Abstract
Neutrophils provide first-line defense against infections and are potent effectors in innate and adaptive immunity. Recently neutrophils have been shown to play important roles in multiple antitumor reactions. A subset of mature neutrophils in human systemic inflammation has been identified as a unique circulating population of myeloid cells, which is capable of inhibiting T cell responses. These neutrophils show unique immunophenotype (CD11c bright/CD62L dim/CD11b bright/CD16 bright). This study reports detection of mature neutrophils with similar immunophenotype in the peripheral blood samples of cancer patients using flow cytometry analysis. This population of neutrophils is not detected in peripheral blood samples of normal controls. Thus this finding suggests the involvement of mature neutrophils in antitumor immunity.
Keywords: Neutrophils, immunosuppressive, cancer, CD62L
Introduction
Neutrophils are the most abundant leukocytes in the circulating blood. They provide the first-line defense against infection and are potent effectors of inflammation [1]. In addition, neutrophils also are engaged in a complex cross-talk with immune and endothelial cells that bridges innate and adaptive immunity [1,2]. Recent studies have suggested that neutrophils are active in immunosurveillance against several tumors [3-6]. Even though many aspects of their biology have been thoroughly investigated, a deep insight into the biological role of neutrophils in antitumor reactions is still needed. Neutrophils could be a perfect weapon for the suppression of tumor growth.
Recent studies have identified myeloid-derived suppressor cells (MDSC) that are potent suppressors of tumor immunity and therefore a significant impediment to cancer immunotherapy [7]. MDSC accumulate in the blood, lymph nodes, and bone marrow and at tumor sites in most patients and experimental animals with cancer and inhibit both adaptive and innate immunity. MDSC are a heterogeneous family of myeloid cells and they are able to suppress T cell activation. In cancer patients, MDSC are typically CD11b+ CD33+ CD34+ CD14- HLA-DR- and can vary in their expression of CD15 and other markers [8-11]. The variation in MDSC phenotype is consistent with the concept that MDSC are a diverse family of cells that are in various intermediate stages of myeloid cell differentiation [7].
Pillay et al identified a subset of mature neutrophils in human systemic inflammation as a unique circulating population of myeloid cells, which is capable of inhibiting T cell responses [12]. These neutrophils show unique immunophenotype (CD11c bright/CD62L dim/CD11b bright/CD16 bright). These cells are observed in humans in vivo during acute systemic inflammation induced by endotoxin challenge or by severe injury. Local release of hydrogen peroxide from the neutrophils into the immunological synapse between the neutrophils and T cells mediated the suppression of T cell proliferation and required neutrophils expression of the integrin Mac-1.
The goal of our study is to evaluate whether the similar subset of neutrophils can be detected in peripheral blood samples of cancer patients, in comparison to normal non-cancer patients.
Materials and methods
Peripheral blood samples were retrieved from the hematology laboratory at the Department of Pathology, UMass Memorial Medical Center, Worcester, MA. This study was approved by the UMass Memorial Medical Center Institutional Review Board.
Samples and patients
Peripheral blood samples were retrieved from 31 cancer patients prior to surgery, radiation, or any systemic chemotherapy. 31 samples from normal healthy patients with routine physical examination were served as controls. There were also 10 samples from patients undergoing surgeries for benign diseases.
Flow cytometry analysis
For all samples, five-color flow cytometry was performed on Beckman Coulter FC-500 instruments (Beckman Coulter, Inc, Miami, FL) using commercially available reagents. All monoclonal antibodies used in the study were from Beckman Coulter, Inc. (Miami, FL) and they included monoclonal antibodies directed against CD45 (clone J. 33, phycoerythrin-Texas Red (ECD) conjugated), CD62L (clone DREG56, fluorescein isothiocyanate (FITC) conjugated), CD16 (clone 3GB, phycoerythrin-cyanine7 (PC7) conjugated), CD11b (clone Bear1, phycoerythrin-cyanine5 (PC5) conjugated), and CD11c (clone BU15, phycoerythrin (PE) conjugated). 100 μL aliquot of whole blood was incubated with the appropriate amount of titrated antibodies for 10 minutes at room temperature in the dark. Then 2.0 ml of VersaLyse solution was added to each tube and then incubated in the dark at room temperature for 15 minutes to lyse the red blood cells. Then the tubes were centrifuge at 400 x g (1,400 rpm) for 5 minutes at room temperature and carefully poured off supernatant. Then 1.0 ml of Sheath Fluid was added to the tubes and centrifuged at 400 x g (1,400 rpm) for 5 minutes at room temperature and carefully pour off supernatant. Each cell pellet was then resuspended in 500 μL of Fixative Solution. Store the samples in the refrigerator until they are analyzed on the flow cytometer. The instrument alignment, sensitivities, and spectral compensation were verified daily by standards, calibrators, procedural controls, and normal peripheral blood samples prior to processing of patient samples. For all cases, at least 15,000 cells acquired for each case and analyzed by gating on neutrophils.
