Abstract
The herpes simplex type 2 (HSV-2) envelope glycoprotein (gD2) was evaluated as a potential antigen candidate for a plasmid DNA (pDNA)-based HSV-2 vaccine. The pDNA was formulated with Vaxfectin, a cationic lipid-based adjuvant, and tested in a murine HSV-2 lethal challenge model. gD2 was expressed as full-length (FL) and secreted (S) gD2 forms. A 0.1 µg pDNA dose was tested to distinguish treatment conditions for survival and a 100 µg pDNA dose was tested to distinguish treatment conditions for reduction in vaginal and latent HSV-2 copies. Vaxfectin-formulated gD2 pDNA significantly increased serum IgG titres and survival for both FL gD2 and S gD2 compared with gD2 pDNA alone. Mice immunized with FL gD2 formulated with Vaxfectin showed reduction in vaginal and dorsal root ganglia (DRG) HSV-2 copies. The stringency of this protection was further evaluated by testing Vaxfectin-formulated FL gD2 pDNA at a high 500 LD50 inoculum. At this high viral challenge, the 0.1 µg dose of FL gD2 Vaxfectin-formulated pDNA yielded 80 % survival compared with no survival for FL gD2 pDNA alone. Vaxfectin-formulated FL gD2 pDNA, administered at a 100 µg pDNA dose, significantly reduced HSV-2 DNA copy number, compared with FL gD2 DNA alone. In addition, 40 % of mice vaccinated with adjuvanted FL pDNA had no detectable HSV-2 viral genomes in the DRG, whereas all mice vaccinated with gD2 pDNA alone were positive for HSV-2 viral genomes. These results show the potential contribution of Vaxfectin-gD2 pDNA to a future multivalent HSV-2 vaccine.
Introduction
Genital infections caused by herpes simplex type 2 (HSV-2) continue to present serious public-health problems throughout the world (Koelle & Corey, 2008; Xu et al., 2006). HSV-2 initially infects genital epithelial cells. Although the primary infection resolves, the virus undergoes retrograde transport into the dorsal root ganglia (DRG) and establishes a permanent latent neuronal infection (Corey et al., 2004). Periodic reactivation produces HSV-2 viral shedding, often without symptoms or lesions, when the virus can be transmitted (Koelle et al., 1992; Wald et al., 2002). HSV-2 has serious medical consequences, including neonatal infection, and is associated with increased susceptibility to human immunodeficiency virus type 1 infection (Chen et al., 2000; Perez et al., 1998; Wald & Corey, 2003). HSV-2 is thus an important vaccine target.
In an attempt to make an effective HSV-2 vaccine, various strategies have been investigated, including live-attenuated virus, inactivated virus and adjuvanted subunit vaccines (Kemble & Spaete, 2007; Koelle & Corey, 2008). The most thoroughly investigated subunit HSV-2 vaccine candidates have been viral envelope glycoproteins (Stanberry et al., 2002). Immunization with adjuvanted recombinant envelope glycoprotein D (gD) elicits neutralizing antibodies (Balachandran et al., 1982; Long et al., 1984) and CD4+ T-cell responses (Fló, 2003). HSV-2 vaccine clinical trials using gD2 have focused on the extracellular domain, lacking the C-terminal transmembrane and cytoplasmic domains of gD2 (Cattamanchi et al., 2008; Koelle & Corey, 2008). To date, adjuvanted gD2 subunit vaccines have shown limited clinical benefits (Belshe et al., 2012; Cohen, 2010; Stanberry et al., 2002). Therefore, there is a need to explore other approaches in vaccine development. Several reports have documented that there are CD4+ and CD8+ T-cell epitopes throughout the gD2 protein (Kim et al., 2008; Koelle et al., 2003), suggesting that full-length (FL) gD2 could potentially improve gD2-based vaccines. Immune response in HSV-2-infected humans and animals showed that T-cell responses are critical for providing protection against both primary and recurrent disease (Koelle & Corey, 2008). DNA vaccination is an attractive approach for developing HSV vaccines because of their potential for producing broad immune responses, including specific CD8+ and CD4+ responses and antibody production (Rajcáni et al., 2005). Plasmid DNA (pDNA) vaccines have been shown to elicit significant cellular immune responses to a variety of bacterial, viral and tumour antigens (reviewed by Donnelly et al., 1997; Sin et al., 1999) and have stimulated protective and therapeutic immune responses in animal HSV models (Bourne et al., 1996; McClements et al., 1996).
The adjuvant activity of Vaxfectin, a cationic lipid-based adjuvant, has been documented extensively for pDNA-based vaccines extending from rodent to non-human primate models of infectious disease (Hartikka et al., 2001; Hermanson et al., 2004; Jimenez et al., 2007; Pan et al., 2008; Reyes et al., 2001). These studies showed that Vaxfectin increased immunogenicity of antigens expressed from pDNA and that animals were protected against infection. Vaxfectin-formulated pDNA-based vaccines have produced protective neutralizing antibody titres and T-cell-specific immune responses in Phase 1 clinical trials of healthy subjects (Smith et al., 2010). In addition, the vaccines were shown to be safe and well tolerated.
In the present study we evaluated the efficacy of Vaxfectin-formulated gD2 vaccines in an intravaginal HSV-2 mouse model using both FL and secreted (S) gD2. End points included quantification of vaginal and DRG viral loads, survival and antibody responses. Vaxfectin-formulated gD2 pDNA showed a higher degree of sterilizing immunity and reduction in latency compared with pDNA alone.
Results
Vaxfectin-formulated gD2 pDNA produces higher antibody titres than gD2 pDNA alone
We compared the immunogenicity of FL gD2 and S gD2 at high (100 µg) and low (0.1 µg) doses with and without Vaxfectin. Previous experiments using 0.1 µg, 3 µg and 100 µg doses showed that only the 0.1 µg dose provided <100 % protection against lethal HSV-2 challenge (unpublished results). Therefore, the 0.1 µg dose was tested to distinguish between treatment conditions for survival and the 100 µg dose was tested for reduction in vaginal and latent HSV-2 copies. IgG serum ELISA titres were measured using gD121–339 protein, which is very similar in sequence to the corresponding region of gD2 (Dolan et al., 1998; Watson, 1983). Results showed that four out of 10 mice seroconverted after three immunizations with 0.1 µg of Vaxfectin-formulated pDNA for both the FL and the S forms of gD2, whereas specific IgG was not observed for non-adjuvanted gD2 pDNA (Fig. 1a). At the 100 µg dose, all animals seroconverted. However, the Vaxfectin-formulated pDNA groups showed significantly higher IgG titres using both forms of gD2 (P = 0.034 for S and P = 0.026 for FL) compared with the non-adjuvanted gD2 pDNA groups (Fig. 1b). Comparison of the FL and S forms of gD2 showed no significant difference in antibody titres between Vaxfectin-formulated pDNAs at either the 0.1 µg or 100 µg pDNA doses at any time point (Fig. 1).
Fig. 1.
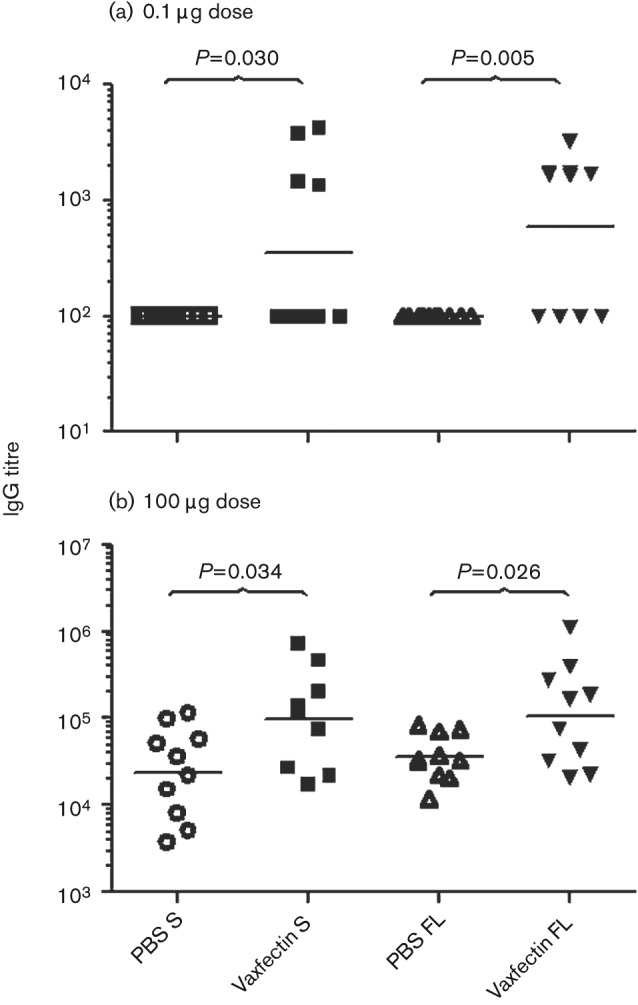
Serum IgG titres from vaccination of BALB/c mice with FL and S gD2 pDNA vaccines, formulated with or without Vaxfectin. Antibodies were measured by ELISA 2 weeks after the third injection. The bars represent geometric mean titres (n = 10 mice per group). Antibody titres were determined from A450 values. Titres <1 : 100 are plotted as 100. (a) Antibody titres at 0.1 µg per dose. (b) Antibody titres at 100 µg per dose. P values as shown.
