Abstract
The nucleus-encoded mitochondrial pyruvate dehydrogenase enzyme complex plays key roles in cellular energy metabolism and acid-base equilibrium. Pyruvate dehydrogenase complex deficiency is due to loss-of-function mutation in one of the five component enzymes, most commonly E1α-subunit. The common clinical presentation ranges from fatal infantile lactic acidosis in newborns to chronic neurological dysfunction. We describe here an unusual presentation of E1α-subunit deficiency presenting as recurrent demyelination, Guillain-Barré syndrome-like demyelinating polyneuropathy at the onset, and ophthalmoplegia in a young infant. The clinical phenotype of the mutation in the patient was unique as compared to the previous reported cases of pyruvate dehydrogenase deficiency. The mother was found to be a mosaic carrier of the mutation. This phenotypic variability of pyruvate dehydrogenase complex deficiency and early suspicion of its unusual neurological manifestations is highlighted. Thiamine and ketogenic diet can be helpful.
Introduction
The nucleus-encoded mitochondrial pyruvate dehydrogenase (PDH) complex plays key roles in cellular energy metabolism and acid-base equilibrium. PDH complex consists of three catalytic enzymes, pyruvate dehydrogenase (E1), dihydrolipoamide transacetylase (E2), dihydrolipoamide dehydrogenase (E3); an additional protein known as E3-binding protein (E3BP) or protein X; and two regulatory enzymes, pyruvate dehydrogenase kinase and phosphatase. The E1-subunit is encoded by the X-linked PDHA1 gene and irreversibly oxidizes pyruvate to acetyl-CoA in presence of thiamine pyrophosphate. PDH deficiencies are largely due to defects in this E1-subunit that result in clinically diverse mitochondrial disorders ranging from fatal infantile lactic acidosis in newborns to chronic neurological dysfunction (Dahl et al. 1992). However, a clinical picture suggestive of recurrent demyelination, as seen in our patient, is an unusual presentation in infancy. The genetic analysis revealed a mutation of PDHA1 gene and the mother was found to be mosaic carrier. The need for identifying unusual neurological presentations of this rare disorder is emphasized.
Case Report
An 18-month-old male child was admitted to our hospital with progressive weakness, loss of previous milestones, ophthalmoplegia, excessive crying, and difficulty in feeding and speaking. There were no seizures or fever. He was born to non-consanguineous parents by emergency caesarian section due to umbilical artery stenosis with compromise of fetomaternal circulation. Antenatal period was normal. His birth weight was 3.1 kg. Subsequently, he was noticed to have developmental delay; he attained social smile at 3 months, neck control at 5 months, cooing at 6 months, reaching out for objects at 7 months, and sitting with support at 8 months. However, when the child was brought to us during his acute illness, he was unable to sit or reach out. Examination showed generalized hypotonia, absence of deep tendon reflexes, mute plantars, and external ophthalmoplegia. He was conscious but irritable and crying excessively. Gag reflex was absent along with difficulty in feeding. He received a 5-day course of pulse methylprednisolone followed by oral steroids in view of a demyelinating illness. The child had an episode of aspiration pneumonia requiring mechanical ventilation and prolonged ICU care for shock and sepsis.
The child had significant past history. At 8 months of age, the baby had upper respiratory infection with mild fever followed by progressive weakness of lower and upper limbs, generalized hypotonia, areflexia, and ophthalmoplegia. He was admitted in a local hospital. Magnetic resonance imaging (MRI) brain was normal. Cerebrospinal fluid (CSF) examination revealed proteins 29 mg/dL, sugar 71 mg/dL (blood sugar 119 mg/dL), cells: 2 lymphocytes/mm3, gram stains, and cultures sterile. Nerve conduction velocity (NCV) was suggestive of motor sensory demyelinating neuropathy (Table 1). Electromyography was suggestive of neurogenic changes with decreased motor units. Repetitive nerve stimulation test was normal. A diagnosis of autoimmune demyelinating polyneuropathy (AIDP) was considered, and he received intravenous immunoglobulin at 2 g/kg after which the weakness gradually improved.
Table 1.
