Abstract
Background: Serious cardiac valve disease and left ventricular hypertrophy occur in most untreated older children with severe mucopolysaccharidosis type I. Although it is assumed that early intervention prevents these processes, evaluation of cardiac findings in these infants has not yet been reported.
Methods: We reviewed echocardiograms of 13 untreated infants < 1 year of age with severe mucopolysaccharidosis type I who had undergone evaluation for hematopoietic cell transplantation. We recorded left ventricular chamber dimensions, septal and posterior wall thicknesses, ventricular function, and aortic sinus diameters. We evaluated mitral and aortic valves for increased thickness, regurgitation, and stenosis.
Results: Average age (7M, 6F) was 221 (range 25–347) days. Left ventricular chamber dimension was ≥2 SD of normal in 3/13; wall thicknesses were ≥2 SD of normal in 2/13 infants. Systolic function was normal. Mitral valves were thickened in all infants; mitral regurgitation was present in 9/13, but significant in only three infants. Aortic valves were thickened in 10/13, but no infant had significant aortic regurgitation. Neither mitral nor aortic stenosis occurred. Aortic roots were dilated to ≥2 SD of normal in 5/13.
Conclusions: Characteristic cardiac features of severe mucopolysaccharidosis type I can be seen in infancy. Mitral and aortic valve thickening are nearly universally present, even in the youngest infants. In 20–30 % of infants, other abnormalities such as left ventricular dilation, increased wall thickness, and mild mitral/aortic regurgitation may occur. Aortic root dilation is a frequent finding. Early intervention with enzyme replacement therapy may minimize the incidence and severity of cardiac findings in these infants.
Summary: Serious cardiac valve disease and left ventricular hypertrophy occur in most untreated older children with severe mucopolysaccharidosis type I. Although it is assumed that early intervention prevents these processes, evaluation of cardiac findings in these infants has not yet been reported. In our study of 13 infants with severe untreated MPS I < 1 year of age, mitral and aortic valve thickening was nearly universally present and aortic root dilation was frequent. Despite this, we found a lower incidence of left ventricular hypertrophy and both a lower incidence and milder expression of mitral and aortic valve dysfunction than previously reported in older children. These findings suggest that earlier intervention, including neonatal screening, may be of benefit to children with severe MPS I.
Introduction
Severe mucopolysaccharidosis type I (MPS I, OMIM # 607014) is a lethal autosomal recessive disorder that is caused by mutation of the genes encoding α-l-iduronidase (IDUA), an enzyme responsible for the degradation of glycosaminoglycan (GAG). GAGs are ubiquitous and essential components of virtually all mammalian tissue (Neufeld and Muenzer 2001). GAG deposition in severe MPS I may occur in the central nervous system leading to blindness, deafness, increased intracranial pressure, and mental retardation; in the joints and bones leading to short stature, dysostosis multiplex, and stiff and poorly mobile joints; and in the airways and soft tissues leading to sleep apnea and other respiratory difficulties. GAG deposition in the heart is associated with myocardial hypertrophy, narrowing of the coronary arteries, aortic root dilation, dilated cardiomyopathy, and thickened aortic and mitral valves resulting in stenosis and/or regurgitation (Braunlin 2012).
The progressive nature of severe MPS I is well established (Neufeld and Muenzer 2001), and it is becoming evident that neither hematopoietic cell transplant (HCT) nor enzyme replacement therapy (ERT) can reverse some pathological processes such as dysostosis multiplex (Weisstein et al. 2004; Sifuentes et al. 2007), corneal clouding (Gillingsrud et al. 1998), and cardiac valve abnormalities (Braunlin et al. 2003, 2006) in older children. The initiative for earlier diagnosis (e.g., newborn screening) and treatment of severe MPS I has been motivated by recent case reports which have shown that ERT, when given to infants less than 1 year of age, may prevent, delay, or attenuate the course of the disease (Gabrielli et al. 2010; McGill et al. 2010; Tylki-Szymanska et al. 2012). The success of this initiative will depend upon the incidence and severity of cardiac pathology found in untreated infants < 1 year of age with severe MPS I; evaluating these issues forms the basis of this study.
