Abstract
Background. Plasmodium falciparum malaria is a common cause of morbidity in African children, but identifying those who are likely to die is problematic. Previous studies suggested that circulating malarial pigment might be a useful predictor of severity, but none were large enough to detect any association with mortality.
Methods. We used thick blood smears performed on admission for 26,296 children hospitalized with P. falciparum at 1 of 6 hospitals in the Severe Malaria in African Children network to assess the prognostic value of pigment-containing granulocytes, monocytes, and parasites.
Results. Although at all but one of the study sites the risk of mortality for subjects presenting with >5 pigmented granulocytes per 200 white blood cells was higher than in subjects with no pigmented granulocytes, adjusted odds ratios estimated through logistic regression, which included other established markers of severe malaria, suggested that associations between pigmented cells and mortality were moderate to nonexistent in most sites. The predictive ability of pigmented cells was low, as measured by the change in the area under the receiver operating characteristic curve of logistic regression models.
Conclusions. Although high levels of pigmented cells were associated with a fatal outcome in some study sites, they were not useful predictors of outcome across Africa.
Giovanni Maria Lancisi, in 1716, was the first to describe the presence of black pigment in human liver, brain, and spleen, but he did not associate these changes with malaria. In 1825, Etienne Bailly reported the dark color of cortical gray matter he observed during autopsies in 1822 of patients from Rome with malarial fever, and Richard Bright confirmed this in his autopsy accounts in 1831. However, these observations of discolored tissues were not extended to the recognition of malarial pigment in corpuscles until Schütz, in 1846, and Meckel von Helmsbach, independently a year later, described brown pigment in the blood of people who died of “pernicious fever.” These early accounts of malarial pigment have recently been reviewed by Poser and Bruyn [1].
It is now recognized that this pigment in patients with malaria, known also as hemozoin, is a degradation product of hemoglobin formed in the process of detoxifying free heme and that it can be found in parasitized erythrocytes, monocytes, and granulocytes of patients with malaria. The formation of malarial pigment (i.e., hemozoin) is a coordinated 2-component process involving both lipids and histidine-rich proteins of Plasmodium falciparum [2].
Malarial pigment has a number of biological effects, some of which may affect immune defense mechanisms. It inhibits macrophage function [3, 4] and dendritic cell functions [5], and it can suppress erythropoiesis [6, 7]. By suppressing prostaglandin E2 production, it leads to tumor necrosis factor overproduction, which in turn is associated with malarial anemia [8].
In addition to these pathophysiological effects, hemozoin in circulating cells may also be useful in diagnosing malaria [9] and in predicting outcome. In 1995, two independent, small studies demonstrated the potential of intraleukocytic pigment as a prognostic factor in malaria [10, 11]. The Gabonese study showed a correlation between the presence of pigment in monocytes and the degree of anemia in children with malaria and a correlation between pigment in neutrophils and parasitemia [10]. The Thai study suggested that the number of pigment-containing neutrophils in adult patients with malaria was a rapid and simple prognostic test [11]. In a follow-up study from Nigeria, the pigment-containing neutrophil count was also a simple marker of disease severity in childhood malaria [12]. However, none of these studies was large enough to detect an association between circulating pigment and mortality.
To obtain more-definitive evidence of the prognostic significance of circulating pigments for, primarily, malaria-associated mortality, our clinical network for Severe Malaria in African Children (SMAC) undertook a large, multicenter study of the prognostic significance of circulating pigment [13]. Because our sites spanned Africa and included a variety of different epidemiological settings, the sample could more completely include the epidemiologic diversity of falciparum malaria in Africa and could generate a sample size large enough to identify any association between circulating pigment and disease outcome. Between the beginning and the completion of the SMAC study, 3 other smaller studies (from Gabon [14], Mali [15], and Kenya [7]) were performed; they reported findings similar to those described in the earlier studies.