Vaxfectin-formulated gD2 pDNA protects against lethal HSV-2 challenge better than gD2 pDNA alone
HSV-2 gD2 pDNA alone or formulated with Vaxfectin was tested at the 0.1 µg and 100 µg doses for protection against lethal intravaginal HSV-2 challenge at 50 LD50. Animals in the negative-control group succumbed to infection by day 8, whereas all animals in the thymidine kinase-negative strain of HSV-2 (TK− HSV-2) positive-control group survived (results not shown). At the 100 µg dose of gD2 pDNA, both Vaxfectin-formulated gD2 pDNA and gD2 pDNA alone (FL and S forms of gD2) induced complete protection against this HSV-2 challenge (results not shown). However, at the 0.1 µg pDNA dose, all animals vaccinated with gD2 pDNA alone died by day 8, whereas Vaxfectin-formulated gD2 pDNA, both FL and S forms, provided 80 and 60 % survival, respectively (P<0.0001 for both groups compared with gD2 pDNA alone; Fig. 2). All surviving animals seroconverted against HSV-2, confirming infection for all treatment groups (results not shown).
Fig. 2.
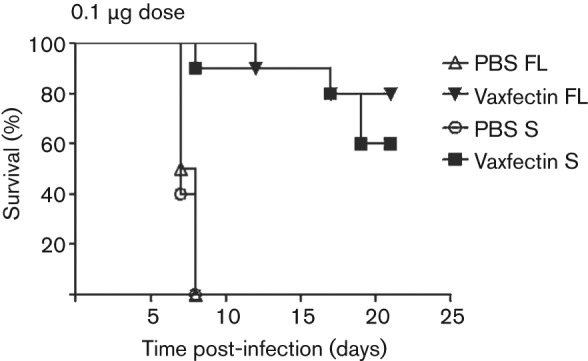
Relationship between adjuvant and survival after HSV-2 challenge with low-dose gD2 pDNA vaccine. BALB/c mice (10 per group) were vaccinated three times intramuscularly with 0.1 µg gD2 pDNA (S and FL). Six days prior to the challenge, 10 animals in each treatment group and five animals in the negative and TK− HSV-2 control groups were treated subcutaneously with 2 mg medroxyprogesterone (Depo-provera). Two weeks after the last vaccination, mice were inoculated vaginally with 50 LD50 of HSV-2. P<0.0001 when Vaxfectin FL is compared with PBS FL; P<0.0001 when Vaxfectin S is compared with PBS S.
Vaxfectin-formulated gD2 pDNA reduces vaginal viral load compared with gD2 pDNA alone
To determine the effect of vaccination on HSV-2 vaginal replication, quantitative PCR (qPCR) for HSV-2 DNA was performed on swabs obtained after inoculation. At the 0.1 µg dose, no significant differences were observed between Vaxfectin-formulated gD2 pDNA, gD2 pDNA alone and the negative-control group (results not shown). However, vaccination with the 100 µg dose of Vaxfectin-formulated gD2 pDNA resulted in lower HSV-2 DNA copy numbers at days 1 and 3 (Fig. 3a, b) and a significantly lower HSV-2 DNA copy number at day 5 compared with gD2 pDNA alone (Fig. 3c; P = 0.024 for S gD2 and P = 0.019 for FL). FL gD2 pDNA alone and Vaxfectin-formulated FL gD2 pDNA produced significantly lower HSV-2 copy numbers at day 3 (P = 0.05 and P = 0.002, respectively; Fig. 3b) and day 5 (P = 0.013 and P = 0.020, respectively; Fig. 3c) than S gD2 pDNA alone and Vaxfectin-formulated S gD2 pDNA.
Fig. 3.
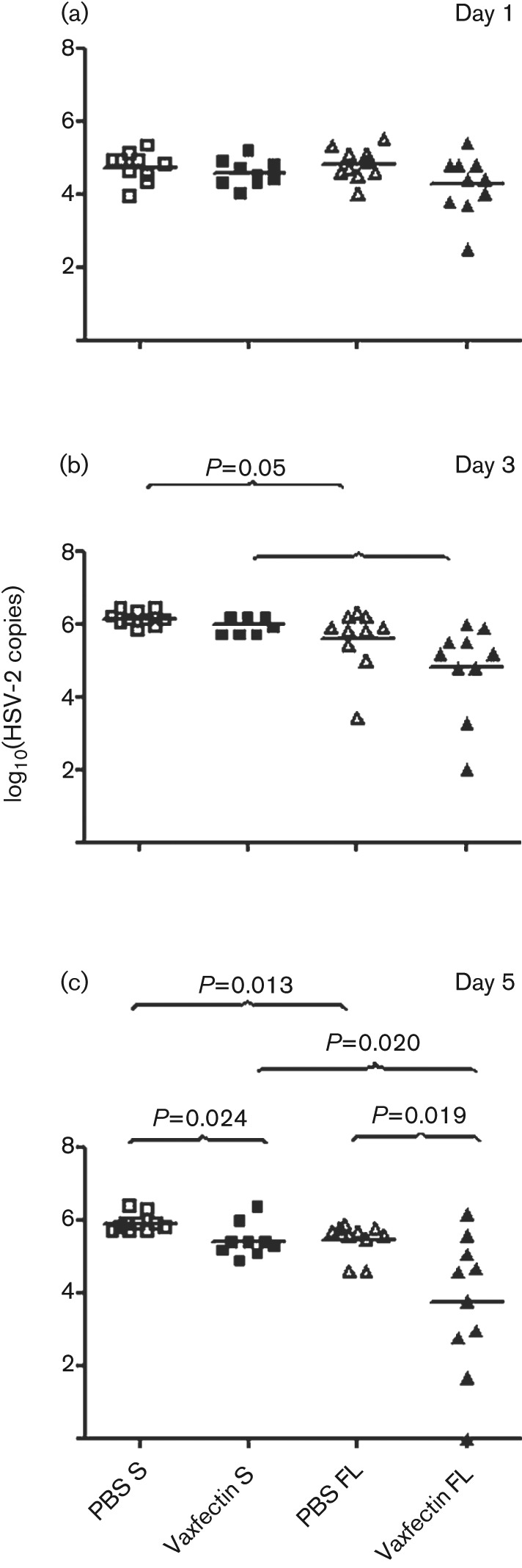
Vaginal HSV-2 DNA copy number on day 1 (a), day 3 (b) and day 5 (c) after vaccination with high-dose gD2 pDNA vaccine followed by lethal challenge with HSV-2. BALB/c mice (10 mice per group) were vaccinated three times intramuscularly with 100 µg gD2 pDNA. At day 14 after the last vaccination, mice were inoculated vaginally with 50 LD50 HSV-2. Vaginal swabs were collected and viral genomes were measured by qPCR for gB HSV-2 DNA. Each symbol represents an individual animal. The mean value for each group is shown by a black horizontal line. Values below the lower limit of detection are indicated as zero. P values as shown.
