Abstract
Delayed recovery of platelet count post allogeneic hematopoietic stem cell transplantation (allo-HSCT) has been associated with worse transplant outcomes. Thrombopoietic agents have been successfully used in immune mediated thrombocytopenia or thrombocytopenia from bone marrow failure syndromes; however, the experience regarding their use after allo-HSCT is limited. Here we report on the safety and efficacy of romiplostim used in 3 consecutive patients with thrombocytopenia post allogeneic transplantation. Two patients had prolonged platelet recovery due to poor graft function while one had secondary failure of platelet recovery, likely immune mediated, post transplantation. Successful use of such agents post-transplant may improve platelet recovery, decrease rates of complications and potentially improve outcomes.
Keywords: Post-transplant thrombocytopenia, romiplostim, allogeneic hematopoietic stem cell transplantation
Introduction
Thrombocytopenia is a common complication after allo-HSCT. Prolonged isolated platelet recovery of more than 90 days has been described in 5-20% of cases [1,2], and may be caused by antibody-mediated platelet destruction, splenic sequestration or delayed production due to impaired megakaryocytic differentiation or viral infections. In addition, the phenomenon of secondary failure of platelet recovery (SFPR) has been proposed by the Seattle group [3] and defined as: A decline of platelet counts below 20,000/microL for 7 consecutive days or requiring transfusion support after achieving sustained platelet counts > or = 50,000/microL without transfusions for 7 consecutive days after HSCT. Several studies have suggested that poor platelet recovery following allo-HSCT is of adverse prognostic significance [4-6]. There is an unmet need to identify effective thrombopoietic agents able to enhance platelet recovery in this setting, with the goal of achieving transfusion independence, thus preventing potentially fatal bleeding complications peri and post allo-HSCT.
Patients and methods
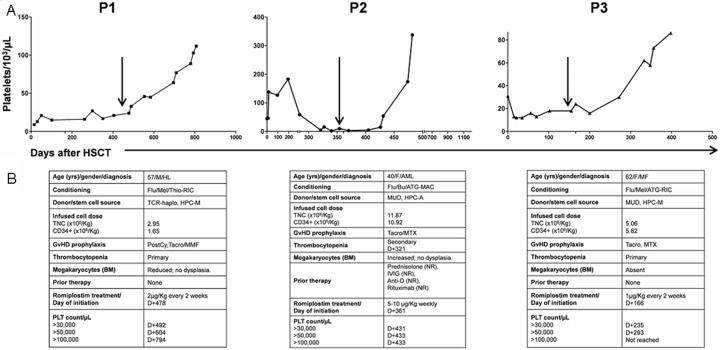
We report here the successful use of romiplostim (Nplate; Amgen, Thousand Oaks, CA) in three consecutive patients with severe thrombocytopenia after allo-HSCT. Romiplostim, a second generation thrombopoiesis-stimulating molecule, was used due to severe symptomatic or persistent thrombocytopenia, from prolonged platelet recovery associated with poor graft function (patients 1 and 3), or due to SFPR, likely associated with immune mediated peripheral platelet consumption (patient 2). The characteristics of the patients and treatment details are summarized in Figure 1.
Figure 1.
A: Platelet recovery for the 3 patients with primary or secondary thrombocytopenia treated with romiplostim after allogeneic hematopoietic stem cell transplantation; B: patient’s characteristics. Arrows indicate Romiplostim administration; P: patient; HSCT: hematopoietic stem cell transplantation; yrs: years; M: male; HL: Hodgkin lymphoma; Flu: fludarabine; Mel: melphalan; Thio: thiotepa; RIC: reduced intensity conditioning; TCR: T cell replete; haplo: haploidentical donor; HPC-M: bone marrow hematopoietic stem cells; TNC: total nucleated cells; PostCy: post-transplant cyclophosphamide; GvHD: graft-versus-host-disease; Tacro: tacrolimus; MMF: mycophenolate mofetil; μg: microgram; Kg: kilogram; D: day; F: female; AML: acute myeloid leukemia; Bu: busulfan; ATG: antithymocyte globulin; MAC: myeloablative conditioning; MUD: matched unrelated donor; HPC-A: peripheral blood hematopoietic stem cells; MTX: methotrexate; NR: no response; IVIG: intravenous immunoglobulin; Anti-D: anti Rho immune globulin; MF: myelofibrosis.
