Abstract
Background: Several studies revealed that MSC from human bone marrow can downregulate graft-versus-host disease (GVHD) after allogeneic HSCT. Methods: Herein we present 50 patients with acute GVHD who got 74 (1-4) MSC infusions for 54 separate episodes of aGVHD. Results: aGVHD was defined as steroid resistant grade IV aGVHD in 42 cases. The major presentation was gastrointestinal GVHD; two (n=18) or more (n=21) systems were involved in the majority of cases. The 1st infusion with MSC was given on day +27 (range, 1 to 136); d+45 (range, +11 to +150) post diagnosis of aGVHD and HSCT, respectively. In 2/3 of the cases treatment was performed with frozen stocked MSCs; in 62 cases early passages (1-3) were used. The median number of infused cells was 1.14±0.47 million per kg in the first injection and up to 4.27 (1.70±1.10) millions in total. The two patients with aggressive liver GVHD received MSCs injections intra hepatic arteries without changes of blood flow or evidence cytolysis, but also without a visible effect. Disease free survival at 3.6 years was 56%. We observed better overall survival in patients with GVHD grade < 4, in responders to the 1st treatment with MSC, and in pediatric group. The multivariate analysis demonstrated independent influence on survival of initial response and younger age. There were no immediate or late toxicity or side effects. Conclusion: Injection of MSCs seems to be a promising and safe treatment of GVHD. The encouraging results obviously should be confirmed in a randomized prospective study.
Keywords: Mesenchymal stromal cells (MSC), mesenchymal stem cells, hematopoietic stem cell transplantation, graft versus host disease, steroid resistance
Introduction
Human mesenchymal stromal cells (MSC), previously known as stem cells, are non-hematopoietic multipotent cells that are able to differentiate along different pathways including the osteogenic, adipogenic, and chondrogenic lineages [1-5].
MSC were first found in bone marrow [6,7] and later on, were isolated and expanded from various other tissues, including adipose, cutaneous, and pulmonary tissues, fetal hepatic, dental pulp [8-17]. These cells have many similarities with various perivascular cells, especially pericytes, which are multilineage stem cells with a gross plasticity potential [17-20]. Pericytes are distributed throughout the body and the association between vascularization and MSC distribution has also been shown [21].
Biological properties and effector mechanisms of MSC are the focus of fundamental research and an object of many potential clinical applications. One of the most pronounced characteristics of these cells are their immuno-suppressive properties, visible both in vitro and in vivo. These findings provided the background for clinical application of MSC in the treatment of graft-versus-host disease (GVHD). The first case of severe steroid-resistant GVHD successfully treated with MSC was described in 2004 [22]. Several other case reports and small series have since published [23-36]. The largest was a multicenter phase I/II study of 55 European patients, published in 2008 on behalf of the Developmental Committee of the European Group for Blood and Marrow transplantation [25].
In totality, fourteen publications presented 183 treated patients with a broad spectrum of effectiveness, showing response rate varying from 0% [32,33] to 100% [34] with estimations based on primary effect and/or overall survival (OS). Although the majority of these papers expressed positive impressions, some of them found that the overall effect was weak, and in some instances, completely undetectable (reviewed by Wernicke et al., 2011) [36]. Despite the fact that clinical use of MSC in post-BMT settings is still far from routine, this approach was recently “considered as a third line treatment option (in UK guidelines, I.R.) but recognize that this is an area of active research and that MSCs may have a greater role in the management of acute GVHD (aGVHD) in the future” [37].
In summary, the information concerning the clinical effectiveness of infusion of MSC for aGVHD is promising, but not uniform and still inconclusive. Herein we present the second large academic multicenter trial following the publication in 2008 result of the European one which presented 55 patient [25] (exception are trials with Prochymal–commercially produced MSC preparation, released as a company report [38] and in an abstract form [39]; no peer-reviewed publication of this trial results is currently available). Our experience was previously presented in EBMT and ASH 2009 annual meetings [40,41]. The goal of the present publication is to update data based on our research and clinical experience and discuss several relevant points.
Materials and methods
Patients
Here we present an update of 50 patients (22 female and 28 male) who were treated for their acute GVHD with MSC from five major Israeli university medical centers. Forty four patients from this group had hematological malignancies. One of them was a patient with X-linked lymphoproliferative disease (XLP) and Burkitt’s lymphoma. The other 5 patients had genetic diseases XLP, sickle cell disease with often abdominal crises, Wiscott-Aldrich syndrome, osteopetrosis and severe combined immune deficiencies, one more patient had severe aplastic anemia (Table 1). All of the patients developed severe steroid resistant acute GVHD, which was defined as disease progression during the first 3 days on 2 mg/kg methylprednisolone or alternatively, due to discerning no improvement for at least 7 days of this treatment [42]. Patients’ median age was 19 (1 to 69) years; half of them (n=25) were considered pediatric (less then 18 y.o.). Treatment with MSC was performed in accordance with Institutional Review Board (IRB) policy and donors and patients, or their legal guardians, provided written informed consent (Hadassah Medical Organization IRB, protocol 0137-08-HMO and Sheba Medical Center IRB, protocol 8370-10-SMC). In total, 74 treatments were administered. Most patients received a single MSC injection, although some received up to 4 injections of MSC (2 patients got 2 treatments twice for the firstly appeared aGVHD and for its exacerbation, 2 patients got 3 injections, 14 patients 2 injections each and the rest 32 patients once). Two cases were a combination of intravenous (i.v.) and intra-arterial (i.a.) injections performed on the same day. They are therefore considered as a single treatment.
Table 1.
HSC and MSC transplantation characteristics
| Diagnoses | ALL-10, JMML-2, XLP-2, AML-15; AML/MDS-7, NHL-4, WAS-1, OP-1, SCID-1, CML-2, CLL-2, SAA-1, MM-1, SCD-1 |
| Age | Median 19 (1 to 69) |
| (children-25, adults-25) | |
| Gender M:F | 28:22 (56%:44%) |
| HSC matching (n=50) | MFD-12 |
| MUD-25 | |
| 9/10-7 | |
| 8/10-2 | |
| haplo-2 | |
| UCB-2 | |
| MSC matching (n=74) | 3rd party, full mismatched-62 |
| 3rd party, haplo-5 | |
| Same donor, haplo-2 | |
| Same donor, matched-5 | |
| aGVHD development | d+9 to d+150 |
| aGVHD severity (maximal) | grade IV-42 |
| grade II-III-8 | |
| Number of systems involved | One-9 |
| Two-18 | |
| More-21 | |
| Other treatments | MP (regular ± high dose)-50, CsA-50, tacrolimus-40, rapamycin-8, MMF-40, ATG-27, anti-CD25 mAb-9, ECP-16 |
| MSC delivery | i.v. only-72 |
| i.a. + i.v.-2 | |
| Average first MSC dose | 1.05 (0.3 to 2.25) x10E6 per kg |
Abbreviations: ALL - acute lymphoblastic leukemia; AML - acute myeloid leukemia; ATG - antithymocyte globulin (Fresenius or Thymoglobulin); CLL - chronic lymphocytic leukemia; CML - chronic myeloid leukemia; ECP - extracorporeal photopheresis; i.a. - intra-arterial injection; i.v. - intravenous; JMML - juvenile myelomonocytic leukemia; MDS - myelodysplastic syndrome; MFD - matched family donor; MM - multiple myeloma; MMF - mycophenolate mofetil; MP - methyl prednisolone; MUD - matched unrelated donor; NHL - non-Hodgkin’s lymphoma; OP - osteopetrosis; SAA - severe aplastic anemia; SCD - sickle cell disease; SCID - severe combined immune deficiency; UCB - umbilical cord blood; WAS - Wiscott Aldrich Syndrome; XLP - X-linked lymphoproliferative disease.