Cell counts
Total leukocyte counts were counted in whole blood using Beckman Coulter LH750 (Beckman Coulter, Inc, Miami, FL). Using the percentages found by flow cytometry and the absolute leukocyte count, the absolute numbers of circulating neutrophil subsets were calculated.
Statistical analysis
Data was summarized using means and standard deviations. Student t tests were applied to test for differences between groups (e.g. cancer patients vs. normal controls; non-cancer surgery patients vs. normal controls; cancer patients vs. non-cancer surgery patients). Statistical significance for all comparisons was based on two-sided comparisons with p < 0.05. Confidence interval is 95%. Data are presented as mean ± standard error of the mean (SEM).
Results
Normal control samples
In order to obtain an accurate and unbiased determination of both percentage and absolute number of subsets of neutrophils in patients, whole blood flow cytometry analysis was performed using a combination of CD45/CD11b/CD11c/CD16/CD62L. 31 normal control samples were analyzed. The neutrophils were gated on based on CD45 intensity and side scatter (Figure 1A). Then the analysis reveals that most of the neutrophils express bright expression of CD11b, CD11c, CD16 and CD62L. The population of cells with dim expression for CD16 represents eosinophils and it is not included in further measurements. The immune suppressive neutrophils exhibit bright expression of CD11b, CD11c, CD16, but dim expression of CD62L as documented in a previous paper by Pillay et al [12]. This subset of neutrophils suppresses lymphocytes activation. A representative histogram from a normal control is shown in Figure 1B. In this example, this subset of neutrophils comprises 0.3% of gated neutrophils and 0.1% of total white blood cells. The absolute count of this subset of neutrophils is 8/mm3. In 31 normal control patient samples, the percentages of this subset of neutrophils in gated neutrophils range from 0.3% to 5.1% with mean of 1.5% (standard error of the mean (SEM): 0.17%) (Figure 2A). The percentages of this subset of neutrophils in total white blood cells range from 0.05% to 1.2% with mean of 0.4% (SEM: 0.05%) (Figure 2B). The absolute counts of this subset of neutrophils range from 4/mm3 to 75/mm3 with mean of 28.1/mm3 (SEM: 3.4/mm3) (Figure 2C).
Figure 1.
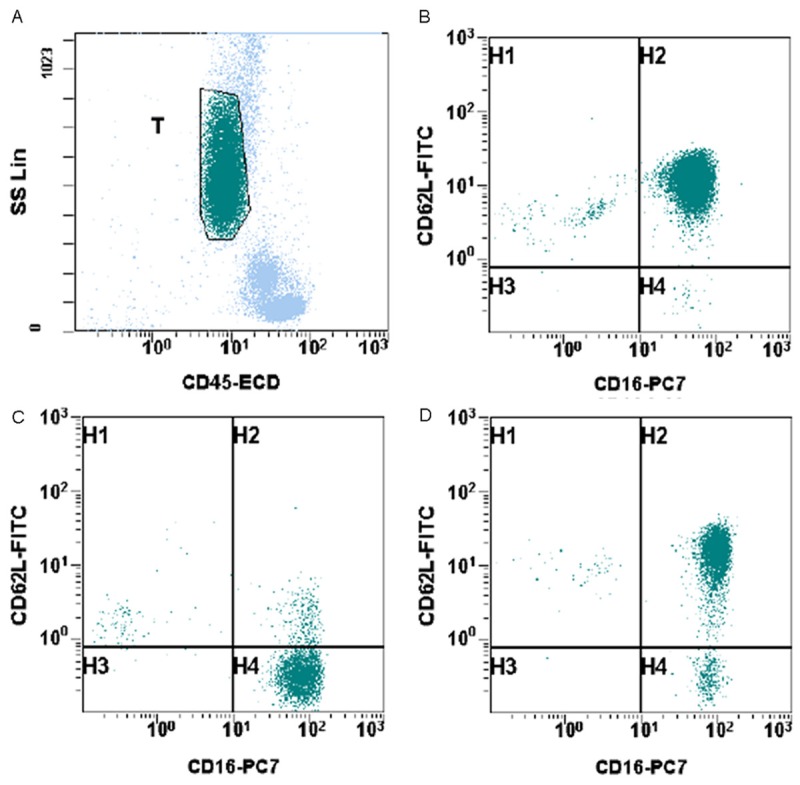
Identification of suppressive neutrophils in peripheral blood samples of cancer patients. A. Neutrophils were selected and gated based on CD45 and side scatter. B. A representative histogram from a normal control. C. A representative histogram from a cancer patient. D. A representative histogram from a non-cancer surgery patient.
