Abstract
In the European Union, the high-concentration capsaicin patch is licensed for the management of neuropathic pain conditions in nondiabetic patients, including postherpetic neuralgia (PHN) and HIV-associated distal sensory polyneuropathy (HIV-DSP). However, in the USA, the Food and Drug Administration approved its use only in PHN patients. Capsaicin is a transient receptor potential vanilloid-1 agonist, which increases the intracellular calcium ion concentration. This triggers calcium-dependent protease enzymes causing cytoskeletal breakdown and leads to the loss of cellular integrity and ‘defunctionalization’ of nociceptor fibres. Efficacy and therapeutic effect has been shown in several clinical studies of PHN and HIV-DSP. The high-concentration capsaicin patch and its practical application are different from low-concentration creams; one application can help for up to 3 months. The process of setting up of a service to use the capsaicin 8% patch is also discussed.
Keywords: Capsaicin, high concentration patch, neuropathic pain, post herpetic neauralgia, HIV, efficacy
Introduction
Capsaicin is derived from hot chilli pepper plants native to the Americas. Chilli peppers are used to spice up cuisines, especially in Mexico and South America. Europeans introduced chilli peppers to Asia and Africa, and they are now an essential ingredient of cuisines in Ethiopia, India, China, Sri Lanka, Thailand, Korea and Malaysia [Bode and Dong, 2011]. The ‘hotness’ of chilli peppers is measured in Scoville heat units, which represent the number of times a chilli extract must be diluted in water for it to lose its heat. Capsaicin scores about 16,000,000, in comparison with jalapenos, which measure about 4500 units.
Chilli was first used in the West as a hot alcoholic pepper extract to treat burning or itching extremities [Turnbull, 1850]. Thresh isolated the pure crystalline form in 1876 [Thresh, 1876]. It has long been available in various formulations as lotions, creams or patches in low concentrations of 0.025% to 0.075%. These products could be purchased over the counter to treat neuropathic and nociceptive musculoskeletal pain, such as postherpetic neuralgia (PHN), diabetic neuropathy, postsurgical pain, osteoarthritis and rheumatic arthritis. Chilli extracts have also been used to treat itching, psoriasis, cluster headache and migraines [Martindale, 1999]. A recent Cochrane review on low-concentration capsaicin in the treatment of neuropathic pain concluded that there were insufficient data to make any treatment recommendations for clinical practice. It also suggested that it had no effect beyond that of placebo [Derry and Moore, 2012]. Due to the nature of interaction with the transient receptor potential vanilloid (TRPV1) receptor, a thermal nociceptor, capsaicin suffered two main disadvantages. Firstly, there were burning sensations and skin reactions that were not tolerated by many, and secondly, multiple daily applications for at least 4–6 weeks were required to see any response, leading to issues with compliance.
These drawbacks were addressed with the new high-concentration capsaicin 8% patch, also known as NGX-4010, which provides rapid, long-lasting pain relief with a single application. Following positive trials in PHN and HIV-associated distal sensory polyneuropathy (HIV-DSP), the European Union (EU) approved its use in peripheral neuropathy in nondiabetic patients, while the US Food and Drug Administration (FDA) approved its use in the USA only to treat PHN.
This article aims to discuss the pharmacology of capsaicin and summarize its efficacy and safety profile based on previous reviews, and to explain a practical way of setting up a service to deliver treatment with the capsaicin 8% patch.
Pharmacodynamics
Capsaicin comes from plants of the genus Capsicum, which belong to the Solanaceae family. Capsaicin is the main pungent chemical, the others being dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin and homocapsaicin [Luo et al. 2011]. The main site of action of capsaicin is the TRPV1 channel. Although the channel is nonselectively activated by many endogenous and exogenous agonists, capsaicin itself is highly selective and potent at this channel.
TRPV1 is a ligand-gated cation channel selectively expressed in the polymodal nociceptive nerve fibres, mainly the C and A delta fibres. It was the first pain transducer to be discovered. It is activated by heat (> 43°) and by acidosis (pH < 6). It is also activated by a number of endogenous agonists like anandamide, N-acetyldopamines, leukotriene B4, long-chain unsaturated fatty acids and, as recent evidence has shown, 9- and 13-hydroxyoctadecadienoic acid [Alawi and Keeble, 2010]. Transient activation of the channel causes depolarization by sodium and calcium ion influx leading to burning, itching or stinging sensations.
In contrast to endogenous chemicals, capsaicin, being a stable compound, has a more persistent rather than transient effect on the channel. Moreover, the calcium:sodium permeability is increased from a baseline of 8:1 to 25:1. There is a massive influx of calcium ions down the electrochemical gradient. In addition, there is also a release of calcium through TRPV1 channels activated on intracellular organelles such as the endoplasmic reticulum. The excess intracellular calcium triggers calcium-dependent protease enzymes causing cytoskeletal breakdown. Microtubule depolymerization causes a halt in fast axonal transport [Chard et al. 1995; Han et al. 2007]. Chloride influx accompanies the influx of positive ions causing osmotic swelling. A TRPV1-independent mechanism also exists by causing direct inhibition of electron-chain transport and subsequent mitochondrial dysfunction [Shimomura et al. 1989]. Thus, multiple mechanisms ultimately lead to loss of cellular integrity and ‘defunctionalization’ of the nociceptor fibres. The nerve fibres retract to a depth at which mitochondrial function is preserved. Immunohistochemical studies have shown that capsaicin produces a highly localized loss of nerve fibres in the epidermis and dermis [Polydefkis et al. 2004].
This replaces the hypothesis from the mid-1980s that substance P depletion is responsible for capsaicin-induced pain relief. It is now known that substance P is one of the many neurotransmitters expressed by nociceptor fibres. Thus, nerve terminal defunctionalization and retraction, as caused by capsaicin, leads to a decrease in all the neuropeptides released by the nerve terminals and substance P is one among them [Anand and Bley, 2011].
