Abstract
Purpose.
To determine the effect of progressive addition lenses (PALs) and single vision lenses (SVLs) on peripheral defocus in myopic children, and to compare the effect of myopic versus hyperopic peripheral defocus on foveal myopia progression.
Methods.
Eighty-four myopic children aged 6 to 11 years with spherical equivalent (SE) cycloplegic autorefraction between −0.75 diopters (D) and −4.50 D were randomly assigned to wear SVLs or PALs. Aberrometry measurements of the eye and spectacles were made centrally, 30° nasally, temporally, and superiorly, and 20° inferiorly on the retina using a Complete Ophthalmic Analysis System for Vision Research (COAS-VR). The association between peripheral defocus and the 1-year change in central myopia was investigated.
Results.
SVLs caused a hyperopic shift in peripheral defocus at all locations (all P ≤ 0.0003). PALs caused a myopic shift in peripheral defocus in three of four locations measured (all P ≤ 0.01) with the greatest shift superiorly due to the PAL addition (−1.04 ± 0.30 D). Superior retinal defocus when wearing either SVLs or PALs was associated with the 1-year change in central myopia. The adjusted 1-year change in central SE myopia was −0.38 D for children with absolute superior myopic defocus (n = 67) and −0.65 D for children with absolute superior hyperopic defocus (n = 17; difference = 0.27 D; P = 0.002).
Conclusions.
PALs caused a myopic shift in peripheral defocus. Superior myopic defocus was associated with less central myopia progression. These data support the continued investigation of optical designs that result in peripheral myopic defocus as a potential way to slow myopia progression. (ClinicalTrials.gov number, NCT00335049.)
Keywords: children, progressive addition lenses, single vision lenses, myopia progression, peripheral defocus
Peripheral defocus has been hypothesized to influence myopia progression in children. We describe peripheral defocus in children with single vision and progressive addition lenses and report on an association between peripheral myopic defocus and slower myopia progression.
Introduction
Clinical trials evaluating the efficacy of progressive addition lenses (PALs) in slowing the progression of myopia in children have generally found statistically significant, but clinically modest reductions in myopia progression.1–5 The mechanism responsible for the PAL treatment effect is not well understood. Hyperopic retinal blur due to a high lag of accommodation during near work is one proposed cause of juvenile-onset myopia progression,6–8 and decreasing hyperopic blur during near work by reducing accommodative lag is one rationale for fitting PALs. While some clinical trials have reported that myopic children with a large lag of accommodation had a greater treatment effect when wearing PALs,3,9 recent clinical trials that restricted enrollment and randomization to myopic children with high accommodative lag did not find clinically meaningful PAL treatment effects.1,5 Studies examining the relationship between accommodative lag and myopia progression have had mixed results with several studies in children finding no association5,10,11 and studies in young adults finding either positive or negative associations.12,13
Hyperopic defocus on the peripheral retina has also been proposed as a risk factor for myopia in humans.14 Uncorrected myopic eyes generally exhibit hyperopic relative peripheral refraction (RPR) in the horizontal ocular meridian, while uncorrected hyperopic eyes typically exhibit myopic RPR.15–22 Experiments in chicks23 and rhesus monkeys24 have provided convincing evidence that large changes in peripheral defocus influence axial eye growth and emmetropization in animal models. Though studies measuring uncorrected peripheral refractive error in myopic children have not found evidence of a meaningful association between relative peripheral refraction and myopia onset or progression,25,26 studies of spectacles or contact lenses that result in large changes in retinal defocus have reported changes in myopia progression. Orthokeratology has been reported to decrease axial elongation in myopic children,27–32 and the myopic shift in peripheral retinal defocus caused by orthokeratology33,34 has been hypothesized to cause the reduction in axial growth. Short term studies have reported that soft bifocal contact lens designs can slow myopia progression,35,36 though long term clinical trial results are not available.
The Study of Theories about Myopia Progression (STAMP) was a 2-year, double-masked randomized clinical trial designed to evaluate two theories of myopia. The first theory hypothesizes that hyperopic retinal blur caused by a high lag of accommodation during near work accelerates axial elongation, and the second theory hypothesizes that mechanical tension created by the crystalline lens or ciliary body restricts equatorial ocular expansion, thereby causing accelerated axial elongation.5,37 Myopic children 6 to 11 years of age with −0.75 diopters (D) to −4.50 D of myopia in each meridian of each eye were randomly assigned to wear single vision lenses (SVLs) or PALs for one year, and all children wore SVLs a second year to evaluate treatment effect permanence. All children had a high accommodative lag and also had near esophoria if their spherical equivalent refractive error was more myopic than −2.25 D.
A small, but statistically significant PAL treatment effect of 0.18 D was found after one year in STAMP, and there was no loss of this treatment effect during the second year of the study when all children wore SVLs.5 Not finding a rebound effect (i.e., loss of the treatment effect) after ceasing PAL wear supports hyperopic defocus-based theories of myopia progression; however, the lack of an association between accommodative lag and myopia progression in STAMP is inconsistent with the small effect being due to reduced foveal blur during near work.5 In addition to the primary outcome of central refractive error, peripheral refractive data were also collected in STAMP to evaluate peripheral defocus and myopia progression.
SVLs typically worn by myopic children38 and adults39 can increase peripheral hyperopic defocus. Because PALs include a near addition, a peripheral myopic shift in defocus is expected in the superior retinal quadrant compared with SVLs when a child looks in primary gaze. If peripheral defocus influences eye growth, differences in the peripheral defocus profile when myopic children wear SVLs versus PALs could be an alternate explanation of the previously reported reductions in myopia progression with PALs. The purpose of this analysis was to determine the effect of both SVLs and PALs on peripheral defocus in myopic children and whether peripheral defocus was associated with myopia progression.
Methods
The study protocol was approved by the Biomedical Sciences institutional review board at The Ohio State University, and the study was conducted in accordance with the tenets of the Declaration of Helsinki. Parents provided written informed consent, and children provided verbal assent.
Data from the 84 children who completed the first year of STAMP are included in these analyses.5 Full details of the overall study design and rationale have been previously published37; the pertinent details are summarized here. The primary outcome in STAMP was the annual change in central spherical equivalent refractive error measured by cycloplegic autorefraction (Grand Seiko WV-500 autorefractor; Grand Seiko Co., Hiroshima, Japan).40,41 Ten measurements of the right eye were made annually, and were averaged using the power vector method described by Thibos et al.42 Measurements were made 30 minutes after instilling the first of two drops of 1% tropicamide, which were separated by 5 minutes. At baseline, children had between −0.75 D and −4.50 D of myopia in each meridian of each eye as determined by cycloplegic autorefraction. Children were randomly assigned to wear either SVLs or PALs (Varilux Ellipse [Essilor of America, Inc., Dallas, TX], a short-corridor design with a +2.00-D add) made of polycarbonate material. The child's spectacle prescription was determined using a standardized most plus (least minus) subjective refraction that yielded the best visual acuity. PALs were fitted at least 2 mm higher than normal while maintaining adequate lens area for clear distance vision and ensuring that the full near power zone was available for near viewing.