Patients, Materials, and Methods
Design. The eligible population comprised all parasitemic children admitted to each of the participating hospitals of the SMAC network. The SMAC network includes sites in 5 countries: Banjul, the Gambia (Medical Research Council Laboratories, Malaria Research Programme, in collaboration with the Royal Victoria Teaching Hospital); Blantyre, Malawi (Blantyre Malaria Project, Queen Elizabeth Central Hospital); Kumasi, Ghana (University of Science and Technology, School of Medical Science); Kilifi, Kenya (Kenya Medical Research Institute for Geographic Medicine); and 2 sites in Gabon, Lambarene and Libreville, both run by the Medical Research Unit of Albert Schweitzer Hospital [13]. A detailed description of the study design and participating sites was provided by Taylor et al. [13, 16].
All children aged <1 to 180 months who were suspected of having malaria and were sick enough to be hospitalized were screened with a thick blood smear for the presence of P. falciparum parasitemia. Consent to join the study was sought from parents/guardians of children with positive blood smears in the appropriate local language. The consent forms were approved by the local ethics committee or institutional review board (IRB), by the Michigan State University IRB as the contract holder, and by the protocol review group at the sponsoring agency, the National Institute of Allergy and Infectious Diseases, Division of Microbiology and Infectious Diseases.
After consent was given, a second finger prick sample of blood was collected, and data on history of present illness and physical findings on admission were recorded on standardized case report forms. Patients were followed throughout their hospitalization, and once results of laboratory tests and the outcome were known, the completed form was submitted to the data entry team at each site.
Malarial pigment assessment. Pigment-containing parasitized erythrocytes (PP), pigmented mononuclear leukocytes (PM), and pigmented granulocytes (PG) were detected and counted by a simplified method validated and described by Lell et al. [17]. In a stained thick blood smear, the number of PP per 200 parasites, the number of PM per 200 mononuclear leukocytes, and the number of PG per 200 granulocytes were counted using a standard laboratory microscopes with 1000-fold magnification.
Definitions of outcomes and other indicators of malaria severity. Our primary outcome was mortality in patients with malaria. We also assessed associations between pigments and severe malaria defined on the basis of data collected at the time of admission on Blantyre coma score, hematocrit, lactate level, glucose level, and other clinical indicators. Children with a Blantyre coma score of ≤2 were classified as having cerebral malaria [18]. Those whose hematocrit was ≤15% or whose hemoglobin level was ≤5 g/dL (in sites where hematocrit was not measured) were categorized as having severe malarial anemia. Children with a lactate level of >5 mmol/L were categorized as having hyperlactatemia, and those with a glucose level of ≤2 mmol/L were classified as having hypoglycemia. Hyperparasitemia was defined as a parasite level of >500,000 parasites/µL.
Other clinical indicators of malaria severity used in these analyses included deep Kussmaul respiration and/or an irregular respiratory rhythm (either of these was interpreted as a sign of respiratory distress) and the inability to sit (interpreted as a sign of prostration). A thorough description of all clinical and laboratory markers is provided elsewhere [13, 19].