Pharmacokinetics
The capsaicin 8% dermal patch is available as a 280 cm2 patch containing 179 mg of capsaicin (640 µg/cm2). It is different from conventional transdermal patches in that it works locally on the skin and there is little systemic absorption. It is extremely lipophilic and therefore is easily absorbed into the epidermal and dermal layers with little affinity for the aqueous blood phase. The amount of drug absorbed is greater with the duration of exposure and also the area applied. The pharmacokinetic data derived from clinical trials in peripheral neuropathy suggest that quantifiable capsaicin concentrations were more often seen in PHN patients than HIV-DSP patients, as the skin of the feet is thicker compared with the rest of the body. Among HIV-DSP patients, they were more often found in those treated for 90 min [European Medicines Agency, 2013]. Blood samples were tested from 173 patients in the trials at varying intervals after patch removal (0, 1, 3, 6 and 24 h). Only 34 (20%) patients had quantifiable levels of capsaicin, and among all the blood samples only 6% were above the lower limit (0.5 mg/ml) of quantifiable plasma capsaicin [Babbar et al. 2009]. The Cmax or mean maximum plasma concentrations after 60- and 90-min applications were 1.38 ng/ml and 2.96 ng/ml, respectively [Babbar et al. 2009]; the Tmax to reach these levels were 1.46 and 1.51 h [Babbar et al. 2009]. Interestingly, after oral ingestion of 26.6 mg of capsaicin, the Cmax was 2.47 ng/ml in a mean time of 47 min [Chaiyasit et al. 2009].
Capsaicin is rapidly metabolized in the liver by cytochrome enzymes. It has a high mean apparent clearance of 54,598 L/h, and is therefore rapidly eliminated with a t1/2 of 1.64 h. Oral capsaicin has an elimination t1/2 of 24.9 min [Jensen et al. 2009]. In vitro studies suggest that capsaicin is metabolized very slowly in the skin [European Medicines Agency, 2013]. A clear clinical advantage of this is that capsaicin lasts for a long time in the skin, which is its site of action, and any systemically absorbed drug is rapidly cleared. Also, because such small quantities of drug reach the systemic circulation, there are no implications for dose adjustments in hepatic or renal failure.
Efficacy and therapeutic uses
In 2009, the FDA and EU approved the use of the capsaicin 8% patch (Qutenza) after four phase III trials [FDA Center for Drug Evaluation and Research, 2009]. These were mainly in-patients with postherpetic neuralgia or HIV-associated neuropathy. As the data in diabetic patients were not sufficiently robust, the EU supported the use of the high-concentration capsaicin patch in neuropathic pain in nondiabetic adults only. The FDA supported the use of Qutenza only for neuropathic pain due to postherpetic neuralgia. In the postmarketing phase, use has been extended to other causes of neuropathic pain such as scar pain, postsurgical pain, cancer-induced neuropathy and localized peripheral neuropathies. Capsaicin is currently undergoing a phase IV trial to compare its efficacy with pregabalin.
Postherpetic neuralgia
The initial pilot study by Backonja and colleagues was a randomized, double-blind study (n = 44) comparing the high-concentration 8% patch with an active control, a low-concentration 0.04% patch [Backonja et al. 2010]. The initial 4-week study period was followed by an open-label extension (n = 24) to 48 weeks during which patients received three further applications of the study drug. The study population included patients older than 18 years, with average numerical pain-rating scores (NPRS) between 3 and 8 and having at least 6 months duration of PHN. If patients used concomitant pain medication these were to be at stable doses for at least 21 days prior to treatment and any patients using more than 60 mg/day of morphine were excluded.
Patients were prepared for the patch with 4% lidocaine cream that was applied for 1 h prior to treatment. The capsaicin 8% patch (NGX-4010) was applied for 60 min to a maximum area of 1000 cm2. In the dose-ranging study, it was applied for 30, 60 and 90 min [Webster et al. 2010]. Treatment-related pain was dealt with by local cooling methods and oral oxycodone (1 mg/ml). In the first week after treatment, hydrocodone bitartrate/acetaminophen (5 mg/500 mg) was allowed as rescue medication up to day 5 only.
Efficacy was assessed by reduction in NPRS scores, percentage of patients with 30% pain relief and the use of questionnaires such as the brief pain inventory, short form, self assessment to treatment and patients’ global impression of change (PGIC), measured using a –3 to +3 point scale (very much worse to very improved); NPRS scores were measured from day –14 to –1 to obtain the average baseline level. The scores in the first week were not included to avoid any confounding effect from the rescue medications. Patients in the pilot study and dose-ranging study who completed the initial period of treatment were enrolled in the open-label extension studies up to 40 weeks and 48 weeks.
The first demonstration of efficacy of the drug was seen in the pilot study [Backonja et al. 2010], which showed a 32.7% reduction in baseline NPRS scores in the NGX-4010 study group compared with a mere 4.4% reduction in the control group (p = 0.003). The reduction of pain scores was seen as early as the first week and the effect seemed to be maintained throughout the study period (4–12 weeks). In the control group pain scores returned to baseline in 2–4 weeks. Table 1 shows that the percentage reduction from baseline pain in all major studies were in a similar range and were maintained up to week 12.
Table 1.