Figure 2.
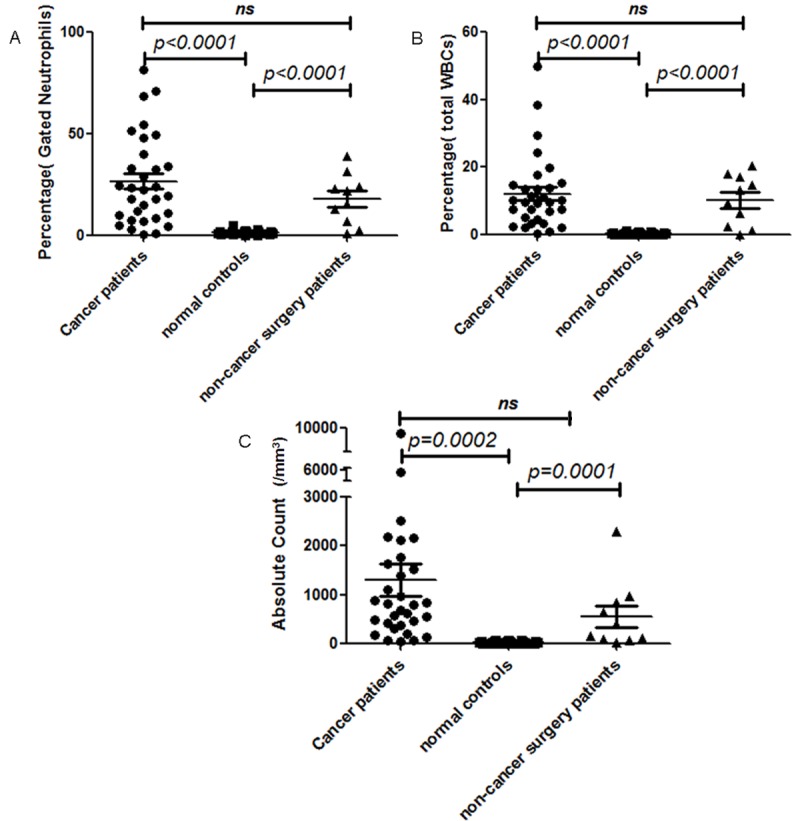
Comparison of gated neutrophil percentages (A), total white blood cell percentages (B), and absolute counts (C) of suppressive neutrophils in normal controls (represented by ■), cancer patients (represented by ●), and non-cancer surgery patients (represented by ▲).
Identification of immune suppressive neutrophils in cancer patients
Peripheral blood samples were retrieved from 31 cancer patients (mean age 62; range 46-88) with newly diagnosed and histologically confirmed solid malignancies. In accordance with American Joint Committee on Cancer (AJCC) Cancer Staging Manual, seventh edition (2010), there are 18 patients with stage I/II disease and 10 patients with stage III/IV disease. In addition, there are 3 patients with glioblastoma multiforme (GBM) in the brain, which do not have TNM designation. Blood samples collected from patients with cancers were obtained prior to surgery, radiation, or any systemic chemotherapy. Patient characteristics are detailed in Table 1.
Table 1.
