Abstract
We diagnosed an adrenomyeloneuropathy (AMN) patient with a double novel missense mutation, c.284C>A (p.A95D) and c.290A>T (p.H97L) in a single ABCD1 allele. In skin fibroblasts from the patient, no ABCD1 protein was detected by immunoblot analysis, and the C24:0 β-oxidation activity was decreased to a level at which the ABCD1 protein was absent. To determine the responsible gene mutation in the patient, we constructed three kinds of mutated ABCD1 gene expression vectors (c.284C>A, c.290A>T or c.284C>A/c.290A>T) and transfected them into CHO cells stably expressing GFP-SKL (CHO/GFP-SKL cells) or CADDS fibroblasts lacking the ABCD1 gene. ABCD1 (p.H97L) displayed the correct peroxisomal localization in CHO/GFP-SKL cells, but ABCD1 (p.A95D) and ABCD1 (p.A95D/p.H97L) were diffuse in the cytosol. Furthermore, ABCD1 (p.H97L) was detected by immunoblot analysis and restored the C24:0 β-oxidation activity in the CADDS fibroblasts, as the wild type ABCD1 did. On the other hand, ABCD1 (p.A95D) and ABCD1 (p.A95D/p.H97L) were not detected and the C24:0 β-oxidation activity was not restored. These results clearly show that c.284C>A is the responsible gene mutation, whereas c.290A>T is a novel polymorphism.
Introduction
X-linked adrenoleukodystrophy (X-ALD) (MIM 300100) is a neurodegenerative disease with an incidence of approximately 1:15,000–30,000 males (Kemp et al. 2001; Takemoto et al. 2002; Suzuki et al. 2005). The biochemical characterization of the disease is based on the accumulation of pathognomonic amounts of saturated very long chain fatty acids (VLCFA, >C22) in tissues, including the brain white matter and the adrenal glands as well as skin fibroblasts (Dubois-Dalcq et al. 1999; Smith et al. 1999; Bezman et al. 2001; Moser et al. 2004). The accumulation of VLCFA in X-ALD has been linked to mutations in the ABCD1 gene [MIM 300371] located at Xq28 (Migeon et al. 1981). The gene codes for an mRNA of 4.3 kb, with a protein of 745 amino acids that is referred to as ABCD1/ALDP (Mosser et al. 1993; Kemp et al. 2011). ABCD1 is a 75 kDa peroxisomal membrane protein that belongs to the ATP-binding cassette protein family and is thought to be involved in the transport of saturated very long chain acyl-CoA into peroxisomes (van Roermund et al. 2008; van Roermund et al. 2011; Morita and Imanaka 2012). Dysfunction of the ABCD1 protein results in a reduction of peroxisomal VLCFA β-oxidation, which leads to the accumulation of VLCFA. Biochemical diagnosis of this disease is based on the measurement of plasma VLCFA. However, it has not yet been made clear how the accumulation of VLCFA in tissues is involved in the pathogenesis and progress of the disease.
X-ALD has a widely varied disease phenotype, including childhood cerebral ALD, adolescent cerebral ALD, adrenomyeloneuropathy (AMN), and Addison’s disease (Moser et al. 2007). The only effective therapy for the cerebral form of ALD is hematopoietic stem cell transplantation at the early stages of the manifestation of the cerebral symptoms. Most AMN patients manifest slowly progressive gait disturbances as the initial symptom. The mean age of onset of AMN is reportedly 30.2 (13–51) years in Japan (Takemoto et al. 2002), and about half of these patients exhibit cerebral involvement approximately 10 years after onset. Therefore, it is necessary to determine the ABCD1 mutations in the presymptomatic diagnosis of X-ALD by extended familial screening of the probands.
Thus far, more than 1,200 ABCD1 gene mutations have been reported by mutation analysis (see the X-ALD mutation database listed at http://www.x-ald.nl). Missense mutations comprise more than 60% of them. Among the missense mutations, about in approximately 50% of them mutant ABCD1 proteins were not detected and in ~15% of them the protein levels were reduced in X-ALD fibroblasts, based on immunofluorescence and/or immunoblot analysis. It is thus likely that most of the mutant ABCD1 are misfolded and do not insert into peroxisomal membrane correctly, which subsequently results in degradation by a protein quality control-related mechanism. We previously showed that certain mutant ABCD1 proteins are degraded by proteasomes or additional protease(s) before or after transport to peroxisomes (Takahashi et al. 2007).
To date, 13 instances of multiple mutations have been reported in the X-ALD database. Among them, only two double mutants (c.38A>C and c.649A>G, p.N13T and p.K217E as well as c. 707G>A and c.1534G>A, p.R236H and p.G512S, respectively) have been partially characterized (Dvorakova et al. 2001; Guimaraes et al. 2002). In this study, we identified a novel double missense mutation, c.284C>A and c.290A>T in the ABCD1 gene in a patient with clinical symptoms compatible with AMN. This mutation in exon 1 of the ABCD1 gene leads to an amino acid exchange of alanine to aspartic acid (p.A95D) and histidine to leucine (p.H97L). In this study, we investigated whether these amino acid substitutions affect the function of the ABCD1 protein. The results show that c.284C>A (p.A95D) is a novel disease-causing mutation and c.290A>T (p.H97L) is a novel polymorphism.