Efficacy of capsaicin 8% in postherpetic neuralgia.
| Study | Duration of patch | % reduction in NPRS scores NGX vs Control | P value |
|---|---|---|---|
| Backonja et al 2010 C102/106 (n=44) | 60 minutes | –32.7 % vs –4.4%(week 2–4) | 0.0003 |
| Webster et al 2010C108 (n=222) | 30, 60, 90 minutes | –26.5% vs –17.3%(week 2–8) | 0.0286 |
| Backonja et al 2008C116 (n=402) | 60 minutes | –29.9% vs –20.4%(week 2–12) | 0.002 |
| Irving et al 2011C117 (n=418) | 60 minutes | –32.3% vs –25%(week 2–12) | 0.017 |
Higher pain relief was seen in those patients not taking concomitant medications in the pilot study, and this was replicated in the subsequent phase III studies. This finding may suggest that patients on previous neuropathic medication could have more treatment-resistant pain and additional pharmacotherapy may have some further therapeutic effect but to a lesser extent. Overall the effect of the capsaicin 8% patch was positive regardless of any concomitant medication used [Backonja et al. 2008].
The pilot study showed that 53% patients had at least a 33% decrease in pain scores from weeks 2 to 4 with the 8% capsaicin versus no such patients in the control group. This further increased to 78% from weeks 9 to 12, again with no patients in the control group. The two 12-week phase III trials had significantly higher responders for 30% pain reduction in the NGX-4010 group (44% versus 33%; p = 0.05 and 47% versus 35%; p = 0.021) [Backonja et al. 2008; Irving et al. 2010] Results for 50% pain reduction were also significantly greater in the capsaicin group (30% versus 21%; p = 0.035) [Irving et al. 2010].
Around 55% of patients in the capsaicin group reported an improvement on the PGIC scale (–3 to +3 scale from very much worse to very much improved) compared with 43% in the control group from weeks 2 to12. Irving and colleagues replicated similar results from weeks 2 to12 (41% versus 26%; p = 0.001) [Backonja et al. 2008; Irving et al. 2010].
McCormack performed an integrated analysis of four drug trials in patients with PHN (n = 1079; n = 597 for capsaicin 8%; n = 482 for controls) [McCormack, 2010]. The reductions in baseline pain scores were significantly higher in the capsaicin group from weeks 2 to12 (31.2% versus 23.9%; p = 0.0002). Significantly more patients achieved at least 30% pain reduction in the capsaicin study group (45% versus 36%; p = 0.0035).
A recent Cochrane review included four studies on the use of the high-concentration patch in PHN (n = 1272). Improvements in PGIC scores were regarded as first-tier evidence. The 8% high-concentration patch was found to be significantly better than the 0.04% control patch. The calculated number needed to treat (NNT) for much or very much improved were 8.8 at 8 weeks (95% confidence interval [CI] 5.3–26.0), and 7.0 at 12 weeks (95% CI 4.6–15.0) [Derry et al. 2013].
Long-term efficacy
Patients who completed the initial double-blind phase entered an open-label extension study [Backonja et al. 2010; Webster et al. 2010]. They received capsaicin 8% patch treatment at intervals of no less than 12 weeks and were followed up until 40 weeks and 48 weeks in the two studies. Similar endpoints of efficacy were sought as before. The median duration of response was found to be 22 weeks and 14% maintained the response for 40 weeks (McCormack et al., 2010, Simpson et al., 2008). The mean percentage reduction in baseline NPRS scores after the first, second and third treatments were –31.4%, –30.0% and –34.1%, respectively [Backonja et al. 2010].
HIV-DSP
DSP in HIV patients can develop either due to the viral load or as a complication of the antiretroviral therapy. This is a common neurological complication and occurs in 29–62% of HIV patients [Simpson et al. 2008]. The efficacy of the high-concentration capsaicin patch has been studied in two phase III trials and an open-label study up to 48 weeks [Clifford et al. 2010; Simpson et al. 2008, 2010].
All patients who had moderate to severe pain from HIV-DSP, lasting for more than 2 months were included in the study. If patients were on antiretroviral therapy doses were required to have been stable for at least 8 weeks. The drug was compared with an active control (low-concentration capsaicin patch 0.04%) as in the PHN studies. The patch was applied for 30, 60 or 90 min.
The mean reduction in NPRS scores at weeks 2–12 in the NGX-4010 group was much higher (22.7%) than in the control group (10.7%) [Simpson et al. 2008]. These were highly significant (p = 0.0026). In individual groups however, significant reductions were seen only in the 30- and 90-min application group but not in the 60-min application group. At least 30% mean reduction in pain was seen in 42%, 24% and 36% of patients in the 30-, 60- and 90-min application groups, respectively. Interestingly, the findings in the 60-min group were not significant. Improvements in PGIC scores were seen in a higher proportion of patients in the capsaicin 8% group (33%) compared with the control (14%). The mean pain reduction scores in the capsaicin 8% group occurred regardless of whether patients were on concomitant neuropathic drugs or neurotoxic antiretroviral therapy. The C119 study did not have adequate power to show significant differences between the capsaicin 8% and control group in each dosage group for the primary and most secondary endpoints [Clifford et al. 2010].
An integrated, pooled analysis of the two phase III trials (n = 239 for capsaicin 8% patch; n = 100 for controls) showed a significantly greater reduction in NPRS scores from weeks 2 to 12 in the high-dose capsaicin group (27.0% versus 15.7%; p = 0.0020) [Backonja et al. 2009]. Likewise a greater proportion of patients in the capsaicin 8% group (39% versus. 23%; p = 0.0051) achieved a greater than 30% reduction in pain.
A recent Cochrane review included two studies of the high-concentration patch in HIV-DSN patients (n = 801). PGIC was a reported outcome in only one study, and based on this the NNT for much or very much improved at 12 weeks was estimated to be 5.8 (95% CI 3.8–12.0). Both studies reported reduction in pain intensity. The NNT for 30% pain-intensity reduction from baseline was 11 [Derry et al. 2013].
Long-term efficacy
A 48-week, open-label study included 52 HIV-DSN patients who had a successful response to the capsaicin 8% patch [Simpson et al. 2010]. Three to four further 60- or 90-min applications were allowed with an interval of at least 12 weeks between the two applications. The capsaicin group showed a 12.4% reduction in baseline NPRS scores by week 48 and as much as 80% of patients reported an improvement in the PGIC scale.
