Abstract
Background: As spinal cord compression at the craniocervical junction (CCJ) is a life-threatening manifestation in patients with mucopolysaccharidosis (MPS) IVA, surgical decompression should be performed before damage becomes irreversible. We evaluated the diagnostic value of several examinations for determining the need for decompression surgery.
Methods: We retrospectively analysed results of clinical neurological examination, somatosensory evoked potential (SEP) and magnetic resonance imaging (MRI) in 28 MPS IVA patients. A scoring system – based on the severity of findings – was used to compare results of patients with and without indication for decompression surgery. Individual test scores and two composite scores were evaluated for their potential to assess severity of CCJ impairment.
Results: Sixteen patients had an indication for surgery; 12 of them had undergone surgery. Twelve patients had no indication for surgery; none had received surgery. Neurological (P = 0.004), MRI (P < 0.001) and atlantoaxial subluxation (P = 0.006) scores, but not SEP and odontoid hypoplasia scores, differed significantly between patients with and without surgical indication. Both the abbreviated CCJ score, i.e. sum of neurological and MRI scores, and the extended CCJ score, i.e. sum of abbreviated CCJ and atlantoaxial subluxation score, discriminated between patients with and without surgical indication (abbreviated: 0–2 points vs 2–5 points, P < 0.001; extended: 0–3 points vs 3–7 points; P < 0.001). Although CCJ instability plays a major role in cervical cord pathology, decompression surgery without occipito-cervical stabilisation may yield good postoperative results.
Conclusions: The abbreviated and extended CCJ scores are objective, transparent and reproducible tools for assessing the CCJ pathology and the need for surgery.
Introduction
Mucopolysaccharidosis IVA (MPS IVA, OMIM #253000) or Morquio A syndrome is a rare metabolic disease caused by deficiency of the lysosomal enzyme N-acetylgalactosamine 6-sulfatase (EC 3.1.6.4), which is involved in the degradation of the glycosaminoglycans (GAGs) chondroitin- 6-sulfate and keratan sulfate. Consequently, partially degraded GAGs accumulate within lysosomes, resulting in progressive cellular damage and dysfunction in multiple tissues and organs, mainly in bone (Muenzer 2011; Tomatsu et al. 2011). Given the excessive deposition of GAGs in bone, the major feature of MPS IVA is skeletal dysplasia, including short trunk dwarfism, odontoid hypoplasia, joint hypermobility/ligamentous laxity, etc. (Muenzer 2011). Odontoid hypoplasia in combination with ligamentous laxity often results in atlantoaxial subluxation/instability, causing narrowing of the spinal canal at the craniocervical junction (CCJ), which is further increased by extradural soft tissue deposition. This leads to compression of the lower brain stem and upper cervical cord. If left untreated, this can ultimately result in irreversible damage of the spinal cord, i.e. myelomalacia. Clinically, it manifests as reduced muscle strength, paresis and ultimately death (Montaño et al. 2007; Muenzer 2011).
As cervical cord compression in MPS IVA can be severely disabling and even life-threatening, it should be addressed before damage becomes irreversible. Surgical stabilisation of the CCJ and decompression are the only means to achieve this goal. However, the risks associated with surgery and anaesthesia should be weighed against the severity of spinal cord compression, the patient’s progression rate and risk of significant permanent spinal cord injury (McLaughlin et al. 2010; Walker et al. 1994). Hence, determining the optimal indications and timing for surgery is crucial. Unfortunately, literature on this topic is scarce. Several diagnostic methods have been described to detect and assess the severity of cervical cord compression in MPS IVA patients, i.e. clinical neurological examination (Takeda et al. 1991), somatosensory evoked potentials (SEPs) (Boor et al. 2000; Takeda et al. 1991) and imaging of the cervical spine/cord – including assessment of atlantoaxial subluxation and odontoid hypoplasia – using X-ray radiography, magnetic resonance imaging (MRI) or computed tomography (CT) (Hughes et al. 1997; Roach et al. 1984; Stevens et al. 1991). However, there is neither consensus on the best diagnostic method, nor on the optimal indications or timing for neurosurgery at the CCJ. Thus, the final decision to perform surgery still depends on the surgeon’s clinical judgement, which is subjective and prone to interobserver variability. Therefore, we aimed at quantifying the individual contribution of each of these diagnostic methods to the decision-making process, i.e. at identifying objective and reproducible criteria to assess the severity of cervical cord compression in MPS IVA patients.