Methods
Patients. This study was reviewed by the Institutional Review Board of the University of Minnesota and deemed an exempted study. The files of the University of Minnesota Bone Marrow Transplant Database were reviewed for patients with severe MPS I who were (1) treated with HCT; (2) had undergone mutation analysis; (3) had an initial echocardiogram obtained at < 1 year of age prior to any therapy (e.g., ERT); and, (4) had an echo available for review. Additional parameters assessed included urinary GAG analysis and leukocyte enzyme analysis. Since 2005, all patients referred for HCT have undergone mutation analysis and cardiac ultrasounds have been digitally recorded and stored. Height and weight were recorded within 24 h of performance of ultrasound and body surface area (BSA) was calculated according to the method of Haycock (Lopez et al. 2010). Cardiac ultrasounds were obtained in un-sedated infants using either Sonos 5500 or iE33 cardiac ultrasound equipment (Phillips Corporation, Bothell, Washington, USA) with 8 and 12 MHz transducers. All echocardiograms were reviewed by two of the authors (EB, JMB) and compared to the reports generated for the medical record. Interpretation differences between the original report and the reviews for this study were minimal and resolved by discussion between the two authors.
Chamber dimensions and function. All studies included two-dimensional imaging and M-mode measurement of left ventricular chamber dimensions in diastole and systole (LVID and LVIS, respectively) and left ventricular septal and posterior wall thicknesses in diastole (IVSd and LVPWd, respectively). Shortening fraction (SF) was calculated following standard guidelines (Lopez et al. 2010). BSA-based Z-scores were determined for each infant’s cardiac dimensions (LVID, LVPWd, and IVSd) using standard methods (http://parameterz.blogspot.com), where, for a given BSA, a Z-score of 0 indicates the mean value, and a Z-score of +2 indicates a value 2 standard deviations greater than expected.
Cardiac valves. Detailed two-dimensional and M-mode imaging of mitral and aortic valves was performed to evaluate for increased thickness, which was graded as absent or present. Spectral and color-flow Doppler interrogation of aortic and mitral valves was performed in standard views (Lai et al. 2006). Mitral and aortic valve regurgitation was qualitatively graded from color-flow images as absent, trace (central color jet <5 % diameter and length of the left atrium or ventricle, respectively), mild (central color jet between 5 % and 20 % diameter and length of left atrium or ventricle, respectively), or moderate (central color jet >20 % diameter and length of left atrium or ventricle, respectively). The presence of mitral or aortic stenosis was determined by comparison of pulsed-Doppler measurement of peak E-wave flow signal across the mitral valve and peak systolic flow signal across the aortic valve to established normal pediatric values (Hatle and Angelsen 1985).
Aortic root. Measurement of the aortic root at the aortic sinuses was performed during peak systole (inner wall to inner wall) by two-dimensional imaging according to ASE guidelines (Lopez et al. 2010). BSA-based Z-scores were calculated from each of these measurements, as previously described.
Statistics. Analysis of differences between groups were made using either Fisher’s exact test for analysis of 2 x 2 contingency tables and unpaired Student t-test for continuous variables with significance found when p < 0.05 (http://graphpad.com).
Results
Between 2005 and 2012, there were 13 infants (6F, 7M) ranging from 25 to 347 (mean 221) days of age who presented for evaluation prior to HCT and all fulfilled the criteria for this study (Table 1). Males were older, but not significantly so, than females (267 ± 67 vs 165 ± 116 days, p = 0.0757). The mean heights of both male and female infants (72.1 ± 2.2 and 65.4 ± 9.0 cm, respectively) were at the 75th percentile for age. The mean weights for males and females (8.8 ± 1.4 kg and 7.3 ± 2.4 kg, respectively) corresponded to the 50th and 75th percentiles for age. Urinary GAG excretion (N = 10) was elevated and leukocyte IDUA levels (N = 13, range 0–2 nmol 4MU/h/mg protein) were markedly low (Table 1) consistent with the diagnosis of severe MPS I. IDUA analysis demonstrated mutations known to be associated with severe MPS I: 7 infants were homozygous for either W402X or Q70X; 2 were compound heterozygous for W402X/Q70X; 2 were compound heterozygous for Q70X/c386-2A>G; and one each with Q70X/35del12 and W402X/Y167X.
Table 1.