Almost all studies used an active control. The possibility of some therapeutic effect in the control group cannot be ruled out. This may have lead to a possible underestimation of the efficacy of the study drug [Irving et al. 2010].
Efficacy in other conditions
Capsaicin has also been studied in other conditions. Although in small numbers, the work done by Bhaskar and colleagues in cancer-associated neuropathic pain showed that almost 71% of patients in this subgroup had 90% pain relief [Bhaskar et al. 2012]. Another observational study showed positive results in patients diagnosed with regional neuropathic pain including postsurgical pain, scar pain and peripheral neuropathy in nondiabetic adults who had been unresponsive to other neuropathic agents. Capsaicin was shown to produce significant reductions in pain scores and functional improvement.
Safety and tolerability
The safety and tolerability of the high-concentration capsaicin patch was evaluated in the phase III trials and in the open-label extension studies that followed [Backonja et al. 2008, 2010; Clifford et al. 2010; Irving et al. 2010; Simpson et al. 2008, 2010; Webster et al. 2010]. Safety assessments included were adverse-events monitoring, vital signs, physical examination including dermal and neurological assessments, treatment-related pain and use of rescue medication [FDA Center for Drug Evaluation and Research, 2009].
In the open-label extension study, which included both PHN and HIV-DSP patients, 98% completed 90% of the treatment [Simpson et al. 2010]. Similar results reflected in other trials suggest that treatment with capsaicin was generally well tolerated. Among the 1327 patients studied in randomized, controlled trials, 883 patients (67%) reported adverse reactions. Most of these were minor application site-related problems that were transient in nature. Only 0.8% of patients in the study group discontinued treatment because of adverse reactions and this compared with 0.6% of patients in the control group [European Medicines Agency, 2013]. The pooled data suggest that the overall dropout rate was only 1.5% [FDA Center for Drug Evaluation and Research, 2009].
Nine deaths were reported but none of these were related to treatment. Serious adverse events were uncommon and almost all events except one were unrelated to treatment [FDA Center for Drug Evaluation and Research, 2009; McCormack, 2010]. There was one case of accelerated hypertension, which possibly could be related to pain associated with the study medication [FDA Center for Drug Evaluation and Research, 2009]. The proportion of patients reporting a change in blood pressure over the course of the phase III studies [Backonja et al. 2008; Irving et al. 2010] was 1.7% in the NGX-4010 group, and 0.7% in the control group [FDA Center for Drug Evaluation and Research, 2009]. On the day of treatment, changes in blood pressure were related to pain caused by the treatment and were usually mild (< 8 mmHg average) and transient [European Medicines Agency, 2013], returning to baseline within 1 h of treatment [Backonja et al. 2008; FDA Center for Drug Evaluation and Research, 2009]. The incidence of cardiac-related adverse events was low and had a similar incidence in the study arm and control arm. However, the risk in patients with pre-existing cardiovascular disease was higher (18%) compared with those without the cardiovascular risk (10.2%) when treated with the capsaicin 8% patch [FDA Center for Drug Evaluation and Research, 2009].
The most common adverse event was problems with the application site such as erythema, pain, oedema and pruritus. Around 96% of PHN patients and 75% of HIV-DSP patients had application-site reactions [Simpson et al. 2010]. Dermal assessment scores for PHN patients were mostly < 2 (definite erythema, readily visible, minimal oedema or minimal popular response), and those for HIV-DSP patients were < 1 (minimal erythema, barely perceptible). There were few patients with scores above 3 or 4 [Simpson et al. 2010]. These reactions were more common in the NGX-4010 group. They peaked just after patch removal (Figure 1) and were transient resolving within 1–3 days [FDA Center for Drug Evaluation and Research, 2009].
Figure 1.
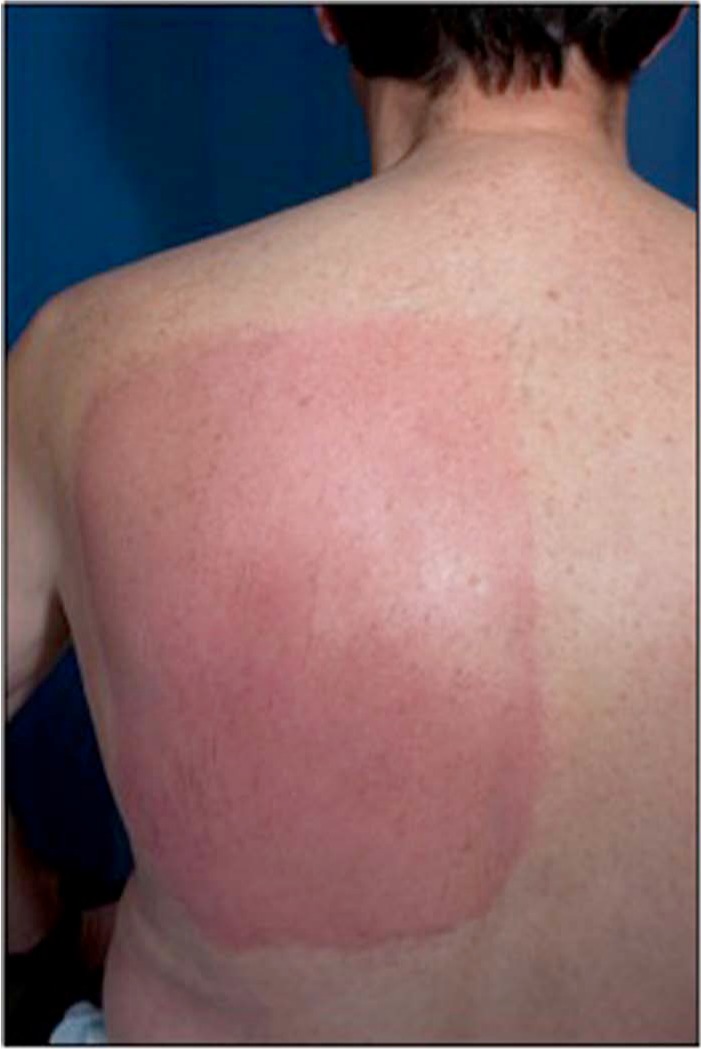
Area of erythema after patch removal.