Results
Patient 1
The first case is a 57-year-old male, with advanced stage Hodgkin lymphoma (HL), who relapsed 9 months following first-line treatment with ABVD (adriamycin, bleomycin, vinblastine and dacarbazine) chemotherapy. The patient had refractory disease despite salvage with a regimen of gemcitabine, navelbine and doxorubicin, and due to unavailability of matched donors he received an allo-HSCT from a haploidentical donor, after fludarabine, melphalan and thiotepa reduced intensity conditioning regimen, and post-transplant cyclophosphamide, tacrolimus and methotrexate for GvHD prevention. Pre-transplant workup did not detect presence of any donor specific HLA antibodies. Neutrophil engraftment occurred at day+23, however, his platelet count failed to recover, persisting below 15-20,000/μL, with transfusion dependence until 1 year post transplant. Serial bone marrow biopsies showed persistently reduced megakaryopoiesis, suggesting reduced platelet production. He had no evidence of viral reactivations or GvHD post allo-HSCT. The patient was started on a trial of romiplostim 16 months post-transplant, at 2 μg/kg every 2 weeks with good tolerability and no adverse side effects. The platelet count started to rise after 11 days, and the patient attained platelet transfusion independence in the first 2 weeks from the beginning of treatment. His romiplostim was discontinued at 11 months post treatment with a sustained platelet count of > 100,000/μL. Bone marrow studies 8 months following romiplostim initiation showed no increased fibrosis.
Patient 2
The second case is a 36-year-old female, with relapsed acute myeloid leukemia (AML) in second remission, who underwent a 10/10 matched unrelated donor allo-HSCT after fludarabine, busulfan and anti-thymocyte globulin (ATG) myeloablative conditioning regimen. GVHD prophylaxis consisted of tacrolimus and methotrexate. She had neutrophil and platelet engraftment (> 50,000/μL) on day+10 and day+12, respectively. Her post-transplant course was uneventful with no evidence of GvHD. However, she developed secondary thrombocytopenia on day+321 post-transplant, following an episode of upper respiratory tract infection, with a decrease in platelet count to less than 10,000/μL. A bone marrow biopsy excluded disease recurrence, and the number of megakaryocytes was found to be increased, suggesting peripheral platelet destruction. Other causes of peripheral platelet destruction including viral etiologies as well as post transplant thrombotic thrombocytopenic purpura were excluded based on negative serological and molecular tests, as well as normal bilirubin, haptoglobin as well as lactate dehydrogenase levels, and absence of schistiocytes on the peripheral blood smear. Severe thrombocytopenia in this case was interpreted as immune mediated (ITP) and standard therapies for ITP were instituted, including high-dose corticosteroids, intravenous immunoglobulin, anti-D and rituximab; however, she did not respond and her platelet counts remained persistently below 10,000/μL, with associated mucosal bleeding and extensive ecchymoses. The patient was started on romiplostim at 5 μg/kg but due to a lack of rise in platelet counts after one month, the dose was increased to 10 μg/kg, with platelet recovery count to more than 100,000/μL after 4 weeks of treatment. Romiplostim treatment was discontinued when the platelet count increased to more than 300,000/μL. The patient currently remains in remission with a normal platelet count, at more than 3 years post-transplant.
Patient 3
The third patient is 62 year old woman with a history of JAK-2 positive myelofibrosis for which she underwent a 10/10 matched unrelated donor allo-HSCT with fludarabine, busulfan and ATG reduced-intensity conditioning. She had rapid engraftment; however, developed recurrence of her disease, necessitating a second transplant a year later, using the same matched unrelated donor, but with a fludarabine, melphalan and ATG reduced-intensity conditioning. She had neutrophil recovery at day+12, however, her platelet count remained below 20,000/μL with transfusion dependency for more than 6 months post-transplant. Her post-transplant course was also complicated by CNS toxoplasmosis on day+98, which was treated with trimethoprim-sulfamethoxazole and subsequently changed to pyrimethamine and clindamycin due to thrombocytopenia and concern for marrow suppression. Her serial bone marrow biopsies post-HSCT showed absent megakaryocytes. She also had no evidence of viral reactivations. This patient developed liver GvHD on day+165. She was started on methylprednisolone at 1 mg/kg with improvement in her transaminitis, and this was tapered over one month. Due to persistent thrombocytopenia despite trimetoprim-sulfamethoxazole discontinuation, on day+166 romiplostim was initiated at 1 μg/kg every 2 weeks. The platelet count recovered to more than 50,000/μL after 18 weeks of treatment. The bone marrow biopsy done 6 months following initiation of romiplostim showed increase in megakaryocytes without increased marrow fibrosis. The patient is alive and well at more than 2 years after her second transplant.