Methods
Patients
Demographic and clinical data were retrospectively collected from all patients with a biochemically confirmed diagnosis of MPS IVA in our department (Villa Metabolica, Mainz), who received at least two of the following assessments, i.e. neurological examination, SEP of the median nerve or MRI of the CCJ. For patients who had undergone at least one surgical intervention at the CCJ in the past, findings from the last preoperative and first postoperative examination were used for evaluation of postoperative outcomes. For patients with a surgical indication who had not yet been operated, the test results that revealed the indication for surgery were used. For patients without indication for decompression surgery, the most recent available test results were used.
Patients were classified as having a rapidly or a slowly progressive phenotype based on body height, physical performance, mobility achieved with orthopaedic support and degree of independence regarding daily activities.
Diagnostic Tests
Clinical neurological examination, registration of SEP of the median nerve and MRI of the CCJ were the main methods used to evaluate the pathology of the CCJ. In addition, atlantoaxial subluxation and odontoid hypoplasia were evaluated using CT or MRI. For each test method, a new scoring system was developed to rate the degree of changes from 0 (normal findings) to 3 (most severe changes) (Table 1). The most severe pathological finding in any examination was used to assess the severity and to determine the score.
Table 1.
Scoring system for pathology of the craniocervical junction and proximal cervical spine. Scoring system for findings of the clinical neurological examination, somatosensory evoked potentials (SEPs) of the median nerve, magnetic resonance imaging (MRI) of the craniocervical junction (CCJ), assessment of atlantoaxial subluxation and development of the dens axis
| Score | Test findings |
|---|---|
| Clinical neurological examination | |
| 0 | Normal neurological findings |
| 1 | Increased deep tendon reflexes, side differences of muscle reflexes |
| 2 | Presence of pyramidal tract signs: Babinski, Oppenheim reflex, clonus |
| 3 | Paresis or weakness of upper and/or lower extremities |
| SEP of the median nerve | |
| 0 | Normal SEP |
| 1 | Extension of at least one of the interpeak latencies N9/P13, N9/N13b or N13a/N20a (more than 2.5 SD) |
| 2 | Lack of P13 and/or N13b (subcortical) |
| 3 | Lack of N20 (cortical) |
| MRI of CCJ (sagittal images) | |
| 0 | No effacement of cerebrospinal fluid (CSF) around the spinal cord |
| 1 | Effacement of CSF on at least one side of the spinal cord |
| 2 | Effacement of CSF in all directions around the spinal cord |
| 3 | Myelomalacia |
| Atlantoaxial subluxation | |
| 0 | Normal position of the atlas |
| 1 | Obvious ventral dislocation of the atlas relative to the axis |
| 2 | Severe ventral dislocation of the atlas relative to the axis, making a regular position of the spinal cord unlikely, regardless of glycosaminoglycan accumulation |
| Development of dens axis | |
| 0 | (Possibly) hypoplastic bony part of dens axis extends completely into the atlas |
| 1 | Bony part of dens axis extends partially into the atlas |
| 2 | Bony part of dens axis does not extend into the atlas |
aAnatomical correlations according to Stöhr et al. (2005):
N9/P13: plexus brachialis – nucleus cuneatus
N9/N13b: plexus brachialis – nucleus cuneatus
N13a/N20: dorsal horn (caudal cervical cord) – cortex
Clinical neurological examination included assessment of easily evaluable and reproducible signs caused by lesions of the pyramidal tract, i.e. deep tendon reflexes and pyramidal signs.
SEP of the median nerve was carried out as described previously (Boor et al. 1998, 2000). As patients with Morquio A syndrome generally have a shorter stature than age-related healthy individuals, not absolute latencies but interpeak latencies between the responses were evaluated and compared with age-specific reference values of our department. Examinations were judged to be pathological in case of significant variation from the age-specific reference values on either right or left side.
For the MRI evaluation, both T1- and T2-weighted images were created. The presence or absence of cerebrospinal fluid (CSF) around the spinal cord could be deduced from the sagittal and axial T2-weighted images. The T2-weighted images (both sagittal and axial) were also examined for increased signal intensity in the cord, suggesting myelomalacia (Lachman et al. 2010). For most patients, images were taken in neutral position of the head. If images were available in flexion and extension of the head, the most severe pathological finding was chosen.