Demographics, urinary GAGs, leukocyte α-l-iduronidase levels, and IDUA mutations of infants <1 year of age with severe untreated MPS I
| Patient | Gender | Age at echo (Days) | u-GAG | IDUA activity (nmol 4MU/h/mg protein) | Mutation 1 | Mutation 2 |
|---|---|---|---|---|---|---|
| 1 | M | 134 | 1645.44* | 0 | Q70X | 35del12 |
| 2 | M | 262 | 114.7** | 0 | W402X | W402X |
| 3 | F | 212 | 135.4** | 0 | W402X | W402X |
| 4 | M | 319 | 172.4** | 0 | Y167X | W402X |
| 5 | M | 323 | 186** | 2 | W402X | W402X |
| 6 | F | 347 | 1300** | 0.7 | W402X | W402X |
| 7 | M | 320 | N/A | 1.1 | Q70X | Q70X |
| 8 | F | 178 | 116** | 0.5 | Q70X | W402X |
| 9 | F | 57 | N/A | 0.6 | Q70X | Q70X |
| 10 | M | 256 | 311** | 0 | Q70X | c386-2A>G |
| 11 | F | 180 | 80.9** | 0.5 | Q70X | W402X |
| 12 | M | 256 | 366-488* | 0 | Q70X | C386-2A>G |
| 13 | F | 25 | N/A | 0.5 | W402X | W402X |
*mg GAG/g creatinine
**mg GAG/mmol creatinine
Mean Z-scores for left ventricular chamber dimensions (LVID), left ventricular wall thicknesses (LVPWd, IVSd), and aortic sinus internal dimensions were all within 2 standard deviations of normal (Table 2); mean shortening fraction was within normal limits. Seven infants had values more than 2 standard deviations greater than normal for one or more of the dimensions (LVID, LVPWd, IVSd, or Aortic Sinus diameter), but none were significantly related to gender, age (≤180 D vs >180 D), or specific mutation (data not shown). The child with the greatest single Z-score (Case 7, LVID-Z-score of 3.37) had a SF at the lower limits of normal, but left ventricular performance appeared depressed and this was confirmed by a depressed measured ejection fraction of 36 %.
Table 2.
Mean and range for Z-scores of left ventricular dimensions and shortening fraction (SF) from cardiac ultrasounds in 13 infants <1 year of age with severe untreated MPS I
| Parameter (number measured) | Mean value | Range of individual values |
|---|---|---|
| Mean Z-score LVID (13) | 1.26 ± 0.86 | 0.45–3.37 |
| Mean Z-score LVPWd (13) | 0.97 ± 1.2 | −1.11–2.61 |
| Mean Z-score IVSd (13) | 0.38 ± 1.05 | −1.47–2.25 |
| Mean Z-score Aortic Sinus (13) | 1.55 ± 0.92 | 0.1–2.48 |
| Mean SF (13) | 35.1 ± 4.8 | 29–45 |
Increased mitral valve thickness was universally present (Table 3, Fig. 1a–c). Despite this finding, mitral regurgitation was either absent or insignificant (trace) in 10/13 (77 %) patients. Significant (mild, or mild to moderate) mitral regurgitation was documented in 3/13 (23 %) infants (Fig. 2a–c), and was found as early as 25 days of age. Mitral valve stenosis was not identified. Normal mitral inflow patterns (E wave >A wave) were present in all infants (data not shown); mean E-wave inflow velocity of 0.85 m/s (equivalent to a peak inflow gradient of 2.9 mmHg) was well within the normal limits of 1.3 m/s (6.8 mmHg) recognized in pediatrics (Hatle and Angelsen 1985).
Table 3.
Summary of mitral/aortic valve findings seen on cardiac ultrasounds in infants <1 year of age with severe untreated MPS I
| Number affected | Doppler velocity mean (range) | |
|---|---|---|
| Mitral valve | ||
| Incidence thickened mitral valve | 13/13 (100 %) | - |
| Incidence of mitral regurgitation None Trace Mild or moderate |
4/13 (31 %) 6/13 (46 %) 3/13 (23 %) |
- |
| Mitral valve stenosis E-wave flow signal (m/s) |
0/13 (0 %) 13 |
0.85 ± 0.16 (0.6–1.1) |
| Aortic valve | ||
| Incidence thickened aortic valve | 10/13 (77 %) | - |
| Incidence aortic regurgitation None Trace |
8/13 (62 %) 5/13 (38 %) |
- |
| Aortic valve stenosis Peak systolic flow signal (m/s) |
0/11 (0 %) 11 |
0.84 ± 0.22 (0.49–1.14) |
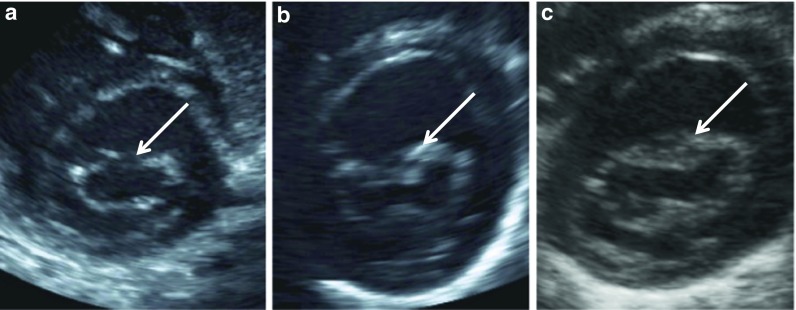
Fig. 1.