Aberrometry was performed on the child's uncorrected right eye under cycloplegia using a validated open-field aberrometer (Complete Ophthalmic Analysis System for Vision Research [COAS-VR]; AMO Wavefront Sciences; Albuquerque, NM).43,44 Nine measurements each were made centrally (line of sight) and at four peripheral retinal locations: 30° nasally, 30° temporally, 30° superiorly, and 20° inferiorly from the line of sight. To ensure accurate fixation in the presence of uncorrected refractive error, children fixated on luminous spot targets. Children turned their heads to view the nasal and temporal targets and turned their eyes to view the superior and inferior targets.45
Aberrometry measurements of the child's assigned right spectacle lens were also made with the spectacles mounted in front of a model eye. Measurement locations through the spectacle lens corresponded to the central and peripheral measurement locations of the child's uncorrected right eye (i.e., along the line of sight and 30° nasally, 30° temporally, 30° superiorly, and 20° inferiorly from the line of sight) while utilizing a standardized central vertex distance of 13 mm. Measurements of the model eye alone were made and subtracted from the combined spectacle and model eye measurements to obtain measurements of the spectacles alone. Because the pupil center in spectacle frames is generally placed above the geometric center of the frame, many spectacle frames are not fitted such that the spectacle lens extends 30° above the line of sight when in primary gaze. For this reason, all inferior retinal measurements of the uncorrected eye and the spectacle lens were made at 20° rather than 30° so that corresponding spectacle measurements were always available. The pupil center of the right spectacle lens was dotted as the child looked across the room in primary gaze after putting on the study spectacles. After removing the spectacles, the four peripheral lens locations were calculated and dotted relative to the pupil center. With the spectacles mounted in front of the model eye, the spectacles were rotated to the appropriate measurement orientations using a goniometer. The lens dots were used to ensure accurate alignment with the aberrometer at each location and were removed prior to making a measurement.
The COAS refractive error data at each ocular and spectacle location were referenced to the corneal plane and converted to power vectors (M, J0, and J45).42 The COAS default method of determining refractive error (non-Seidel) and a 2-mm analysis diameter was used because RPR data analyzed by this method using the COAS have been previously validated against RPR measurements made with the Grand Seiko autorefractor,46 which also uses a circular measurement beam of approximately the same diameter.40 Each aberrometry spot pattern was visually inspected to ensure that it was not distorted or compromised due to a blink. RPR of the uncorrected eye at each peripheral location was calculated by taking the difference between the mean spherical equivalent at the peripheral location and the mean central spherical equivalent. Spectacle relative peripheral defocus (RPD) at each peripheral location (i.e., the change in the eye's RPD caused by wearing the spectacle lens) was calculated by subtracting the central spherical equivalent defocus of the lens from the peripheral spherical equivalent defocus of the lens at each location. The ocular and spectacle lens measurements at each location were combined to obtain the eye's peripheral defocus (M) and RPD through the spectacle lens in primary gaze.
Analyses were performed using statistical software (STATA 12.1; StataCorp, College Station, TX; and SAS 9.3; SAS Institute, Inc., Cary, NC). Repeated-measures ANOVA were used to determine whether RPR of the uncorrected eye, the RPD experienced when wearing spectacles, and astigmatism differed by retinal location or lens type (SVL or PAL). When analyzing spectacle RPD, the central spherical equivalent power of the spectacle lens was included as a covariate to determine whether the power of the lens was associated with the change in RPD caused by the spectacle lens. When appropriate, post hoc t-test comparisons were performed using the method described by Tukey and the appropriate mean square error from the model. T-tests were used to determine whether RPR or RPD at a specific peripheral location was significantly different than zero. Statistical significance at the alpha less than 0.05 level was determined using P values adjusted for multiple comparisons using the method described by Benjamini and Hochberg.47
Multiple linear regression was used to model the 1-year change in spherical equivalent refractive error. Potential covariates included baseline RPR, factors found to have a significant association with myopia progression (baseline age, sex, and ethnicity), treatment group, and baseline refractive error. Because myopic peripheral defocus is hypothesized to slow myopia progression and hyperopic defocus to increase myopia progression, peripheral retinal defocus at each location while wearing spectacles was added to the model as a dichotomous variable (hyperopic versus myopic peripheral defocus).
Results
The mean (±SD) age of the 84 children was 9.9 ± 1.3 years, and 44 children (52%) were female. The mean (±SD) cycloplegic spherical equivalent refractive error of the children was −1.96 ± 0.78 D (range: −0.83 to −4.02 D). Forty-three children were assigned to wear SVLs and 41 to PALs. Because non-Hispanic, white children made up the majority of the children enrolled (66%), children were grouped by whether they were a non-Hispanic, white child when evaluating ethnicity in statistical models.5
RPR (Uncorrected Eye)
Refractive error (M, J0, and J45) and RPR by retinal location at baseline are shown in Table 1. There was no difference in RPR between the SVL and PAL groups (P = 0.98). RPR did differ by retinal location (P < 0.0001); however, these differences did not depend on whether the child was assigned to the SVL or PAL group (location by lens type interaction; P = 0.78). Because RPR did not differ by spectacle group, RPR at each location was averaged across groups when determining whether RPR was significantly different than zero at each retinal location.
Table 1. .
Mean (±SD) M, J0, J45, and Relative Peripheral Refraction (RPR) of the Uncorrected Eye at Baseline by Retinal Location for Children Assigned to the SVL and PAL Groups
|
Uncorrected Eye |
Retinal Location |
||||
|
Horizontal Meridian |
Central |
Vertical Meridian |
|||
|
30° Temporal |
30° Nasal |
30° Superior |
20° Inferior |
||
| M, D | |||||
| SVL group | −1.72 ± 1.02 | −1.81 ± 0.94 | −2.36 ± 0.97 | −2.76 ± 1.15 | −2.79 ± 1.02 |
| PAL group | −1.54 ± 1.08 | −1.60 ± 0.83 | −2.15 ± 0.68 | −2.46 ± 1.13 | −2.67 ± 1.07 |
| J0, D | |||||
| SVL group | −0.97 ± 0.39 | −0.46 ± 0.34 | +0.08 ± 0.25 | +1.09 ± 0.40 | +0.48 ± 0.29 |
| PAL group | −1.04 ± 0.37 | −0.45 ± 0.33 | +0.04 ± 0.23 | +1.04 ± 0.38 | +0.53 ± 0.45 |
| J45, D | |||||
| SVL group | +0.05 ± 0.29 | −0.10 ± 0.16 | +0.04 ± 0.16 | +0.39 ± 0.25 | −0.26 ± 0.32 |
| PAL group | −0.07 ± 0.31 | −0.07 ± 0.24 | +0.02 ± 0.16 | +0.42 ± 0.24 | −0.32 ± 0.32 |
| RPR, D* | |||||
| SVL group | +0.64 ± 0.74 | +0.55 ± 0.60 | – | −0.40 ± 0.92 | −0.42 ± 0.80 |
| PAL group | +0.61 ± 0.78 | +0.55 ± 0.58 | – | −0.30 ± 0.94 | −0.51 ± 0.90 |
After averaging across spectacle groups, RPR at all four retinal locations were significantly different than zero at the alpha < 0.05 level using the Benjamini-Hochberg method to correct for multiple comparisons. Values for each location were averaged across group because differences in RPR by retinal location did not depend on the spectacle group (P = 0.79).
There was a significant difference between RPR measured in the horizontal meridian of the eye versus the vertical meridian of the eye (i.e., there was a horizontal/vertical asymmetry in RPR). The mean RPR at each peripheral location was significantly different than zero (all P ≤ 0.0007). In the horizontal meridian of the eye, the mean (±SD) RPR was hyperopic (nasal retina = +0.56 ± 0.59 D; temporal retina = +0.63 ± 0.76 D) while in the vertical meridian, the mean (±SD) RPR was myopic (superior retina = −0.36 ± 0.93 D; inferior retina = −0.48 ± 0.84 D).