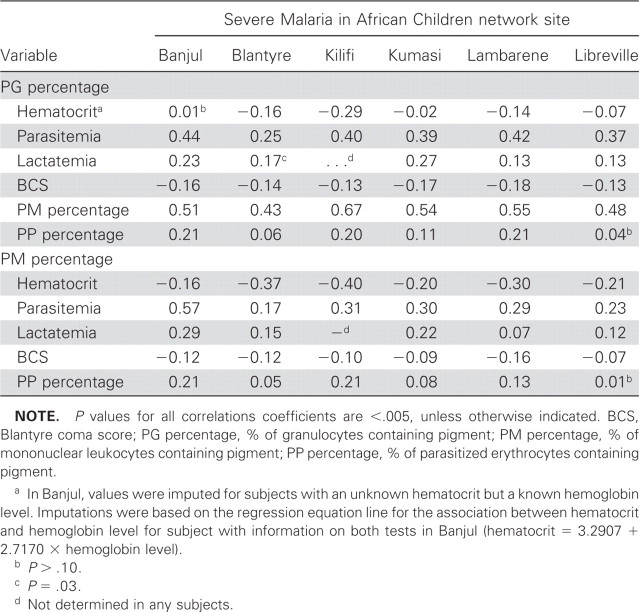
Statistical analyses. Descriptions of demographic, clinical, and laboratory profiles and mortality outcomes by site were based on means (and standard deviations), for normally distributed values; medians (and interquartile ranges), for skewed values; and proportions, for categorical variables. Comparison of continuous variables across sites was based on analysis of variance and Kruskal-Wallis tests. Proportions were compared by Pearson χ2 tests. Because of heterogeneity across sites, all analyses were site specific. Spearman correlation coefficients were used to describe associations of PG, PP, and PM with markers of severe malaria.
To study the prognostic value of pigment for malaria mortality, we estimated odds ratios (ORs) and C statistics (corresponding to the areas under the receiver operating characteristic [ROC] curves) in logistic regression models including death as the outcome. The area under the ROC curve is a function of the ability of a predictor to discriminate persons who survived from those who died, because it is a function of the sensitivity and false-positive fractions of a test. It varies from 0.5 (for the poorest predictors) to 1 (for perfect predictors). We also assessed the ability of pigment to predict malaria deaths in conjunction with other predictors on the basis of the change in the C statistic from models excluding and those including pigment, adjusting for other predictors. In logistic regression models, PG and PM were categorized as 0% of cells, 0.5%–2.5% of cells, 3%–5% of cells, and >5% of cells. Because very few subjects presented with PP, the effect of PP was estimated on the basis of PP detection versus no PP detection. Clinical, demographic, and laboratory variables were selected for the models if their P values in Wald tests (approximately equal to likelihood ratio test in large populations) were less than .01 in at least 3 sites, if they impacted the coefficients of pigment variables, or if they increased the C statistic by ≥0.01 units in at least 3 sites. These analyses were performed separately for each site, because interactions of study sites with all other covariates included in the models (particularly with pigment variables) were statistically significant. We chose to report multivariable models including both PG and PM because conclusions based on those models were comparable to those based on models including PG and PM separately.
All P values are 2-tailed. Statistical analyses were performed using SAS, version 9 (SAS Institute), and S-Plus 7 for Windows (Insightful).
Results
General description. From December 2000 through May 2005, a total of 26,389 patients were enrolled. Of these, 93 (0.4%) were excluded from further analysis, primarily because of missing age data, an age of >180 months, or an out-of-range weights-for-age value plus an unknown date of birth. The analysis data set thus included 26,296 subjects. Demographic characteristics, medical history, clinical presentation, and laboratory findings on admission varied across the 6 sites (table 1). A total of 205 subjects absconded, and an additional 55 subjects had missing outcomes. There were 1129 deaths (overall mortality, 4.3%) among the 26,036 subjects with observed outcomes. Between-site differences were statistically significant (P<.001) for all variables except sex. Mortality rates were highest in Banjul (9.5%); intermediate in Libreville (5.1%), Kumasi (4.5%), and Kilifi (3.6%); and lowest in Blantyre (2.5%) and Lambarene (1.4%).
Table 1.

Demographic, clinical, and laboratory characteristics and outcomes of African children with malaria
The distribution of pigmented cells also varied across the 6 sites (P<.001 for each) (table 1). Overall, 37% of subjects had PG, and the median percentage of cells with pigment was low (2% of granulocytes). Sixty-three percent of subjects had PM, and again, the median percentage of cells with pigment was low (4% of mononuclear leukocytes). Patients in Libreville and Lambarene had the highest percentages of granulocytes and mononuclear leukocytes that contained pigment. PP was a rare finding, present in only 3% of patients overall, among whom a median of 5% of parasitized erythrocytes were pigmented.