MSC preparation and quality control
Isolation and culture of MSC from bone marrow
Bone marrow aspirate was obtained from the iliac crest in amounts of 60 to 120 ml, and was gently passed through a nylon cell strainer, a 21 gauge needle and a 23 gauge needle. Cells were re-suspended in DMEM supplemented with 15% fetal bovine serum, 1% glutamine and 1% antibiotics (3 ×108 cells per flask). Cells were plated into 25 cm2 culture bottles maintained at 37°C in a humidified atmosphere with 5% CO2. In some cases BM-MNCs were obtained by Ficoll gradient (1.077 g/dl) (Lymphoprep, cat. 1114547, Fresenius Kabi Norge AS, Norway), re-suspended with media culture (107 cells/flask) and plated on 75 cm2 flasks. Medium was replaced two times a week. When cells reached confluence, they were trypsinized, re-suspended in medium and re-plated for expansion.
MSC donors serology (blood group, a set of infections) were performed according to conventional requirements for HSCT donors.
Sterility for gram positive, gram negative organisms, anaerobes and fungi was done from passage used for treatment, before and after cell freezing.
Cells’ karyotype (FISH) were tested in a limited number of cultures prior to injection based on data of stability in maintaining normal diploid karyotype of human MSCs throughout at least 10 early passages [43-45] no chromosomal abnormalities were detected.
Aspect and morphology
Culturing was performed under light microscopy control. MSC culture demonstrated fibroblast-like appeared cell layer in all cases.
FACS analysis
Culture-expanded MSC were characterized phenotypically by flow cytometry (Becton-Dickinson). Fluorescein isothiocyanate (FITC), phycoerythrin (PE) or APC conjugated antibodies against CD45 (<5%; <1% in case of use of MSC from an original mismatched donor), CD105 (70-95%), CD90 (70-95%), CD3 (<5%; in case of use of MSC from an original mismatched HSC donor, CD3 absolute number should not exceed 1 x10E4 cells per kg of recipient body weight). HLA-DR (<5%), HLA-class 1 (>80%) were used in the majority of cases, as well as CD4, CD8, CD56, Stro1, CD117, CD13, CD14, CD34, CD73, CD9, and CD166. Trypsin-EDTA (Biological Industries, Beit-Haemek, Israel), and washed cells were incubated with antibodies and analyzed by using the Cell Quest Software to detect surface antigens.
Cryopreservation and thawing procedure
MSC were frozen using freezing medium containing DMEM low glucose with FBS 80% and DMSO 10%. Samples were defrost not completely in a water bath 37 degrees, re-suspended in DMEM low glucose, 35% FBS, 1% Glutamine, with addition of penicillin, streptomycin and nystatin, 1% of each (Biological Industries, Beit-Haemek, Israel), spinned down 800 rpm 10 minutes then washed twice with PBS.
Mitogen response suppression of expanded cells was performed in the 6 samples. Different concentrations of MSC were incubated with or without lymphocytes (105 cells/300 mcl) and with or without PHA (3 mg/ml) in triplicate in 96-well plates for 72 hours. The plates were pulsed with 1 μCI per well 3H thymidine (3HT) during the final 18 h. In vitro lymphocyte proliferation was evaluated by measuring 3HT incorporation in cells harvested over fiberglass filters on a Microbeta scintillation counter (Packard). The MSCs suspension from each donor demonstrated similar immuno-suppressive properties in vitro (reported by Barkats et al., 2007) [46].
MSC transfusion
Cells were centrifuged, washed once with NS and were re-suspended in 15-20 cc of NS immediately before infusion to avoid major agglutination. They were slowly injected into a central line along with the flow of NS. Intra-arterial treatment with MSC was performed in two cases of severe liver GVHD following haploidentical transplantation into the right and left hepatic arteries under imaging control, using a previously reported technique [47,48]. All treatments were performed without premedication in parallel to 3 days of treatment with azithromycin for Mycoplasma infection prophylaxis.
Cells were produced in a sterile box in accordance with GMP regulations in done in 2 of 5 participating centers Hadassah (for 42 patients) and Sheba, Tel Hashomer (for 9 patients; one patients got two treatments using cells from both centers).
Statistics
For statistical analysis, we used t-test and lineal correlations (Excell for MAC v.14.1.4, 2011), chi-square and Fisher’s exact test using software available online [49,50]. Survival analysis was assessed by using the Kaplan–Meier methods (log-rank test) and Cox regression for univariate and multivariate analysis in PASW Statistics 18.0 and MedCalc version 12.4. Plots were performed using MedCalc version 12.4. All tests were two-sided. Binary logistic regression was analyzed using PASW Statistics 18.0. The p-values less than 0.05 were considered as statistically significant.
Results
HSCT characteristics
The HSCT source distributed as follows: the vast majority of patients (n=44) were transplanted using conventional high resolution of tissue typing (HLA) MFD (10/10) or MUD 9 or 10 out of 10 matched; two from 8/10 MUD; two received transplantations from unrelated 6/6 UCB and two from a haploidentical parent (Table 1). In 41 cases, mobilized PBSC were used and in 7 cases, harvested BM was used.
Forty-five patients received myeloablative while 5 received reduced intensity conditioning; 32 patients received fludarabine based conditioning while thirteen received TBI containing protocols. Median TNC was 9.5 x10E8 (from 1.72 to 25.0) (excluding haplo and UCB unit). The number of CD34+ cells was 9.05 x10E6 (from 1.17 to 39.0) and the number of CD3+ was 3.79 x10E8 per kg (from 0.3 to 4.6); one patient who got haplo BMT after positive selection of CD34+ received extended number (2 x10E5) CD3+ cells per kg of body weight.