RPD When Wearing Spectacles
The refractive error and RPD of the eyes in primary gaze when wearing the assigned spectacles (SVLs or PALs) are shown in Table 2. Differences in RPD by retinal location depended on spectacle lens type (i.e., location by spectacle type interaction; P < 0.0001). For children wearing SVLs, RPD in the horizontal meridian of the eye was more hyperopic than RPD in the vertical meridian (all P < 0.05; Tukey). RPD values in the horizontal meridian of SVL-wearing eyes were significantly more hyperopic than zero (both P < 0.0001).
Table 2. .
Mean (±SD) Aberrometry-Based M, J0, J45, and Relative Peripheral Defocus (RPD) by Retinal Location for Children Assigned to the SVL and PAL Groups While Wearing Their Assigned Spectacles During the First Year of the Study
|
Corrected Eye |
Retinal Location |
||||
|
Horizontal Meridian |
Central |
Vertical Meridian |
|||
|
30° Temporal |
30° Nasal |
30° Superior |
20° Inferior |
||
| M, D | |||||
| SVL group | +0.46 ± 0.80 | +0.32 ± 0.65 | −0.32 ± 0.29 | −0.38 ± 0.97 | −0.53 ± 0.87 |
| PAL group | −0.08 ± 0.87 | −0.35 ± 0.63 | −0.61 ± 0.42 | −1.95 ± 1.03 | −0.62 ± 0.87 |
| J0, D | |||||
| SVL group | −0.83 ± 0.37 | −0.37 ± 0.34 | +0.11 ± 0.18 | +0.90 ± 0.42 | +0.38 ± 0.32 |
| PAL group | −0.81 ± 0.36 | 0.00 ± 0.36 | +0.15 ± 0.19 | +1.35 ± 0.38 | +0.38 ± 0.45 |
| J45, D | |||||
| SVL group | +0.09 ± 0.25 | −0.09 ± 0.18 | +0.05 ± 0.12 | +0.37 ± 0.25 | −0.20 ± 0.30 |
| PAL group | +0.61 ± 0.42 | −0.50 ± 0.31 | −0.06 ± 0.17 | +0.07 ± 0.27 | −0.26 ± 0.30 |
| RPD, D | |||||
| SVL group | +0.78 ± 0.76* | +0.64 ± 0.66* | – | −0.07 ± 0.98 | −0.22 ± 0.84 |
| PAL group | +0.53 ± 0.82* | +0.26 ± 0.58* | – | −1.35 ± 0.97* | −0.01 ± 0.99 |
For relative peripheral defocus values, the value is significantly different than zero at the alpha < 0.05 level after correcting for multiple comparisons using the Benjamini-Hochberg method.
As expected, superior retinal RPD with PALs (mean ± SD = −1.35 ± 0.97 D) was more myopic than with SVLs (−0.07 ± 0.98 D) due to the inferior near addition of the PALs (P < 0.05; Tukey). On average, RPD at the nasal retinal location was also relatively more myopic (less hyperopic) with PALs than SVLs (P < 0.05; Tukey). No significant difference between groups was found at the other peripheral locations. While the superior RPD of PAL-wearing children was myopic (P < 0.0001), the RPD measured in the horizontal meridian of PAL-wearing eyes was hyperopic (both P ≤ 0.01).
Change in RPD Caused by SVLs and PALs
The spectacle power measured at each lens location (M, J0, and J45) and spectacle RPD (the change in RPD caused by the spectacles) are shown in Table 3. Although there was not a significant difference in spherical equivalent refractive error between children assigned to the two groups (P = 0.26), the mean central lens power measured in the PAL group (−1.55 D) was significantly less myopic than the SVL group (−2.05 D; P = 0.007). The less minus central power measured in PALs is consistent with the PALs being fitted higher than normal to encourage use of the PAL addition.
Table 3. .
Mean (±SD) Aberrometry-Based Spectacle Power (M, J0, J45) by Lens Location and the Spectacle Relative Peripheral Defocus (i.e., the Change in Relative Peripheral Defocus [RPD] Due to the Spectacles) When SVLs and PALs Were Worn During the First Year of the Study.
|
Spectacle Power, D |
Retinal Location Affected by Spectacles |
||||
|
Horizontal Meridian |
Central |
Vertical Meridian |
|||
|
30° Temporal, Nasal Lens |
30° Nasal, Temporal Lens |
30° Superior, Inferior Lens |
20° Inferior, Superior Lens |
||
| M | |||||
| SVLs | −2.18 ± 1.00 | −2.13 ± 0.97 | −2.05 ± 0.94 | −2.38 ± 1.06 | −2.25 ± 1.00 |
| PALs | −1.46 ± 0.74 | −1.25 ± 0.71 | −1.55 ± 0.70 | −0.50 ± 0.78 | −2.05 ± 0.77 |
| J0 | |||||
| SVLs | −0.14 ± 0.17 | −0.09 ± 0.16 | −0.03 ± 0.18 | +0.19 ± 0.20 | +0.10 ± 0.21 |
| PALs | −0.23 ± 0.22 | −0.45 ± 0.25 | −0.11 ± 0.20 | −0.30 ± 0.21 | +0.15 ± 0.20 |
| J45 | |||||
| SVLs | −0.04 ± 0.13 | −0.01 ± 0.13 | −0.01 ± 0.11 | +0.02 ± 0.14 | −0.06 ± 0.12 |
| PALs | −0.68 ± 0.21 | +0.43 ± 0.17 | +0.08 ± 0.14 | +0.35 ± 0.18 | −0.06 ± 0.14 |
| Spectacle RPD (change due to spectacles) | |||||
| SVLs | +0.14 ± 0.14* | +0.08 ± 0.14* | – | +0.33 ± 0.20* | +0.20 ± 0.20* |
| PALs | −0.08 ± 0.21* | −0.30 ± 0.15* | – | −1.04 ± 0.30* | +0.50 ± 0.31* |
For spectacle relative peripheral defocus change values, the value is significantly different than zero at the alpha < 0.05 level after correcting for multiple comparisons using the Benjamini-Hochberg method.
The changes in RPD caused by each type of spectacle lens (SVL or PAL) depended on the location measured (P < 0.0001; location by lens type interaction). On average, SVLs caused a statistically significant hyperopic increase in RPD at all locations, though the increase was clinically small in some locations (all P ≤ 0.0003). PALs caused a statistically significant myopic shift in RPD in three of the four locations (all P ≤ 0.01): superior retina (−1.04 D), nasal retina (−0.30 D), and temporal retina (−0.08 D). Inferiorly, PALs caused a significant hyperopic shift in RPD of 0.50 D (P < 0.0001).
The difference between the shift in RPD caused by PALs versus SVLs is shown in Figure 1 and was statistically significant at each location (all P < 0.05; Tukey). Compared with SVLs, PALs caused a myopic shift in RPD in the superior, nasal, and temporal retinal locations. PALs caused a hyperopic RPD shift compared with SVLs in the inferior retinal location. The largest myopic shift in RPD between PALs and SVLs was at the superior retinal location (mean ± SE = −1.37 ± 0.06 D) due to the near addition on the inferior portion of PALs.
Figure 1. .