Surgery
The indication for decompression surgery was made on clinical grounds, focusing on signs and symptoms of upper motor neuron lesion and compromised lower brain stem function, especially central apnoea. MRI findings and SEP were also taken into account. Posterior decompression surgery consisted of partial laminectomy of C1-C3 in combination with enlargement of the foramen magnum (if indicated), and removal of the thickened ligaments without opening the dura. Additional stabilisation of the cervical spine was performed if needed.
Statistics
Statistical analysis was done using IBM SPSS 19. The Fisher Exact, Mann-Whitney, Wilcoxon and sign tests were used for ordinal variables, continuous variables, ordinal data, and for comparison of pre- and postoperative outcomes for both ordinal and continuous variables, respectively. A P-value < 0.05 was considered statistically significant.
Results
Demographics and Baseline Characteristics
The study included 28 patients with a biochemically and/or genetically confirmed diagnosis of MPS IVA (16 males, 12 females) (Table 2). Median age at the time of analysis was 12.9 years (range 5–49). Twelve patients had undergone at least one neurosurgical intervention at the CCJ in the past. Three patients underwent posterior decompression surgery plus cervical spine stabilisation, while nine patients did not need stabilisation. Four patients had an indication for cervical cord surgery but had not yet been operated, either because of recent worsening of their situation or because of anaesthesia risks. Another 12 patients did not (yet) have an indication for decompression surgery.
Table 2.
Demographic information at last follow-up (N = 28)
| N (%) | |
|---|---|
| Gender | |
| Male | 16 (57%) |
| Female | 12 (43%) |
| Age | |
| < 18 years (children) | 21 (75%) |
| ≥ 18 years (adults) | 7 (25%) |
| Phenotypical disease severity | |
| Slowly progressive (attenuated) phenotype | 8 (28%) |
| Rapidly progressive (severe) phenotype | 20 (72%) |
| Neurosurgery at the craniocervical junction (CCJ) | |
| History of neurosurgery at the CCJ (≥1 intervention) | 12 (43%) |
| Indication for neurosurgery at the CCJ, but not yet operated | 4 (14%) |
| No indication for neurosurgery at the CCJ | 12 (43%) |
N number of patients
All 16 patients with an indication for surgery were children (< 18 years) at the time of surgery or at the time of finding the indication, except for one 39-year-old woman. Their median age at that time was 7.9 years (range 2–39), which is lower than the median age of 11.9 years of the 12 patients without surgical indication at last follow-up (range 5–20; 10 children, 2 adults). All 16 patients who needed surgery – except for the 39-year-old woman – had a rapidly progressive phenotype, compared to only 5/12 patients without surgical indication.
Comparison of Cervical Cord Pathology in Patients With and Without Surgical Indication
The scores of diagnostic tests to assess the severity of cervical cord compression were compared between patients with and without an indication for neurosurgery at the CCJ. The outcomes are summarised in Table 3. For SEP of the median nerve, results were comparable between patients with and without surgical indication. In contrast, for neurological examination and MRI of the CCJ, pathological findings were more common and more severe among patients who needed neurosurgery. As only few preoperative images were available for assessment of atlantoaxial subluxation and odontoid hypoplasia, some postoperative images were used as well (only for patients who did not undergo stabilisation). Although we could not establish a scoring system for atlantoaxial subluxation based on clear objective criteria, such as atlanto-dens interval, due to the different ages of our patients, subluxation was significantly more common in patients who needed spinal cord decompression. In contrast, development of the bony part of the dens axis (N = 27) did not differ significantly between both patient groups (data not shown; P = 0.634).
Table 3.