Materials and Methods
Materials
[1-14C]lignoceric acid (53 mCi/mmol) was purchased from Moravek Biochemicals (Brea, CA). ECL Plus, a Western blotting detection system, and Fluorolink Cy3 labeled goat anti-rabbit IgG, were purchased from GE Healthcare (Buckinghamshire, England). The mouse anti-human ALDP/ABCD1 monoclonal antibody (MAB2162) was purchased from Millipore (Billerica, MA). The rabbit anti-PMP70/ABCD3 antibody was raised against the COOH-terminal 15 amino acids of rat PMP70/ABCD3 (Imanaka et al. 1996). The rabbit anti-catalase antibody was purchased from Rockland Immunochemicals (Gilbertsville, PA).
Cell Culture
CHO-K1 cells were cultured in F12 medium with 10% FCS containing streptomycin and penicillin at 37°C and 5% CO2. CHO/GFP-SKL cells, which were prepared by the transfection of a pEGFP/SKL vector into CHO cells, were cultured in the same medium containing G418. Human skin fibroblasts from a healthy individual, an ALD patient and a contiguous ABCD1 DXS1357E deletion syndrome (CADDS) patient were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FCS. CADDS were characterized by deletions that extended into the promoter region of ABCD1 and the neighboring gene (DXS1357E) (Corzo et al. 2002).
ABCD1 Gene Analysis
PCR products of the ABCD1 gene were amplified by means of a modification of the method of Boehm et al. (Boehm et al. 1999) using genomic DNA extracted from blood of the patient that covered all of the coding regions and exon-intron junctions of the ABCD1 gene. DNA analysis was based on informed consent.
VLCFA Analysis
VLCFA in serum from the patient were determined using gas chromatography/mass spectrometry (GC/MS), as described previously (Takemoto et al. 2003).
Plasmid Constructions and Transfection
pcDNA4His/ABCD1 was prepared as described previously (Takahashi et al. 2007). The human ABCD1 gene was amplified with a PCR protocol using pcDNA3.1+/ABCD1 (kindly provided by Dr. K. Kamijo, Tohoku University) as the template. The amplification product was cloned into a pcDNA4HisMax vector by TA-cloning, as described in the manufacturer’s instructions. pcDNA4/mutant ABCD1 vectors with the target mutations (c.284 C>A and/or c290 A>T) were prepared with a QuickChange Site-Directed mutagenesis kit (Stratagene, La Jolla, CA) using the pcDNA4/ABCD1 plasmid as the template. The mutations in the constructions were confirmed by DNA sequencing on an ABI PRISM 310 DNA sequencer (Perkin Elmer Life Science, Wellesley, MA). The primers used for the generation of the mutant ABCD1 constructs were as follows: the ABCD1-C284A-F primer: gagacggggctgctggacctgcactcgg, the ABCD1-C284A-R primer: ccgagtgcaggtccagcagccccgtctc, the ABCD1-A290T-F primer: gctgctggccctgctctcggccgccttgg, the ABCD1-A290T-R primer: ccaaggcggccgagagcagggccagcag, the ABCD1-C284A, A290T-F primer: gagacggggctgctggacctgctctcggccgccttg, and the ABCD1-C284A, A290T-R primer: caaggcggccgagagcaggtccagcagccccgtctc.
Transfection of the pcDNA4/mutant ABCD1 vectors into CHO/GFP-SKL cells and human skin fibroblasts was performed using Effectene Transfection Reagent (Qiagen, Valencia, CA) and by electroporation with a Gene Pulser (Bio-Rad), respectively (Takahashi et al. 2007). Two or three days after transfection, cells were fixed for immunofluorescence analysis and harvested for immunoblot analysis or fatty acid β-oxidation assay.
Immunofluorescence
Immunofluorescence analysis was performed as described previously (Takahashi et al., 2007). The cells were fixed with 3% formaldehyde and permeabilized with 1% Triton X-100 or 30 μM digitonin. The primary antibodies used were a rabbit anti-human catalase polyclonal antibody or a rabbit anti-rat ABCD3 polyclonal antibody and a mouse anti-human ABCD1 monoclonal antibody.
Fatty Acid β-Oxidation
β-oxidation was measured essentially as described by Watkins et al. (1991). Human skin fibroblasts were suspended in 0.25 M sucrose containing 1 mM EDTA, and 10 mM Tris–HCl, pH 8.0 were incubated with [1-14C]lignoceric acid solubilized in 0.05 ml of α-cyclodextrin (10 mg/ml) in 10 mM Tris–HCl, pH 8.0 for 60 min at 37°C. The reactions were terminated by the addition of 0.05 ml of 1 N KOH and perchloric acid was added to a final concentration of 6% and the samples were maintained at 4°C for at least 3 h. The mixture was centrifuged at 2,000 rpm for 5 min and the supernatant was subjected to Folch partitioning. The aqueous phase was removed for scintillation counting.
Other Methods
Immunoblotting was performed as described previously using ECL Plus Western blotting detection reagent (Kurisu et al. 2003). The protein concentration was determined by the Lowry method (Lowry et al. 1951) using bovine serum albumin as the standard.