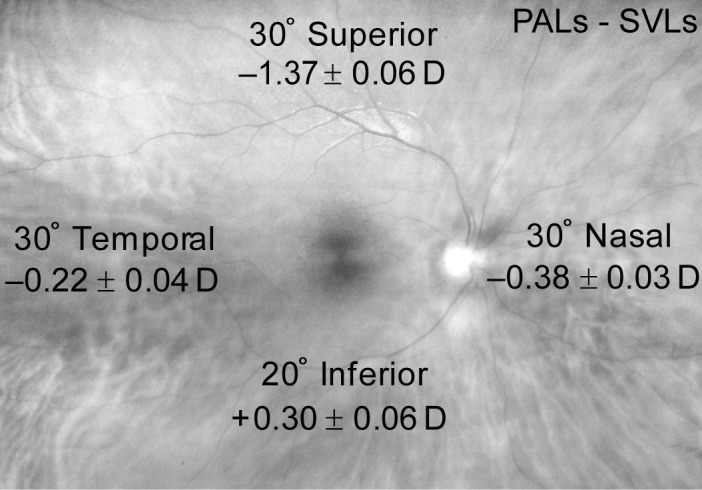
The difference in spectacle RPD (i.e., the change in the eye's RPD caused by the spectacle lens) between PALs and SVLs by retinal location in Year 1 of the study. Values are mean ± SE.
The prescribed spherical equivalent power of the spectacle lens was associated with the change in RPD caused by the lens. Spectacle lenses with more minus power were associated with a relatively greater hyperopic shift in RPD (P < 0.001). This association did not depend on lens type (SVL or PAL) or lens location (i.e., there were no significant two-way or three-way interactions with lens power; all P ≥ 0.39). For every diopter of spherical equivalent minus power in the spectacle lens, the shift in RPD caused by the lens was 0.07 D more hyperopic (95% confidence interval [CI]: 0.03 to 0.10 D). Although PALs resulted in a myopic shift in RPD in multiple lens locations compared with SVLs, the magnitude of the peripheral myopic shift decreased slightly (i.e., was relatively more hyperopic) as the spectacle lens increased in minus power.
Peripheral Astigmatism (J0 and J45)
The mean amounts of J0 and J45 in the uncorrected eye did not differ between the SVL and PAL group (both P > 0.36; Table 1). As expected, the magnitude of J0 was greater in all off-axis locations than centrally (all P > 0.05; Tukey).
Because the sign of the astigmatism component (J0 or J45) indicates the orientation of the astigmatism (e.g., 90° vs. 180° for J0), the absolute value of J0 and J45 measured in the spectacle lenses was examined to evaluate differences in the magnitude of astigmatism induced by each lens type. The change in J0 and J45 caused by the spectacle lens at each location differed by lens type (P < 0.0001; Table 3). The magnitude of J0 and J45 in SVLs did not differ across the lens locations (all P > 0.05; Tukey). PALs induced significantly more J45 astigmatism in the horizontal meridian than SVLs (all P < 0.05; Tukey), which was expected because these locations on the PAL are outside the distance and near viewing areas of the lens.
Peripheral Defocus and Myopia Progression
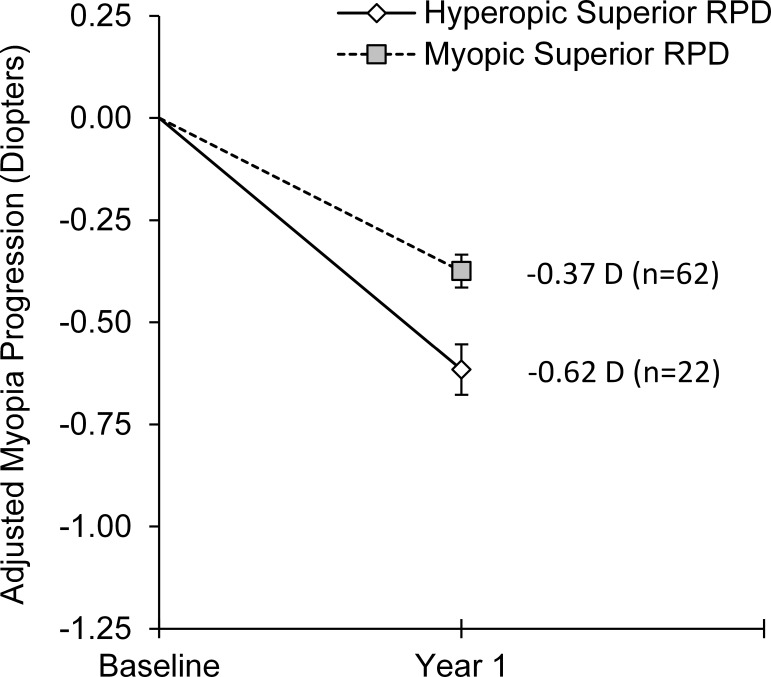
During year one of the study when children were randomly assigned to wear either PALs or SVLs, there was no association between nasal, temporal, or inferior retinal RPD and the 1-year change in central spherical equivalent refractive error (all P ≥ 0.58; Table 4); however, superior retinal RPD was significantly associated with the 1-year change in central refractive error (P = 0.001). No interaction was found between treatment group and superior RPD (P = 0.35) or RPD at any other location (all P ≥ 0.13), which suggests that the effect of peripheral defocus was consistent across all children regardless of treatment group assignment. We therefore assessed the 1-year change in refractive error as a function of peripheral defocus for the sample as a whole. Although only half of the children wore PALs, nearly half of the SVL-wearing children had myopic superior RPD such that 74% of the children experienced superior myopic RPD with their spectacles. Children with myopic superior RPD had 0.24 D less central myopia progression after 1 year than children with hyperopic superior RPD (P = 0.001; 95% CI = 0.10 to 0.39 D; Fig. 2). The 1-year change in refractive error was not associated with baseline RPR superiorly (P = 0.25) or any other peripheral location (all P ≥ 0.14), providing evidence that the reduction in myopia progression associated with superior myopic defocus when wearing spectacles was not due to some aspect of baseline eye shape.
Table 4. .