Findings of diagnostic tests to evaluate the pathology of the craniocervical junction (CCJ) in patients without (−; results of last follow-up) and with (+; preoperative results) an indication for neurosurgery at the CCJ
| Diagnostic test | Surgical indication | N | Percentage of patients with score | P (− vs +) | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| Clinical neurological examination | – | 12 | 67 | 33 | 0 | 0 | 0.004 |
| + | 14 | 7a | 57 | 21 | 14 | ||
| Somatosensory evoked potentials of the median nerve | – | 12 | 83 | 17 | 0 | 0 | 0.571 |
| + | 10 | 90 | 10 | 0 | 0 | ||
| Magnetic resonance imaging | – | 10 | 80 | 20 | 0 | 0 | < 0.001 |
| + | 14 | 7a | 14 | 43 | 36 | ||
| Atlantoaxial subluxation assessment | – | 11 | 82 | 18 | 0 | – | 0.006 |
| + | 14 | 21 | 36 | 43 | – | ||
Explanation of scoring system for each test: see Table 1
aPathology scores 0 for clinical neurological examination and magnetic resonance imaging were not observed in a single patient
For some patients, MRI pictures of the CCJ were taken in several positions of the head, i.e. in neutral position, flexion and/or extension (Fig. 1). From these images, one can see that compression of the cervical cord depends on the position of the head. During flexion of the head, compression is obvious, with no visible CSF around the spinal cord. In contrast, in neutral position of the head, the cervical cord is surrounded by a minimal amount of CSF. During extension, the space available for the spinal cord is even more increased.
Figure 1.
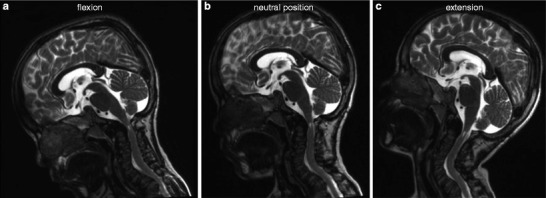
Impact of position of the head on cervical cord compression. T2-weighted magnetic resonance imaging of the craniocervical junction during (a) flexion, (b) neutral position and (c) extension of the head of an 8-year-old boy with severe MPS IVA, who underwent decompression surgery when he was 4 years old. Spinal cord compression depends on the position of the head and is the most obvious during flexion
Composite CCJ Scores for Defining the Need for Neurosurgery
Two composite CCJ scores were developed to evaluate whether combining the results of the individual examinations could improve the assessment of severity of cervical cord compression. The abbreviated CCJ score is the sum of the pathology scores for neurological examination and MRI and ranges from 0 to 6. The extended CCJ score is the sum of the abbreviated CCJ score and the atlantoaxial subluxation score and ranges from 0 to 8. SEP and odontoid hypoplasia scores were not included, as they did not differ significantly between both patient groups. Both scores were compared between patients with and without an indication for surgery.
Both scores could discriminate between patients with and without surgical indication (both P < 0.001). Indeed, in patients without surgical indication, abbreviated and extended CCJ scores ranged from 0 to 2 (median 0.5) and from 0 to 3 (median 1), respectively, while in patients with surgical indication, they ranged from 2 to 5 (median 4) and from 3 to 7 (median 5). Thus, in none of the patients requiring surgery, neurological examination and MRI findings were normal (score 0) at the same time.
Impact of Cervical Cord Decompression
To assess if surgery had an impact on the test results, we compared the pre- and postoperative scores of the three main diagnostic procedures and the abbreviated CCJ score. Differences between pre- and postoperative findings were not statistically significant. For neurological examination and for the abbreviated CCJ score, there was a trend towards improvement, with 45% (P = 0.219) and 78% (P = 0.180) of patients showing postoperative improvement, respectively.
Discussion
This retrospective study aimed at quantifying the relative utility of individual methods to assess the severity of CCJ impairment, in order to establish objective and reproducible criteria regarding the need for neurosurgery, allowing comparison between different centres. Clinical neurological examination, MRI and atlantoaxial subluxation appeared to be reliable methods to determine the indication for cervical cord surgery, with MRI being the most sensitive one, in line with a previous publication (Hughes et al. 1997). In contrast, SEP of the median nerve in neutral position of the head could not discriminate between patients with and without a surgical indication (Takeda et al. 1991), as opposed to a previous report (Boor et al. 2000). Similarly, the degree of odontoid hypoplasia appeared not to be a good criterion to determine the need for surgery in MPS IVA patients, as suggested previously (Hughes et al. 1997; Stevens et al. 1991). This is not surprising, as transition from cartilage to bone in the dens axis might not be completed before the age of 8 years, and 11/28 patients in our study were < 8 years old. Furthermore, cervical cord compression may also occur in the absence of bony odontoid hyperplasia (Shukla et al. 2011). The abbreviated and extended CCJ scores – combining MRI, neurological examination and atlantoaxial subluxation scores – appeared to have a higher discriminatory power than the individual tests.