Case Report
We report a 65-year-old male patient. His first reported symptom had been gait difficulty, which first began when he was 40 years old. An original spasticity of the lower extremities increased very slowly. At the age of 60, the patient developed a urinary excretion disturbance. At the age of 65, neurological examination revealed a spasticity of the lower extremities with increased deep tendon reflexes and a positive Babinski sign, with no sensory symptoms. He was still able to walk with a cane. Neuropsychological examination found normal cognitive function. His family history revealed that his nephew had been diagnosed with childhood cerebral ALD at the age of 7. He has suffered from adrenal insufficiency and has been received adrenal hormone therapy for more than 20 years. In addition, his mother and sister had developed spastic paraplegia. They had been diagnosed as being symptomatic female heterozygotes for X-ALD, due to the presence of increased VLCFA levels in the serum.
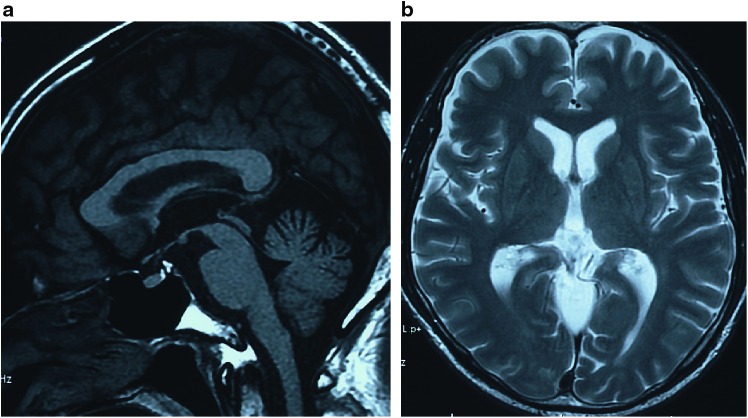
Routine laboratory studies were normal. The plasma concentrations of ACTH and cortisol were normal and tests for antinuclear antibodies were negative. Serology for syphilis, HTLV-I, or HIV was negative. The VLCFA ratios of C24:0/C22:0, C25:0/C22:0, and C26:0/C22:0 were markedly elevated in plasma from the patient (1.24, 0.044 and 0.046) compared to the control (1.05 ± 0.16, 0.024 ± 0.006 and 0.012 ± 0.005), respectively. Motor and sensory nerve conduction studies revealed a slightly decreased conduction velocity in the posterior tibial nerves on both sides. Somatosensory evoked potentials recorded after median and posterior tibial nerve stimulation displayed a prolonged central sensory conduction time bilaterally. Magnetic resonance imaging (MRI) of the brain revealed mild cerebellar atrophy. On T2-weighted images, no white matter abnormalities were found (Fig. 1). An MRI of the spinal cord revealed mild atrophy in the upper thoracic spinal cord.
Fig. 1.
An MRI image in the X-ALD patient. (a) A T1-weighted midsagittal image shows mild atrophy of the cerebellum. (b) A T2-weighted axial image shows there are no definite signal intensity changes in the cerebral white matter
Results and Discussion
Analysis of ABCD1 Gene in the Patient