Association between pigmented cells and other biological markers of severe malaria. Table 2 explores whether pigmented cells were associated with any severe clinical manifestation that could contribute to the subject's death. The percentages of granulocytes and mononuclear leukocytes containing pigment were each correlated with parasitemia (Spearman rank correlation coefficients, 0.25–0.44 and 0.17–0.57, respectively), with the weakest correlations observed in Blantyre (table 2). The percentage of mononuclear leukocytes containing pigment was negatively correlated with hematocrit (Spearman rank correlation coefficients, −0.16 to −0.40). The percentage of parasitized erythrocytes containing pigment was not correlated with any other marker of disease. Correlations between pigmented cells and glucose level were near 0 and did not reach statistical significance in any site.
Table 2.
Spearman rank correlation coefficients for associations between pigmented cells and other disease markers in African children with malaria by site.
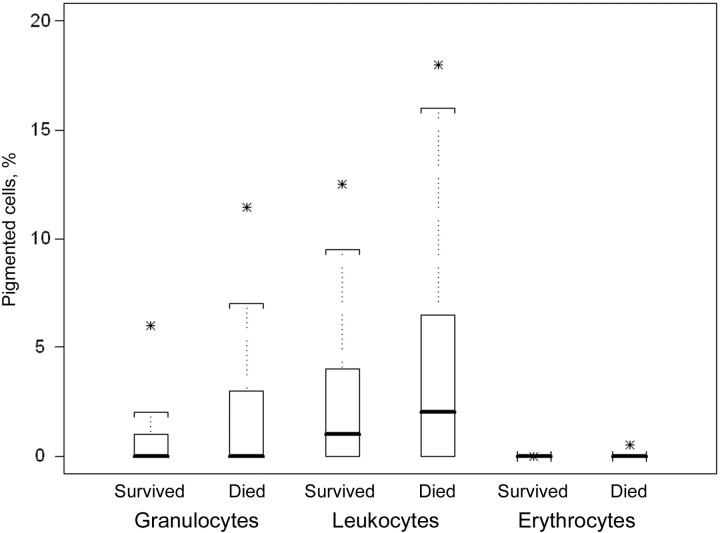
Association between pigment in cells and mortality. The distributions of PG and PM in subjects with malaria who died and survived were skewed with medians close to 0, although counts of these cells were slightly higher among subjects who died (figure 1). In models that included pigment variables and study sites, interactions with study sites were statistically significant (P of interaction with site was <.001 for PG, .002 for PM, and .02 for PP). Therefore, all analyses for the association with mortality were performed separately for each site.
Figure 1.
Distribution of malaria pigment in granulocytes, mononuclear leukocytes, and parasitized erythrocytes from African children who survived or died from malaria. Comparisons between children who survived and those who died reached statistical significance in each group (P<.001 for each comparison). Solid bars, median values; upper hinges, 75th percentiles; lower hinges, 25th percentiles; whiskers, most-extreme values within 1.5 × interquartile range; stars, 95th percentiles.
In crude models, the risk of mortality increased with an increase in PG, reaching statistical significance in all sites except Kilifi (table 3). Despite these statistically significant differences, the predictive ability of PG for mortality (as measured by C statistics, or areas under the ROC curves) was low in most sites. The risk of mortality tended to increase with an increase in PM only in Banjul and Blantyre, with less consistent patterns at the other sites (table 3); the predictive ability was low. The presence of PP was associated with increased mortality at 3 study sites (Blantyre, Kumasi, and Lambarene), but again, the predictive ability was low.
Table 3.

Mortality risk and distribution of cells with malaria pigment among African children with malaria.