Adjusted 1-Year Difference in Central Myopia Progression (D) by Type of Peripheral Defocus (Myopic vs. Hyperopic) at Each Peripheral Location When Wearing Either SVLs or PALs During Year 1 of the Study
| Study Year 1 (SVLs or PALs) |
Type of Peripheral Defocus, Number of Children |
Progression Difference Between Groups,* Myopic–Hyperopic |
|||
|
Retinal Location |
Hyperopic |
Myopic |
Mean ± SE |
P
Value |
95% CI for Difference |
| Relative peripheral defocus (RPD) | |||||
| 30° Superior | 22 | 62 | 0.24 ± 0.07 | 0.001 | 0.10 to 0.39 D |
| 20° Inferior | 40 | 44 | −0.01 ± 0.07 | 0.91 | −0.15 to 0.13 D |
| 30° Nasal | 63 | 21 | −0.04 ± 0.08 | 0.58 | −0.20 to 0.11 D |
| 30° Temporal | 71 | 13 | −0.02 ± 0.09 | 0.84 | −0.21 to 0.17 D |
| Absolute peripheral defocus (as measured by aberrometer) | |||||
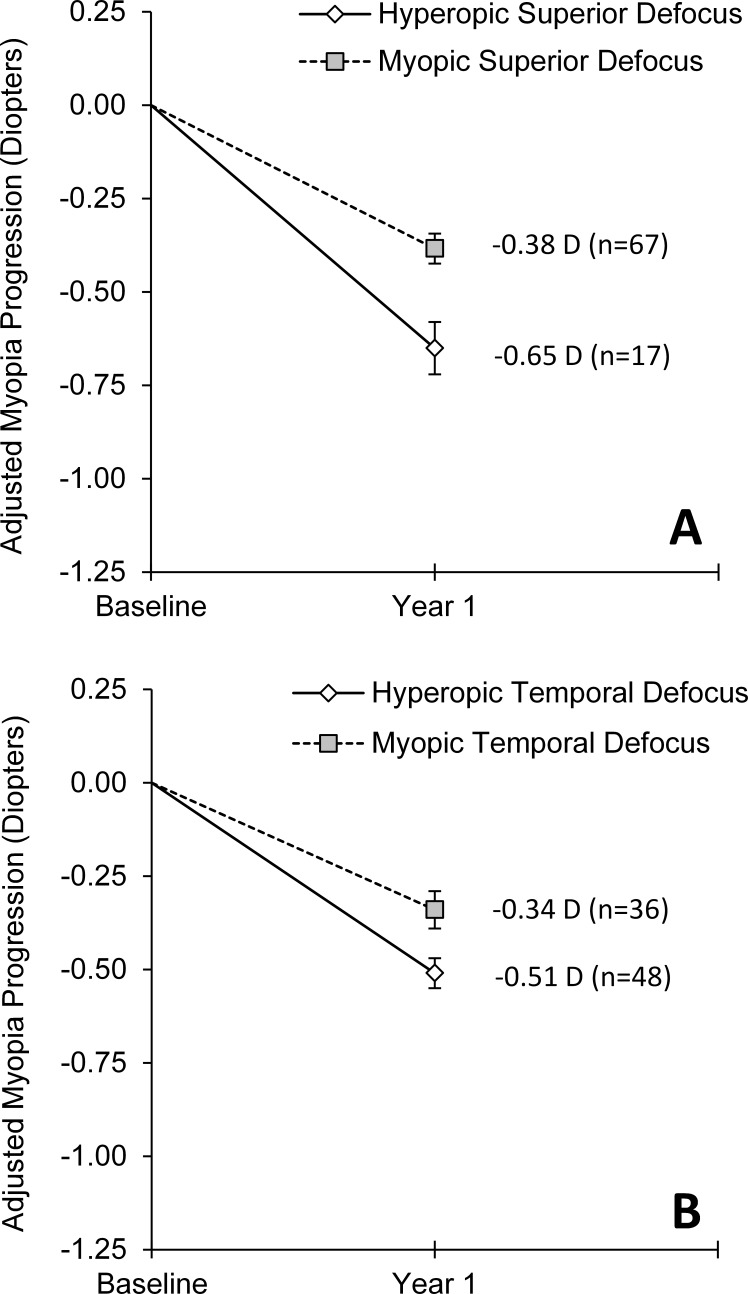
| 30° Superior | 17 | 67 | 0.27 ± 0.08 | 0.002 | 0.11 to 0.43 D |
| 20° Inferior | 21 | 63 | 0.08 ± 0.08 | 0.34 | −0.09 to 0.25 D |
| 30° Nasal | 40 | 44 | 0.09 ± 0.07 | 0.17 | −0.04 to 0.22 D |
| 30° Temporal | 48 | 36 | 0.18 ± 0.07 | 0.01 | 0.04 to 0.31 D |
All differences adjusted for baseline refractive error, age, sex, and ethnicity. Positive difference indicates slower myopia progression in children with myopic peripheral defocus compared to children with hyperopic peripheral defocus.
Figure 2. .
Mean 1-year change in central spherical equivalent refractive error for children with hyperopic RPD on the superior retina and children with myopic RPD on the superior retina during the first study year when children wore either SVLs or PALs. Annual progression is adjusted for baseline refractive error, baseline age, sex, and ethnicity. Error bars represent SE.
Using absolute peripheral defocus during year one (i.e., the amount of defocus as measured by the aberrometer as opposed to relative defocus), both superior and temporal retinal defocus were significantly associated with the 1-year change in central refractive error (both P ≤ 0.01; Table 4). There was again no interaction found between treatment group and absolute superior defocus (P = 0.62), temporal defocus (P = 0.53), or peripheral defocus nasally or inferiorly (both P ≥ 0.16). Eighty percent of children experienced myopic superior retinal defocus and had significantly less central myopia progression compared with children with hyperopic superior retinal defocus (0.27 D; P = 0.002; 95% CI = 0.11 to 0.43 D; Fig. 3A). Myopic temporal retinal defocus (experienced by 43% of children) was also associated with significantly less central myopia progression than hyperopic temporal retinal defocus by 0.18 D (P = 0.01; 95% CI = 0.04 to 0.31 D; Fig. 3B). There was not a significant association between nasal or inferior retinal defocus and the change in central refractive error (both P ≥ 0.17).
Figure 3. .
Mean 1-year change in central spherical equivalent refractive error during the first study year (wearing either SVLs or PALs) for children with absolute peripheral defocus (as measured by the aberrometer) that was hyperopic versus myopic on the (A) superior retina and (B) temporal retina. Annual progression is adjusted for baseline refractive error, baseline age, sex, and ethnicity. Error bars represent SE.
Because myopic superior defocus was consistently associated with slower myopia progression than hyperopic superior defocus during year one of the study when children wore either SVLs or PALs, we evaluated whether the amount of myopic superior defocus was associated with myopia progression in a dose-dependent manner. When limiting analyses to the 62 children with myopic superior RPD, there was no association between the amount of superior myopic RPD and the 1-year change in myopia (P = 0.67). Likewise, when analyzing only the 67 children with myopic superior absolute defocus, no association was found between the amount of superior myopic defocus and the 1-year change in myopia (P = 0.27). While we found that myopic superior peripheral defocus inhibited the progression of myopia, we did not find evidence that myopic peripheral defocus slowed progression in a dose-dependent manner.
We also examined whether superior defocus was significantly associated with the 1-year change in central spherical equivalent refractive error during year two of the study when all children wore SVLs. PALs caused a significant amount of superior myopic defocus in year one. Removal of the PAL in year two caused a significant superior peripheral hyperopic shift. When all children wore SVLs in year two, neither the mean (±SD) amount of superior RPD (+0.19 ± 1.00 D), nor superior absolute defocus (−0.16 ± 1.00 D) were statistically different than zero. However, roughly half of the children still experienced some amount of superior myopic defocus, though a much smaller amount than with PALs, because superior relative peripheral myopia is often found in myopic children. Of the 82 children available for analysis in year two of the study, 36 children (44%) had myopic superior RPD and progressed (mean ± SE) −0.31 ± 0.06 D compared with those with hyperopic superior RPD (−0.44 ± 0.06 D), a mean difference in progression between the myopic and hyperopic group of 0.12 D that was not statistically significant (P = 0.16; Table 5). When considering absolute superior defocus, 41 children (50%) had myopic superior defocus and progressed −0.34 ± 0.06 D compared with those with hyperopic superior defocus (−0.43 ± 0.06), a difference in progression of 0.09 D that again was not statistically significant (P = 0.30; Table 5).
Table 5. .