Genomic DNA was extracted from the patient’s white blood cells. DNA sequence analysis demonstrated two novel, non-synonymous variants in the ABCD1 gene (c.284C>A, c.290A>T, leading to p.A95D, p.H97L changes, respectively). Another missense mutation (p.H97P) has been reported in amino acid residue in the X-ALD database, but the mutation in c.284C>A (p.A95D) and c.290A>T (p.H97L) has not been previously reported. p.A95D and p.H97L are located at a distance of only one amino acid residue and are located in TMD1, as deduced from a hydrophobicity plot.
Expression of the ABCD1 Proteins and VLCFA β-Oxidation in Fibroblasts from the X-ALD Patient
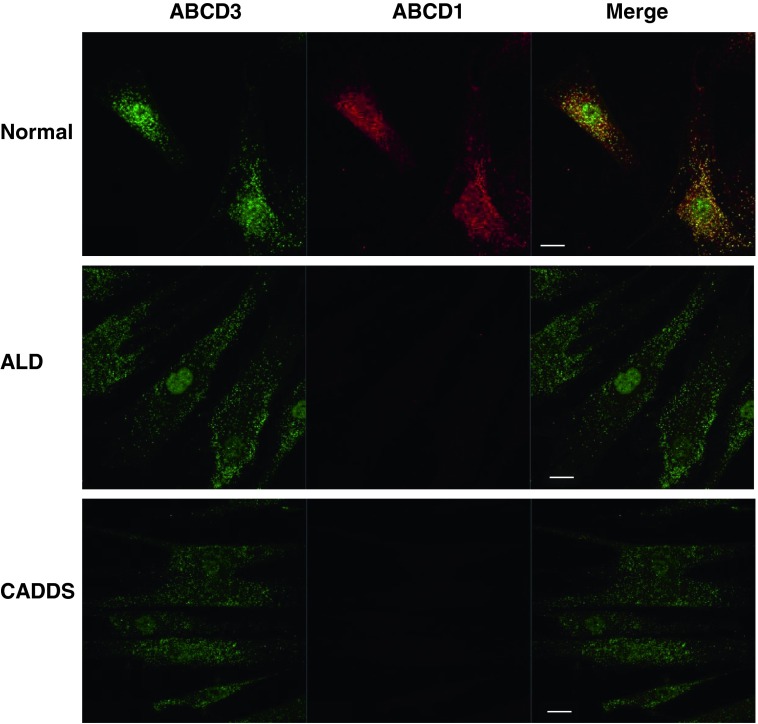
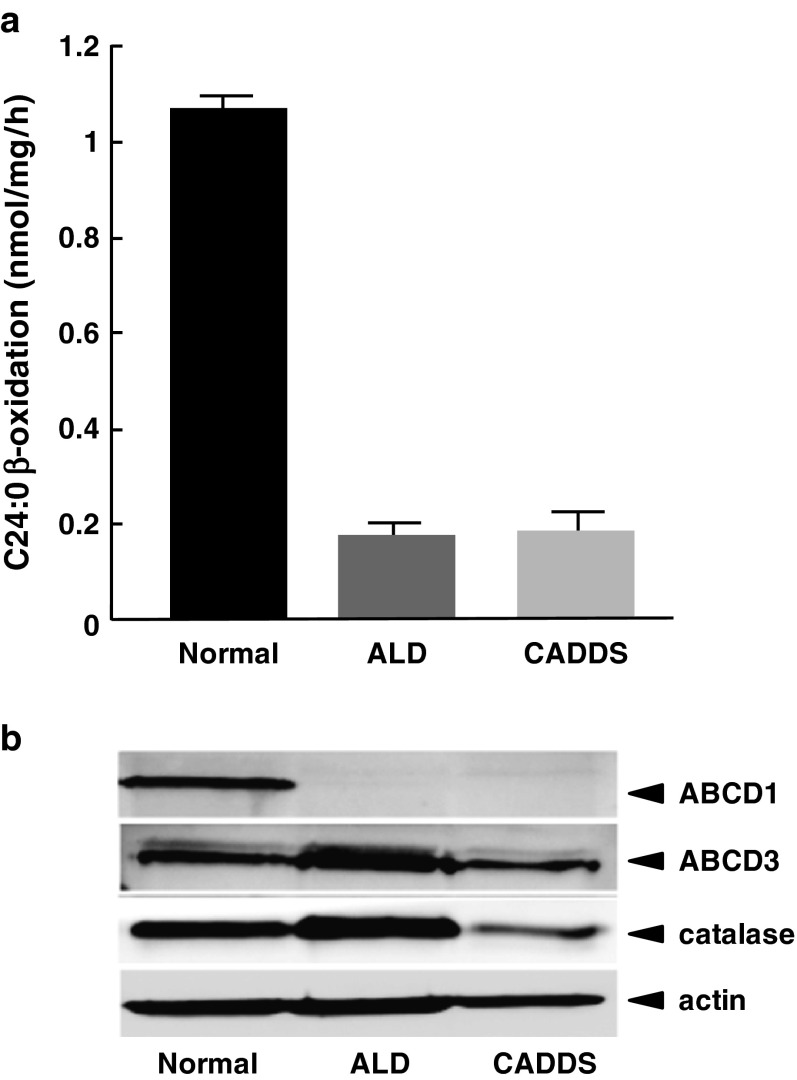
We first analyzed the expression of the mutant ABCD1 protein in skin fibroblasts from the patient by immunofluorescence analysis. Peroxisomes were stained with an anti-ABCD3 antibody that recognized the ABCD3 protein, which is a main peroxisomal membrane protein. In this experiment, CADDS fibroblasts were used as negative control because they lack the ABCD1 gene. As shown in Fig. 2, normal, X-ALD, and CADDS fibroblasts exhibited ABCD3-positive immunofluorescent dots (green), suggesting the presence of intact peroxisomes in these cells. In normal fibroblasts, ABCD1-positive dots were detected and superimposed with peroxisomes. In contrast, no immunofluorescent dots were observed in X-ALD fibroblasts or CADDS fibroblasts. In the X-ALD fibroblasts, the C24:0 β-oxidation activity (0.18 ± 0.02 nmol/mg/h) was less than 20% of the normal fibroblasts (1.07 ± 0.05 nmol/mg/h), and this activity was similar to that of CADDS fibroblasts (0.19 ± 0.03 nmol/mg/h) (Fig. 3a), while the ABCD1 protein in the X-ALD fibroblasts was scarcely detected by immunoblot analysis. Taken together, the mutant ABCD1 protein with the double mutation (p.A95D and p.H97L) was not functional, because the mutant ABCD1 protein was degraded in the cytosol, resulting in a reduction of peroxisomal fatty acid β-oxidation.
Fig. 2.
Immunofluorescence analysis of ABCD1 expression in normal, X-ALD, and CADDS fibroblasts. Expression of mutant ABCD1 proteins in fibroblasts was analyzed by immunofluorescence analysis. Normal, X-ALD, and CADDS fibroblasts were stained with anti-ABCD3 and anti-ABCD1 antibodies followed by secondary antibodies conjugated with the dye reagents. Fluorescent dots were observed under confocal microscopy. Bar = 20 μm. ABCD1 protein was not detected in X-ALD and CADDS fibroblasts
Fig. 3.