When interpreting these results, it should be noted that the classification of patients into two groups, i.e. those with and without surgical indication, was dependent on the decision of a single neurosurgeon. Although this surgeon was very experienced in judging the need for surgery, this decision remained highly subjective and might have been taken too early or too late (i.e. in those patients who already had myelomalacia). However, it is currently the only available standard against which the new scoring system could be tested. Indeed, the only other existing scoring system for evaluating cervical cord involvement is solely based on MRI findings and was not developed to determine the optimal timing for surgery (Castro et al. 2008; Lachman et al. 2010). Future research will be needed to confirm the validity of our scoring system, by comparing the (long-term) outcomes of patients with and without a surgical indication according to the CCJ scores.
Based on our observations, we propose relative and absolute indications for neurosurgery at the CCJ (Table 4). Importantly, when interpreting this table, evolution of the severity scores over time, i.e. rapidity of progression, should also be taken into account. Thus, pyramidal tract signs and CSF effacement around the spinal cord might be considered as relative indications for surgery when stable over time – requiring close monitoring – but they might require immediate surgical intervention in case of rapid aggravation. This is a limitation of the current study. Therefore, it might be interesting for future studies to add a ‘longitudinal’ or ‘time’ component to the new scoring system, allowing intervention in earlier stages, when pathology is still reversible.
Table 4.
Proposed absolute and relative indications for neurosurgery at the craniocervical junction (CCJ) in patients with MPS VIA. Outcomes of diagnostic methods suggesting the need for decompression surgery are indicated
| Diagnostic methoda | Relative indication | Absolute indication |
|---|---|---|
| Clinical neurological examination | Score 2 (pyramidal tract signs) | Score 3 (paresis or limb weakness) |
| MRI of CCJ | Score 2 (absence of CSF around spinal cord) | Score 3 (myelomalacia) |
| Abbreviated CCJ score | Score 2 | Score ≥ 3 |
| Extended CCJ score | Score 3 | Score ≥ 4 |
aThe most extensive diagnostic method available for a single patient should be considered. Extended CCJ score is more extensive than abbreviated CCJ score, while abbreviated CCJ score is more extensive than MRI of CCJ or clinical neurological examination alone
In addition, our study showed that flexion-extension imaging is indispensable to detect CCJ instability in all MPS IVA patients – unless there is a severe risk of aggravating the patient’s conditions – as cervical cord compression might only be noticed in flexion and not in neutral position of the head (Fig. 1) (Roach et al. 1984; Stevens et al. 1991). We preferred MRI over other methods for flexion-extension imaging such as CT and X-ray radiography, because it directly visualises the spinal cord and uses non-ionising radiation, which is especially advantageous in children. Moreover, plain flexion-extension X-ray films might be difficult to interpret. Indeed, overlap between the thickened skull and the very small C1-C2 vertebrae is common in MPS IVA patients and might lead to delayed detection of instability, when myelopathy is already present (Ransford et al. 1996). As many MPS IVA patients are children at the time of diagnosis, very short sequence MRI and limited flexion-extension movements were required to minimise the risks of general anaesthesia and damaging the spinal cord by prolonged flexion of the neck.
Finally, to our knowledge, this is the first study in MPS IVA patients showing that cervical laminectomy for cervical spine decompression is feasible without posterior occipito-cervical fixation/fusion, leading to improvement in abbreviated CCJ score in 78% of patients. Indeed, given the major role of instability/hypermobility of the CCJ in the pathophysiology of spinal cord damage (Ashraf et al. 1991), (prophylactic) cervical fusion has always been preferred as surgical intervention, either with or without posterior decompression, with variable success rates (Ain et al. 2006; Kopits et al. 1972; Lipson 1977; Northover et al. 1996; Ransford et al. 1996; White et al. 2009). However, metal stabilisation may cause artefacts on MRI or CT scan, thereby hindering future monitoring, while bone grafts may complicate revision surgery in case of relapse. In addition, stabilisation severely limits neck motion (White et al. 2009). Therefore, in our study, stabilisation was only performed in three patients. The (short-term) success of laminectomy-only suggests that, next to CCJ instability, permanent cervical cord compression due to extradural soft tissue thickening plays a role in spinal cord damage (Stevens et al. 1991), thereby confirming the mixed pathophysiology of CCJ lesions (White et al. 2009). However, comparison of our results with those of previous studies applying cervical stabilisation is very difficult, as the severity of neurological impairment before surgery might differ between studies. Therefore, a prospective long-term follow-up study comparing laminectomy with cervical stabilisation will be needed to confirm our findings.