Pain associated with treatment was common in the NGX-4010 group. Around 36% of patients reported a 30% increase in baseline pain scores compared with 13% in controls [Simpson et al. 2010]. The pain increased on days 0 and 1 and from day 2 onwards it started working downwards [Simpson et al. 2010]. In PHN patients, depending on the number of treatments, the proportion of patients reporting pain varied from 35% to 48% and the mean dose of oxycodone used ranged from 12.2 mg to 17.1 mg. In the HIV-DSP group 32–46% patients complained of pain during the four repeat treatments and the mean dose of oxycodone used was 12.3–31.7 mg depending on the cycle of treatment. The use of rescue medication from day 0 to day 5 was higher in HIV-DSP patients but was the same as PHN patients by day 5.
Neurological and sensory assessments were carried out clinically as well as using quantitative sensory testing. Most subjects reported no changes and the minority who did, reported an improvement or return to normal sensation [Simpson et al. 2010]. Other uncommon adverse events reported were coughing and sneezing, caused by aerosolization of the drug from the patch. Nausea was thought to be associated with the use of opioid rescue medication. Erythema was more common in PHN patients while diarrhoea, loss of weight and throat infections were more common in the HIV-DSP patients.
In conclusion, the drug was well tolerated with application-site problems being the most frequently encountered problems, but these were self-limiting and transient. None of the adverse events were related to multiple exposures and no cumulative toxicity was reported.
Setting up the delivery of treatment with the capsaicin 8% patch
The first uses of capsaicin 8% patches in clinical practice outside clinical trials were started in the UK in June 2010 at the Christie NHS Foundation Trust, Manchester, UK; by this time the patch had already been in use in Germany for several months. The process of setting up a service model for the delivery of treatment with the capsaicin 8% patch and a practical description of the treatment process with high-concentration capsaicin patches are described below.
The licensing agreement with the UK Medicines and Healthcare Products Regulatory Agency for the product stipulated that the clinical practitioners delivering the treatment should undergo a two-step training programme.
Initial training involves clinical information of the mode of action, efficacy results from integrated pivotal phase III studies and a summary of the overall safety data. This is followed by a practical application workshop to understand the administration procedure as well as demonstrating competency in the administration of the patch using placebo patches.
Live training involves the use of the capsaicin 8% patches in a clinical setting under the supervision of an experienced trainer and also sign-off the competency to be able to counsel patients appropriately before, during and after administration of the capsaicin 8% patches.
Currently there is online training following which a live training or supervision of the application procedure may be arranged. The service has been set up in most instances in the UK with one or more trained operators, mainly nurses, as a day-case service in an appropriate clinical setting, which is usually a room with adequate ventilation, be it in a theatre or day-unit setting.
Pretreatment visit and counselling
Patients deemed appropriate for the treatment are counselled prior to the day of treatment in clinic about what to expect and are also given appropriate patient information leaflets outlining the treatment procedure and postprocedural care at home. Any questions or concerns raised by the patient or a family member are addressed to their satisfaction. The patient is requested not to shave or remove any body hair from the area to be treated within 48 h prior to treatment to prevent any breach of skin integrity at the site of application; any troublesome hair that prevents close contact of the patch to the skin is carefully snipped using scissors by the operator immediately before the procedure. The patients are also informed that, despite pretreatment with local anaesthetic cream or gel, they may or may not experience erythema and a burning sensation, but that this is transient and will not cause any damage to the skin. It is also explained to the patients that a lack of burning sensation and erythema at the application site is not an indicator of the potential efficacy of the patch. The burning sensation and pain associated with the treatment are often described by the patient as a ‘deep heat, like a bad sunburn’ and they are reassured that experience shows it settles down within 48 h to 72 h; most patients only require localized dry-cooling methods or simple analgesics to manage their localized pain and discomfort. Patients are recommended to bring their own rescue medication used for the flare up of their neuropathic pain on the day of treatment and audiovisual equipment to listen to music or watch an entertainment programme during treatment. This distraction may help to make the experience less uncomfortable. The patients are also advised that someone else should drive them home, as the treatment-associated discomfort or the analgesics taken to manage the pain may impair their driving ability.
On the day of the treatment, every effort is made to keep the patient relaxed and, again, reassured about the procedure. They are encouraged to have a relative present if they so wish and also to use audiovisual equipment or reading materials to keep them suitably distracted from the discomfort of the treatment. Some centres use communal bays where patients can interact during treatment, but due to the set up in our centre we have individual treatment bays, which are well ventilated and have a window with an external view.
The capsaicin 8% patch and area to be treated
The capsaicin 8% patch measures 14 cm × 20 cm (280 cm2) and contains a total of 179 mg of capsaicin (640 µg/cm2). A maximum of four patches can be used at any given time to treat an area of 1120 cm2 [Astellas Pharma Europe Ltd, 2012]. We have found in our clinical practice that most areas in the trunk or periphery require 1–2 patches, whilst treatment of both feet for peripheral neuropathy often requires 3–4 patches. The patch and cleansing gel are stored below 25°C and an unopened patch has a shelf life of 4 years. The patch is removed from the sealed pack only prior to the application and should be used within 2 h. The patch can be used to treat localized neuropathic pain in all areas of the body with the exception of the face, above the hairline on the scalp and on or near the mucous membranes of the face or perineum.
