;64:526–529. doi: 10.1128/aem.64.2.526-529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardella TJ, Rubin D, Abou-Samra AB, Keutmann HT, Potts JT, Jr, Kronenberg HM, Nussbaum SR. Expression of human parathyroid hormone-(1–84) in Escherichia coli as a factor X-cleavable fusion protein. J. Biol. Chem. 1990;265:15854–15859. [PubMed] [Google Scholar]
- 15.Terpe K. Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl. Microbiol. Biotechnol. 2006;72:211–222. doi: 10.1007/s00253-006-0465-8. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Lin J, Liu M, Tao X, Wei D, Ma X, Yang S. Large scale preparation of recombinant human parathyroid hormone 1–84 from Escherichia coli. Prot. Express. and Purif. 2007;54:212–219. doi: 10.1016/j.pep.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Rolz C, Pellegrini M, Mierke DF. Molecular characterization of the receptor-ligand complex for parathyroid hormone. Biochemistry. 1999;38:6397–6405. doi: 10.1021/bi9829276. [DOI] [PubMed] [Google Scholar]
- 18.Whitmore L, Wallace B. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32:W668–W673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrow JA, Segall ML, Lund-katz S, Phillips MC, Knapp M, Rupp B, Weisgraber K. Differences in Stability among the Human Apolipoprotein E Isoforms Determined by the Amino-Terminal Domain. Biochemistry. 2000;39:11657–11666. doi: 10.1021/bi000099m. [DOI] [PubMed] [Google Scholar]
- 20.Savitzky A, Golay MJE. Smoothing and differentiation of data by a simplified least squares procedure. Anal. Chem. 1964;36:1627–1639. [Google Scholar]
- 21.McCoy J, LaVallie E. Expression and Purification of Thioredoxin Fusion Proteins. Curr. Prot. Mol. Bio. 2001;28:16.8.1–16.8.14. doi: 10.1002/0471142727.mb1608s28. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrini M, Royo M, Rosenblatt M, Chorev M, Mierke DF. Addressing the Tertiary Structure of Human Parathyroid Hormone-(1–34)*. J. Biol. Chem. 1998;273:10420–10427. doi: 10.1074/jbc.273.17.10420. [DOI] [PubMed] [Google Scholar]
- 23.Barbier J-R, Gardella TJ, Dean T, Maclean S, Potetinova Z, Whitfield JF, Willick GE. Backbone-methylated Analogues of the Principle Receptor Binding Region of Human Parathyroid Hormone. J. Biol. Chem. 2005;280:23771–23777. doi: 10.1074/jbc.M500817200. [DOI] [PubMed] [Google Scholar]
- 24.Zull J, Smith SK, Wiltshire R. Effect of Methionine Oxidation and Deletion of Amino-terminal Residues on the Conformation of Parathyroid Hormone: Circular Dichroism Studies. J. Biol. Chem. 1990;285:5671–5676. [PubMed] [Google Scholar]
- 25.Scian M, Marin M, Bellanda M, Tou L, Alexander J, Rosenblatt M, Chorev M, Peggion E, Mammi S. Backbone dynamics of human parathyroid hormone (1–34): flexibility of the central region under different environmental conditions. Biopolymers. 2006;84:147–160. doi: 10.1002/bip.20355. [DOI] [PubMed] [Google Scholar]
- 26.Li Y. Recombinant production of antimicrobial peptides in Escherichia coli: A review. Protein Express. Purif. 2011;80:260–267. doi: 10.1016/j.pep.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Bellot G, Pascal R, Mendre C, Urbach S, Mouillac B, Demene H. Expression, purification and NMR characterization of the cyclic recombinant form of the third intracellular loop of the vasopressin type 2 receptor. Prot. Express. Purif. 2011;78:131–138. doi: 10.1016/j.pep.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Kapust RB, Tozser J, Copeland TD, Waugh DS. The P1’ specificity of tobacco etch virus protease. Biochem. and Biophys. Res. Comm. 2002;294:949–955. doi: 10.1016/S0006-291X(02)00574-0. [DOI] [PubMed] [Google Scholar]
- 29.Cai M, Huang Y, Kazuasu S, Clore GM, Gronenborn A, Craigie R. An efficient and cost-effective isotope labeling protocol for proteins expressed in Escherichia coli. J. Biomol. NMR. 1998;11:97–102. doi: 10.1023/a:1008222131470. [DOI] [PubMed] [Google Scholar]