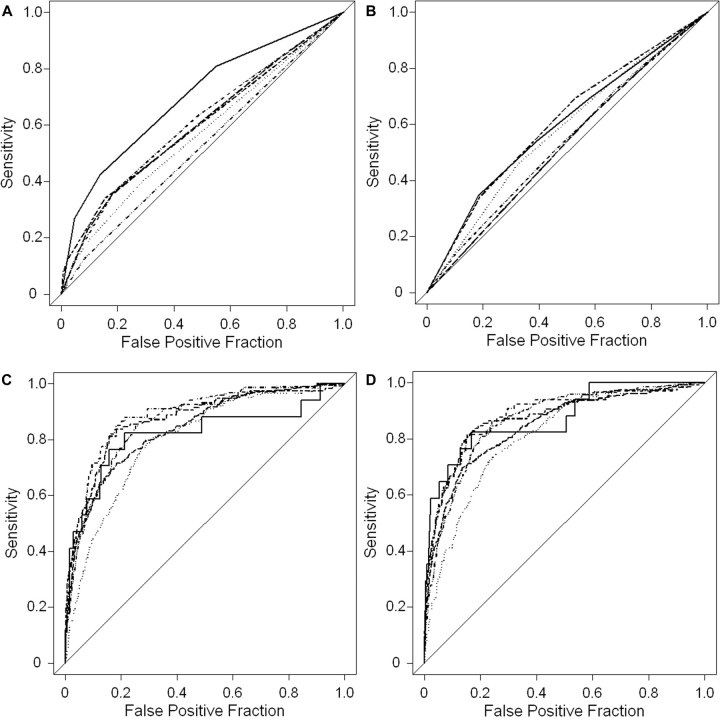
In models including both PG and PM, relevant predictors of mortality in at least 3 sites included hyperlactatemia (lactate level, >5 mmol/L), coma (Blantyre coma score, ≤2), deep breathing, prostration, hypoglycemia (glucose level, <2.2 mmol/L), and lower weight-for-age z score (table 4). For most sites, although crude ORs suggested that increasing PG levels were associated with an increased risk of mortality (table 3), adjusted models showed that the risk of mortality for subjects in whom ≤5% of granulocytes were pigmented was similar to that for subjects with no PG (table 4). However, in Blantyre, Kumasi, and Lambarene, the risk of mortality for subjects in whom >5% of granulocytes were pigmented was statistically significantly higher than that for subjects with no PG. After controlling for PM and the other predictors of mortality, there was a statistically significant association between PM and reduced mortality in Banjul, Kilifi, and Kumasi (table 4). Despite these statistically significant associations, the cumulative ability of PG and PM to predict mortality was relatively low in all areas except Lambarene. In particular, the predictive ability of the multivariable logistic regression models increased only slightly when categorized PG and PM were added to the model containing hyperlactatemia, coma, deep breathing, prostration, hypoglycemia, and weight-for-age z scores (the change in the C statistic was ≤0.01 in all sites except Lambarene, where it was 0.05). These results are confirmed by the ROC curves presented in figure 2.
Table 4.
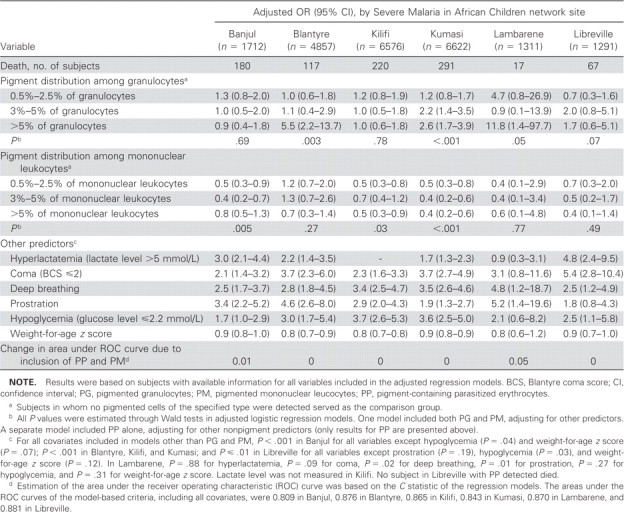
Findings of logistic regression analysis to determine adjusted odds ratios (ORs) of the associations between pigmented cells and mortality among African children with malaria.