Vaxfectin-formulated FL gD2 pDNA reduces viral latency in DRG compared with gD2 pDNA alone
The level of HSV-2 viral latency was determined by measuring the HSV-2 DNA copy number in the DRG, normalized per host mouse cell. Lumbar and sacral DRG from surviving animals were pooled from each group, at 90 days after viral challenge. Vaccination with the 100 µg pDNA dose of Vaxfectin-formulated FL gD2 pDNA produced significantly lower DRG HSV-2 latency compared with survivors vaccinated with the same dose of gD2 pDNA alone (P = 0.007; Fig. 4). Six of the 10 animals vaccinated with Vaxfectin-formulated FL gD2 pDNA had no detectable HSV-2 DNA in their DRG, compared with only one of nine survivors vaccinated with FL gD2 pDNA alone. When pDNA encoding the S form of gD2 was used, no significant difference was observed with or without Vaxfectin (Fig. 4). The FL form of Vaxfectin-formulated gD2 pDNA produced significantly lower levels of latent DRG HSV-2 DNA than the S form (P = 0.028; Fig. 4). There was no difference between the two forms of non-adjuvanted gD2 (P = 0.227).
Fig. 4.
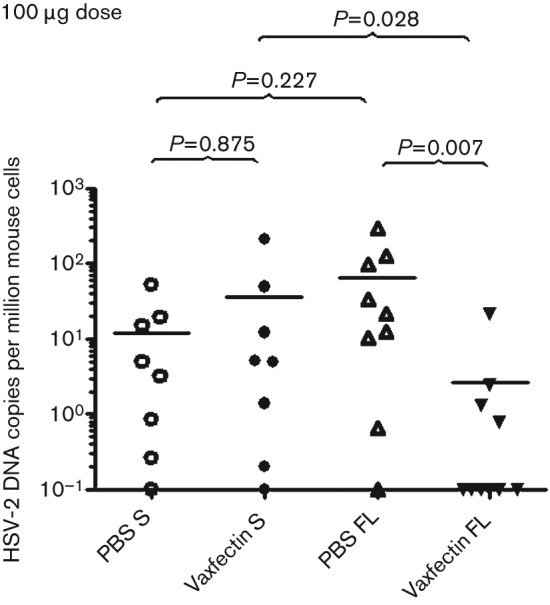
HSV-2 DNA copy number in DRG from mice surviving HSV-2 challenge following completion of pDNA vaccinations. BALB/c mice (10 per group) were vaccinated three times intramuscularly with 100 µg gD2 pDNA (S and FL) per dose. At day 14 after vaccination, mice were inoculated vaginally with 50 LD50 HSV-2. DRGs were collected 90 days after challenge and HSV-2 genomes were measured by qPCR assay. Each symbol represents an individual animal. The mean value for each group is shown by a black horizontal line. Viral genome numbers were normalized using mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) DNA. Values below the lower limit of detection are indicated as 0.1. P values as shown.
Vaxfectin-formulated FL gD2 pDNA produces high IgG titres irrespective of dose and protects better against HSV-2 lethal challenge than gD2 pDNA alone
Vaxfectin-formulated FL gD2 pDNA was selected for further evaluation, as both acute vaginal and latent DRG HSV-2 DNA copy numbers were lower for the FL vaccine compared with Vaxfectin-formulated S gD2 pDNA. Vaxfectin-formulated FL gD2 pDNA again produced higher IgG titres compared with gD2 pDNA alone at both the 0.1 µg and the 100 µg doses (P<0.001 for both comparisons). A dose–response relationship was observed, such that all animals seroconverted at the high (100 µg) but not at the low (0.1 µg) dose (results not shown). To determine the strength of protection, mice were challenged with both 50 and 500 LD50 HSV-2. At the 0.1 µg dose of gD2 pDNA, Vaxfectin-formulated FL gD2 pDNA provided significantly better survival compared with FL gD2 pDNA at both 50 and 500 LD50 of HSV-2 (P<0.0001 and P = 0.006, respectively; Fig. 5a, b). After challenge of vaccinated mice with 50 LD50 of HSV-2, all mice vaccinated with Vaxfectin-formulated FL gD2 pDNA were protected, whereas only one of 10 mice vaccinated with gD2 pDNA alone was protected. Moreover, when the viral challenge dose was increased 10-fold to 500 LD50, 80 % of mice vaccinated with the Vaxfectin-formulated FL gD2 pDNA still survived, compared with 0 % of mice vaccinated with gD2 pDNA (Fig. 5b). At the 100 µg pDNA dose, all mice survived both viral challenge doses regardless of the inclusion of Vaxfectin (results not shown).
Fig. 5.
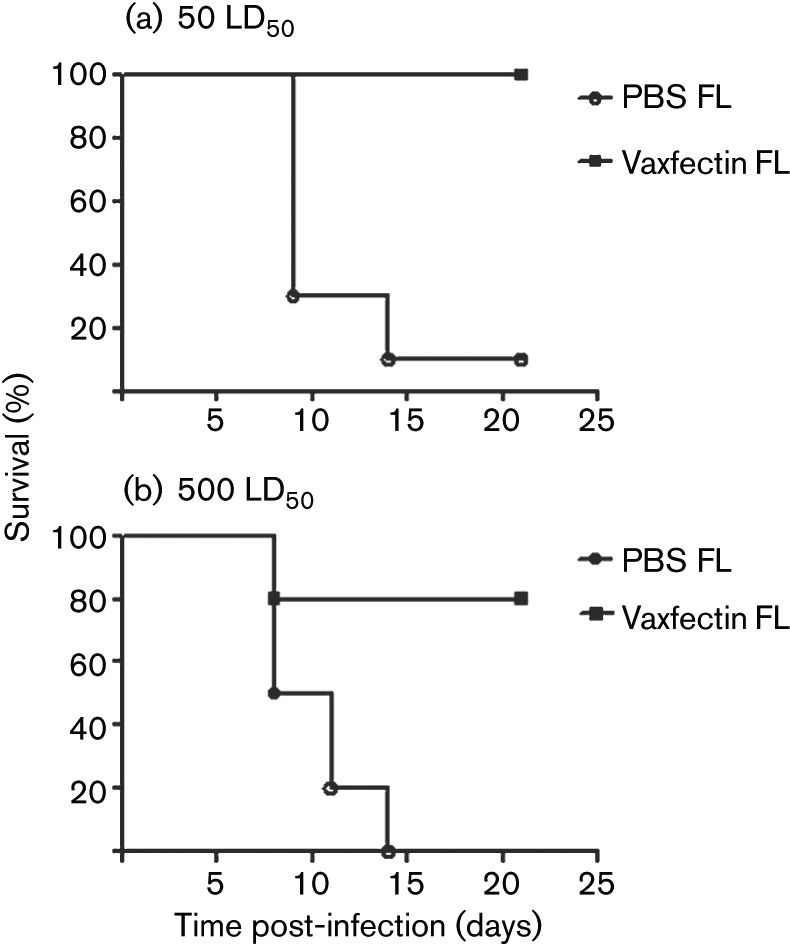
Inclusion of Vaxfectin improves survival after low-dose FL gD2 vaccination and standard- or high-dose HSV-2 challenge. BALB/c mice (10 per group) were vaccinated three times intramuscularly with 0.1 µg FL gD2 pDNA. Six days prior to the challenge, 10 animals in each treatment group and five animals in the negative and TK− HSV-2 control groups were treated subcutaneously with 2 mg medroxyprogesterone. Two weeks after the last vaccination, mice were inoculated vaginally with 50 LD50 (a) or 500 LD50 (b) HSV-2. P<0.0001 when Vaxfectin FL is compared with PBS FL at 50 LD50; P = 0.006 when Vaxfectin FL is compared with PBS FL at 500 LD50.