In all cases, GVHD prophylaxis was based on cyclosporin A (CsA) from d=-4 or d=-1 and included its combinations with MTX on d+1, +3 and +6 (n=8), with ATG on days -4 to -1 (either Fresenius 5 mg/kg/d or Thymoglobulin 2.5 mg/kg/d) for days from -4 to -1 (n=7). For 10 patients, CsA was combined with MMF from d+1. Patients who received T cell depleted PBSC were not given medicamentous GVHD prophylaxis. There were no graft failures by definition.
GvHD severity and treatment before MSC
Acute GVHD started from day +6 to day +72 following BMT (median d+17) excluding three patients in whom aGVHD was provoked later on by DLI (d+150, d+360, d+415). In another 3 cases, the disease manifested itself in a hyper-acute form prior to the engraftment (on d+6, d+8 and d+8).
The major presentation which determined overall severity and governed the subsequent course of the disease was gastrointestinal (GI) GVHD (Table 1). In most of the patients, GI GVHD was defined as grade 4, with a full clinical picture of it as follows: out of 42 (84%) patients, in two cases competitive diagnosis was viral gastroenteritis (RV and CMV). Skin GVHD grade 2 or more was diagnosed in 33 (67%) of the cases, nine of them a grade 4. Liver symptoms presented in 28 of the (58%) patients. Two cases were determined as presumably VOD. Liver GVHD of grade 2 or more was seen in 24 patients. In 3 patients liver involvement was defined as GVHD grade 4. Overall aGVHD severity was defined as grade 4 in 39 patients, grade 3 in 6 patients and grade 2 in 5 patients.
There was a range of treatments used prior to the trial with MSC, including, methyl prednisolone (MP) in all patients (high dose and/or i.a. injections), mycophenolate mofetil (MMF), methotrexate (MTX), azathioprine, extracorporeal photopheresis (ECP), serotherapy, change of calcineurin inhibitors (Table 1). Only five patients showed a temporary and not pronounced partial response.
MSC treatment
Data regarding treatment of GVHD with MSC is presented Table 1. The 1st treatment with MSC was performed between 11 (hyperacute GVHD) and 180 days following BMT (or DLI) (median 47 days), and between days 1 and 136 (median 27 days) following diagnosis of acute GVHD.
In 50 patients we treated 54 separate episodes of GVHD. The numbers of treatments varied from 1 to 4 and in total 74 treatments were performed.
In two third of the cases (48/74 injections) treatment was performed with frozen stocked MSC. The remainders of the treatments were performed with fresh expanded cells. Three patients received both frozen and fresh cells (in separate treatments). In 30 cases passage one was used for the treatment, in 28 passages two, in 12 passage three, and late passages (six or seven) where used in one case each. The median number of infused cells was 1.0 (from 0.3 to 3.1, with an average of 1.14±0.47) x10E6 per kg of patient body weight in the first injection and up to 4.27 (1.70±1.10) x10E6 in total.
An initial response was seen in 33 out of 50 patients (66%) but complete resolution of symptoms was documented in only 17 (34%) of them. In 2 cases, after 2 treatments for each one, with a complete and a very good partial response, we saw recurrence of GVHD. It the first case exacerbation was provoked by rotavirus infection and was successfully treated with 2 other injections of MSC.
The second of these two patients, after haplo BMT, had complete effect from MSC and was discharged from the hospital after two treatments with MSC without signs of GVHD (on continuous medicamentous immunosuppressive treatment). Later on, when treatment started being tapered down, the patient began to lose weight and had severe diarrhea, following which, bilirubin and liver enzymes started to grow. Intensified treatment consisted of steroids, tacrolimus, MMF, ECP, anti-CD25 monoclonal antibodies (daclizumab) including i.v. injection of 3rd party MSC, but no improvements occurred. Twenty-five days later under radiological control, MSC suspension was injected both into the right and left hepatic arteries (Figure 1). There was no evidence of microembolization due to MSCs suspension infusion-either changes of blood flow after injection (Figure 1) or deterioration of liver function tests during consecutive days (cytolysis). However, no positive clinical or laboratory effects were not noticed either and 3 weeks later the patient died from liver and then multi-organ failure. Similar intra-arterial delivery was done for one more patients with hepatic and GI GVHD, also without a clear response.
Figure 1.
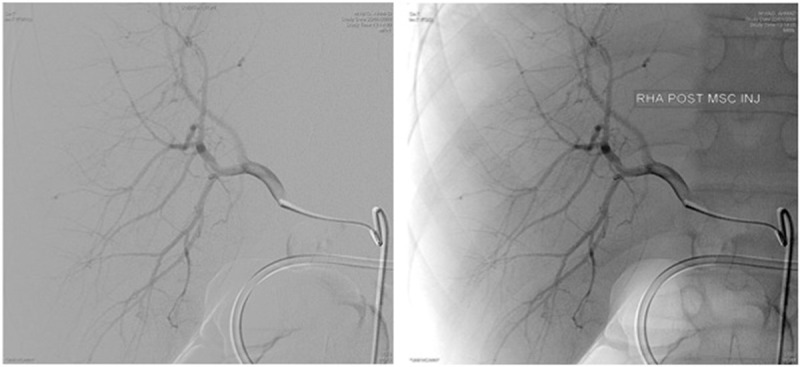
Digital subtraction angiography (DSA) before (left) and after (right) intra-arterial injection of MSC suspension into the right hepatic artery; no changes in flow after injection were identified.
Several patients who received MSCs (mostly in the beginning of the trial) had extremely severe diseases, with very short life expectancy and therefore received therapy on a desperate basis. Five of them died in less than a week and are therefore considered as non-evaluable.
Survival analysis
Figure 2 presents overall survival and DFS, when cause of death was not directly associated with acute GVHD: in total seven patients died from relapse of NHL (1 patient), pulmonary aspergillosis with alveolar hemorrhage (2 patients), septic shock with multiorgan failure (2 patients), intracranial bleeding (1 patient), and pneumonia (1 patient). All these seven patients had complete (n=4) or good partial (n=3) response for MSC injection; one at the time of death (26 months after HSCT, 25 months after beginning of aGVHD and 24 months after treatment with MSC) had residual limited chronic GVHD. Therefore, estimated DFS at 3.6 years is 56%.
Figure 2.