Figure 2.
Receiver operating characteristic curves assessing pigment and other predictors as prognostic factors for mortality. Predictions were based on site-specific logistic regression models using only pigmented granulocytes (PG; A), only pigmented mononuclear leukocytes (PM; B), other relevant predictors but no pigment variables (C), and including PG, PM, plus other relevant predictors (D). Other relevant predictors included hyperlactatemia, coma, deep breathing, prostration, hypoglycemia, and weight-for-age z scores (table 4). · · · ·, Banjul; — – —, Blantyre; — · · · —, Kilifi; — —— —, Kumasi; ———, Lambarene; — · —, Libreville.
Inclusion of additional predictors in models for any of the sites did not affect appreciably either the ORs or confidence intervals (CIs) for the effects of circulating pigment on mortality. Similarly, exclusion of nonsignificant predictors from models of specific sites did not alter these estimates. Exclusion from the models variables that resulted in large numbers of missing subjects in Banjul, Blantyre, Lambarene, and Libreville did not affect these conclusions. Further separation of the PG category of >5% of cells into 5.5%–10% of cells and >10% of cells did not appreciably affect results. Dichotomization of PG into no PG detection and any PG detection yielded adjusted ORs that were close to 1 and did not reach statistical significance at any site. This lack of association between the presence of any PG and mortality in the dichotomized PG analysis was likely a result of the high contribution of subjects with low levels of pigmented granulocytes (i.e., those with pigment in 0.5%–2.5% of cells and those with pigment in 3%–5% of cells) to the overall risk among subjects with PG detected, because the percentage of granulocytes with pigment was <5% in most subjects.
Because of the small percentage of subjects (3%) with PP, separate adjusted models that included PP (and not PG and PM) were required. The adjusted odds of mortality were increased in subjects with PP in Kumasi (OR, 3.9; 95% CI, 1.8–8.3; P<.001) and in Banjul (OR, 2.2; 95% CI, 1.1–4.7; P=.04). The addition of PP terms also led to negligible changes in the predictive abilities of the regression models (changes in predictive abilities were ≤.01 for all sites).
Association between pigmented cells and mortality in the different clinical manifestations. To identify subgroups of children in which PG and PM could predict malaria-associated mortality, we compared the association of mortality and pigmented cells between subjects with and subjects without each severe malaria syndrome (i.e., hypoglycemia, severe anemia, hyperparasitemia, hyperlactatemia, and deep breathing). Because of small numbers of subjects, Lambarene was excluded from these analyses, since many models for this site did not converge. The ORs were similar between subjects with and subjects without each syndrome, and interaction terms were not statistically significant in regression models predicting death. Furthermore, the differences in the C statistics for PG between subgroups of subjects were generally small and not statistically significant, suggesting that PG and PM were not good surrogate markers for mortality even in these distinct subgroups. Models did not always converge in these analyses because of the small number of deaths in some subgroups.
Discussion
Simple, reliable, and rapidly assessable markers of mortality in patients with malaria and disease severity are needed to complement the diagnosis of malaria, which still relies primarily on blood film examination. On the basis of preliminary studies, circulating malaria pigment was an appealing potential marker of adverse outcome in severe disease because its assessment did not require additional resources. The SMAC network represented an opportunity to test definitively the usefulness of enumerating circulating pigmented cells in persons with malaria across Africa. Our hypothesis was that the level of circulating malaria pigment could complement assessment with other objective markers of disease severity, such as blood glucose, lactate, and hemoglobin levels.