Vaccination with Vaxfectin-formulated FL gD2 pDNA reduces vaginal HSV-2 DNA copies compared with gD2 pDNA alone
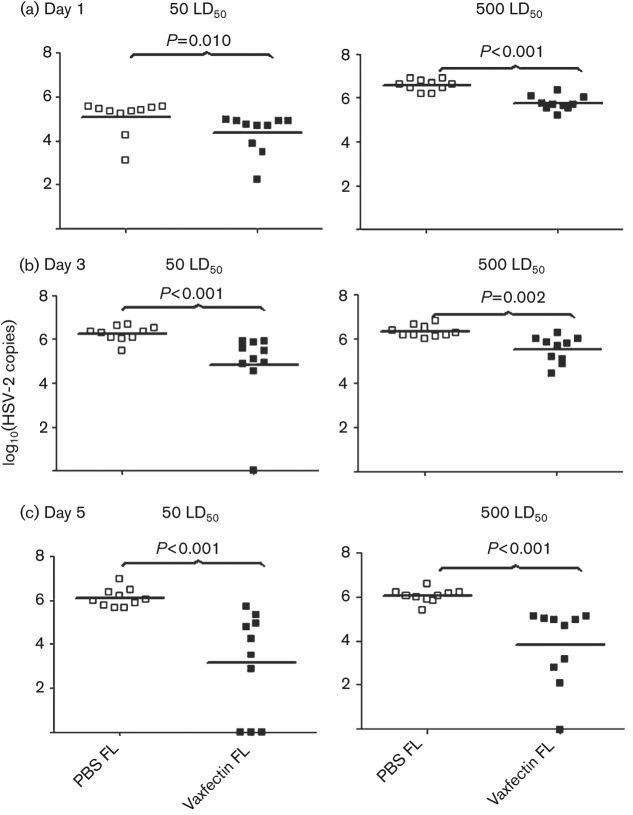
The amount of vaginal HSV-2 DNA at days 1, 3 and 5 was measured by qPCR. Vaccination with 100 µg Vaxfectin-formulated FL gD2 pDNA reduced HSV-2 DNA copy number significantly at both 50 LD50 or 500 LD50 HSV-2 challenge doses at each time point compared with vaccination with the same dose of FL gD2 DNA administered without adjuvant (Fig. 6). As noted above, all animals survived viral challenge at this vaccine dose, but the viral load data clearly differentiate the efficacy of Vaxfectin-formulated vs non-adjuvanted vaccine. By day 5, three mice challenged with 50 LD50 and one mouse challenged with 500 LD50 had no detectable vaginal HSV-2 DNA (Fig. 6c). The vaginal HSV-2 copy number detected on day 5 for mice vaccinated with Vaxfectin-formulated FL gD2 pDNA did not differ statistically between the 50 and 500 LD50 HSV-2 challenge doses, showing the effectiveness of the vaccine at the highest viral challenge dose.
Fig. 6.
Vaginal HSV-2 DNA copy number on day 1 (a), day 3 (b) and day 5 (c) following HSV-2 challenge. BALB/c mice (10 per group) were vaccinated three times intramuscularly with 100 µg FL gD2 pDNA (□, PBS FL; ▪, Vaxfectin FL). At day 14 after the last vaccination, mice were inoculated vaginally with 50× LD50 (left graphs) or 500× LD50 (right graphs) of HSV-2. Vaginal swabs were collected and viral genomes were measured by qPCR for HSV-2 DNA. Each symbol represents an individual animal. The mean value for each group is shown by a black horizontal line. Values below the lower limit of detection are listed as zero.
Vaxfectin-formulated FL gD2 pDNA significantly reduces latent DRG HSV-2 DNA copy number compared with gD2 pDNA alone
Animals vaccinated with 100 µg gD2 pDNA dose and surviving HSV-2 vaginal challenge were examined for HSV-2 DNA copies in DRG. Mice vaccinated with Vaxfectin-formulated FL gD2 pDNA had significantly lower DRG HSV-2 DNA copy number at both the 50 and the 500 LD50 HSV-2 challenge doses, compared with gD2 alone (P = 0.002 and P = 0.005, respectively; Fig. 7). Seven out of 10 mice vaccinated with Vaxfectin-formulated FL gD2 pDNA showed no detectable HSV-2 DNA copy number at 50 LD50, compared with one of 10 mice vaccinated with gD2 pDNA alone (Fig. 7a). Notably, at 500 LD50 of HSV-2, four out of 10 animals had no detectable HSV-2 DNA copy number, whereas all mice vaccinated with gD2 pDNA alone had detectable HSV-2 DNA in their lumbosacral DRG (Fig. 7b). These results are consistent with vaginal HSV-2 DNA levels (Fig. 6), showing efficacy of the Vaxfectin-formulated FL gD2 vaccine in preventing HSV-2 viral latency, even at a viral challenge of 500 LD50.
Fig. 7.
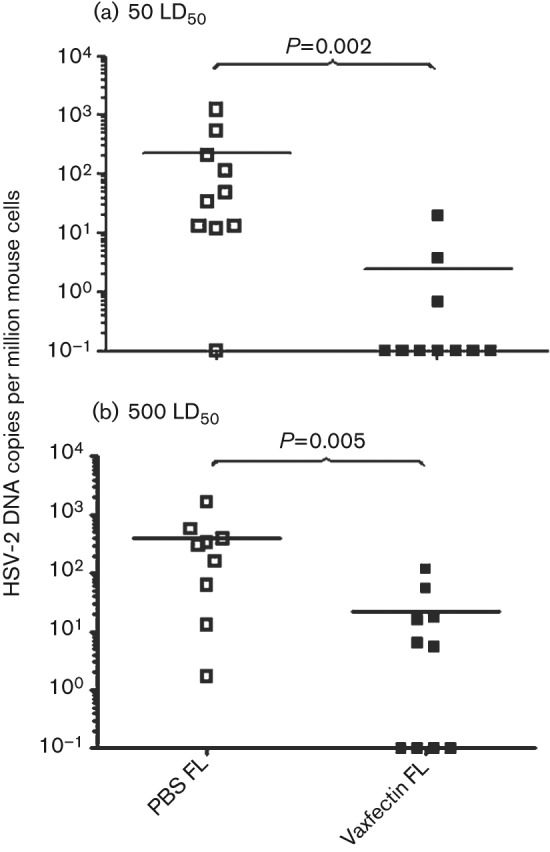
DRG HSV-2 DNA copy number following HSV-2 challenge. BALB/c mice (10 per group) were vaccinated three times intramuscularly with 100 µg gD2 pDNA. At day 14 after vaccination, mice were inoculated vaginally with 50 LD50 (a) or 500 LD50 (b) HSV-2. DRGs were collected 90 days after challenge and viral genome was detected using a qPCR assay. Each symbol represents an individual animal. The mean value for each group is shown by a black horizontal line. Viral genome numbers were normalized using mouse GAPDH DNA. Values below the lower limit of detection are indicated as 0.1.
Discussion
Subunit vaccines consisting of recombinant envelope glycoproteins gB2 and gD2, or gD2 alone with different adjuvants have undergone efficacy testing in clinical trials (Belshe et al., 2012; Corey et al., 1999; Stanberry et al., 2002). While strong neutralizing antibodies were induced, efficacy end points were not met (Belshe et al., 2012; Cohen, 2010; Corey et al., 1999). An effective prophylactic HSV-2 vaccine is the most likely option for the successful control of the spread of genital herpes. End points in Phase 3 trials have shifted from prevention of infection in early studies (Corey et al., 1999) to prevention of clinical disease in more recent studies (Stanberry et al., 2002). At the population level, control will be assisted by a reduction in symptomatic and asymptomatic HSV-2 shedding, which is responsible for HSV-2 transmission. Therefore the induction of immune responses which could reduce reactivation and shedding is arguably a valuable end point for a preventative vaccine, even if the vaccine is not sterilizing and vaccines seroconvert to HSV-2.
Two different forms of gD2 were first compared: FL gD21–393 and S gD2, a secreted form of gD2 comprising aa 1–340. The S form lacks the transmembrane and cytoplasmic domains (Higgins et al., 2000), similar to the recombinant gD protein adjuvanted with MF59 (Langenberg et al., 1995) or alum and monophosphorylated lipid A (MPL) (Bernstein et al., 2005). The use of a pDNA vaccine provides the advantage of allowing the evaluation of a full-length form, FL gD21–393, which includes CD4 and CD8 epitopes distributed throughout the protein (BenMohamed et al., 2003; Chentoufi et al., 2008; Kim et al., 2008; Koelle et al., 2003). There was no difference in the antibody response or survival (Figs 1 and 2) between these two forms of gD2. While there could be a difference in neutralizing antibody titres between the two forms of gD, this was not explored further due to previous studies which showed that antibodies are not required for protection from lethal challenge by either HSV-2 pDNA vaccines or by the live-attenuated virus used as a positive control vaccine in the HSV-2 vaginal challenge model (Iijima et al., 2008; Nass et al., 2001). There was a clear difference in HSV-2 replication in the vagina (Fig. 3) and in DRG (Fig. 4). The host mechanisms controlling virus replication after FL gD2 pDNA vaccination remain undefined. It is possible that quantitative functional differences in antigen-specific CD4+ or CD8+ T-cell responses correlate with the improvements observed with Vaxfectin and such studies are planned for the future.