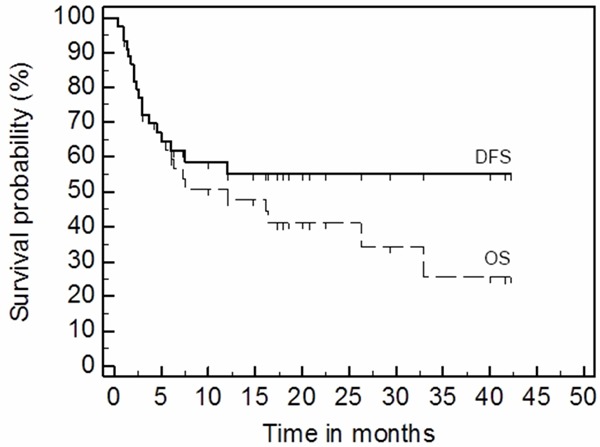
Estimated GVHD free survival (DFS) and overall survival (OS) for 43 months.
Stratification according to GVHD severity showed significantly inferior survival rates in patients with severe grade 4 forms of GVHD (Figure 3, logrank test p=0.0014, Cox’s univariate analysis HR=4.472, p=0.096).
Figure 3.
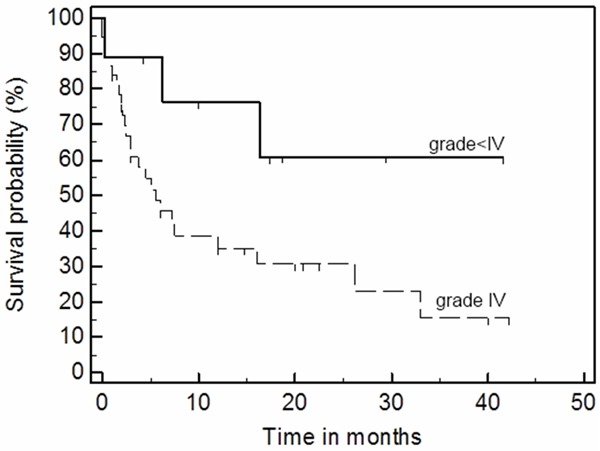
Overall survival is inferior in GVHD patients with grade IV compare to grade <IV (II and III) after MSC treatment (p=0.0014, logrank test).
The stratification of patients according to a response is presented in Figure 4, which demonstrates clear differences in 6 months survival between responders versus not-responders (logrank test p=0.000). Comparison of responders (complete and partial) to non-responders shows a statistically significantly greater overall GVHD severity (t-test, p=0.031) and liver GVHD (p=0.044) as well as earlier GVHD onset (p=0.049) and time of MSC treatment from BMT (p=0.031). However, there were no significant differences in time from GVHD development and beginning of treatment with MSC (p=0.7) between the two groups of the responders and non-responders.
Figure 4.
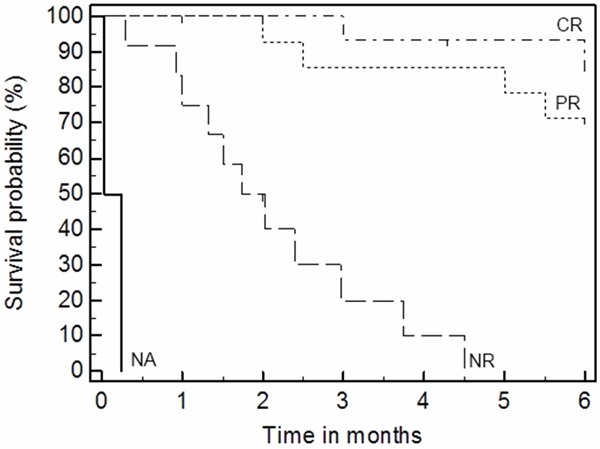
Overall survival (6 months) is superior in those who demonstrated a response versus nonresponders (p=0.000, logrank test). CR - complete response; PR - partial response; NR - no response; NA - not evaluable cases.
The evaluation of the effect the age is shown in Figure 5. The pediatric group (age <18 years) demonstrated higher survival rates compare to the adult group (logrank test p=0.011, univariate Cox’s p=0.006). There was a significant difference between pediatric and adult groups in the number of infused MSCs both total (p=0.009) and at the first treatment (p=0.018); they were higher in pediatric patients.
Figure 5.
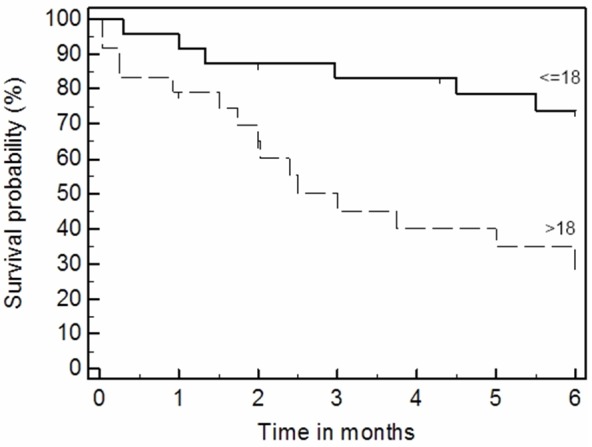
Overall survival (6 months) is superior in pediatric group of patients; p=0.011, logrank test.
Analysis of additional characteristics of MSC treatment demonstrated that the number of passage (1st, 2nd or 3rd for the first treatment) is not significantly associated either with response (chi-square, p=0.206) or OS (p=0.137). The number of injections, whether one, two or more than that also did not show any association with response (p=0.629) and OS (p=0.857). We found no negative influence of cell freezing on OS and 6 months survival (p=0.748). In fact, there was slightly superior response rate with the frozen cells (chi-square, p=0.046). In addition, there was a shorter period of time between HSCT and the beginning of treatment with MSC in non-responders, (37±20 vs 57±29 d, t-test p=0.029) which was mainly associated with a tendency to the earlier GVHD onset in this group (18±10 vs 26±17, p=0.077; r=0.59) but not with a slightly shorter time period between GVHD and the first MSC injection (21±16 vs 33±23, p=0.109; r=-0.01: all results are in days, cases with onset on after d+100 after BMT or treatment after d+150, as well as patient with NA response were excluded from the analysis).
Multivariate analysis
Cox regression multivariate analysis demonstrated significant independent influence on 6 months survival of initial response, partial or complete (HR 29.4, 95% confidential interval (CI) 7.84-110.10, p=0.000), and younger age (HR 1.059, 95% CI 1.023-1.097, p=0.001). Additional analysis of response predictors showed the severity of liver involvement is a significant poor predictor (grade from 0 to 4; OR 2.546, 95% CI 1.132-5.728, p=0.024). A possible interpretation of this could be the previously described domination of GI GVHD in the vast majority of severe cases. Therefore, additional liver involvement plays a role as a strong independent factor (Table 2).
Table 2.