Take-Home Message
Clinical neurological examination, magnetic resonance imaging and evaluation of atlantoaxial subluxation are the best diagnostic methods to assess the severity of cervical cord compression in patients with MPS IVA; they can be combined into two composite scores which can help to determine the need and optimal timing for decompression surgery.
Authors’ Contributions
Christian Möllmann was involved in designing the study and collecting and interpreting the data. He was involved in drafting the manuscript and revising it critically for important intellectual content. This publication is part of Christian Möllmann’s MD thesis, entitled ‘Die kraniozervikale Stenose bei Mukopolysaccharidose IVA (M. Morquio A)’, defended at the Johannes Gutenberg-Universität in Mainz in 2012.
Christian Lampe was involved in designing the study and in analysing and interpreting the data, especially SEP data. He was involved in drafting the manuscript and revising it critically for important intellectual content.
Wibke Müller-Forell was involved in performing and interpreting the MRI examinations and in revising the manuscript.
Maurizio Scarpa, Paul Harmatz and Michael Beck were involved in analysing and interpreting the data and in revising the manuscript for important intellectual content.
Manfred Schwarz has been involved in the interpretation of data, drafting the manuscript and revising it critically for important intellectual content.
Christina Lampe was involved in designing the study and in collecting, analysing and interpreting the data. She was involved in drafting the manuscript and revising it critically for important intellectual content. Christina Lampe accepts full responsibility for the work and the conduct of the study, had access to all data and controlled the decision to publish.
All authors have given final approval of this version to be published.
Competing Interests
Christian Möllman received travel grants from BioMarin Europe Ltd.
Christian Lampe received travel grants from BioMarin Europe Ltd.
Wibke Müller-Forell has no conflicts of interest.
Maurizio Scarpa provided consulting services to BioMarin Pharmaceutical Inc, Novato, CA, USA. He has also received research grants, participated in advisory boards, and received speakers’ honoraria and travel support from BioMarin.
Paul Harmatz has provided consulting services to BioMarin Pharmaceutical Inc, Novato, CA, USA. He has also received research grants, participated in advisory boards, and received speakers’ honoraria and travel support from BioMarin.
Manfred Schwarz received travel grants and speakers’ fee from BioMarin Europe Ltd.
Michael Beck provided consulting services to BioMarin Pharmaceutical Inc, Novato, CA, USA. He also received research grants, participated in advisory boards, and received speakers’ honoraria and travel support from BioMarin.
Christina Lampe received travel grants, speakers’ fee, participated in advisory boards, provided consulting services and received article processing charge from BioMarin Europe Ltd and Shire.
Funding
The authors are grateful to Ismar Healthcare NV for their writing assistance, which was funded by BioMarin Europe Ltd. The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Ethical Approval
Ethical approval was not required for this retrospective study.
Patient Consent Statement
All patients included in the study gave informed consent.
Footnotes
Competing interests: Competing interests declared by authors can be found at the end of this chapter
Contributor Information
Christina Lampe, Email: christina.lampe@unimedizin-mainz.de.