Adjusted 1-Year Difference in Central Myopia Progression (D) by Type of Peripheral Defocus (Myopic Versus Hyperopic) on the Superior Retina During Year 2 of the Study When All Children Wore SVLs
|
Study Year 2 (All Wore SVLs)
Retinal Location |
Type of Peripheral Defocus, Number of Children |
Progression Difference Between Groups,* Myopic–Hyperopic |
|||
|
Hyperopic |
Myopic |
Mean ± SE |
P
Value |
95% CI for Difference |
|
| Relative peripheral defocus (RPD) | |||||
| 30° Superior | 46 | 36 | 0.12 ± 0.09 | 0.16 | −0.05 to 0.30 D |
| Absolute peripheral defocus (as measured by aberrometer) | |||||
| 30° Superior | 41 | 41 | 0.09 ± 0.09 | 0.30 | −0.08 to 0.26 D |
Differences adjusted for baseline refractive error, age, sex, and ethnicity. Positive difference indicates slower myopia progression in children with myopic peripheral defocus compared to children with hyperopic peripheral defocus.
Discussion
PALs result in a small, statistically significant reduction in myopia progression in children.1–5 The rationale for fitting myopic children with bifocal spectacles or PALs has traditionally been to reduce hyperopic blur during near work by decreasing accommodative lag.6–8 Based on more recent work in primate animal models,24,48 it has been hypothesized that peripheral hyperopic blur in the human eye results in accelerated axial elongation and that optical treatments that decrease or eliminate hyperopic retinal defocus slow myopia progression in children. Multiple optical treatments that manipulate peripheral defocus are currently being investigated including novel spectacle lens designs,49 orthokeratology lenses,27,28,30 and various bifocal and novel soft contact lens designs.35,36,50 We compared the effect of SVLs and PALs on peripheral defocus when myopic children wear their glasses, and we determined the change in RPD attributable to each type of spectacle lens. We also examined the effect of peripheral defocus on the 1-year progression of myopia.
Effect of Spectacle Lenses on Peripheral Defocus
The uncorrected myopic eyes in our study had relative peripheral hyperopia in the horizontal meridian and relative peripheral myopia in the vertical meridian.37 Hyperopic RPR in the horizontal meridian of uncorrected myopic eyes is consistent with previous studies.15,16,18,20 Though some studies have reported more hyperopic RPR 30° in the periphery in adults than we found in this study,39,51 our mean amount of myopia was lower. Although we found that vertical RPR was myopic, recent studies have reported both myopic and hyperopic RPR in the vertical meridian of myopic eyes.15,21,52,53 Though differences in measurement methods exist between the studies, the disparity between study results suggests that the vertical meridian RPR among myopic eyes is variable.
Previous studies have reported that SVLs cause a hyperopic shift in RPD in the horizontal meridian of the eye, usually when moderate amounts of myopia were being corrected.38,39,51 In the present study, we found that SVLs caused a hyperopic shift in RPD in both the horizontal and vertical meridians with the largest changes in the vertical meridian. Compared with SVLs, the shift in RPD caused by PALs was more myopic in three of the four peripheral locations with the largest myopic shift in RPD corresponding to the PAL add corridor. We also found a significant association between the shift in RPD caused by spectacle lenses and the amount of central myopia being corrected. Regardless of lens type, lenses that corrected higher amounts of central myopia resulted in a more hyperopic RPD shift than lower powered lenses of the same type. Putting this result into context for each lens type, a −4.00 D PAL resulted in a smaller myopic shift in RPD than a −1.00 D PAL, and a −4.00 D SVL caused a more hyperopic shift in RPD than a −1.00 D SVL. This result is consistent with a previous report that SVLs correcting higher amounts of myopia resulted in a greater hyperopic shift in RPD than SVLs correcting lower amounts of myopia.38 If greater amounts of peripheral hyperopic defocus result in more rapid myopia progression,36,49 this finding has implications for both standard SVL and specialty spectacle lens design. It is not advantageous for lenses to create more peripheral hyperopic defocus when a practitioner increases lens power to compensate for increased myopic refractive error.
Peripheral Defocus and Myopia Progression
The peripheral myopic shift in RPD caused by PALs could provide an alternate explanation for the previously reported small decreases in myopia progression with PALs.1–5 Although only half of the children in the current study wore PALs during year one, roughly 75% of children experienced some amount of superior myopic defocus when wearing their assigned correction due to variability in peripheral defocus in the vertical meridian of the uncorrected eye. Children with superior myopic defocus had significantly less central myopia progression than children with superior hyperopic defocus. Although PALs resulted in a large myopic shift in superior peripheral defocus, the change at the other peripheral locations was much smaller (<0.50 D). The lack of an association when analyzing nasal and inferior defocus and the inconsistent result temporally when analyzing RPD and absolute peripheral defocus in year one may indicate that larger doses of defocus above a threshold value are needed to have an appreciable effect on human myopia progression. Because the superior retina is where the greatest range of defocus values were measured due to the PAL addition, this finding provides support for the continued investigation of optical designs that create a large amount of myopic peripheral defocus in all retinal quadrants. Contact lenses would be the most advantageous method of consistently delivering a peripheral myopic defocus profile to the retina because the contact lens stays fairly centered with eye movements.
The amount of peripheral myopic defocus necessary to have a meaningful effect is unclear. One possibility is that a dose-response relationship exists where greater amounts of peripheral myopic defocus result in greater reductions in myopia progression. Another possibility is that there is not a dose-response relationship and that any amount of myopic peripheral defocus above some threshold acts as a “stop” signal to slow myopia progression. If there is no dose-response relationship, perhaps greater reductions in myopia progression are possible as more peripheral locations experience myopic defocus. Although hyperopic superior defocus was associated with faster central myopia progression, we did not find evidence that greater amounts of myopic peripheral defocus slowed central myopia progression more than lesser amounts of myopic peripheral defocus in year one. When limiting analyses to only children with myopic superior defocus in year one, there was no association between the amount of peripheral defocus and the 1-year change in myopia (all P ≥ 0.27).
Interestingly, we did not find a significant association between superior defocus and myopia progression in year two when all children wore SVLs. One might have expected to see an increase in myopia progression due to the superior hyperopic shift in defocus after children in PALs switched to SVLs. Despite the significant hyperopic shift in superior defocus with the cessation of PAL wear in year two, there was only a 30% decrease in the percentage of children with myopic superior defocus. Many uncorrected myopic eyes have relative peripheral myopia under ordinary circumstances.15,37 The large percentage of children still experiencing even small amounts of superior myopic defocus combined with slower progression in general due to increased age made any association in year two difficult to detect. It is important to note that this study was not powered to specifically examine this dose-response question, as it was not the primary outcome. It will be important for future studies with a larger sample to utilize interventions that result in a large range of peripheral defocus in order to further examine this question.
Two longitudinal studies of myopic children have not found a meaningful association between uncorrected RPR and myopia onset or progression, and a cross-sectional study in adults has raised the question of whether uncorrected RPR is associated with changes in refractive error or is a consequence of refractive error.25,26,54 We also did not observe a significant association between uncorrected RPR at baseline and myopia progression even though a significant association was found between myopic peripheral defocus and progression in year one when wearing an optical correction that resulted in large changes in retinal defocus, similar to the previously cited studies involving orthokeratology. Because optical corrections (spectacles and contact lenses) can alter peripheral defocus, it will be important for future progression studies to determine peripheral defocus when wearing correction.