Cancer patient characteristics in the study
| Number of patients (n) | 31 |
|---|---|
| Mean age in years (range) | 62 (46-88) |
| Gender | |
| Male | 4 |
| Female | 27 |
| Cancer diagnosis | |
| Colon | 1 |
| Breast | 5 |
| Pancreatic | 2 |
| Lung | 1 |
| Melanoma | 1 |
| Ovarian | 8 |
| Uterine | 9 |
| Brain | 3 |
| Bladder | 1 |
| AJCC clinical cancer stage | |
| I/II | 18 |
| III/IV | 10 |
| Brain cancer | 3 |
In cancer patients, freshly drawn whole blood samples were analyzed by flow cytometry analysis using a combination of CD45/CD11b/CD11c/CD16/CD62L. The neutrophils were gated on based on CD45 intensity and side scatter. Then the analysis reveals that some neutrophils express bright expression of CD11b, CD11c, CD16 and CD62L. But there is increased number of immune suppressive neutrophils, which is characterized by bright expression of CD11b, CD11c, CD16, but dim expression of CD62L. A representative example from a cancer patient is showed in Figure 1C. In this example, this subset of neutrophils comprises 87.7% of gated neutrophils and 10.5% of total white blood cells. The absolute count of this subset of neutrophils is 891/mm3. In 31 cancer patient samples, the percentages of this subset of neutrophils in gated neutrophils range from 0.5% to 87.7% with mean of 26.8% (SEM: 3.95%) (Figure 2A). The percentages of this subset of neutrophils in total white blood cells range from 0.4% to 49.9% with mean of 10.3% (SEM: 2.36%) (Figure 2B). The absolute counts of this subset of neutrophils range from 49/mm3 to 9189/mm3 with mean of 1312/mm3 (SEM: 328.3/mm3) (Figure 2C). This finding is statistically significant when compared to normal controls in regards to neutrophil percentage (p < 0.0001) (Figure 2). However, the cancer stages do not seem to correlate with the number of immune suppressive neutrophils detected in peripheral blood samples.
Identification of immune suppressive neutrophils in non-cancer surgery patients
There were also 10 samples from patients undergoing surgeries for benign diseases, which include uterine leiomyomata, ovarian cystic benign neoplasms, and brain abscess. There is also increased number of immune suppressive neutrophils, which is characterized by bright expression of CD11b, CD11c, CD16, but dim expression of CD62L. A representative example is demonstrated in Figure 1D. In this example, this subset of neutrophils comprises 7.4% of gated neutrophils and 2.6% of total white blood cells. The absolute count of this subset of neutrophils is 73/mm3. In 10 non-cancer surgery patient samples, the percentage of this subset of neutrophils in gated neutrophils range from 1.2% to 39.1% with mean of 18.0% (SEM: 3.92%) (Figure 2A). The percentages of this subset of neutrophils in total white blood cells range from 0.1% to 20.4% with mean of 10.3% (SEM: 2.36%) (Figure 2B). The absolute counts of this subset of neutrophils range from 17/mm3 to 2299/mm3 with mean of 559.8/mm3 (SEM: 222.1/mm3) (Figure 2C). This finding is statistically significant when compared to normal controls (Figure 2). However, when compared to cancer patients, the difference is not statistically significant.
Discussion
Pillay et al has reported the identification of a mature neutrophil subset with immune suppressive function, which suppresses T cell activation in a Mac-1-dependent manner [12]. In their study, this subset of neutrophils exhibit unique immunphenotype of CD11c bright/CD62L dim/CD11b bright/CD16 bright. They have shown that this unique subset of neutrophils was detected in patients suffering systemic inflammation. They also demonstrated that these neutrophils displayed regulatory properties. In various murine models of cancer, sepsis, and transplantation, neutrophils were found to be part of MDSCs. This heterogeneous group of cells, suppressing lymphocyte activation, consists mainly of immature neutrophils and monocytes.
Here our study detects the same neutrophil subset in peripheral blood samples from cancer patients. Our work is the first to our knowledge to show a distinct neutrophil phenotype CD11c bright/CD62L dim/CD11b bright/CD16 bright in cancer patients. The percentage and absolute number of this subset of neutrophils is statistically significantly increased in cancer patients when compared to normal controls. There is also increased number of neutrophils in non-cancer surgery patients when compared to normal controls. The percentages and absolute numbers of neutrophils seem to be increased in cancer patients when compared to non-cancer surgery patients, but they are not statistically significant.