We found that, although circulating pigmented cells were associated with a fatal outcome in some study sites, they were not useful markers of fatal outcome for individual patients. Only an increased PG level (>5% of granulocytes) was associated with mortality, an effect noted in 5 sites. However, after accounting for other clinical manifestations and laboratory markers of severity, this association remained significant only in 3: Blantyre, Kumasi, and Lambarene. Even when malaria was categorized by other indicators of disease severity, such as coma score and metabolic markers, no additional useful associations emerged between circulating pigmented cells and outcome in any of the subgroups.
Our findings that higher PG values are associated with higher parasite levels confirm a previous demonstration of this phenomenon [10]. Because the circulation half-life of granulocytes is relatively short (i.e., ≤24 h), these cells may thus serve as real-time markers of parasite growth and maturation. In contrast, the degree of anemia correlates best with increased PM; these white cells live longer and may indicate more-chronic infection. This association is also consistent with earlier findings [10]. In this study, we found relatively few PP, which may be due to the fact that all slide readings were performed on thick blood smears, where erythrocytes are lysed. However, when thin films of samples obtained at the same time were examined, intraerythrocytic pigment was very rarely present. In our study, the association of PP with outcome was poor.
The SMAC study included a broad spectrum of parasitemic children sick enough to be admitted to a hospital, whereas some of the earlier studies focused on patients with specific complications of severe malaria. Only 37% of the SMAC patients had pigmented granulocytes, compared with 54%–100% of subjects in previous studies, and only 63% of the SMAC population had pigmented mononuclear leukocytes, compared with 70%–100% in the former studies. The percentage of parasitized erythrocytes in the SMAC patients was also lower, with median values ranging from 1% to 5%. The discrepancies between the SMAC patient population and the patients included in the previous studies may reflect the fact that a proportion of the SMAC population had only moderately severe malaria.
In contrast to all previously published studies, this study was large enough to use mortality as an end point. There was significant heterogeneity between sites: higher proportions of patients had PM and PG in Lambarene and Libreville than in the other SMAC sites. However, despite site differences in the prognostic ability of pigments and several clinical manifestations across sites, conclusions were consistent in all 6 sites with regard to pigments. The crude ORs indicated an association between pigmented cells and disease severity in most sites, but the C statistics were low (close to 0.5), suggesting that presence of pigment was a weak prognostic factor. The associations disappeared when adjusted ORs were estimated in models that included more clinical and laboratory features. This large study highlights the limits of using circulating pigmented blood cells as a prognostic marker of malaria in individual patients across Africa.
Footnotes
Potential conflicts of interest: none reported.
Financial support: Institute of Allergy and Infectious Diseases, National Institutes of Health (grant AI45955). The funder had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, or approval of the manuscript.
C.V. had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author contributions: data collection, analysis, writing of manuscript (P.G.K.); analysis and interpretation of results, writing of manuscript, editing assistance, review of manuscript (C.V., D.W.); data collection, editing assistance, review of manuscript (M.A.M., C.O., S.I., M.K., C.R.N., T.A., M.P., K.B.); editing assistance, review of manuscript (S.K.); protocol development, data collection (L.B., S.M.); data collection, writing of manuscript, editing assistance, review of manuscript, study supervision, obtaining of funding (T.T.).