One might wonder whether some other aspect of the PALs other than the manipulation of peripheral defocus is responsible for the PAL treatment effect. When allowing treatment group assignment (PAL or SVL) to compete in models where peripheral defocus was significantly associated with central myopia progression, the treatment group assignment was no longer significant (i.e., treatment group no longer explained the reduction in central myopia progression when peripheral defocus was allowed to compete in the same model). As previously reported, we also did not find a significant association between accommodative lag and the progression of myopia.5
A limitation of this study is that our calculation of peripheral refraction measures during spectacle wear assumes the child is looking in primary gaze with distance fixation through the center of the assigned spectacle lens, which may not reflect the child's usage of the spectacles in all situations. Peripheral defocus values through other lens locations were not made; however, the significant association between superior peripheral defocus and central myopia progression, where PALs resulted in the greatest difference in peripheral defocus compared with SVLs, warrants further investigation and confirmation in future studies. To overcome this study limitation and to better evaluate the hypothesis that peripheral myopic defocus slows myopia progression, future studies could utilize contact lenses. Orthokeratology lenses, which cause peripheral myopic defocus,33 were recently shown to slow myopia progression in a clinical trial.31 Early evidence has also suggested that center–distance soft bifocal contact lenses may slow myopia progression,35,55 though long term randomized clinical trials are needed. To determine whether myopic peripheral defocus is the mechanism responsible for the slowed progression reported with these contact lens modalities, future studies are needed that measure the peripheral defocus created by these contact lens designs. Clinical trials are also needed to determine whether any slowing of myopia progression continues to build with subsequent years of lens wear. These data would provide a better understanding of how peripheral defocus might influence myopia progression and would provide valuable information to guide the optimization of optical designs.
In summary, PALs resulted in a myopic shift in peripheral defocus with the largest myopic shift on the superior retina due to the PAL add corridor. Children with myopic superior defocus had significantly less myopia progression. These findings support the continued investigation of optical designs that create peripheral myopic defocus in all retinal quadrants as a means of slowing the progression of myopia.
Acknowledgments
The authors thank the following study personnel for their contributions to this research:
Data and Safety Monitoring Committee: Mark A. Bullimore, MCOptom, PhD (2006–2012; chair); Leslie Hyman, PhD (2006–2011), and Melvin L. Moeschberger, PhD (2006–2012).
Masked examiners: Bradley Dougherty, OD, MS (2007–2010), Kerri McTigue (2008–2010), Donald O. Mutti, OD, PhD (2008–2010), Kathryn Richdale, OD, PhD (2007–2010), Eric Ritchey, OD, PhD (2007–2010), and Aaron Zimmerman, OD, MS (2007–2008).
Opticians: Melissa Button (2007–2010), Aaron Chapman (2006–2007), Melissa Hill (2006–2008), Brandy Knight (2008–2010), Scott Motley (2007–2009), and Jeff Rohlf (2006–2010).
Optometry Coordinating Center: Lisa Jones-Jordan, PhD (Director, 2005–2012), G. Lynn Mitchell, MAS (Biostatistician, 2005–2012), Loraine Sinnott, PhD (Biostatistician, 2005–2012), Linda Barrett (Data Entry; 2005–2007), Austen Tanner (Data Entry, 2005–2010), Melanie Schray (Database Management, 2005–2010).
Supported by grants from the National Institutes of Health (Grant K12-EY015447), Essilor of America, Inc., and an Ezell Fellowship sponsored by the American Optometric Foundation Presidents Circle (DAB).
Disclosure: D.A. Berntsen, Essilor of America, Inc. (F); C.D. Barr, None; D.O. Mutti, Essilor of America, Inc. (F); K. Zadnik, Essilor of America, Inc. (F)
References
- 1. Correction of Myopia Evaluation Trial 2 Study Group for the Pediatric Eye Disease Investigator Group Progressive-addition lenses versus single-vision lenses for slowing progression of myopia in children with high accommodative lag and near esophoria. Invest Ophthalmol Vis Sci. 2011; 52: 2749–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gwiazda J, Hyman L, Hussein M, et al. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003; 44: 1492–1500 [DOI] [PubMed] [Google Scholar]
- 3. Hasebe S, Ohtsuki H, Nonaka T, et al. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008; 49: 2781–2789 [DOI] [PubMed] [Google Scholar]
- 4. Yang Z, Lan W, Ge J, et al. The effectiveness of progressive addition lenses on the progression of myopia in Chinese children. Ophthalmic Physiol Opt. 2009; 29: 41–48 [DOI] [PubMed] [Google Scholar]
- 5. Berntsen DA, Sinnott LT, Mutti DO, Zadnik K. A randomized trial using progressive addition lenses to evaluate theories of myopia progression in children with a high lag of accommodation. Invest Ophthalmol Vis Sci. 2012; 53: 640–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic Physiol Opt. 1999; 19: 126–133 [DOI] [PubMed] [Google Scholar]
- 7. Goss DA, Hampton MJ, Wickham MG. Selected review on genetic factors in myopia. J Am Optom Assoc. 1988; 59: 875–884 [PubMed] [Google Scholar]
- 8. Goss DA, Rainey BB. Relationship of accommodative response and nearpoint phoria in a sample of myopic children. Optom Vis Sci. 1999; 76: 292–294 [DOI] [PubMed] [Google Scholar]
- 9. Gwiazda JE, Hyman L, Norton TT, et al. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci. 2004; 45: 2143–2151 [DOI] [PubMed] [Google Scholar]
- 10. Berntsen DA, Sinnott LT, Mutti DO, Zadnik K. Accommodative lag and juvenile-onset myopia progression in children wearing refractive correction. Vision Res. 2011; 51: 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weizhong L, Zhikuan Y, Wen L, Xiang C, Jian G. A longitudinal study on the relationship between myopia development and near accommodation lag in myopic children. Ophthalmic Physiol Opt. 2008; 28: 57–61 [DOI] [PubMed] [Google Scholar]
- 12. Allen PM, O'Leary DJ. Accommodation functions: co-dependency and relationship to refractive error. Vision Res. 2006; 46: 491–505 [DOI] [PubMed] [Google Scholar]
- 13. Rosenfield M, Desai R, Portello JK. Do progressing myopes show reduced accommodative responses? Optom Vis Sci. 2002; 79: 268–273 [DOI] [PubMed] [Google Scholar]
- 14. Hoogerheide J, Rempt F, Hoogenboom W. Acquired myopia in young pilots. Ophthalmologica. 1971; 163: 209–215 [DOI] [PubMed] [Google Scholar]
- 15. Atchison DA, Pritchard N, Schmid KL. Peripheral refraction along the horizontal and vertical visual fields in myopia. Vision Res. 2006; 46: 1450–1458 [DOI] [PubMed] [Google Scholar]
- 16. Millodot M. Effect of ametropia on peripheral refraction. Am J Optom Physiol Opt. 1981; 58: 691–695 [DOI] [PubMed] [Google Scholar]
- 17. Logan NS, Gilmartin B, Wildsoet CF, Dunne MC. Posterior retinal contour in adult human anisomyopia. Invest Ophthalmol Vis Sci. 2004; 45: 2152–2162 [DOI] [PubMed] [Google Scholar]