The vaccine potency can be increased by formulating an adjuvant with an antigen to boost the immune system. Adjuvants, such as MF59 and alum with MPL, have been used to adjuvant split vaccines containing recombinant truncated envelope glycoproteins. Both of these adjuvants increase the humoral immune response, but have not been shown to increase cell-mediated immunity (Shiver et al., 2002; Ulmer et al., 1999). In this study, Vaxfectin, a cationic lipid adjuvant, was added to improve immunogenicity of the HSV-2 gD pDNA. Vaxfectin was initially developed to enhance immune responses to pDNA-based vaccines (Sullivan et al., 2010). Numerous mouse, rabbit, dog and non-human primate studies have demonstrated the ability of Vaxfectin to boost antibody titres and cell-mediated immunity, resulting in durable responses. (Hartikka et al., 2001; Hermanson et al., 2004; Jimenez et al., 2007; Margalith & Vilalta, 2006; Reyes et al., 2001). As an example, a study in infant and juvenile macaques showed protection against a measles virus challenge 1 year after vaccination (Pan et al., 2008). Moreover, Vaxfectin-formulated pDNA encoding H5 haemagglutinin was able to protect mice and ferrets against a lethal viral challenge. This same Vaxfectin-formulated vaccine candidate was tested in two phase 1 clinical trials producing durable immune responses that resulted in protective neutralizing antibody titres and induction of T-cell-specific immune response (Jimenez et al., 2007; Smith et al., 2010). Unlike the alum/MPL HSV-2 split vaccine, which did not prevent infection in a recently completed phase 3 clinical trial (Aumakhan et al., 2010; Belshe et al., 2012), expression of the FL gD will provide the complete repertoire of T-cell epitopes (Hosken et al., 2006) and Vaxfectin should further boost the cell-mediated immune response against these epitopes.
In the present study, we have shown that both FL and S forms of gD2 formulated with Vaxfectin showed significantly higher antibody titres, compared with gD2 alone (Fig. 1). Previous studies demonstrated the importance of antibodies in the protection of neuronal tissues against HSV (Bourne et al., 2002; Klein, 1980; Simmons & Nash, 1985). However, review of our results shows that some animals that failed to seroconvert were nonetheless protected from death at the 0.1 µg dose of Vaxfectin-formulated FL gD2 (Figs 1 and 2). This apparent discrepancy may be related to limited sensitivity of our IgG assay or to a protective role for CD4+ T-cells, as demonstrated previously in some pDNA-HSV-2 challenge studies (Manickan et al., 1995; Nass et al., 2001; Sin et al., 2000, 2001).
Our results also showed that vaginal and DRG levels of HSV-2 DNA were reduced in mice vaccinated with Vaxfectin-formulated gD2 pDNA compared with gD2 pDNA alone (Figs 3 and 4). Notably, in our initial experiment, at the low dose of gD2 (0.1 µg), no animals vaccinated with either form of gD2 seroconverted after three injections, whereas in the Vaxfectin-formulated gD2 pDNA groups, seroconversion occurred in 50 % of the animals (Fig. 1a). Interestingly, survival for the 0.1 µg dose was extremely dependent on the inclusion of Vaxfectin (Fig. 2), regardless of the form of gD2 pDNA used. Vaxfectin-formulated FL gD2 pDNA reduced vaginal and DRG HSV2 DNA copies significantly, compared with Vaxfectin-formulated S gD2 pDNA (Figs 3 and 4). Some end points were clearly dependent on the length of the gD2 construct used, with FL gD2 performing better than S gD2 either with or without Vaxfectin as measured by vaginal HSV-2 DNA loads on day 5 (Fig. 3).
Mouse and guinea pig infection models (BenMohamed et al., 2003; Bourne et al., 2005; Sawtell, 1998; Sawtell et al., 1998, 2001) suggest that the intensity of viral reactivation corresponds to the number of HSV-2-infected neurons in the DRG and the intensity of infection per neuron, such that non-sterilizing vaccines that perturb natural infection to reduce ganglionic latency could rationally be expected to have a beneficial effect on prevention of latency. Formulation of gD pDNA with Vaxfectin resulted in lower DRG latent HSV-2 loads, correlating with lower vaginal replication; this is consistent with a model of pathogenesis in which intense local replication precedes and predicts access of virus to the ganglia. These data suggest that an optimized HSV-2 vaccine could improve the natural history of HSV-2 shedding, and reduce the transmission of HSV-2 in the population.
Based on comparison of FL and S gD2, and data indicating that Vaxfectin increased immune responses and protection, the stringency of the experimental system was enhanced by increasing the challenge dose of HSV-2 10-fold (500 LD50). Notably, at a 0.1 µg pDNA dose of FL gD2, Vaxfectin-formulated gD2 pDNA still provided a high level of protection against both standard and high-dose challenge in mice (Fig. 5). The results showed that the 100 µg dose of FL gD2 pDNA formulated with Vaxfectin significantly reduced vaginal and DRG HSV-2 DNA copy number equally well after either 50 or 500 LD50 HSV-2 challenge doses (Figs 6 and 7). This indicates that the Vaxfectin-formulated vaccine generates a strong effect that can control primary infection and limit subsequent latent HSV-2 infection in mice. These results suggest that a non-sterilizing vaccine can still have a beneficial influence on both acute vaginal replication, which can be expected to correlate with the intensity of genital symptoms in humans undergoing primary genital herpes infections, as well as the level of DRG latency, which arguably is correlated directly with HSV reactivation.
Overall, the Vaxfectin-formulated vaccine expressing FL gD2 in a mouse HSV-2 model demonstrated excellent immunogenicity, increased protection with low doses of gD2 pDNA vaccine, and led to significant reduction in the vaginal and ganglion HSV-2 DNA copies. While truncated gD2 protein with MPL and alum (MPL-alum) has been disappointing in a recent clinical trial, the benefits seen with expression of FL gD2 formulated with Vaxfectin adjuvant in this report indicate that gD2, combined with other HSV-2 subunits targeting T-cell responses (Muller et al., 2009), may be a viable preventative vaccine candidate. Future studies will evaluate such multivalent vaccines to determine whether a Vaxfectin-adjuvanted DNA vaccine has prophylactic and therapeutic activity in the guinea pig model of genital HSV-2.
Methods
Mice.
Female BALB/cJ mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and used at 5–6 weeks of age. Mice were housed in pathogen-free conditions. Protocols were approved by the University of Washington Institutional Animal Care and Use Committee.
Plasmid DNA (pDNA), formulations and vaccination.
The HSV-2 gD2 sequence was used as either the full-length (FL) gene or a secreted (S) form (aa 1–340) (Dolan et al., 1998).
HSV-2 genes were codon-optimized using proprietary algorithms developed at Vical. DNA was synthesized by GeneArt (Regensburg) and sequenced to threefold redundancy. Sequences encoding the FL or S form were inserted into expression plasmid VR1012 (Hartikka et al., 1996) that contains a constitutive hCMV (human cytomegalovirus) immediate-early enhancer/promoter. pDNA was manufactured as previously described (Vilalta et al., 2009). The expression of FL gD2 and S gD2 was confirmed (results not shown) by flow cytometry (Kask et al., 2010). Briefly, COS-7 cells were transfected with FL gD2, S gD2, or empty vector VR1012. At 48 h, trypsin-harvested cells were permeabilized for S gD2, but not for FL gD2, as described previously (Jing et al., 2009). Cells were stained with mouse mAb anti-gD 2C10 (Santa Cruz Biotechnology) or isotype control at 1 µg ml−1 and washed. Bound IgG was detected with allophycocyanin-conjugated goat anti-mouse IgG (Biolegend). After washing and fixation, cells were analysed on a FacsCanto II cytometer (Becton Dickinson).
pDNA was formulated with Vaxfectin as first described by Hartikka et al. (2001). Briefly, both GAP-DMORIE [(±)-N-(3-aminopropyl)-N,N-dimethyl-2,3-bis(cis-9-tetradecenyloxy)-1-propanaminium bromide] and DPyPE (aminopropyl-dimethyl-myristoleyloxy-propanaminium bromide-diphytanoylphosphatidyl-ethanolamine) were resuspended in chloroform, mixed in 1 : 1 molar ratio, aliquotted into vials and dried to create Vaxfectin dry lipid film. On the day of injection, the lipid film vials were resuspended in 1 ml 0.9 % saline. pDNA was prepared in 0.9 % saline, 20 mM sodium phosphate, pH 7.2. The pDNA was formulated with Vaxfectin by gently streaming the lipid into pDNA at a 1 : 1 equal volume dilution, yielding 1 mg pDNA ml−1 and 1.09 mg Vaxfectin ml−1. The final pDNA nucleotide : cationic lipid molar ratio was 4 : 1. For the 1 mg ml−1 high gD2 pDNA dose, the formulation was injected undiluted. The formulation was diluted with PBS to yield the 1 µg ml−1 low gD2 pDNA dose.