References
- 1.Poser CM, Bruyn GW. An illustrated history of malaria. Midsomer Norton, United Kingdom: Parthenon; 1999. [Google Scholar]
- 2.Tripathi AK, Garg SK, Tekwani BL. A physiochemical mechanism of hemozoin (beta-hematin) synthesis by malaria parasite. Biochem Biophys Res Commun. 2002;290:595–601. doi: 10.1006/bbrc.2001.6231. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzer E, Turrini F, Ulliers D, Giribaldi G, Ginsburg H, Arese P. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med. 1992;176:1033–41. doi: 10.1084/jem.176.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prada J, Malinowski J, Muller S, Bienzle U, Kremsner PG. Hemozoin differentially modulates the production of interleukin 6 and tumor necrosis factor in murine malaria. Eur Cytokine Netw. 1995;6:109–12. [PubMed] [Google Scholar]
- 5.Skorokhod OA, Alessio M, Mordmuller B, Arese P, Schwarzer E. Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: a peroxisome proliferator-activated receptor-gamma-mediated effect. J Immunol. 2004;173:4066–74. doi: 10.4049/jimmunol.173.6.4066. [DOI] [PubMed] [Google Scholar]
- 6.Giribaldi G, Ulliers D, Schwarzer E, Roberts I, Piacibello W, Arese P. Hemozoin- and 4-hydroxynonenal-mediated inhibition of erythropoiesis: possible role in malarial dyserythropoiesis and anemia. Haematologica. 2004;89:492–3. [PubMed] [Google Scholar]
- 7.Casals-Pascual C, Kai O, Cheung JO, et al. Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood. 2006;108:2569–77. doi: 10.1182/blood-2006-05-018697. [DOI] [PubMed] [Google Scholar]
- 8.Keller CC, Davenport GC, Dickman KR, et al. Suppression of prostaglandin E2 by malaria parasite products and antipyretics promotes overproduction of tumor necrosis factor-alpha: association with the pathogenesis of childhood malarial anemia. J Infect Dis. 2006;193:1384–93. doi: 10.1086/503047. [DOI] [PubMed] [Google Scholar]
- 9.Grobusch MP, Hanscheid T, Kramer B, et al. Sensitivity of hemozoin detection by automated flow cytometry in non- and semi-immune malaria patients. Cytometry B Clin Cytom. 2003;55:46–51. doi: 10.1002/cyto.b.10039. [DOI] [PubMed] [Google Scholar]
- 10.Metzger WG, Mordmuller BG, Kremsner PG. Malaria pigment in leucocytes. Trans R Soc Trop Med Hyg. 1995;89:637–8. doi: 10.1016/0035-9203(95)90423-9. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen PH, Day N, Pram TD, Ferguson DJ, White NJ. Intraleucocytic malaria pigment and prognosis in severe malaria. Trans R Soc Trop Med Hyg. 1995;89:200–4. doi: 10.1016/0035-9203(95)90496-4. [DOI] [PubMed] [Google Scholar]
- 12.Amodu OK, Adeyemo AA, Olumese PE, Gbadegesin RA. Intraleucocytic malaria pigment and clinical severity of malaria in children. Trans R Soc Trop Med Hyg. 1998;92:54–6. doi: 10.1016/s0035-9203(98)90952-x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor T, Olola C, Valim C, et al. Standardized data collection for multi-center clinical studies of severe malaria in African children: establishing the SMAC network. Trans R Soc Trop Med Hyg. 2006;100:615–22. doi: 10.1016/j.trstmh.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luty AJ, Perkins DJ, Lell B, et al. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect Immun. 2000;68:3909–15. doi: 10.1128/iai.68.7.3909-3915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyke KE, Diallo DA, Dicko A, et al. Association of intraleukocytic Plasmodium falciparum malaria pigment with disease severity, clinical manifestations, and prognosis in severe malaria. Am J Trop Med Hyg. 2003;69:253–9. [PubMed] [Google Scholar]
- 16.Olola CH, Missinou MA, Issifou S, et al. Medical informatics in medical research—the Severe Malaria in African Children (SMAC) Network's experience. Methods Inf Med. 2006;45:483–91. [PubMed] [Google Scholar]
- 17.Lell B, Missinou MA, Issifou S, et al. Assessment of a simplified method for counting leukocytic malaria pigment. Am J Trop Med Hyg. 2005;73:588–92. [PubMed] [Google Scholar]
- 18.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–59. [PubMed] [Google Scholar]
- 19.Newton C, Valim C, Krishna S, et al. The prognostic value of measures of acid/base balance in pediatric falciparum malaria, compared with other clinical and laboratory parameters. Clin Infect Dis. 2005;41:948–57. doi: 10.1086/432941. [DOI] [PMC free article] [PubMed] [Google Scholar]