VLCFA β-oxidation activity and ABCD1 protein expression in X-ALD fibroblasts. VLCFA β-oxidation activity in the normal, ALD, βand CADDS fibroblasts was measured using [1-14C]C24:0 as substrate (a). Results are the means ± S.D.; n = 3. Expression of ABCD1 proteins in each of the cells was analyzed by immunoblot analysis (b). The C24:0 β-oxidation was less than 20% of the normal fibroblasts and the ABCD1 protein was not detected in the X-ALD fibroblasts
To investigate in detail which of the missense mutations is a cause of X-ALD phenotype, we investigated the expression level, subcellular localization, and restoration of defected β-oxidation activity by ABCD1 (p.A95D), ABCD1 (pH97L), and ABCD1 (p.A95D/p.H97L). CHO/GFP-SKL cells are useful for the analysis of peroxisomal localization of an expressed protein. On the other hand, CADDS fibroblasts lacking the ABCD1 protein are beneficial to examine whether mutant ABCD1 is functional by transfection with the corresponding mutant ABCD1 gene.
Impact of the p.A95D or p.H97L Substitution on the Stability of ABCD1 Protein
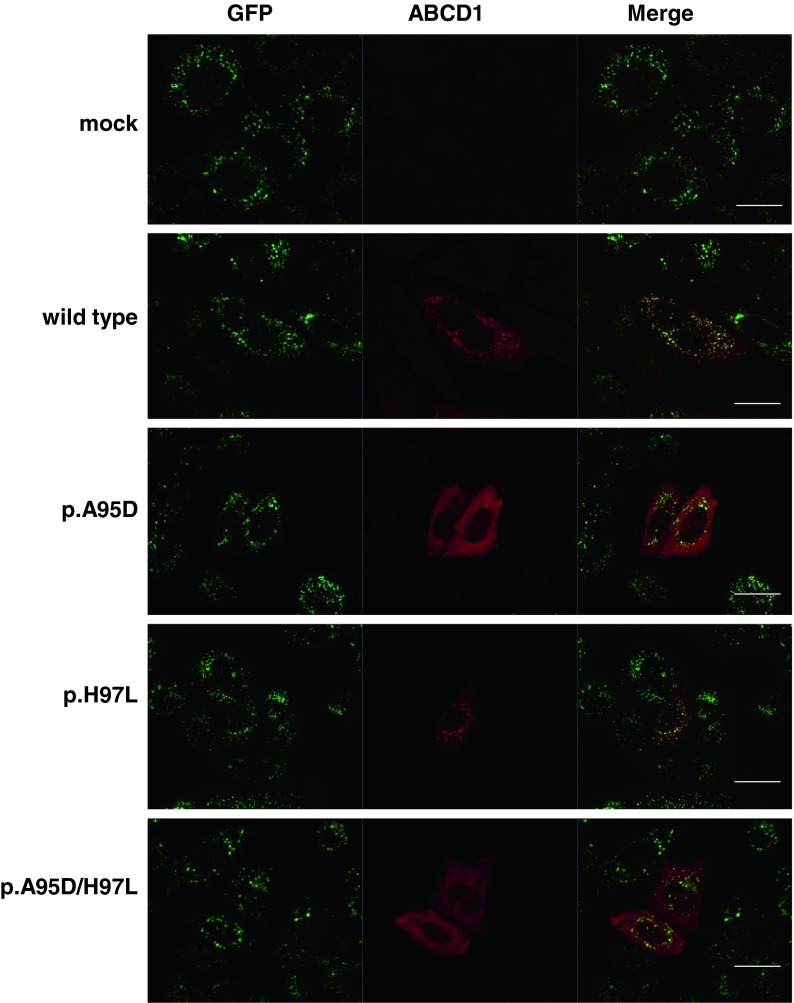
To determine which missense mutation causes the ABCD1 protein instability, we expressed each mutant ABCD1 protein with the p.A95D, p.H97L, or p.A95D/p.H97L mutation in CHO/GFP-SKL cells. The cells express GFP in fusion with the serine-lysine-leucine (SKL) sequence at the C-terminal, which is a targeting signal for peroxisomal localization. As shown in Fig. 4, peroxisomes were detected as green fluorescent dots in the CHO/GFP-SKL cells. Three mutant ABCD1 proteins were transiently expressed in the CHO/GFP-SKL cells and subjected to immunofluorescence analysis with an anti-human ABCD1 antibody. When wild type (WT) ABCD1 was expressed, ABCD1-positive dots (indicated by the red dots) were detected and superimposed with green fluorescent dots, suggesting that WT ABCD1 correctly localized to peroxisomes. Like the WT ABCD1, the mutant ABCD1 (p.H97L) also localized to peroxisomes. In contrast, when the mutants ABCD1 (p.A95D) or ABCD1 (p.A95D/p.H97L) were expressed in CHO/GFP-SKL cells, fluorescent dots were not formed, and the fluorescence was diffuse in the cytosol. Since the mutants ABCD1 (p.A95D) and ABCD1 (p.A95D/p.H97L) were scarcely detected by immunoblot analysis in fibroblasts (as shown in Figs. 3b and 5b), it is likely that these diffused fluorescence signals were the result of the non-degraded mutant ABCD1 proteins due to the overexpression in CHO/GFP-SKL cells. Taken together, the instability of the mutant ABCD1 with the double mutation is caused by the missense mutation at c.284C>A (p.A95D) but not c.290A>T (p.H97L).