Electrophysiological data of the patient at the time of initial presentation
| Patient | cut-off values for age (mean-2.5SD) | |
|---|---|---|
| Motor NCV (m/s) | ||
| Median | 13.51 | 26.22 |
| Peroneal | 18.81 | 25.4 |
| Tibial | 5.12 | 24.75 |
| F-wave latency (peroneal) | 17.62 | 21 |
| Sensory NCV (m/s) | ||
| Sural | 14.05 | 20.52 |
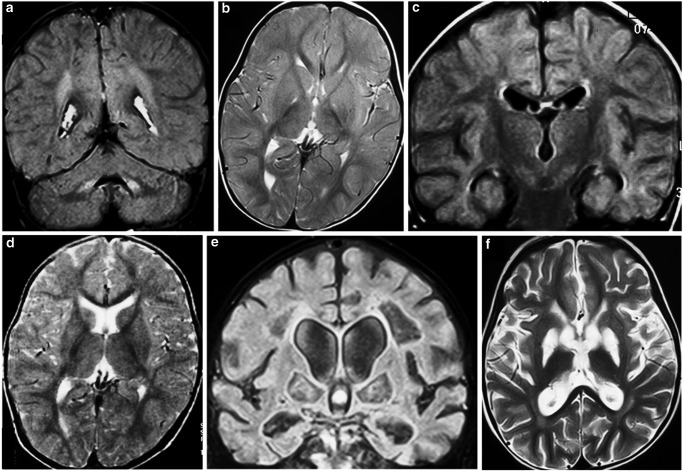
At 15 months of age, the child was again admitted in a local hospital with generalized weakness, ptosis, and regression of milestones. MRI brain showed T2/FLAIR hyperintensities involving bilateral periventricular areas and the hilus of the dentate nucleus in the cerebellum suggestive of demyelination (Fig. 1a and b). NCV showed no evidence of conduction blocks or dispersion. Conduction velocities of all motor and sensory nerves were altered, consistent with sequelae of earlier attack of AIDP. CSF examination revealed no cells and proteins 31 mg%. CSF-PAGE was positive for oligoclonal bands. The child received a 5-day course of pulse methylprednisolone in view of acute demyelinating encephalomyelitis followed by oral steroids. He showed rapid recovery but again deteriorated and was then brought to our hospital.
Fig. 1.
(a and b): MRI brain (FLAIR and T2) at the age of 15 months (second deterioration) shows hyperintense signal involving bilateral hilum of dentate nucleus, superior and middle cerebellar peduncles, and periventricular white matter suggestive of demyelination. These areas were hypo- to isointense on T1 images and showed no restriction on diffusion weighted or any enhancement post-contrast. Rest of the supratentorial brain parenchyma is normal in signal intensity and gray-white matter differentiation. Bilateral basal ganglia, thalami, and corpus callosum are normal. (c and d): MRI brain (FLAIR and T2) at the age of 18 months (first presentation to us) shows subtle T2/FLAIR hyperintensities in bilateral periventricular parietooccipital white matter. These showed no evidence of diffusion restriction or post-contrast enhancement. Bilateral basal ganglia, thalami, and corpus callosum are normal. (e and f): MRI brain (FLAIR and T2) at the age of 22 months (follow-up at 1 year after the illness) shows bilateral symmetrical linear areas of gliotic change in bilateral periventricular white matter, centrum semiovale, and globus pallidi. No diffusion restriction was seen. Corpus callosum is thinned out in body portion. Ventricular system is dilated with dilated 3rd and 4th ventricles. Bilateral thalami appear normal
In the current admission at 18 months of age, laboratory investigations showed pH 7.29, serum ammonia 64 μmol/L (9–33 μmol/L), and lactate 7.7 mmol/L (0.7–2.1 mmol/L). CSF lactate was 25.02 mmol/L. Fundus examination was normal. MRI brain revealed non-enhancing subtle T2/FLAIR hyperintensities in bilateral periventricular parietooccipital white matter (Fig. 1c and d). The mutational analysis for mitochondrial disease was negative. In view of recurrent peripheral and central demyelination, ophthalmoplegia and waxing-waning course, genetic analysis for PDH deficiency was sent which showed hemizygosity for the c.412C>T, p.Leu138Phe mutation in exon 4 of the PDHA1 gene. His mother was found to be mosaic for this mutation. The patient was started on oral thiamine and ketogenic diet. Supportive care was continued in form of physiotherapy and tracheostomy tube care. Follow-up MRI brain 1 year after the illness showed symmetrical gliotic changes in bilateral periventricular white matter and globus pallidi with communicating hydrocephalus, thinned-out corpus callosum, and cerebral atrophy (Fig. 1e and f).