Once the area to be treated is confirmed by the patient and operator, the area is traced using a skin-marking pen; if there is any doubt about the area to be treated, it is recommended that the area be slightly overtreated rather than undertreated as some patients experience suboptimal analgesia if any part of the area has been missed (Figure 2). We also recommend using a transparency sheet to document the area to be treated as it not only makes it easier to cut the capsaicin 8% patches in the most economical fashion, but also acts as a record to compare the size of the treatment area for subsequent treatments. We have noted alongside other operators that there is shrinkage of the area of allodynia and hyperpathia with subsequent treatments and a record of the size and shape of the initial treated area is often useful.
Figure 2.
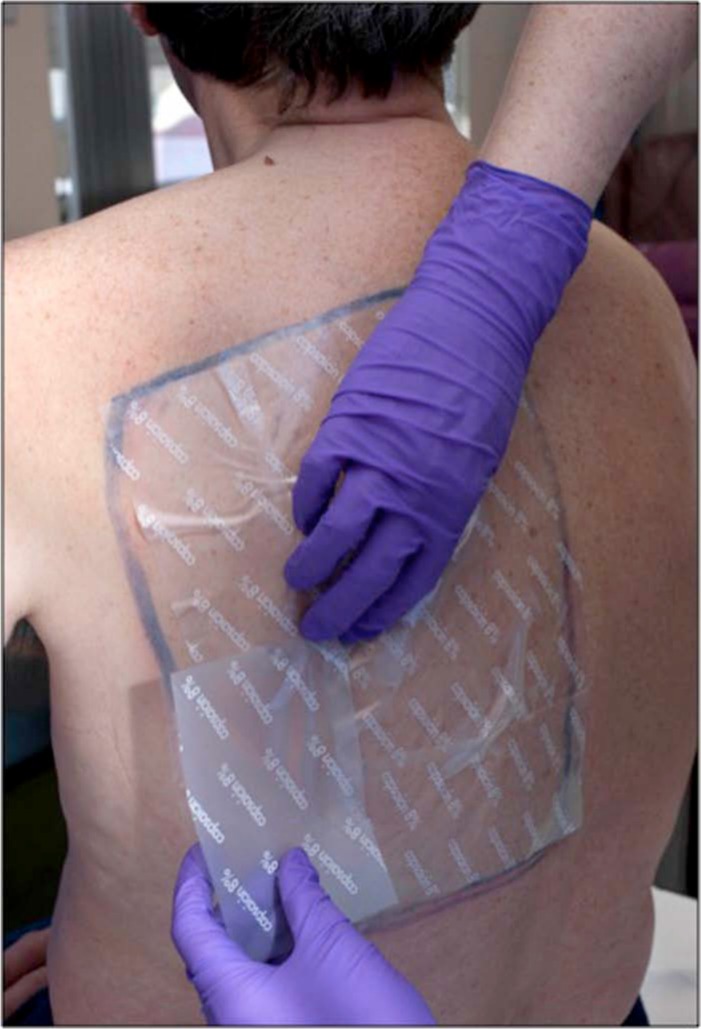
Application of 8% capsaicin patches on the traced area.
Pretreatment with local anaesthetic cream
The current Summary of Product Characteristics recommendations for the capsaicin 8% patch state that the topical local anaesthetic cream should be applied to the entire treatment area as well as the surrounding 1–2 cm for a time specified by the manufacturer [European Medicines Agency, 2013]. Many practitioners have observed that a large number of patients find the application of the local anaesthetic and its removal prior to the patch application far more distressing, particularly if they had significant allodynia in the region. The recently concluded multicentre LIFT study has demonstrated that the use of even a low-dose analgesic like tramadol 50 mg prior to the patch application can effectively reduce the treatment-related discomfort comparable with the application of a topical local anaesthetic [Astellas Pharma Europe Ltd, 2012]. These findings substantiate what most experienced operators have observed as well as reducing the duration of the treatment, significantly enabling more patients to be treated while simultaneously bringing in cost benefits as well as more efficient utilization of resources and manpower.
Patch application procedure
The use of good-fitting nitrile gloves is important to ensure ease of application; latex gloves are not suitable for this purpose as capsaicin can diffuse through them and contaminate the operators’ hands [Astellas Pharma Europe Ltd, 2012]. The application of the patch to cleaned and dried skin is performed in a manner such as to minimize aerosolization as well as to ensure good adhesion. The tracing of the area to be treated is used as a guide to cut the patches and after application they are kept in place by wrapping the area with cling-film, bandages or socks. Sandbags and weights have also been used to put pressure on the patch to ensure good contact with the underlying skin. The treatment of hands and feet are particularly time consuming and cutting the capsaicin 8% patch into little strips, wrapping them around individual digits and covering the web-spaces have given better results (Figure 3). Areas of mucous membranes or sensitive areas like the nipple in close proximity to the treated area can be covered with barrier tape to prevent the patch from coming into contact.
Figure 3.
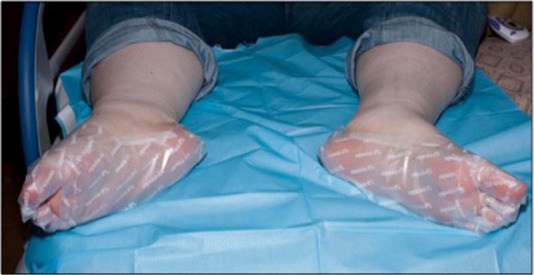
Patch application on extremities.