Fig. 4.
Expression of mutant ABCD1 proteins in CHO/GFP-SKL cells. CHO/GFP-SKL cells were transfected with empty vector, pcDNA4/WT ABCD1 (wild type), or pcDNA4/mutant ABCD1 (p.A95D, p.H97L, or p.A95D/p.H97L). After the transfection, cells on the coverslips were fixed and subjected to immunofluorescence analysis. The expressed GFP-SKL proteins that localized in the peroxisomes were detected as green fluorescent dots. The expressed ABCD1 proteins were detected using an anti-human ABCD1 antibody followed by secondary antibodies conjugated with the dye reagent. Bar = 20 μm. The mutant ABCD1 (p.H97L) was localized to peroxisomes, but the mutants ABCD1 (p.A95D) and ABCD1 (p.A95D/p.H97L) were diffuse in the cytosol
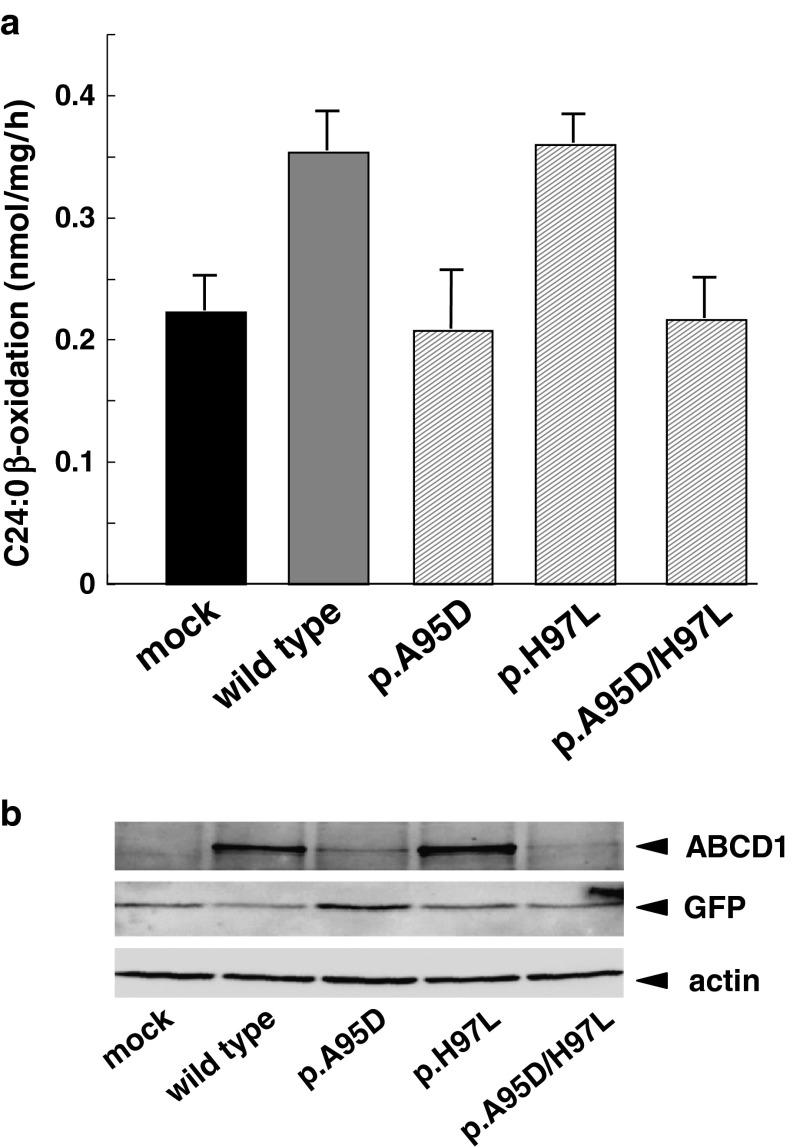
Fig. 5.