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Ain MC, Chaichana KL, Schkrohowsky JG. Retrospective study of cervical arthrodesis in patients with various types of skeletal dysplasia. Spine. 2006;31:E169–E174. doi: 10.1097/01.brs.0000202758.61848.61. [DOI] [PubMed] [Google Scholar]
- Ashraf J, Crockard HA, Ransford AO, Stevens JM. Transoral decompression and posterior stabilisation in Morquio’s disease. Arch Dis Child. 1991;66:1318–1321. doi: 10.1136/adc.66.11.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boor R, Goebel B, Taylor MJ. Subcortical somatosensory evoked potentials after median nerve stimulation in children. Eur J Paediatr Neurol. 1998;2:137–143. doi: 10.1016/S1090-3798(98)80029-9. [DOI] [PubMed] [Google Scholar]
- Boor R, Miebach E, Brühl K, Beck M. Abnormal somatosensory evoked potentials indicate compressive cervical myelopathy in mucopolysaccharidoses. Neuropediatrics. 2000;31:122–127. doi: 10.1055/s-2000-7495. [DOI] [PubMed] [Google Scholar]
- Castro S, Ayres-Basto M, Rodrigues E, Campos MM, Guimarães J, Leão-Teles E. Vertebro-medular imaging findings in mucopolysaccharidosis types II and IV. J Inherit Metab Dis. 2008;31(1):109. [Google Scholar]
- Hughes DG, Chadderton RD, Cowie RA, Wraith JE, Jenkins JPR. MRI of the brain and craniocervical junction in Morquio’s disease. Neuroradiology. 1997;39:381–385. doi: 10.1007/s002340050429. [DOI] [PubMed] [Google Scholar]
- Kopits SE, Perovic MN, McKusick V, Robinson RA, Bailey JA., III Congenital atlantoaxial dislocations in various forms of dwarfism. J Bone Joint Surg Am. 1972;54-A:1349–1350. [Google Scholar]
- Lachman R, Martin KW, Castro S, Basto MA, Adams A, Teles EL. Radiologic and neuroradiologic findings in the mucopolysaccharidoses. J Pediatr Rehabil Med. 2010;3:109–118. doi: 10.3233/PRM-2010-0115. [DOI] [PubMed] [Google Scholar]
- Lipson SJ. Dysplasia of the odontoid process in Morquio’s syndrome causing quadriparesis. J Bone Joint Surg Am. 1977;59:340–344. [PubMed] [Google Scholar]
- McLaughlin AM, Farooq M, Donnelly MB, Foley K. Anaesthetic considerations of adults with Morquio's syndrome - a case report. BMC Anesthesiol. 2010;10:2. doi: 10.1186/1471-2253-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- Muenzer J. Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 2011;50(Suppl 5):v4–v12. doi: 10.1093/rheumatology/ker394. [DOI] [PubMed] [Google Scholar]
- Northover H, Cowie RA, Wraith JE. Mucopolysaccharidosis type IVA (Morquio syndrome): a clinical review. J Inherit Metab Dis. 1996;19:357–365. doi: 10.1007/BF01799267. [DOI] [PubMed] [Google Scholar]
- Ransford AO, Crockard HA, Stevens JM, Modaghegh S. Occipito-atlanto-axial fusion in Morquio-Brailsford syndrome. A ten-year experience. J Bone Joint Surg Br. 1996;78:307–313. [PubMed] [Google Scholar]
- Roach JW, Duncan D, Wenger DR, Maravilla A, Maravilla K. Atlanto-axial instability and spinal cord compression in children–diagnosis by computerized tomography. J Bone Joint Surg Am. 1984;66:708–714. [PubMed] [Google Scholar]
- Shukla D, Arvind S, Devi BI. Myelopathy in a dwarf: a case of Morquio’s syndrome without odontoid hypoplasia. Neurol India. 2011;59:126–127. doi: 10.4103/0028-3886.76861. [DOI] [PubMed] [Google Scholar]
- Stevens JM, Kendall BE, Crockard HA, Ransford A. The odontoid process in Morquio-Brailsford’s disease. The effects of occipitocervical fusion. J Bone Joint Surg Br. 1991;73:851–858. doi: 10.1302/0301-620X.73B5.1910048. [DOI] [PubMed] [Google Scholar]
- Stöhr M, Dichgans J, Büttner U, Hess CW. Evozierte Potenziale: SEP – VEP – AEP –EKP – MEP. Berlin: Springer 4 Auflage; 2005. [Google Scholar]
- Takeda E, Hashimoto T, Tayama M, et al. Diagnosis of atlantoaxial subluxation in Morquio's syndrome and spondyloepiphyseal dysplasia congenita. Acta Paediatr Jpn. 1991;33:633–638. doi: 10.1111/j.1442-200X.1991.tb01877.x. [DOI] [PubMed] [Google Scholar]
- Tomatsu S, Montaño AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr Pharm Biotechnol. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- Walker RWM, Darowski M, Morris P, Wraith JE. Anaesthesia and mucopolysaccharidoses. A review of airway problems in children. Anaesthesia. 1994;49:1078–1084. doi: 10.1111/j.1365-2044.1994.tb04360.x. [DOI] [PubMed] [Google Scholar]
- White KK, Steinman S, Mubarak SJ. Cervical stenosis and spastic quadriparesis in Morquio disease (MPS IV). A case report with twenty-six-year follow-up. J Bone Joint Surg Am. 2009;91:438–442. doi: 10.2106/JBJS.H.00148. [DOI] [PubMed] [Google Scholar]