Discussion
In this report we describe the efficacy of romiplostim, a second-generation thrombopoietic growth factor, in the treatment of three consecutively treated patients with persistent thrombocytopenia post allo-HSCT. These patients were treated with romiplostim due to severe thrombocytopenia for which no other standard therapy was available. Although thrombocytopenia was immune-mediated only in one of our patients (patient 2), all 3 patients responded to treatment achieving the goal of transfusion independence (Figure 1).
Second generation thrombopoietin mimetics have been proven to be efficacious in the treatment of patients with immune mediated thrombocytopenia [7-11] and two agents, romiplostim and eltrombopag, have been approved by the US Food and Drug Administration for use in these settings. Previous reports have also documented the efficacy of these agents in several patients with thrombocytopenia related to hepatitis C-related liver cirrhosis [12] with low-risk myelodysplastic syndromes [13] and more recently, with aplastic anaemia refractory to immune-suppressive therapy [14]. Literature on the use of these agents in the post-transplant setting has been sparse and limited to single case reports or case series [15-18]. In the largest series reported to date, Calmettes et al. [16] used romiplostim in the management of 7 patients with severe SFPR. The use of romiplostim was well tolerated, and all patients achieved platelet transfusion independence, (and a platelet count > 50,000/μL) at a median time of 54 days after treatment institution. Our findings support the utility of thrombomimetic agents in the treatment of severe thrombo-cytopenia following allo-HSCT. Of note, contrarily to the patients treated by Calmettes et al. [16], 2 out of the 3 patients in our series had prolonged primary platelet recovery following transplantation, rather than SPFR, but still responded to romiplostim achieving transfusion independence shortly after the initiation of romiplostim. Romiplostim administration was well tolerated, without need for dose adjustments or drug discontinuation. Importantly, bone marrow biopsies performed before and after romiplostim administration, did not display any increase in marrow fibrosis, a known adverse event described with the use of thrombopoietic agents [19]. This included the one patient who had myelofibrosis prior to transplantation. Regarding the optimal dosing of romiplostim, our findings, together with those reported series by Calmettes et al. [16] suggest that in the majority of patients, low doses (between 1 to 5 μg/kg) are sufficient to elicit haematological responses. Despite the limited number of patients, this report adds to the very limited literature supporting the use of these agents after allo-HSCT and help form the basis for prospective studies in this setting. In conclusion, our report suggests the utility and safety of romiplostim for the treatment of thrombocytopenia of different causes in patients who received an allo-HSCT. Prospective studies are needed to evaluate the role of these agents after hematopoietic stem cell transplantation.
Disclosure of conflict of interest
None.