C24:0 β-oxidation activity in CADDS fibroblasts expressing mutant ABCD1 proteins. C24:0 β-oxidation activity in the CADDS fibroblasts expressing mutant ABCD1s was analyzed as in Fig. 3. CADDS fibroblasts were transfected with empty vector, pcDNA4/WT ABCD1 (wild type) or each pcDNA4/mutant ABCD1 (p.A95D, p.H97L, p.A95D/p.H97L). After 3-day transfection, the cells were harvested for determination of the C24:0 β-oxidation activities (a) and immunoblotting (b). Results are means ± S.D.; n = 3. The mutant ABCD1 (p.H97L) was expressed in CADDS fibroblasts and the C24:0 β-oxidation activity was recovered as wild type ABCD1. In contrast, the mutants ABCD1 (p.A95D) and ABCD1 (p.A95D/p.H97L) were scarcely expressed and the C24:0 β-oxidation activities were not recovered
Impact of the p.A95D or p.H97L Substitution on VLCFA β-Oxidation Activity
In contrast to the mutant ABCD1 (p.A95D), which was degraded in cytosol, the mutant ABCD1 (p.H97L) was correctly localized in peroxisomes. To determine whether the mutant ABCD1 (p.H97L) is functional, we next expressed the mutant ABCD1 proteins in the CADDS fibroblasts lacking the ABCD1 protein. In the CADDS fibroblasts, the C24:0 β-oxidation activity was approximately 0.2 nmol/mg/h, as shown in Figs. 2 and 5a. When WT ABCD1 was expressed in the CADDS fibroblasts, the C24:0 β-oxidation activity was increased to approximately 0.35 nmol/mg/h (Fig. 5a). Similar results were observed by expressing mutant ABCD1 (p.H97L). As expected, no increase was observed by expressing mutant ABCD1 (p.A95D) or ABCD1 (p.A95D/p.H97L). These results are in good agreement with the results that WT ABCD1 and mutant ABCD1 (p.H97L) were clearly detected by immunoblot analysis, but mutant ABCD1 (p.A95D) and ABCD1 (p.A95D/p.H97L) were only faintly detected (Fig. 5b). The transfection efficiencies for the ABCD1 variants were 20–30%. Taken together, we conclude that mutation at c.284C>A (p.A95D) is the cause of the X-ALD phenotype, but the mutation at c.290A>T (p.H97L) is a genetic polymorphism.
Concerning multiple mutations of ABCD1, some of the features have been characterized in two cases: ABCD1 (p.N13T/p.K217E) (Dvorakova et al. 2001) and ABCD1 (p.R236H/p.G512S) (Guimaraes et al. 2002). In the case of ABCD1 (p.N13T/p.K217E), p.K217E was suggested to be the disease-causing mutation and p.N13T a polymorphism, since p.K217E, but not p.N13T, failed to restore defective VLCFA β-oxidation in X-ALD fibroblasts. However, the expression level of p.K217E was not examined. In the case of ABCD1 (p.R236H/p.G512S), p.G512S has already described as being associated with X-ALD, but it has not yet been determined whether p.R236H is a disease-causing mutation (Guimaraes et al. 2002). No data was reported on the expression of p.R236H, p.G512S, or p.R236H/p.G512S.
With regard to ABCD1 mutation, there is a cluster of mutations associated with X-ALD in TMD1: p.A95D (in this study), p.H97P, p.S98P, p.S98L, p.S98W, p.A99D (the X-ALD mutation database listed at http://www.x-ald.nl). In contrast, p.H97L in this study was shown to be a polymorphism. It has been suggested that post-translated ABCD1 associates with Pex19p in the cytosol targets the peroxisomal membranes and is inserted into the membranes through Pex3p. The NH2-terminal Pex19p-binding region (aa.68-82) and TMD1 are involved in the targeting of the peroxisomal membranes (Landgraf et al. 2003; Halbach et al. 2005). It is of interest to determine whether and how each mutation in the cluster in TMD1 disturbs the targeting.
In any event, for the determination of the disease-causing mutations of ABCD1 an extended familial screening of the probands is needed for effective bone marrow transplantation at the early stages of the cerebral symptoms. In addition, polymorphism analysis is required for the determination of the genetic status of at-risk individuals. The protocol presented here is useful for identifying the genetic polymorphisms and improving the quality of genetic counseling in the prenatal diagnosis.
Acknowledgments
This research was supported in part by a Grant-in-Aid for Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan, and for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20590054, 22590060, 23590072). Pacific Edit reviewed the manuscript prior to submission.
Footnotes
Competing interests: None declared
References
- Bezman L, Moser AB, Raymond GV, et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol. 2001;49:512–517. doi: 10.1002/ana.101. [DOI] [PubMed] [Google Scholar]
- Boehm CD, Cutting GR, Lachtermacher MB, Moser HW, Chong SS. Accurate DNA-based diagnostic and carrier testing for X-linked adrenoleukodystrophy. Mol Genet Metab. 1999;66:128–136. doi: 10.1006/mgme.1998.2779. [DOI] [PubMed] [Google Scholar]
- Corzo D, Gibson W, Johnson K, et al. Contiguous deletion of the X-linked adrenoleukodystrophy gene (ABCD1) and DXS1357E: a novel neonatal phenotype similar to peroxisomal biogenesis disorders. Am J Hum Genet. 2002;70:1520–1531. doi: 10.1086/340849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M, Feigenbaum V, Aubourg P. The neurobiology of X-linked adrenoleukodystrophy, a demyelinating peroxisomal disorder. Trends Neurosci. 1999;22:4–12. doi: 10.1016/S0166-2236(98)01319-8. [DOI] [PubMed] [Google Scholar]