Multivariate analysis of predictors for (A) overall survival (6 months) and (B) initial response (complete or partial) for MSC treatment
| A. Cox multivariate analysis of 6 months OS after 1st MSC treatment | |||
|
| |||
| Predictor | HR | 95.0% CI for HR | Sig. |
|
| |||
| Age in years | 1.059 | 1.023-1.097 | 0.001 |
| Skin GVHD grade (0-4) | 0.940 | 0.618-1.428 | 0.770 |
| Gut GVHD grade (0-4) | 0.950 | 0.360-2.504 | 0.917 |
| Liver GVHD grade (0-4) | 0.794 | 0.537-1.174 | 0.248 |
| Overall GVHD grade (0-4) | 1.341 | 0.182-9.894 | 0.774 |
| Time to the 1st MSC treatment after HSCT | 1.000 | 0.987-1.013 | 0.993 |
| Time to the 1st MSC after GVHD onset | 0.953 | 0.905-1.004 | 0.071 |
| Clinical response for MSC treatment | 29.374 | 7.837-110.100 | 0.000 |
|
| |||
| B. Response analysis by binary logistic regression | |||
|
| |||
| Predictor | OR | 95.0% CI for OR | Sig. |
|
| |||
| Age in years | 1.009 | 0.951-1.071 | 0.767 |
| Time to the 1st MSC treatment after BMT | 0.914 | 0.797-1.047 | 0.195 |
| Skin GVHD grade (0-4) | 0.694 | 0.208-2.314 | 0.552 |
| Gut GVHD grade (0-4) | 0.513 | 0.103-2.565 | 0.417 |
| Liver GVHD grade (0-4) | 2.546 | 1.132-5.728 | 0.024 |
| Number of MSC per kg cells (all treatments) | 0.868 | 0.301-2.502 | 0.794 |
| 1st MSC dose after GvHD onset | 1.060 | 0.925-1.213 | 0.402 |
Abbreviations: HR - Hazard Ratio; OR - Odds Ratio; CI - confidential Interval; in bold - significant ones.
Safety
Procedures were safe in the treated group. We did not identify any visible side effects of MSCs transplantation from any of the 74 procedures performed, either immediately or later on.
Discussion
Herein we present our experience in the treatment of 50 patients with severe steroid-resistant forms of aGVHD with MSC. This is a second major series of patients published following the presentation of data of European clinical trial in 2008 [25]. Patients had different basic diseases and underwent different types of transplant. Additionally, as shown above, in a group of patients suffered from severe aGVHD, we succeeded in obtaining a positive response to the treatment in ⅔ of the cases and reached a complete resolution of symptoms in ⅓ of them. Recently, Wernicke and co-authors [36] summarized all cases presented in peer reviewed publications (14 papers). With addition of a paper published in Chinese at 2013 (Zhao et al.) [51], the total number of patients presented in scientific literature up till now is 205. The number of responders (PR+CR) from amongst these patients was 73.6% (n=151), which is the same as our group 73.3% (n=33, when 5 NA patient are excluded). Very similar results were seen in those four papers, where the number of treated cases was highest: 73.7% (n=19, steroids refractory chronic GVHD) [23], 70.9% (n=55) [25] and 93.5% (n=31) [29] and 72.7% (n=22) [51]. To date no prospective randomized studies have been published in peer reviewed journals.
The only exception from relative similarity of the results obtained from academic institutions represents a company sponsored study of the product Prochymal (Osiris Therapeutics, Inc., Columbia, MD, USA). Results were presented as a press release and in the abstract form only [38,39], but not as a peer reviewed publication. Results of this study were analyzed this year by J Galipeau in Cytotherapy Journal [52]. The trial was designed as a randomized and placebo controlled. This study showed an opposite to quoted above publications outcome and did not meet the endpoint criteria. The major explanation of negative result offered in Galipeau review was a technological one: cell product used in Osiris Therapeutics, Inc. trial underwent during preparation a very high “proliferative pressure” compare to European studies as well as presented here where number of cell doses was never extend 10 (in our case 1-5) what is 3 logs less then in Osiris product (10,000 doses from a single donor) [52]. In case of our trial maximal number cells we harvested after expansion from a single bone marrow collection was 400 x10E6 MSC which was enough for 1 to 7 patients (in case of sick children lower total MSC number was infused each time). We did not find any correlation of used passage number with effectiveness (p=0.137 for passages 1-3 vs. >3), but resent publication from Karolinska (Sweden) suggested that treatment with early-passage MSCs improves survival [53].
Overall, our data confirmed those published by other groups. In most of the papers authors and experts have expressed a favorable opinion. However there are a few new nuances that can be pointed out based on our own data and experience.
It is known that steroid resistance in cases of GVHD does not respond well to the majority of known treatments and that all patients are receiving combined therapy consisting of calcineurin inhibitors, MMF, a range of monoclonal (anti-CD25, anti-CD3, anti-TNF etc.) or polyclonal (ATG) antibodies, ECP, etc. As mentioned earlier, in the majority of previous cases and according to our experience, most, if not all of those patients who received MSC treatment were given one of several combinations of agents in addition to steroids, both prior to, as well as during the time of the MSC injections. In our series, all patients continued to receive various types of immunosuppressive treatments, and we presume that this happened in other series as well, since MSC is not considered as the first or even the second line of treatment against GVHD. This raises an important issue: how to separate the effects of MSC in the context of combined immunosuppressive therapies, which is often difficult to achieve in single patients, since coincidence can never be fully excluded (post hoc non ergo propter hoc). It is well established that steroid-resistant grade 4 aGVHD has an extremely poor prognosis with a mortality rate estimated at 80% [54] or more. In contrast, data presented here demonstrate much better overall and aGVHD free survival (Figure 3). Therefore, the use of MSC in complex treatment of aGVHD probably has an additional impact which contributes to phenomenological improvement of survival.
It is obvious that MSC injection is not the kind of intervention that should cause other treatments to be stopped. Moreover, we would like to stress that MSC should be added in addition to ongoing complex treatments and that only later on medication based immunosuppression can be tapered down and stopped. This message was not clear until the present although it was assumed. On the other hand, if treatment with MSC is usually performed when patients are loaded with immunosuppressive and anti-inflammatory medicines the sensitivity of MSC to them should be established. There is a growing amount of information concerning the interaction of immunosuppressive agents with known/supposed MSC effects/pathways and its influence on the final effect. However, this information is still fragmented and conflicting [22,55,56]. Effects can vary and be dependent on concentration of acting agents as well as on MSC proportions, T cells activation models, presence of MФ, inflammation activity etc. In vitro [55] and of course in vivo, the effects are even more complex. Therefore, since at present, MSC effects on cytotoxic T cells and their effectiveness in treating aGVHD is becoming more clear-cut, in future studies, additional emphasis should be placed on this kind of treatment in combined therapies of steroid-resistant forms of GVHD.