MDSCs producing high arginase have been found in renal cell carcinoma patients [9]. In human with metastatic cancer disease, arginase I-mediated suppression of lymphocytes was reported [9,13]. Arginase I depletes the microenvironment of L-arginine, which is essential for various T cell functions. Despite the fact that neutrophil-T cell interactions have been described in lymphoid organs in mice [14,15], it is still unknown whether human neutrophils enter lymphoid organs and come into close contact with T cells. In many acute and chronic inflammatory conditions, however, neutrophils and T cells are present in the tissues. As neutrophilic inflammation frequently results in tissue damage, neutrophil-induced inhibition of T cell proliferation might be essential to limit T cell activation and, thereby, maintain tolerance in these inflammatory conditions. The exact role for neutrophils in immune tolerance in humans remains to be examined; however, it is clear that neutrophil association with solid tumors is detrimental for disease outcome, possibly through suppressing immunogenic antitumor responses.
Our data also corroborated with previous work by Diaz-Montero CM et al, which reported that circulating MDSC levels in cancer patients of all stages are significantly higher when compared to normal volunteers [16]. They found that percentages and absolute numbers of MDSC increased with clinical cancer stage and extensive tumor burden. Their study provided evidence that MDSC is a clinically important mediator of tumor-mediated immune suppression in patients with solid tumors. Abnormal accumulation of MDSC is an important mechanism of T cell unresponsiveness in cancer patients. Future cancer vaccine trials should therefore take into account circulating MDSC in patients as their presence may significantly decrease their clinical effectiveness. Pharmacologic strategies to reduce levels of MDSC in patients should be tested in future clinical trials. However, it is unknown whether reduction of MDSC in either number or partial reversal of their immunosuppressive properties is possible in cancer patients and whether this would significantly delay tumor progression. This may be a clinically viable strategy to significantly improve the efficacy of immunotherapy in cancer patients.
In conclusion, we provide evidence of identification of a unique subset of neutrophils in peripheral blood samples from cancer patients, which have been shown to have immune suppressive capacity. Understanding of the functions of this neutrophil subset in human cancers might provide novel therapeutic strategies to target immune suppression in inflammation and cancer.
Disclosure of conflict of interest
None.
References
- 1.Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339–345. doi: 10.1182/blood.v97.2.339. [DOI] [PubMed] [Google Scholar]
- 2.Colombo MP, Modesti A, Parmiani G, Forni G. Local cytokine availability elicits tumor rejection and systemic immunity through granulocyte-T-lymphocyte cross-talk. Cancer Res. 1992;52:4853–4857. [PubMed] [Google Scholar]
- 3.Lichtenstein A. Granulocytes as possible effectors of tumor immunity. In: Oettgen HF, editor. Human Cancer Immunology. Philadelphia, PA: Saunders; 1990. pp. 731–747. [Google Scholar]
- 4.Midorikawa Y, Yamashita T, Sendo F. Modulation of the immune response to transplanted tumors in rats by selective depletion of neutrophils in vivo using a monoclonal antibody: abrogation of specific transplantation resistance to chemical carcinogen-induced syngeneic tumors by selective depletion of neutrophils in vivo. Cancer Res. 1990;50:6243–6247. [PubMed] [Google Scholar]
- 5.Matsumoto Y, Saiki I, Murata J, Okuyama H, Tamura M, Azuma I. Recombinant human granulocyte colony-stimulating factor inhibits the metastasis of hematogenous and non-hematogenous tumors in mice. Int J Cancer. 1991;49:444–449. doi: 10.1002/ijc.2910490323. [DOI] [PubMed] [Google Scholar]
- 6.Colombo MP, Ferrari G, Stoppacciaro A, Parenza M, Rodolfo M, Mavilio F, Parmiani G. Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. J Exp Med. 1991;173:889–897. doi: 10.1084/jem.173.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J. Clin. Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 9.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patient: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 10.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients’ CD4+ T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2008;57:1493–1504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers J, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abadie V, Badell E, Douillard P, Ensergueix D, Leenen PJ, Tanguy M, Fiette L, Saeland S, Gicquel B, Winter N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood. 2005;106:1843–1850. doi: 10.1182/blood-2005-03-1281. [DOI] [PubMed] [Google Scholar]
- 15.Maletto BA, Ropolo AS, Alignani DO, Liscovsky MV, Ranocchia RP, Moron VG, Pistoresi-Palencia MC. Presence of neutrophil-bearing antigen in lymphoid organs of immune mice. Blood. 2006;108:3094–3102. doi: 10.1182/blood-2006-04-016659. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