During the application period the patient is fully monitored, as there may be a transient rise in blood pressure due to treatment-associated discomfort. For this reason, patients with uncontrolled hypertension are deferred from treatment until their blood pressure is suitably controlled with appropriate antihypertensives. Following patch application, it is removed by gently rolling inwards to minimize aerosolization. Any residual capsaicin is removed from the area by using the proprietary cleansing gel included in the treatment pack, which is left on for at least 1 min before removal with a dry gauze and the area washed with soap and tepid water. The patch and all the linen, gauze and gloves are immediately disposed off in a sealed waste-disposal plastic bag to avoid contamination with capsaicin. Patients are advised to avoid touching the treated area in the days following treatment as well as ensuring that there is no contact with contaminated clothes/linen. Care should also be taken to ensure that family members and pets avoid coming into contact with the treated area.
Management of treatment-associated discomfort
Most patients tolerate the procedure very well and distraction strategies with audiovisual equipment and interactions/reassurance from the operator are sufficient to alleviate most of the distress. However, patients are encouraged to ask for analgesia, be it simple analgesics like paracetamol or opioids like tramadol or oxycodone. Patients are encouraged to bring their usual rescue analgesia and if they prefer to use that, its use is documented during the procedure. The best method for alleviating the burning sensation is the use of dry cooling; a chilled cool pack wrapped in linen is applied over the treated area. Questions have been raised about possible interference with the mechanism of action of capsaicin with localized cooling, so it is recommended that it be used after treatment; however, we have used it in some cases towards the end of the treatment period to alleviate patient distress due to the burning sensation. Wet compresses are not recommended prior to treatment as they interfere with patch adhesion and postprocedure they can cause leaching of the capsaicin from the deeper layers of the skin. Patients who have had their feet treated for peripheral neuropathy are recommended to wrap their feet in cling-film or a plastic bag and immerse in water at room temperature after 24 h post-treatment as many patients found that to be comforting. Most of the symptoms settle down within 48–72 h and it is extremely rare for symptoms to persist after 5 days. Some patients may need reassurance that once the pain has settled, there may be some underlying numbness due to their pre-existing neuropathy.
Follow up of patients
Patients are routinely followed up by telephone at 24 h and 72–96 h postprocedure to ensure that they are supported as well as to address any queries. They are advised to maintain a pain diary to monitor their pain scores and improvement in function, and are reviewed in the clinic at 4 weeks and 8 weeks, during which time systemic analgesics including opioid and neuropathic drugs are down-titrated or even tapered off if possible. Even though the patch application can be repeated every 3 months, we do not routinely advocate it and wait for the symptoms to recur before offering the next treatment. This has been shown to vary between 3 months and 15 months on average, depending on the underlying pathology [Bhaskar et al. 2012]. The patients are on regular follow up and have the details of the pain service to contact for advice as well as booking an appointment for a consultation with a view to planning a repeat treatment.
Capsaicin patch-application equipment checklist
Capsaicin 8% patches
Topical anaesthetic cream/gel
Cleansing gel/gauze/wipes
Skin-marker pen
Transparency tracing sheet
Appropriately sized nitrile gloves (three pairs) for the operator and assistant; note that latex gloves are not suitable as the capsaicin can diffuse through them
Bandages/cling-film/socks (if feet are being treated)
Scissors
Soap and running water
Hair dryer/blower
Monitoring equipment – SpO2
Clinical waste-disposal bag
Application procedure for the capsaicin 8% patch
Identify the area to be treated; ensure that the skin is intact and unbroken.
Using the skin-marking pen, mark the area to be treated; if any hair is to be removed, it should be clipped close to the skin using a pair of scissors.
The area to be treated is traced on to the tracing sheet clearly labelling the cephalocaudal and right-left orientation.
Topical anaesthetic cream (EMLA or 4% lidocaine) is applied for a period according to the manufacturer’s instruction – usually for 1 h.
After the stipulated time, remove the topical anaesthetic cream and dry the skin carefully; if the patient has significant allodynia over the area to be treated, the use of a hair dryer is often helpful to dry and warm the area to facilitate adhesion.
Capsaicin 8% patches are cut to shape and orientation based on the tracing sheet template of the treatment area.
Apply the capsaicin patches to the treatment area ensuring close adhesion to the skin; bandaging, cling-film or socks may be used to facilitate the adhesion. The patch is left in place for 30 min if the feet are being treated or 60 min for the rest of the body.
After the treatment duration has been completed, the capsaicin 8% patch is removed by carefully rolling inwards to reduce the risk of aerosolization.
Clean the treated area of any residual capsaicin on the skin with the cleansing gel and allow the area to dry spontaneously.
Postprocedural instructions for patients
Counselling about avoiding contamination of clothes and towels with capsaicin at home.
Care to be taken to avoid contact of the treated area particularly with children and pets.
Delayed onset of burning sensation and pain a few hours after the treatment once the effect of the local anaesthetic has worn off.
Use of localized dry cooling and analgesics for managing pain and discomfort.
To avoid hot baths/showers, exposure of the treated area to direct sunlight and vigorous exercise for 24–48 h or until the acute symptoms have settled down.
To ensure that pre-existing prescribed analgesia and neuropathic pain medications are not abruptly discontinued without medical advice and supervision, even if there is dramatic pain relief, to avoid withdrawal effects.
To contact the treatment unit or patient’s GP if there is any concerns of adverse side effects.
Contact details of the treatment unit.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Ganesan Baranidharan, Leeds Teaching Hospitals NHS Trust, Leeds Pain and Neuromodulation Centre, Seacroft Hospital, Leeds LS14 6UH, UK.
Sangeeta Das, Leeds Teaching Hopsitals NHS Trust, Leeds Pain and Neuromodulation Centre, Leeds, UK.
Arun Bhaskar, The Christie NHS Foundation Trust, Pain Medicine, Manchester, UK.