Mice were injected bilaterally with 50 µl intramuscular injection yielding a total volume of 100 µl per mouse. Mice were vaccinated three times on days 0, 14 and 28. Negative-control animals received no vaccine. Positive-control animals were immunized by intravaginal infection at day 14 with 0.5×106 p.f.u. TK− HSV-2 strain 333 after Depo-provera (Pharmacia) pre-treatment (Coen et al., 1989).
Anti-gD121–339 and anti-HSV-2 antibody measurement.
Recombinant HSV-1 gD protein, expressing aa 21–339, was purchased from Meridian Life Sciences. This protein has a primary sequence that is 89 % identical to that of gD2 (Dolan et al., 1998; Watson, 1983) and was used because long polypeptide versions of gD2 are not commercially available. ELISAs were performed as described previously (Muller et al., 2009). Briefly, high-binding 96-well flat-bottom ELISA plates (Corning Costar) were coated with 250 ng gD121–339 ml−1 0.1 M Na2CO3 (pH 9.6) buffer overnight at 4 °C. Subsequently, serial threefold dilutions of sera starting at 1 : 100 were added overnight at 4 °C. Total IgG was detected by addition of biotinylated goat anti-mouse Fc (Biodesign), followed by streptavidin–HRP (Pierce) and TMB peroxidase substrate (KPL). Plates were read at A450 using a Victor3 1420 plate reader (Perkin Elmer). Assays were done in duplicate and mean A450 values were used in subsequent calculations.
To calculate relative antibody titres, pooled serum from gD121–339-immunized mice was used as a positive control. In order to generate gD121–339 immune sera, four mice were immunized with 30 µg gD121–339 protein, mixed with Titermax adjuvant (CytRx Corporation), prepared according to the manufacturer’s instructions. The animals were primed intramuscularly, followed by intraperitoneal boosts at 2 week intervals. Two weeks after the last immunization, the sera were collected and pooled for use as a standard in the ELISA. The dilution factor at which an A450 value was two times above the background was assigned as the end-point relative titre. To calculate relative anti-gD121–339 IgG titres for each sample, we included a standard curve with the positive-control sera beginning at 1 : 2700 on each ELISA plate. Dilutions of the unknown sera with A450 values falling within the linear portion of the positive-control A450 vs dilution standard curve were used to calculate relative anti-gD121–339 IgG titres.
To confirm that animals surviving the HSV-2 challenge seroconverted to HSV-2, binding IgG antibodies to whole HSV-2 by ELISA were measured. Briefly, UV-treated HSV-2 stock (below) was diluted 1 : 1000 in 0.1 M Na2CO3 (pH 9.6) buffer and coated overnight at 4 °C. Mouse sera were applied to duplicate wells at 1 : 100 dilutions and binding IgG was detected as above. The raw A450 was measured and animals with a mean A450 greater than 2× naïve sera controls were considered seropositive.
Virus challenge.
Six days prior to the challenge, the animals were treated subcutaneously with 2 mg medroxyprogesterone (Depo-provera; Pharmacia) to confer susceptibility to infection (Parr et al., 1994). Two weeks after the third vaccination, the animals were challenged intravaginally with 1.55×104 or 1.55×105 p.f.u. of HSV-2 strain 186, corresponding to 50 LD50 or 500 LD50, respectively, determined in preliminary experiments in age-matched animals. The virus was thawed immediately before use and diluted in PBS and 0.1 % normal mouse serum prior to administration. Animals were anaesthetized with ketamine and xylazine. Vaginas were cleaned using a sterile calcium alginate fibre-tipped urethral swab (14-959-78; Thermo Fisher Scientific). Ten microlitres of diluted virus was gently injected into the vagina with a hand-held pipette and a 20 µl tip. Animals were placed in a supine position and awakened after several minutes. The animals were monitored twice daily, for 21 days, for signs of disease and survival. Animals with hind-limb paralysis, urine staining of the perineum, or other pre-morbid signs or symptoms were euthanized.
HSV-2 replication.
On days 1, 3 and 5 post-infection, vaginal swabs were collected to assess the relative extent of virus replication. Using Copan polyester urethral swabs (185CS01; Copan), the vaginas of unanaesthetized animals were swabbed by gently turning the swab through 360°. Swabs were stored in digestion buffer (100 mM KCl, 10 mM Tris pH 8.0, 25 mM EDTA, 05 % Nonidet P-40) at −80 °C prior to use. DNA extraction and qPCR for HSV-2 DNA copy number were performed as described by Wald et al. (2003).
Animals that survived HSV-2 challenge were examined for virus replication in DRG 90 days after challenge. DRG from the bilateral lumbar and sacral regions were dissected as described by Malin et al. (2007) and collected into sterile tubes on dry ice. Pooled ganglia from each animal were washed carefully into 200 µl digestion buffer. DNA extraction and HSV-2 DNA copy number measurement were performed as above. We also estimated mouse cell number by measuring the mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) DNA copy number and dividing by two. Mouse DNA genomes were measured with a qPCR primer-/probe cocktail (part 4308313) amplifying the GAPDH gene (ABI). Results are presented as HSV-2 DNA copies per million mouse cells.
Statistical analysis.
Wilcoxon rank sum test or Student’s t-test was used to compare log10 IgG titres, and to compare log10 vaginal HSV-2 DNA copy numbers between groups. Kaplan–Meier curves were generated to compare survival in days between groups. Survival distributions were compared between groups using the log-rank test. All statistical analysis was performed using sas 9.2 for PC. A two-sided P-value <0.05 was considered statistically significant. No multiple comparison adjustments were made.
Acknowledgements
The authors would like to acknowledge Hong Xie for assistance with HSV PCR. This work was supported by NIH STTR grant AI065015.