Discussion
A subset of PDH-deficient patients present with an atypical course with intermittent neurological symptoms often triggered by infectious illnesses, like intermittent ataxia (Debray et al. 2008), recurrent acute dystonia (Head et al. 2004), extrapyramidal movement disorders, and episodic peripheral weakness mimicking Guillain-Barré syndrome (GBS) (Strassburg et al. 2006; Debray et al. 2006). Such an early initial presentation of PDH deficiency clinically mimicking GBS is quite rare. Remarkably, the first episode in our patient occurred at the age of 8 months, while classic GBS is unusual in this age group (Hughes and Cornblath 2005). This suggests that acute “GBS-like” weakness at the outset is a potentially reversible and probably under-recognized manifestation of PDH deficiency and should be diagnosed early by nerve conduction studies. Defects in cellular energy metabolism lead to a reduced synthesis of adenosine triphosphate and pathological accumulation of lactate, H+, and free radicals which possibly impair myelination and other vital functions of nervous tissue (Strassburg et al. 2006). External ophthalmoplegia, considered the hallmark of mitochondrial disorders in adults, was also an important clue in our patient (Jackson et al. 1995).
Treatment of most patients with PDH deficiency has been disappointing and no intervention specific to this disease has been evaluated in randomized controlled trials (Patel et al. 2012). As the symptoms are due to energy depletion from inefficient ATP production and lactate accumulation, ketogenic diet helps by providing the brain with an alternate “ketone” fuel and minimizing lactate accumulation by limiting carbohydrate intake. By the same mechanism, PDH deficiency usually responds to thiamine and ketogenic diet (Naito et al. 2002). A possible additional benefit of the diet is increased brain insulin-like growth factor receptor expression and neuronal glucose transporter expression (Cheng et al. 2003). Reports of a few children with PDH deficiency whose clinical course improved dramatically while following a high-fat diet are consistent with this postulate and have resulted in the incorporation of such diets in the routine care of many patients (Wexler et al. 1997; Kossoff et al. 2009). Nutritional mixtures of various cofactors and vitamins have been tried, usually, with limited biochemical rationale. Recommendations of the International Ketogenic Diet Study Group (Kossoff et al. 2009) states that the diet is the treatment of choice for two distinct disorders of brain energy metabolism: GLUT-1 deficiency syndrome and PDH deficiency. Unfortunately, despite this, our patient deteriorated. This could have been, perhaps, due to the initiation of the diet at a later age when the child presented to us at 18 months.
The metabolic causes of demyelinating polyneuropathy in young children are limited such as lysosomal disorders (metachromatic leukodystrophy, Krabbe disease), beta-mannosidosis, sialidosis, Gaucher disease, Niemann-Pick C, Refsum and other peroxisomal disorders, mitochondrial disorders like MNGIE, or rarely acute liver or renal failure. Acute polyneuropathies mimicking GBS have been described in acute attacks of porphyrias (Sedel et al. 2007), tyrosinemia type I (Mitchell et al. 1990), and in PDH deficiency (Debray et al. 2006). Treatable causes of metabolic neuropathies in children worth remembering are biotinidase deficiency, homocysteine remethylation defects, ornithine aminotransferase deficiency, vitamin E deficiency, serine deficiency, Wilson disease, and cerebrotendinous xanthomatosis (Garcia-Cazorla et al. 2009) though these are not exclusively demyelinating type of neuropathies.
The diagnosis in our patient was confirmed after mutation analysis which revealed hemizygosity for the c.412C>T mutation in exon 4 of the PDHA1 gene causing substitution of Leucine for 138 Phenylalanine (L138F). The mosaicism for the mutation in the mother can be explained by the fact that a subset of heterozygous females carries an E1-subunit mutation but have mild or no symptoms at all, presumably because of an X-inactivation pattern favoring expression of the normal X chromosome and resulting in a mosaic population of cells (Brown et al. 1994). The mutation has been described before in a 20-year-old male with a different clinical presentation of Leigh’s disease, absence of lactic acidemia, and basal ganglia changes on neuroimaging (Cameron et al. 2004). Our patient was unique to have recurrent demyelination as a manifestation of this mutation in PDH deficiency at such an early age.
Conclusion
PDH deficiency should be considered in patients with unexplained recurrent acute neurological symptoms with lactic acidemia. Long-term prognosis and outcome remain uncertain though thiamine and ketogenic diet are useful.
Acknowledgments
None
Take-Home Message
PDH deficiency should be considered in patients with unexplained recurrent acute neurological symptoms with lactic acidemia.
Author Contribution
Pratibha Singhi is clinician-in-charge, reviewed neuroradiology and electrophysiological data, and also reviewed the manuscript; Linda De Meirleir helped in the diagnosis and genetic studies; Willy Lissens helped in mutational analysis; Sunit Singhi is clinician-in-charge of pediatric intensive care; and Arushi Gahlot Saini helped in patient management, draft of manuscript, and literature search.
Author Guarantor
Dr P Singhi (corresponding author)
Conflict of Interest
None
Financial Disclosure
None
Ethical Approval
Manuscript is a retrospective case report that does not require ethics committee approval at the institution.