One of the vital questions for clinicians to answer is the selection of patients and predictors. Until recently it was shown that acute gastrointestinal GVHD responds better than other types of GVHD. Because of this opinion, the selection of patients in this study was geared towards severe gastrointestinal GVHD. This selection obviously brought some bias to the results and the role and severity of gastrointestinal symptoms was reduced and did not sound as significant. On the other hand, we also selected other than severe GI GVHD factors which could be considered as prognostic ones. The severity of liver disease in our group was demonstrated as an important factor associated with a poor response to MSC injections. However the presence of a complete or partial response in conjunction with a younger age displayed an independent association with superior survival. Multivariate analysis supports the earlier impression that these two factors appear crucial to OS and GVHD DFS.
We found that the only significant difference between the pediatric and adult patient groups was in the number of cells per kg of BW, which was higher in the group aged <18 y.o. It must be mentioned that the best published results were obtained in MD Anderson with a partial response rate of 93.5% and a complete response rate of 77.4% in a representative group of adult patients (n=31) [29]. There are two major differences which distinguish the presented patient group. Firstly, 68% of patients suffered from GVHD grade 2 and only 9.7% grade 4. Theretofore, the relative condition of the patients was far less severe in comparison to other studies. The second major difference was the use of higher MSC doses and a more intensive regimen: injections at 3 day intervals of 2 x10E6 or 8 x10E6 cells per kg. There were no significant differences associated with the use of two doses. Nevertheless, each of these doses together is higher than the believed empirical working dose of 1 x10E6 MSC per kg. Tandem injections can play a role in better results, as well. Very high doses and continuous treatment (from 6 x10E6 to 108 x10E6 per kg in up to 21 infusions per patient in twice weekly or weekly basis) was used in 12 pediatric patients with a response rate of 100% (58% complete); the group was represented by patients with grade 3-4 GVHD, treated in different hospitals on a compassionate basis [31]. Our earlier in vitro data also showed that the immunosuppressive effect of hMSC is dose dependent [46]. Therefore, in our group differences in injected MSC doses between children and adults can also influence on different success rate in pediatric and adult groups, despite it being difficult to prove based on our data.
As a rule, deep immunosuppression, either primary (e.g., in the event of T cell depleted graft) or secondary (due to GVHD itself and anti GVHD treatment) leads to infectious complications, especially giving rise to opportunistic infections. In the recent publication of Karlsson et al. [57] it was nicely demonstrated how MSC has a different effect on alloreactivity in GVHD and immune response to viral infections such as CMV and EBV. In the group presented here, we did not see cases of EBV or CMV reactivation directly associated with MSC infusion(s). According to our knowledge, a breakout of opportunistic infections associated with MSC treatment was not described in reviewed literature in any clinical setting. In these terms, earlier introduction of MSC can be more safely compared to serotherapy, which usually, despite reasonable rates of clinical response, does not greatly increase OS, which is mainly due to an increasing rate of viral infections [58-64].
At present, effects and mechanisms of MSC action presented in permanently published multiples original articles and reviews. For inhibition of inflammation, MSC can use multiple mechanisms. The direct cell-to-cell contact or the paracrine regulation is the more studied “theory” and is the better understood [65,66]. Ringdent et al., [28] demonstrated the presence of MSC donor DNA in the target organ (intestine). We twice tried to find a trace of MSC DNA in situ and did not succeed (data not shown). Our preliminary animal (mice) data shows that in cases of intravenous transfusion, the majority of cells are trapped in the lungs, the first parenchymal organ they meet in circulation. Only a few of them (even after pretreatment with nitroprusside) can pass the lung barrier and make their way into different tissues at the time when early effects can be already visible (data not shown). Therefore, we have hypothesized that local delivery, by bypassing lungs, can augment the effects of MSC. To augment the topical effects of MSC they can be delivered into vessels which supplied damaged area. We specifically used the hepatic arteries in 2 patients with liver GVHD. Our major cause for concern in such cases was relate to the MSC size which is several times larger than all blood cells and their pronounced adhesive properties which cause the formation of agglutinates in several minutes. However, in both cases, we did not encounter either changes in the macrocirculation seen at digital subtraction angiography immediately after injections, or an increase in liver enzymes in the following days. Unfortunately, we saw no dramatic positive effect from such procedures and one of the 2 patients died soon after the procedure due to grade 4 acute GVHD after haploidentical HSCT. Similar pilot results were published later in a series of 3 patients who received MSC intra-arterial injections for GI GVHD [33]. One of the impressions from in whole 5 described cases is that intra-arterial delivery does not demonstrate advantages in comparison to intravenous delivery.
In conclusion, MSC demonstrates a clear and pronounced effect in the complex treatment of severe, steroid-resistant forms of aGVHD and can definitely be successfully used to treat it. Younger age and initial response predict a better prognosis, while presence of liver GVHD was associated with an inferior response to MSC. MSC might be a safe and effective treatment for certain groups of patients with aGVHD who do not respond to standard immunosuppressive therapies. Optimal timing, doses and regimens of this treatment as well as extracorporeal expansion regimens approaches need to be evaluated.
Disclosure of conflict of interest
None.