References
- Alawi K., Keeble J. (2010) The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther 125: 181–195 [DOI] [PubMed] [Google Scholar]
- Anand P., Bley K. (2011) Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth 107: 490–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astellas Pharma Europe Ltd (2012) Tolerability of QUTENZATM when Applied after Pre-treatment with Lidocaine or Tramadol in Subjects with Peripheral Neuropathic Pain – A Randomized, Multi-center, Assessor-blinded Study. Chertsey, UK [Google Scholar]
- Babbar S., Marier J., Mouksassi M., Beliveau M., Vanhove G., Chanda S., et al. (2009) Pharmacokinetic analysis of capsaicin after topical administration of a high-concentration capsaicin patch to patients with peripheral neuropathic pain. Ther Drug Monit 31: 502–510 [DOI] [PubMed] [Google Scholar]
- Backonja M., Irving A., Webster R. (2009) Efficacy of Qutenza_ (NGX-4010), a high concentration capsaicin cutaneous patch, in patients with peripheral neuropathic pain: results of integrated analyses. Eur J Neurol 3: 1046 [Google Scholar]
- Backonja M., Malan T., Vanhove G., Tobias J.; C102/106 Study Group (2010) NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an open-label extension. Pain Med 11: 600–608 [DOI] [PubMed] [Google Scholar]
- Backonja M., Wallace M., Blonsky E., Cutler B., Malan P., Rauck R., et al. (2008) NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurol 7: 1106–1112 [DOI] [PubMed] [Google Scholar]
- Bhaskar A., England J., Lowe J. (2012) Management of neuropathic pain (NP) using the capsaicin 8% patch in patients with cancer. Presented at the European Association for Palliative Care, Norway Poster 465. [Google Scholar]
- Bode A., Dong Z. (2011) The two faces of capsaicin. Cancer Res 71: 2809–2814 [DOI] [PubMed] [Google Scholar]
- Chaiyasit K., Khovidhunkit W., Wittayalertpanya S. (2009) Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J Med Assoc Thai 92: 108–113 [PubMed] [Google Scholar]
- Chard P., Bleakman D., Savidge J., Miller R. (1995) Capsaicin-induced neurotoxicity in cultured dorsal root ganglion neurons: involvement of calcium-activated proteases. Neuroscience 65: 1099–1108 [DOI] [PubMed] [Google Scholar]
- Clifford D., Simpson D., Brown S., Moyle G., Brew B., Conway B. (2010) A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of painful HIV-associated distal sensory polyneuropathy. 17th Conference on Retroviruses and Opportunistic Infections, San Francisco [Google Scholar]
- Derry S., Moore R. (2012) Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev 9: CD010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry S., Sven-Rice A., Cole P., Tan T., Moore R. (2013) Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2: CD007393. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (2013) Summary of Product Characteristics: Qutenza 179 mg cutaneous patch. [http://tinyurl.com/4y9hahb
- FDA Center for Drug Evaluation and Research (2009) Medical Review: Qutenza. Silver Spring, MD: [http://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022395s000Medr.pdf] [Google Scholar]
- Han P., McDonald H., Bianchi B., Kouhen R., Vos M., Jarvis M., et al. (2007) Capsaicin causes protein synthesis inhibition and microtubule disassembly through TRPV1 activities both on the plasma membrane and intracellular membranes. Biochem Pharmacol 73: 1635–1645 [DOI] [PubMed] [Google Scholar]
- Irving G., Backonja M., Dunteman E., Blonsky E., Vanhove G., Lu S., et al. (2010) A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Med 12: 99–109 [DOI] [PubMed] [Google Scholar]
- Jensen T., Madsen C., Finnerup N. (2009) Pharmacology and treatment of neuropathic pains. Curr Opin Neurol 22: 467–474 [DOI] [PubMed] [Google Scholar]
- Luo X., Peng J., Li Y. (2011) Recent advances in the study on capsaicinoids and capsinoids. Eur J Pharmacol 650: 1–7 [DOI] [PubMed] [Google Scholar]
- Martindale W. (1999) Reynolds J. (ed.), The Extra Pharmacopoeia/Martindale, 32nd edition. London: The Pharmaceutical Press [Google Scholar]
- McCormack P. (2010) Capsaicin dermal patch: in non-diabetic peripheral neuropathic pain. Drugs 70: 1831–1842 [DOI] [PubMed] [Google Scholar]
- Polydefkis M., Hauer P., Sheth S., Sirdofsky M., Griffin J., McArthur J. (2004) The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain 127: 1606–1615 [DOI] [PubMed] [Google Scholar]
- Shimomura Y., Kawada T., Suzuki M. (1989) Capsaicin and its analogs inhibit the activity of NADH-coenzyme Q oxidoreductase of the mitochondrial respiratory chain. Arch Biochem Biophys 270: 573–577 [DOI] [PubMed] [Google Scholar]
- Simpson D., Brown S., Tobias J.; NGX-4010 C107 Study Group (2008) Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology 70: 2305–2313 [DOI] [PubMed] [Google Scholar]
- Simpson D., Gazda S., Brown S., Webster L., Lu S., Tobias J., et al. (2010) Long-term safety of NGX-4010, a high-concentration capsaicin patch, in patients with peripheral neuropathic pain. J Pain Symptom Manage 39: 1053–1064 [DOI] [PubMed] [Google Scholar]
- Thresh J. (1876) Isolation of capsaicin. The Pharmaceutical Journal and Transactions 6: 941–947 [Google Scholar]
- Turnbull A. (1850) Tincture of capsaicin as a remedy for chilblains and toothache. Dublin Free Press 1: 95–96 [Google Scholar]
- Webster L., Malan T., Tuchman M., Mollen M., Tobias J., Vanhove G. (2010) A multicenter, randomized, double-blind, controlled dose finding study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. J Pain 11: 972–982 [DOI] [PubMed] [Google Scholar]