References
- Aumakhan B., Hardick A., Quinn T. C., Laeyendecker O., Gange S. J., Beyrer C., Cox C., Anastos K., Cohen M. & other authors (2010). Genital herpes evaluation by quantitative TaqMan PCR: correlating single detection and quantity of HSV-2 DNA in cervicovaginal lavage fluids with cross-sectional and longitudinal clinical data. Virol J 7, 328 10.1186/1743-422X-7-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. (1982). Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun 37, 1132–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe R. B., Leone P. A., Bernstein D. I., Wald A., Levin M. J., Stapleton J. T., Gorfinkel I., Morrow R. L., Ewell M. G. & other authors (2012). Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366, 34–43 10.1056/NEJMoa1103151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BenMohamed L., Bertrand G., McNamara C. D., Gras-Masse H., Hammer J., Wechsler S. L., Nesburn A. B. (2003). Identification of novel immunodominant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus glycoprotein D that confer protective immunity. J Virol 77, 9463–9473 10.1128/JVI.77.17.9463-9473.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein D. I., Aoki F. Y., Tyring S. K., Stanberry L. R., St-Pierre C., Shafran S. D., Leroux-Roels G., Van Herck K., Bollaerts A., Dubin G., Glaxo Smith Kline Herpes Vaccine Study Group (2005). Safety and immunogenicity of glycoprotein D-adjuvant genital herpes vaccine. Clin Infect Dis 40, 1271–1281 10.1086/429240 [DOI] [PubMed] [Google Scholar]
- Bourne N., Milligan G. N., Schleiss M. R., Bernstein D. I., Stanberry L. R. (1996). DNA immunization confers protective immunity on mice challenged intravaginally with herpes simplex virus type 2. Vaccine 14, 1230–1234 10.1016/S0264-410X(96)00027-8 [DOI] [PubMed] [Google Scholar]
- Bourne N., Pyles R. B., Bernstein D. I., Stanberry L. R. (2002). Modification of primary and recurrent genital herpes in guinea pigs by passive immunization. J Gen Virol 83, 2797–2801 [DOI] [PubMed] [Google Scholar]
- Bourne N., Milligan G. N., Stanberry L. R., Stegall R., Pyles R. B. (2005). Impact of immunization with glycoprotein D2/AS04 on herpes simplex virus type 2 shedding into the genital tract in guinea pigs that become infected. J Infect Dis 192, 2117–2123 10.1086/498247 [DOI] [PubMed] [Google Scholar]
- Cattamanchi A., Posavad C. M., Wald A., Baine Y., Moses J., Higgins T. J., Ginsberg R., Ciccarelli R., Corey L., Koelle D. M. (2008). Phase I study of a herpes simplex virus type 2 (HSV-2) DNA vaccine administered to healthy, HSV-2-seronegative adults by a needle-free injection system. Clin Vaccine Immunol 15, 1638–1643 10.1128/CVI.00167-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Ballard R. C., Beck-Sague C. M., Dangor Y., Radebe F., Schmid S., Weiss J. B., Tshabalala V., Fehler G. & other authors (2000). Human immunodeficiency virus infection and genital ulcer disease in South Africa: the herpetic connection. Sex Transm Dis 27, 21–29 10.1097/00007435-200001000-00005 [DOI] [PubMed] [Google Scholar]
- Chentoufi A. A., Zhang X., Lamberth K., Dasgupta G., Bettahi I., Nguyen A., Wu M., Zhu X., Mohebbi A. & other authors (2008). HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol 180, 426–437 [DOI] [PubMed] [Google Scholar]
- Coen D. M., Kosz-Vnenchak M., Jacobson J. G., Leib D. A., Bogard C. L., Schaffer P. A., Tyler K. L., Knipe D. M. (1989). Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A 86, 4736–4740 10.1073/pnas.86.12.4736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (2010). Immunology. Painful failure of promising genital herpes vaccine. Science 330, 304 10.1126/science.330.6002.304 [DOI] [PubMed] [Google Scholar]
- Corey L., Langenberg A. G., Ashley R., Sekulovich R. E., Izu A. E., Douglas J. M., Jr, Handsfield H. H., Warren T., Marr L. & other authors (1999). Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282, 331–340 10.1001/jama.282.4.331 [DOI] [PubMed] [Google Scholar]
- Corey L., Wald A., Celum C. L., Quinn T. C. (2004). The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr 35, 435–445 10.1097/00126334-200404150-00001 [DOI] [PubMed] [Google Scholar]
- Dolan A., Jamieson F. E., Cunningham C., Barnett B. C., McGeoch D. J. (1998). The genome sequence of herpes simplex virus type 2. J Virol 72, 2010–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly J. J., Ulmer J. B., Liu M. A. (1997). DNA vaccines. Life Sci 60, 163–172 10.1016/S0024-3205(96)00502-4 [DOI] [PubMed] [Google Scholar]
- Fló J. (2003). Co-immunization with plasmids coding the full length and a soluble form of glycoprotein D of HSV-2 induces protective cellular and humoral immune response in mice. Vaccine 21, 1239–1245 10.1016/S0264-410X(02)00476-0 [DOI] [PubMed] [Google Scholar]
- Hartikka J., Sawdey M., Cornefert-Jensen F., Margalith M., Barnhart K., Nolasco M., Vahlsing H. L., Meek J., Marquet M. & other authors (1996). An improved plasmid DNA expression vector for direct injection into skeletal muscle. Hum Gene Ther 7, 1205–1217 10.1089/hum.1996.7.10-1205 [DOI] [PubMed] [Google Scholar]
- Hartikka J., Bozoukova V., Ferrari M., Sukhu L., Enas J., Sawdey M., Wloch M. K., Tonsky K., Norman J. & other authors (2001). Vaxfectin enhances the humoral immune response to plasmid DNA-encoded antigens. Vaccine 19, 1911–1923 10.1016/S0264-410X(00)00445-X [DOI] [PubMed] [Google Scholar]
- Hermanson G., Whitlow V., Parker S., Tonsky K., Rusalov D., Ferrari M., Lalor P., Komai M., Mere R. & other authors (2004). A cationic lipid-formulated plasmid DNA vaccine confers sustained antibody-mediated protection against aerosolized anthrax spores. Proc Natl Acad Sci U S A 101, 13601–13606 10.1073/pnas.0405557101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Herold K. M., Arnold R. L., McElhiney S. P., Shroff K. E., Pachuk C. J. (2000). Plasmid DNA-expressed secreted and nonsecreted forms of herpes simplex virus glycoprotein D2 induce different types of immune responses. J Infect Dis 182, 1311–1320 10.1086/315879 [DOI] [PubMed] [Google Scholar]
- Hosken N., McGowan P., Meier A., Koelle D. M., Sleath P., Wagener F., Elliott M., Grabstein K., Posavad C., Corey L. (2006). Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J Virol 80, 5509–5515 10.1128/JVI.02659-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N., Linehan M. M., Zamora M., Butkus D., Dunn R., Kehry M. R., Laufer T. M., Iwasaki A. (2008). Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med 205, 3041–3052 10.1084/jem.20082039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez G. S., Planchon R., Wei Q., Rusalov D., Geall A., Enas J., Lalor P., Leamy V., Vahle R. & other authors (2007). Vaxfectin-formulated influenza DNA vaccines encoding NP and M2 viral proteins protect mice against lethal viral challenge. Hum Vaccin 3, 157–164 10.4161/hv.3.5.4175 [DOI] [PubMed] [Google Scholar]
- Jing L., McCaughey S. M., Davies D. H., Chong T. M., Felgner P. L., De Rosa S. C., Wilson C. B., Koelle D. M. (2009). ORFeome approach to the clonal, HLA allele-specific CD4 T-cell response to a complex pathogen in humans. J Immunol Methods 347, 36–45 10.1016/j.jim.2009.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask A. S., Chen X., Marshak J. O., Dong L., Saracino M., Chen D., Jarrahian C., Kendall M. A., Koelle D. M. (2010). DNA vaccine delivery by densely-packed and short microprojection arrays to skin protects against vaginal HSV-2 challenge. Vaccine 28, 7483–7491 10.1016/j.vaccine.2010.09.014 [DOI] [PubMed] [Google Scholar]
- Kemble G., Spaete R. (2007). Herpes simplex vaccines. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis, chapter 69. Edited by Arvin A., Campadelli-Fiume G., Mocarski E., Moore P. S., Roizman B., Whitley R., Yamanishi K. Cambridge: Cambridge University Press; [PubMed] [Google Scholar]
- Kim M., Taylor J., Sidney J., Mikloska Z., Bodsworth N., Lagios K., Dunckley H., Byth-Wilson K., Denis M. & other authors (2008). Immunodominant epitopes in herpes simplex virus type 2 glycoprotein D are recognized by CD4 lymphocytes from both HSV-1 and HSV-2 seropositive subjects. J Immunol 181, 6604–6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J. (1980). Effect of immune serum on the establishment of herpes simplex virus infection in trigeminal ganglia of hairless mice. J Gen Virol 49, 401–405 10.1099/0022-1317-49-2-401 [DOI] [PubMed] [Google Scholar]
- Koelle D. M., Corey L. (2008). Herpes simplex: insights on pathogenesis and possible vaccines. Annu Rev Med 59, 381–395 10.1146/annurev.med.59.061606.095540 [DOI] [PubMed] [Google Scholar]