- Dvorakova L, Storkanova G, Unterrainer G, et al. Eight novel ABCD1 gene mutations and three polymorphisms in patients with X-linked adrenoleukodystrophy: The first polymorphism causing an amino acid exchange. Hum Mutat. 2001;18:52–60. doi: 10.1002/humu.1149. [DOI] [PubMed] [Google Scholar]
- Guimaraes CP, Lemos M, Sa-Miranda C, Azevedo JE. Molecular characterization of 21 X-ALD Portuguese families: identification of eight novel mutations in the ABCD1 gene. Mol Genet Metab. 2002;76:62–67. doi: 10.1016/S1096-7192(02)00023-9. [DOI] [PubMed] [Google Scholar]
- Halbach A, Lorenzen S, Landgraf C, Volkmer-Engert R, Erdmann R, Rottensteiner H. Function of the PEX19-binding site of human adrenoleukodystrophy protein as targeting motif in man and yeast.PMP targeting is evolutionarily conserved. J Biol Chem. 2005;280:21176–21182. doi: 10.1074/jbc.M501750200. [DOI] [PubMed] [Google Scholar]
- Imanaka T, Shiina Y, Takano T, Hashimoto T, Osumi T. Insertion of the 70-kDa peroxisomal membrane protein into peroxisomal membranes in vivo and in vitro. J Biol Chem. 1996;271:3706–3713. doi: 10.1074/jbc.271.7.3706. [DOI] [PubMed] [Google Scholar]
- Kemp S, Pujol A, Waterham HR, et al. ABCD1 mutations and the X-linked adrenoleukodystrophy mutation database: role in diagnosis and clinical correlations. Hum Mutat. 2001;18:499–515. doi: 10.1002/humu.1227. [DOI] [PubMed] [Google Scholar]
- Kemp S, Theodoulou FL, Wanders RJ, et al. Mammalian peroxisomal ABC transporters: from endogenous substrates to pathology and clinical significance. Br J pharmacol. 2011;164:1753–1766. doi: 10.1111/j.1476-5381.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurisu M, Morita M, Kashiwayama Y, et al. Existence of catalase-less peroxisomes in Sf21 insect cells. Biochem Biophys Res Commun. 2003;306:169–176. doi: 10.1016/S0006-291X(03)00913-6. [DOI] [PubMed] [Google Scholar]
- Landgraf P, Mayerhofer PU, Polanetz R, Roscher AA, Holzinger A. Targeting of the human adrenoleukodystrophy protein to the peroxisomal membrane by an internal region containing a highly conserved motif. Eur J Cell Biol. 2003;82:401–410. doi: 10.1078/0171-9335-00331. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Migeon BR, Moser HW, Moser AB, Axelman J, Sillence D, Norum RA. Adrenoleukodystrophy: evidence for X linkage, inactivation, and selection favoring the mutant allele in heterozygous cells. Proc Nati Acad Sci U S A. 1981;78:5066–5070. doi: 10.1073/pnas.78.8.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Imanaka T. Peroxisomal ABC transporters: structure, function and role in disease. Biochim Biophys Acta. 2012;1822:1387–1396. doi: 10.1016/j.bbadis.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Moser HW, Fatemi A, Zackowski K, et al. Evaluation of therapy of X-linked adrenoleukodystrophy. Neurochem Res. 2004;29:1003–1016. doi: 10.1023/B:NERE.0000021245.12181.90. [DOI] [PubMed] [Google Scholar]
- Moser HW, Mahmood A, Raymond GV. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. 2007;3:140–151. doi: 10.1038/ncpneuro0421. [DOI] [PubMed] [Google Scholar]
- Mosser J, Douar AM, Sarde CO, et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- Smith KD, Kemp S, Braiterman LT, et al. X-linked adrenoleukodystrophy: genes, mutations, and phenotypes. Neurochem Res. 1999;24:521–535. doi: 10.1023/A:1022535930009. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Takemoto Y, Shimozawa N, et al. Natural history of X-linked adrenoleukodystrophy in Japan. Brain Dev. 2005;27:353–357. doi: 10.1016/j.braindev.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Morita M, Maeda T, et al. Adrenoleukodystrophy: subcellular localization and degradation of adrenoleukodystrophy protein (ALDP/ABCD1) with naturally occurring missense mutations. J Neurochem. 2007;101:1632–1643. doi: 10.1111/j.1471-4159.2007.04457.x. [DOI] [PubMed] [Google Scholar]
- Takemoto Y, Suzuki Y, Horibe R, Shimozawa N, Wanders RJ, Kondo N. Gas chromatography/mass spectrometry analysis of very long chain fatty acids, docosahexaenoic acid, phytanic acid and plasmalogen for the screening of peroxisomal disorders. Brain Dev. 2003;25:481–487. doi: 10.1016/S0387-7604(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Takemoto Y, Suzuki Y, Tamakoshi A, et al. Epidemiology of X-linked adrenoleukodystrophy in Japan. J Hum Genet. 2002;47:590–593. doi: 10.1007/s100380200090. [DOI] [PubMed] [Google Scholar]
- van Roermund CW, Visser WF, Ijlst L, et al. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 2008;22:4201–4208. doi: 10.1096/fj.08-110866. [DOI] [PubMed] [Google Scholar]
- van Roermund CW, Visser WF, Ijlst L, Waterham HR, Wanders RJ. Differential substrate specificities of human ABCD1 and ABCD2 in peroxisomal fatty acid β-oxidation. Biochim Biophys Acta. 2011;1811:148–152. doi: 10.1016/j.bbalip.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Watkins PA, Ferrell EV, Jr, Pedersen JI, Hoefler G. Peroxisomal fatty acid β-oxidation in HepG2 cells. Arch Biochem Biophys. 1991;289:329–336. doi: 10.1016/0003-9861(91)90419-J. [DOI] [PubMed] [Google Scholar]