Footnotes
Competing interests: None declared
References
- Brown GK, Otero LJ, LeGris M, Brown RM. Pyruvate dehydrogenase deficiency. J Med Genet. 1994;31:875–879. doi: 10.1136/jmg.31.11.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JM, Levandovskiy V, Mackay N, Tein I, Robinson BH. Deficiency of pyruvate dehydrogenase caused by novel and known mutations in the E1alpha subunit. Am J Med Genet A. 2004;131:59–66. doi: 10.1002/ajmg.a.30287. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Kelley B, Wang J, Strauss D, Eagles DA, Bondy CA. A ketogenic diet increases brain insulin-like growth factor receptor and glucose transporter gene expression. Endocrinology. 2003;144:2676–2682. doi: 10.1210/en.2002-0057. [DOI] [PubMed] [Google Scholar]
- Dahl HH, Brown GK, Brown RM, et al. Mutations and polymorphisms in the pyruvate dehydrogenase E1 alpha gene. Hum Mutat. 1992;1:97–102. doi: 10.1002/humu.1380010203. [DOI] [PubMed] [Google Scholar]
- Debray FG, Lambert M, Vanasse M, et al. Intermittent peripheral weakness as the presenting feature of pyruvate dehydrogenase deficiency. Eur J Pediatr. 2006;165:462–466. doi: 10.1007/s00431-006-0104-5. [DOI] [PubMed] [Google Scholar]
- Debray FG, Lambert M, Gagne R, et al. Pyruvate dehydrogenase deficiency presenting as intermittent isolated acute ataxia. Neuropediatrics. 2008;39:20–23. doi: 10.1055/s-2008-1077084. [DOI] [PubMed] [Google Scholar]
- Garcia-Cazorla A, Wolf N, Serrano M, et al. Inborn errors of metabolism and motor disturbances in children. J Inherit Metab Dis. 2009;32:618–629. doi: 10.1007/s10545-009-1194-9. [DOI] [PubMed] [Google Scholar]
- Head RA, de Goede CG, Newton RW, et al. Pyruvate dehydrogenase deficiency presenting as dystonia in childhood. Dev Med Child Neurol. 2004;46:710–712. doi: 10.1111/j.1469-8749.2004.tb00986.x. [DOI] [PubMed] [Google Scholar]
- Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Schaefer JA, Johnson MA, Morris AA, Turnbull DM, Bindoff LA. Presentation and clinical investigation of mitochondrial respiratory chain disease. A study of 51 patients. Brain. 1995;118(Pt 2):339–357. doi: 10.1093/brain/118.2.339. [DOI] [PubMed] [Google Scholar]
- Kossoff E, Zupec-Kania B, Amark P, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50:304–317. doi: 10.1111/j.1528-1167.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- Mitchell G, Larochelle J, Lambert M, et al. Neurologic crises in hereditary tyrosinemia. N Engl J Med. 1990;322:432–437. doi: 10.1056/NEJM199002153220704. [DOI] [PubMed] [Google Scholar]
- Naito E, Ito M, Yokota I, et al. Thiamine-responsive pyruvate dehydrogenase deficiency in two patients caused by a point mutation (F205L and L216F) within the thiamine pyrophosphate binding region. Biochim Biophys Acta. 2002;1588:79–84. doi: 10.1016/S0925-4439(02)00142-4. [DOI] [PubMed] [Google Scholar]
- Patel K, O'Brien T, Subramony S, Shuster J, Stacpoole P. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Molecular Genetics Metab. 2012;105:34–43. doi: 10.1016/j.ymgme.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedel F, Barnerias C, Dubourg O, Desguerres I, Lyon- Caen O, Saudubray JM. Peripheral neuropathy and inborn errors of metabolism in adults. J Inherit Metab Dis. 2007;30:642–653. doi: 10.1007/s10545-007-0684-x. [DOI] [PubMed] [Google Scholar]
- Strassburg HM, Koch J, Mayr J, Sperl W, Boltshauser E. Acute flaccid paralysis as initial symptom in 4 patients with novel E1alpha mutations of the pyruvate dehydrogenase complex. Neuropediatrics. 2006;37:137–141. doi: 10.1055/s-2006-924555. [DOI] [PubMed] [Google Scholar]
- Wexler I, Hemalathu S, McConnell J, et al. Outcome of pyruvate dehydrogenase deficiency treated with ketogenic diets: studies in patients with identical mutations. Neurology. 1997;49:1655–1661. doi: 10.1212/WNL.49.6.1655. [DOI] [PubMed] [Google Scholar]