- Koelle D. M., Benedetti J., Langenberg A., Corey L. (1992). Asymptomatic reactivation of herpes simplex virus in women after the first episode of genital herpes. Ann Intern Med 116, 433–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle D. M., Liu Z., McClurkan C. L., Cevallos R. C., Vieira J., Hosken N. A., Meseda C. A., Snow D. C., Wald A., Corey L. (2003). Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci U S A 100, 12899–12904 10.1073/pnas.2131705100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg A. G., Burke R. L., Adair S. F., Sekulovich R., Tigges M., Dekker C. L., Corey L. (1995). A recombinant glycoprotein vaccine for herpes simplex virus type 2: safety and immunogenicity [corrected]. Ann Intern Med 122, 889–898 [DOI] [PubMed] [Google Scholar]
- Long D., Madara T. J., Ponce de Leon M., Cohen G. H., Montgomery P. C., Eisenberg R. J. (1984). Glycoprotein D protects mice against lethal challenge with herpes simplex virus types 1 and 2. Infect Immun 43, 761–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin S. A., Davis B. M., Molliver D. C. (2007). Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc 2, 152–160 10.1038/nprot.2006.461 [DOI] [PubMed] [Google Scholar]
- Manickan E., Rouse R. J., Yu Z., Wire W. S., Rouse B. T. (1995). Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J Immunol 155, 259–265 [PubMed] [Google Scholar]
- Margalith M., Vilalta A. (2006). Sustained protective rabies neutralizing antibody titers after administration of cationic lipid-formulated pDNA vaccine. Genet Vaccines Ther 4, 2 10.1186/1479-0556-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements W. L., Armstrong M. E., Keys R. D., Liu M. A. (1996). Immunization with DNA vaccines encoding glycoprotein D or glycoprotein B, alone or in combination, induces protective immunity in animal models of herpes simplex virus-2 disease. Proc Natl Acad Sci U S A 93, 11414–11420 10.1073/pnas.93.21.11414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. J., Dong L., Vilalta A., Byrd B., Wilhelm K. M., McClurkan C. L., Margalith M., Liu C., Kaslow D. & other authors (2009). Herpes simplex virus type 2 tegument proteins contain subdominant T-cell epitopes detectable in BALB/c mice after DNA immunization and infection. J Gen Virol 90, 1153–1163 10.1099/vir.0.008771-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass P. H., Elkins K. L., Weir J. P. (2001). Protective immunity against herpes simplex virus generated by DNA vaccination compared to natural infection. Vaccine 19, 1538–1546 10.1016/S0264-410X(00)00380-7 [DOI] [PubMed] [Google Scholar]
- Pan C. H., Jimenez G. S., Nair N., Wei Q., Adams R. J., Polack F. P., Rolland A., Vilalta A., Griffin D. E. (2008). Use of Vaxfectin adjuvant with DNA vaccine encoding the measles virus hemagglutinin and fusion proteins protects juvenile and infant rhesus macaques against measles virus. Clin Vaccine Immunol 15, 1214–1221 10.1128/CVI.00120-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr M. B., Kepple L., McDermott M. R., Drew M. D., Bozzola J. J., Parr E. L. (1994). A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest 70, 369–380 [PubMed] [Google Scholar]
- Perez G., Skurnick J. H., Denny T. N., Stephens R., Kennedy C. A., Regivick N., Nahmias A., Lee F. K., Lo S. C. & other authors (1998). Herpes simplex type II and Mycoplasma genitalium as risk factors for heterosexual HIV transmission: report from the heterosexual HIV transmission study. Int J Infect Dis 3, 5–11 10.1016/S1201-9712(98)90088-1 [DOI] [PubMed] [Google Scholar]
- Rajcáni J., Mosko T., Rezuchová I. (2005). Current developments in viral DNA vaccines: shall they solve the unsolved? Rev Med Virol 15, 303–325 10.1002/rmv.467 [DOI] [PubMed] [Google Scholar]
- Reyes L., Hartikka J., Bozoukova V., Sukhu L., Nishioka W., Singh G., Ferrari M., Enas J., Wheeler C. J. & other authors (2001). Vaxfectin enhances antigen specific antibody titers and maintains Th1 type immune responses to plasmid DNA immunization. Vaccine 19, 3778–3786 10.1016/S0264-410X(01)00090-1 [DOI] [PubMed] [Google Scholar]
- Sawtell N. M. (1998). The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J Virol 72, 6888–6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell N. M., Poon D. K., Tansky C. S., Thompson R. L. (1998). The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J Virol 72, 5343–5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell N. M., Thompson R. L., Stanberry L. R., Bernstein D. I. (2001). Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J Infect Dis 184, 964–971 10.1086/323551 [DOI] [PubMed] [Google Scholar]
- Shiver J. W., Fu T. M., Chen L., Casimiro D. R., Davies M. E., Evans R. K., Zhang Z. Q., Simon A. J., Trigona W. L. & other authors (2002). Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415, 331–335 10.1038/415331a [DOI] [PubMed] [Google Scholar]
- Simmons A., Nash A. A. (1985). Role of antibody in primary and recurrent herpes simplex virus infection. J Virol 53, 944–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin J. I., Ayyavoo V., Boyer J., Kim J., Ciccarelli R. B., Weiner D. B. (1999). Protective immune correlates can segregate by vaccine type in a murine herpes model system. Int Immunol 11, 1763–1773 10.1093/intimm/11.11.1763 [DOI] [PubMed] [Google Scholar]
- Sin J., Kim J. J., Pachuk C., Satishchandran C., Weiner D. B. (2000). DNA vaccines encoding interleukin-8 and RANTES enhance antigen-specific Th1-type CD4+ T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. J Virol 74, 11173–11180 10.1128/JVI.74.23.11173-11180.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin J. I., Kim J. J., Zhang D., Weiner D. B. (2001). Modulation of cellular responses by plasmid CD40L: CD40L plasmid vectors enhance antigen-specific helper T cell type 1 CD4+ T cell-mediated protective immunity against herpes simplex virus type 2 in vivo. Hum Gene Ther 12, 1091–1102 10.1089/104303401750214302 [DOI] [PubMed] [Google Scholar]
- Smith L. R., Wloch M. K., Ye M., Reyes L. R., Boutsaboualoy S., Dunne C. E., Chaplin J. A., Rusalov D., Rolland A. P. & other authors (2010). Phase 1 clinical trials of the safety and immunogenicity of adjuvanted plasmid DNA vaccines encoding influenza A virus H5 hemagglutinin. Vaccine 28, 2565–2572 10.1016/j.vaccine.2010.01.029 [DOI] [PubMed] [Google Scholar]
- Stanberry L. R., Spruance S., Cunningham A. L., Bernstein D. I., Mindel A., Sacks S., Tyring S., Aoki F. Y., Slaoui M. & other authors (2002). Glycoprotein-D–adjuvant vaccine to prevent genital herpes. N Engl J Med 347, 1652–1661 10.1056/NEJMoa011915 [DOI] [PubMed] [Google Scholar]
- Sullivan S. M., Doukas J., Hartikka J., Smith L., Rolland A. (2010). Vaxfectin: a versatile adjuvant for plasmid DNA- and protein-based vaccines. Expert Opin Drug Deliv 7, 1433–1446 10.1517/17425247.2010.538047 [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., DeWitt C. M., Chastain M., Friedman A., Donnelly J. J., McClements W. L., Caulfield M. J., Bohannon K. E., Volkin D. B., Evans R. K. (1999). Enhancement of DNA vaccine potency using conventional aluminum adjuvants. Vaccine 18, 18–28 10.1016/S0264-410X(99)00151-6 [DOI] [PubMed] [Google Scholar]
- Vilalta A., Shlapobersky M., Wei Q., Planchon R., Rolland A., Sullivan S. (2009). Analysis of biomarkers after intramuscular injection of Vaxfectin-formulated hCMV gB plasmid DNA. Vaccine 27, 7409–7417 10.1016/j.vaccine.2009.08.075 [DOI] [PubMed] [Google Scholar]
- Wald A., Corey L. (2003). How does herpes simplex virus type 2 influence human immunodeficiency virus infection and pathogenesis? J Infect Dis 187, 1509–1512 10.1086/374976 [DOI] [PubMed] [Google Scholar]
- Wald A., Zeh J., Selke S., Warren T., Ashley R., Corey L. (2002). Genital shedding of herpes simplex virus among men. J Infect Dis 186 (Suppl. 1), S34–S39 10.1086/342969 [DOI] [PubMed] [Google Scholar]
- Wald A., Huang M. L., Carrell D., Selke S., Corey L. (2003). Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis 188, 1345–1351 10.1086/379043 [DOI] [PubMed] [Google Scholar]
- Watson R. J. (1983). DNA sequence of the herpes simplex virus type 2 glycoprotein D gene. Gene 26, 307–312 10.1016/0378-1119(83)90203-2 [DOI] [PubMed] [Google Scholar]
- Xu F., Sternberg M. R., Kottiri B. J., McQuillan G. M., Lee F. K., Nahmias A. J., Berman S. M., Markowitz L. E. (2006). Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296, 964–973 10.1001/jama.296.8.964 [DOI] [PubMed] [Google Scholar]