References
- 1.Prockop DJ. Marrow stomal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Caplan Al. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Bianco P, Gehron RP. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 7.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 8.Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG. Cells isolated from adult humanskeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg. 1999;65:22–26. [PubMed] [Google Scholar]
- 9.Warejcka DJ, Harvey R, Taylor BJ, Young HE, Lucas PA. A population of cells isolated from rat heart capable of differentiating into several mesodermal phenotypes. J Surg Res. 1996;62:233–42. doi: 10.1006/jsre.1996.0201. [DOI] [PubMed] [Google Scholar]
- 10.Gronthos S, Zannettino AC, Graves SE, Ohta S, Hay SJ, Simmons PJ. Differential cell surface expression of the STROP-1 and alkaline phosphatase antigens on discrete development stages in primary cultures of human bone cells. J Bone Miner Res. 1999;14:47–56. doi: 10.1359/jbmr.1999.14.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Grigoriadis AE, Heersche JN, Aubin JE. Differentiation of muscle, cartilage, and bone from progenitor cells present in a bone derived clonal cell population: effect of dexamethason. J Cell Biol. 1998;106:2139–2151. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loncar D. Ultrastructural analysis of differentiation of rat endoderm in vitro: Adipose vascular-stromal cells induce endoderm differentiation, which in turn induces differentiation of vascular-stromal cells into chondrocytes. J Submicrosc Cytol Pathol. 1992;24:509–519. [PubMed] [Google Scholar]
- 13.Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105–10. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 14.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 15.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 16.Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099–1100. [PubMed] [Google Scholar]
- 17.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 18.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 19.Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 20.Covas DT, Anepucci RA, Fontes AM, Silva WA Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 21.da Silva Meirelles L, Sand TT, Harman RJ, Lennon DP, Caplan AI. MSC frequency correlates with blood vessel density in equine adipose tissue. Tissue Eng Part A. 2009;15:221–229. doi: 10.1089/ten.tea.2008.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 23.Weng JY, Du X, Geng SX, Peng YW, Wang Z, Lu ZS, Wu SJ, Luo CW, Guo R, Ling W, Deng CX, Liao PJ, Xiang AP. Mesenchymal stem cell as salvage treatment for refractory chronic GVHD. Bone Marrow Transplant. 2010;45:1732–1740. doi: 10.1038/bmt.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller I, Kordowich S, Holzwarth C, Isensee G, Lang P, Neunhoeffer F, Dominici M, Greil J, Handgretinger R. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis. 2008;40:25–32. doi: 10.1016/j.bcmd.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 25.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 26.Zhang LS, Liu QF, Huang K, Zhang Y, Fan ZP, Huang SL. [Mesenchymal stem cells for treatment of steroid-resistant chronic graft-versus-host disease] . Zhonghua Nei Ke Za Zhi. 2009;48:542–546. [PubMed] [Google Scholar]
- 27.von Bonin M, Stolzel F, Goedecke A, Richter K, Wuschek N, Holig K, Platzbecker U, Illmer T, Schaich M, Schetelig J, Kiani A, Ordemann R, Ehninger G, Schmitz M, Bornhäuser M. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant. 2009;43:245–251. doi: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- 28.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, Omazic B, Aschan J, Barkholt L, Le Blanc K. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 29.Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M, Monroy R, Uberti J. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Fang B, Song Y, Lin Q, Zhang Y, Cao Y, Zhao RC, Ma Y. Human adipose tissue-derived mesenchymal stromal cells as salvage therapy for treatment of severe refractory acute graft-vs. -host disease in two children. Pediatr Transplant. 2007;11:814–817. doi: 10.1111/j.1399-3046.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 31.Prasad VK, Lucas KG, Kleiner GI, Talano JA, Jacobsohn D, Broadwater G, Monroy R, Kurtzberg J. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant. 2010;17:534–541. doi: 10.1016/j.bbmt.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Lim JH, Lee MH, Yi HG, Kim CS, Kim JH, Song SU. Mesenchymal stromal cells for steroid-refractory acute graft-versus-host disease: a report of two cases. Int J Hematol. 2010;92:204–207. doi: 10.1007/s12185-010-0606-9. [DOI] [PubMed] [Google Scholar]
- 33.Arima N, Nakamura F, Fukunaga A, Hirata H, Machida H, Kouno S, Ohgushi H. Single intra-arterial injection of mesenchymal stromal cells for treatment of steroid-refractory acute graft-versus-host disease: a pilot study. Cytotherapy. 2010;12:265–268. doi: 10.3109/14653240903390795. [DOI] [PubMed] [Google Scholar]
- 34.Fang B, Song Y, Liao L, Zhang Y, Zhao RC. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant Proc. 2007;39:3358–3362. doi: 10.1016/j.transproceed.2007.08.103. [DOI] [PubMed] [Google Scholar]
- 35.Lucchini G, Introna M, Dander E, Rovelli A, Balduzzi A, Bonanomi S, Salvadè A, Capelli C, Belotti D, Gaipa G, Perseghin P, Vinci P, Lanino E, Chiusolo P, Orofino MG, Marktel S, Golay J, Rambaldi A, Biondi A, D'Amico G, Biagi E. Platelet- lysate-expanded mesenchymal stromal cells as a salvage therapy for severe resistant graft-versus-host disease in a pediatric population. Biol Blood Marrow Transplant. 2010;16:1293–1301. doi: 10.1016/j.bbmt.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Wernicke CM, Grunewald TG, Juenger H, Kuci S, Kuci Z, Koehl U, Mueller I, Doering M, Peters C, Lawitschka A, Kolb HJ, Bader P, Burdach S, von Luettichau I. Mesenchymal stromal cells for treatment of steroid-refractory GvHD: a review of the literature and two pediatric cases. Int Arch Med. 2011;4:27. doi: 10.1186/1755-7682-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, Scarisbrick JJ, Taylor PC, Hadzic N, Shaw BE, Potter MN on behalf of the Haemato-oncology Task Force of the British Committee for Standards in Haematology and the British Society for Blood and Marrow Transplantation. Diagnosis and management of acute graft-versus-host disease. Br J Haematol. 2012;158:30–45. doi: 10.1111/j.1365-2141.2012.09129.x. [DOI] [PubMed] [Google Scholar]
- 38.Prochymal® phase clinical trial For the treatment of Steroid-Refractory Acute GvHD. From (accessed on 15 May 2013): http://www.osiris.com/clinical_prochymal_piii_gvhd.php.