References
- 1.Nash RA, Gooley T, Davis C, Appelbaum FR. The Problem of Thrombocytopenia after Hematopoietic Stem Cell Transplantation. Oncologist. 1996;1:371–380. [PubMed] [Google Scholar]
- 2.Bielski M, Yomtovian R, Lazarus HM, Rosenthal N. Prolonged isolated thrombocytopenia after hematopoietic stem cell transplantation: morphologic correlation. Bone Marrow Transplant. 1998;22:1071–1076. doi: 10.1038/sj.bmt.1701499. [DOI] [PubMed] [Google Scholar]
- 3.Bruno B, Gooley T, Sullivan KM, Davis C, Bensinger WI, Storb R, Nash RA. Secondary failure of platelet recovery after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2001;7:154–162. doi: 10.1053/bbmt.2001.v7.pm11302549. [DOI] [PubMed] [Google Scholar]
- 4.Wagner JL, Flowers ME, Longton G, Storb R, Martin P. Use of screening studies to predict survival among patients who do not have chronic graft-versus-host disease at day 100 after bone marrow transplantation. Biol Blood Marrow Transplant. 2001;7:239–240. doi: 10.1053/bbmt.2001.v7.abbmt070239. [DOI] [PubMed] [Google Scholar]
- 5.First LR, Smith BR, Lipton J, Nathan DG, Parkman R, Rappeport JM. Isolated thrombocytopenia after allogeneic bone marrow transplantation: existence of transient and chronic thrombocytopenic syndromes. Blood. 1985;65:368–374. [PubMed] [Google Scholar]
- 6.Bolwell B, Pohlman B, Sobecks R, Andresen S, Brown S, Rybicki L, Wentling V, Kalaycio M. Prognostic importance of the platelet count 100 days post allogeneic bone marrow transplant. Bone Marrow Transplant. 2004;33:419–423. doi: 10.1038/sj.bmt.1704330. [DOI] [PubMed] [Google Scholar]
- 7.Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, Rodeghiero F, Chong BH, Wang X, Berger DP. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363:1889–1899. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 8.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM, Sanz MA, Liebman HA, Slovick FT, de Wolf JT, Bourgeois E, Guthrie TH Jr, Newland A, Wasser JS, Hamburg SI, Grande C, Lefrere F, Lichtin AE, Tarantino MD, Terebelo HR, Viallard JF, Cuevas FJ, Go RS, Henry DH, Redner RL, Rice L, Schipperus MR, Guo DM, Nichol JL. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371:395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 9.Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, Arning M, Stone NL, Bussel JB. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377:393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 10.Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, Salama A, Jenkins JM, Roychowdhury D, Mayer B, Stone N, Arning M. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:641–648. doi: 10.1016/S0140-6736(09)60402-5. [DOI] [PubMed] [Google Scholar]
- 11.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113:2161–2171. doi: 10.1182/blood-2008-04-150078. [DOI] [PubMed] [Google Scholar]
- 12.McHutchison JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M, Berg T, Gordon SC, Campbell FM, Theodore D, Blackman N, Jenkins J, Afdhal NH. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227–2236. doi: 10.1056/NEJMoa073255. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, Fenaux P, Sekeres MA, Becker PS, Boruchov A, Bowen D, Hellstrom-Lindberg E, Larson RA, Lyons RM, Muus P, Shammo J, Siegel R, Hu K, Franklin J, Berger DP. Safety and efficacy of romiplostim in patients with lower-risk myelodysplastic syndrome and thrombocytopenia. J. Clin. Oncol. 2010;28:437–444. doi: 10.1200/JCO.2009.24.7999. [DOI] [PubMed] [Google Scholar]
- 14.Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, Parikh AR, Soto S, Biancotto A, Feng X, Lozier J, Wu CO, Young NS, Dunbar CE. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11–19. doi: 10.1056/NEJMoa1200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck JC, Burke MJ, Tolar J. Response of refractory immune thrombocytopenia after bone marrow transplantation to romiplostim. Pediatr Blood Cancer. 2010;54:490–491. doi: 10.1002/pbc.22332. [DOI] [PubMed] [Google Scholar]
- 16.Calmettes C, Vigouroux S, Tabrizi R, Milpied N. Romiplostim (AMG531, Nplate) for secondary failure of platelet recovery after allo-SCT. Bone Marrow Transplant. 2011;46:1587–1589. doi: 10.1038/bmt.2011.179. [DOI] [PubMed] [Google Scholar]
- 17.Gangatharan SA, Cooney JP. Persistent thrombocytopenia post auto-SCT for AML treated with romiplostim in a patient with HIV. Bone Marrow Transplant. 2011;46:1280–1281. doi: 10.1038/bmt.2010.298. [DOI] [PubMed] [Google Scholar]
- 18.Bollag RJ, Sterett M, Reding MT, Key NS, Cohn CS, Ustun C. Response of complex immune-mediated thrombocytopenia to romiplostim in the setting of allogeneic stem cell transplantation for chronic myelogenous leukemia. Eur J Haematol. 2012;89:361–364. doi: 10.1111/j.1600-0609.2012.01832.x. [DOI] [PubMed] [Google Scholar]
- 19.Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood. 2009;114:3748–3756. doi: 10.1182/blood-2009-05-224766. [DOI] [PubMed] [Google Scholar]