- 18. Seidemann A, Schaeffel F, Guirao A, Lopez-Gil N, Artal P. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am A Opt Image Sci Vis. 2002; 19: 2363–2373 [DOI] [PubMed] [Google Scholar]
- 19. Mutti DO, Hayes JR, Mitchell GL, et al. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007; 48: 2510–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mutti DO, Sholtz RI, Friedman NE, Zadnik K. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000; 41: 1022–1030 [PubMed] [Google Scholar]
- 21. Schmid GF. Variability of retinal steepness at the posterior pole in children 7–15 years of age. Curr Eye Res. 2003; 27: 61–68 [DOI] [PubMed] [Google Scholar]
- 22. Verkicharla PK, Mathur A, Mallen EA, Pope JM, Atchison DA. Eye shape and retinal shape, and their relation to peripheral refraction. Ophthalmic Physiol Opt. 2012; 32: 184–199 [DOI] [PubMed] [Google Scholar]
- 23. Irving EL, Callender MG, Sivak JG. Inducing ametropias in hatchling chicks by defocus–aperture effects and cylindrical lenses. Vision Res. 1995; 35: 1165–1174 [DOI] [PubMed] [Google Scholar]
- 24. Smith EL III, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009; 49: 2386–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mutti DO, Sinnott LT, Mitchell GL, et al. Relative peripheral refractive error and the risk of onset and progression of myopia in children. Invest Ophthalmol Vis Sci. 2011; 52: 199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sng CC, Lin XY, Gazzard G, et al. Change in peripheral refraction over time in Singapore Chinese children. Invest Ophthalmol Vis Sci. 2011; 52: 7880–7887 [DOI] [PubMed] [Google Scholar]
- 27. Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol. 2009; 93: 1181–1185 [DOI] [PubMed] [Google Scholar]
- 28. Cho P, Cheung SW, Edwards M. The longitudinal orthokeratology research in children (LORIC) in Hong Kong: a pilot study on refractive changes and myopic control. Curr Eye Res. 2005; 30: 71–80 [DOI] [PubMed] [Google Scholar]
- 29. Kakita T, Hiraoka T, Oshika T. Influence of overnight orthokeratology on axial elongation in childhood myopia. Invest Ophthalmol Vis Sci. 2011; 52: 2170–2174 [DOI] [PubMed] [Google Scholar]
- 30. Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: refractive and biometric changes. Invest Ophthalmol Vis Sci. 2012; 53: 5060–5065 [DOI] [PubMed] [Google Scholar]
- 31. Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012; 53: 7077–7085 [DOI] [PubMed] [Google Scholar]
- 32. Hiraoka T, Kakita T, Okamoto F, Takahashi H, Oshika T. Long-term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5-year follow-up study. Invest Ophthalmol Vis Sci. 2012; 53: 3913–3919 [DOI] [PubMed] [Google Scholar]
- 33. Kang P, Swarbrick H. Peripheral refraction in myopic children wearing orthokeratology and gas-permeable lenses. Optom Vis Sci. 2011; 88: 476–482 [DOI] [PubMed] [Google Scholar]
- 34. Queiros A, Gonzalez-Meijome JM, Jorge J, Villa-Collar C, Gutierrez AR. Peripheral refraction in myopic patients after orthokeratology. Optom Vis Sci. 2010; 87: 323–329 [DOI] [PubMed] [Google Scholar]
- 35. Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology. 2011; 118: 1152–1161 [DOI] [PubMed] [Google Scholar]
- 36. Sankaridurg P, Holden B, Smith E III, et al. Decrease in rate of myopia progression with a contact lens designed to reduce relative peripheral hyperopia: one-year results. Invest Ophthalmol Vis Sci. 2011; 52: 9362–9367 [DOI] [PubMed] [Google Scholar]
- 37. Berntsen DA, Mutti DO, Zadnik K. Study of theories about myopia progression (STAMP) design and baseline data. Optom Vis Sci. 2010; 87: 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin Z, Martinez A, Chen X, et al. Peripheral defocus with single-vision spectacle lenses in myopic children. Optom Vis Sci. 2010; 87: 4–9 [DOI] [PubMed] [Google Scholar]
- 39. Backhouse S, Fox S, Ibrahim B, Phillips JR. Peripheral refraction in myopia corrected with spectacles versus contact lenses. Ophthalmic Physiol Opt. 2012; 32: 294–303 [DOI] [PubMed] [Google Scholar]
- 40. Bailey MD, Twa MD, Mitchell GL, Dhaliwal DK, Jones LA, McMahon TT. Repeatability of autorefraction and axial length measurements after laser in situ keratomileusis. J Cataract Refract Surg. 2005; 31: 1025–1034 [DOI] [PubMed] [Google Scholar]
- 41. Choong YF, Chen AH, Goh PP. A comparison of autorefraction and subjective refraction with and without cycloplegia in primary school children. Am J Ophthalmol. 2006; 142: 68–74 [DOI] [PubMed] [Google Scholar]
- 42. Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997; 74: 367–375 [DOI] [PubMed] [Google Scholar]
- 43. Cheng X, Himebaugh NL, Kollbaum PS, Thibos LN, Bradley A. Validation of a clinical Shack-Hartmann aberrometer. Optom Vis Sci. 2003; 80: 587–595 [DOI] [PubMed] [Google Scholar]
- 44. Salmon TO, West RW, Gasser W, Kenmore T. Measurement of refractive errors in young myopes using the COAS Shack-Hartmann aberrometer. Optom Vis Sci. 2003; 80: 6–14 [DOI] [PubMed] [Google Scholar]
- 45. Radhakrishnan H, Charman WN. Peripheral refraction measurement: does it matter if one turns the eye or the head? Ophthalmic Physiol Opt. 2008; 28: 73–82 [DOI] [PubMed] [Google Scholar]
- 46. Berntsen DA, Mutti DO, Zadnik K. Validation of aberrometry-based relative peripheral refraction measurements. Ophthalmic Physiol Opt. 2008; 28: 83–90 [DOI] [PubMed] [Google Scholar]
- 47. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995; 57: 289–300 [Google Scholar]
- 48. Smith EL III. Prentice Award Lecture 2010: a case for peripheral optical treatment strategies for myopia. Optom Vis Sci. 2011; 88: 1029–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sankaridurg P, Donovan L, Varnas S, et al. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optom Vis Sci. 2010; 87: 631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walline JJ, Jones-Jordan LA, Greiner KL, McVey M. The effects of soft bifocal contact lenses on myopia progression in children (E-Abstract 110642). Optom Vis Sci. 2011; 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tabernero J, Vazquez D, Seidemann A, Uttenweiler D, Schaeffel F. Effects of myopic spectacle correction and radial refractive gradient spectacles on peripheral refraction. Vision Res. 2009; 49: 2176–2186 [DOI] [PubMed] [Google Scholar]
- 52. Chen X, Sankaridurg P, Donovan L, et al. Characteristics of peripheral refractive errors of myopic and non-myopic Chinese eyes. Vision Res. 2010; 50: 31–35 [DOI] [PubMed] [Google Scholar]
- 53. Ehsaei A, Mallen EA, Chisholm CM, Pacey IE. Cross-sectional sample of peripheral refraction in four meridians in myopes and emmetropes. Invest Ophthalmol Vis Sci. 2011; 52: 7574–7585 [DOI] [PubMed] [Google Scholar]
- 54. Jaeken B, Artal P. Optical quality of emmetropic and myopic eyes in the periphery measured with high-angular resolution. Invest Ophthalmol Vis Sci. 2012; 53: 3405–3413 [DOI] [PubMed] [Google Scholar]
- 55. Walline JJ, Jones-Jordan LA. Axial elongation with corneal reshaping, soft bifocal, and spherical contact lens wear (E-Abstract 120062). Optom Vis Sci. 2012; 89 [Google Scholar]