- 39.Martin PJ, Uberti JP, Soiffer RJ, Klingemann H, Waller EK, Daly AS, Herrmann RP, Kebriaei P. Prochymal improves response rates in patients with steroid-refractory acute graft versus host disease (SR-GVHD) involving the liver and gut: results of a randomized, placebo-controlled, multicenter phase III trial In GVHD. Biology of Blood and Marrow Transplantation. 2010;16:S169–S170. [Google Scholar]
- 40.Resnick IB, Stepensky P, Shapira MY, Barkatz C, Elkin G, Gurevich O, Prigozhina T, Pikarsky E, Waldman E, Amar A, Samuel S, Shapira M, Weintraub M, Or R. Treatment of Severe Acute Graft-Versus-Host Disease with Mesenchymal Stromal Cells. Blood. 2009:114. (abstract 2249) [Google Scholar]
- 41.Stepensky P, Barkatz C, Or R, Shapira MY, Aker M, Weintraub M, Resnick IB. Immunossupressive effect of MSC can be used for severe acute forms of GVHD in pediatrics: a single center experience. Bone Marrow Transplant. 2008;42(Suppl s2):s122. [Google Scholar]
- 42.Martin PJ, Schoch G, Fisher L, Byers V, Appelbaum FR, McDonald GB, Storb R, Hansen JA. A retrospective analysis of therapy for acute graft-versus-host disease: secondary treatment. Blood. 1991;77:1821–1828. [PubMed] [Google Scholar]
- 43.Mareschi K, Ferrero I, Rustichelli D, Aschero S, Gammaitoni L, Aglietta M. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006;97:744–754. doi: 10.1002/jcb.20681. [DOI] [PubMed] [Google Scholar]
- 44.Izadpanah R, Kaushal D, Kriedt C, Tsien F, Patel B, Dufour J, Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229–4238. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrero I, Mazzini L, Rustichelli D, Gunetti M, Mareschi K, Testa L, Nasuelli N, Oggioni GD, Fagioli F. Bone marrow mesenchymal stem cells from healthy donors and sporadic amyotrophic lateral sclerosis patients. Cell Transplant. 2008;17:255–266. doi: 10.3727/096368908784153940. [DOI] [PubMed] [Google Scholar]
- 46.Barkats C, Resnick I, Mancuta D, Weiss L, Rebstein I, Kurkalli B, Elkin G, Mizrachi L, Slavin S, Gurevitch O, Resnick IB. Mesenchymal stem cells from umbilical cord vein, placenta, and bone marrow demonstrate similar phenotype and immunosuppressive activity which is not mediated by apoptosis. Haematologica. 2007;92:404. [Google Scholar]
- 47.Shapira MY, Bloom AI, Or R, Sasson T, Nagler A, Resnick IB, Aker M, Zilberman I, Slavin S, Verstanding A. Intra-arterial catheter directed therapy for severe graft-versus-host disease . Br J Haematol. 2002;119:760–764. doi: 10.1046/j.1365-2141.2002.03923.x. [DOI] [PubMed] [Google Scholar]
- 48.Bloom AI, Shapira MY, Or R, Sasson T, Resnick IB, Zilberman I, Verstandig A, Aker M, Slavin S, Muszkat M. Intrahepatic arterial administration of low-dose methotrexate in patients with severe hepatic graft-versus-host disease: an open-label, uncontrolled trial. Clin Ther. 2004;26:407–414. doi: 10.1016/s0149-2918(04)90036-7. [DOI] [PubMed] [Google Scholar]
- 49.Online Chi-square statistics. (accessed on 15 May 2013). From: http://www.physics.csbsju.edu/stats/contingency_NROW_NCOLUMN_form.html.
- 50.Online Chi-square statistics. (accessed on 15 May 2013). From: http://www.sph.emory.edu/~cdckms/ctab-logbased-exact.html.
- 51.Zhao K, Huang F, Peng YW, Zhou HS, Fan ZP, Zhang X, Guo XT, Xu N, Sun J, Xiang P, Liu QF. [Clinical observation of mesenchymal stem cell as salvage treatment for refractory acute graft-versus-host disease] . Zhonghua Xue Ye Xue Za Zhi. 2013 Feb;34:122–6. [PubMed] [Google Scholar]
- 52.Galipeau J. The mesenchymal stromal cells dilemma—does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 53.von Bahr L, Sundberg B, Lonnies L, Sander B, Karbach H, Hagglund H, Ljungman P, Gustafsson B, Karlsson H, Le Blanc K, Ringden O. Long-Term Complications, Immunologic Effects, and Role of Passage for Outcome in Mesenchymal Stromal Cell Therapy. Biol Blood Marrow Transplant. 2012;18:557–564. doi: 10.1016/j.bbmt.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 54.Apperley J, Masszi T. Graft versus host disease. In: Appley J, Carreras E, Gluckman E, Masszi T, editors. Haematopoietic Stem Cell Transplantation. 6th edition. ESH-EBMT Handbook; 2012. p. 233. [Google Scholar]
- 55.Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, Frassoni F, Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 56.Nifontova I, Svinareva D, Petrova T, Drize N. Sensitivity of mesenchymal stem cells and their progeny to medicines used for the treatment of hematoproliferative diseases. Acta Haematol. 2008;119:98–103. doi: 10.1159/000120440. [DOI] [PubMed] [Google Scholar]
- 57.Karlsson H, Samarasinghe S, Ball LM, Sundberg B, Lankester AC, Dazzi F, Uzunel M, Rao K, Veys P, Le Blanc K, Ringdén O, Amrolia PJ. Mesenchymal stem cells exert differential effects on alloantigen and virus-specific T-cell responses. Blood. 2008;112:532–541. doi: 10.1182/blood-2007-10-119370. [DOI] [PubMed] [Google Scholar]
- 58.Remberger M, Aschan J, Barkholt L, Tollemar J, Ringdén O. Treatment of severe acute graft-versus-host disease with anti-thymocyte globulin. Clin Transplant. 2001;15:147–153. doi: 10.1034/j.1399-0012.2001.150301.x. [DOI] [PubMed] [Google Scholar]
- 59.Wolff D, Roessler V, Steiner B, Wilhelm S, Weirich V, Brenmoehl J, Leithaeuser M, Hofmeister N, Junghanss C, Casper J, Hartung G, Holler E, Freund M. Treatment of steroid-resistant acute graft-versus-host disease with daclizumab and etanercept. Bone Marrow Transplant. 2005;35:1003–1010. doi: 10.1038/sj.bmt.1704929. [DOI] [PubMed] [Google Scholar]
- 60.Busca A, Locatelli F, Marmont F, Ceretto C, Falda M. Recombinant human soluble tumor necrosis factor receptor fusion protein as treatment for steroid refractory graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2007;82:45–52. doi: 10.1002/ajh.20752. [DOI] [PubMed] [Google Scholar]
- 61.Kennedy GA, Butler J, Western R, Morton J, Durrant S, Hill GR. Combination antithymocyte globulin and soluble TNFalpha inhibitor (etanercept) +/- mycophenolate mofetil for treatment of steroid refractory acute graft-versus-host disease. Bone Marrow Transplant. 2006;37:1143–1147. doi: 10.1038/sj.bmt.1705380. [DOI] [PubMed] [Google Scholar]
- 62.Khoury H, Kashyap A, Adkins DR, Brown RA, Miller G, Vij R, Westervelt P, Trinkaus K, Goodnough LT, Hayashi RJ, Parker P, Forman SJ, DiPersio JF. Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant. 2001;27:1059–1064. doi: 10.1038/sj.bmt.1703032. [DOI] [PubMed] [Google Scholar]
- 63.Arai S, Margolis J, Zahurak M, Anders V, Vogelsang GB. Poor outcome in steroid-refractory graft-versus-host disease with antithymocyte globulin treatment. Biol Blood Marrow Transplant. 2002;8:155–160. doi: 10.1053/bbmt.2002.v8.pm11939605. [DOI] [PubMed] [Google Scholar]
- 64.MacMillan ML, Weisdorf DJ, Davies SM, DeFor TE, Burns LJ, Ramsay NK, Wagner JE, Blazar BR. Early antithymocyte globulin therapy improves survival in patients with steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:40–46. doi: 10.1053/bbmt.2002.v8.pm11858189. [DOI] [PubMed] [Google Scholar]
- 65.Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N. A critical role of IFN-gamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–857. doi: 10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- 66.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]