(a–c). Two-dimensional cardiac ultrasound images of mitral valve in diastole from (a) normal 7 month old infant; (b) 25 day old infant with severe untreated MPS I, and (c) 256 day old infant with severe untreated MPS I. Note increased thickening of mitral valve, especially in central portion of leaflets (arrows) when compared to normal valve
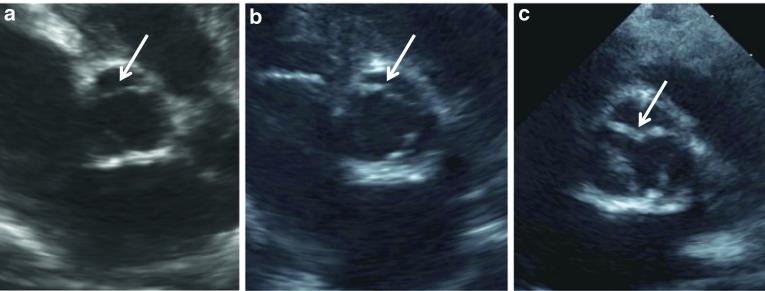
Fig. 2.
(a–c). Color Doppler images from the left ventricle showing moderate mitral regurgitation (arrows) in: (a) a 212 day infant; and mild mitral valve regurgitation from: (b) a 212 day old infant and (c) a 25 day old infant, all with untreated severe MPS I
Increased aortic valve thickness occurred in 10/13 infants (77 %) (Table 4, Fig. 3a–c), but aortic regurgitation was either absent (8 infants; 62 %) or insignificant/trace (5 infants; 38 %) (Fig. 4). Aortic valve stenosis was not present. The mean value for peak flow across the aortic valve of 0.84 m/s (equivalent to a gradient of 2.8 mmHg) was well within normal limits of 1.8 m/s (13 mmHg) recognized in pediatrics (Hatle and Angelsen 1985).
Table 4.
Comparison of mean Z-scores of cardiac dimensions and cardiac function between infants >180 days and those ≤180 days of age with severe untreated MPS I
| Parameter | ≤ 180 D (N = 5) | > 180 D (N = 8) | p-value |
|---|---|---|---|
| Age | 114.8±70.7 | 286.9±46.6 | 0.0002 |
| BSA | 0.33±0.05 | 0.44±0.03 | 0.0005 |
| Mean Z-score LVID | 0.97±0.66 | 1.44±0.96 | 0.3568 |
| SF | 33.6±2.7 | 36±5.7 | 0.4043 |
| Mean Z-score LVPWd | 0.57±1.27 | 1.22±1.17 | 0.3727 |
| Mean Z-score IVSd | −0.42±0.93 | 0.88±0.80 | 0.0215 |
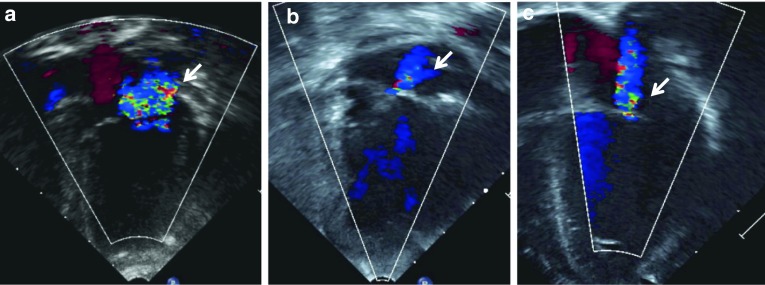
Fig. 3.
(a–c). Two-dimensional short axis images of: (a) normal aortic valve in 3 month old infant; and thickened aortic valves in (b) 25 day old infant and (c) 256 day old infant, both with severe untreated MPS I. Compare the thin, nearly transparent aortic leaflets (arrow) in (a) with those of (b) and (c)
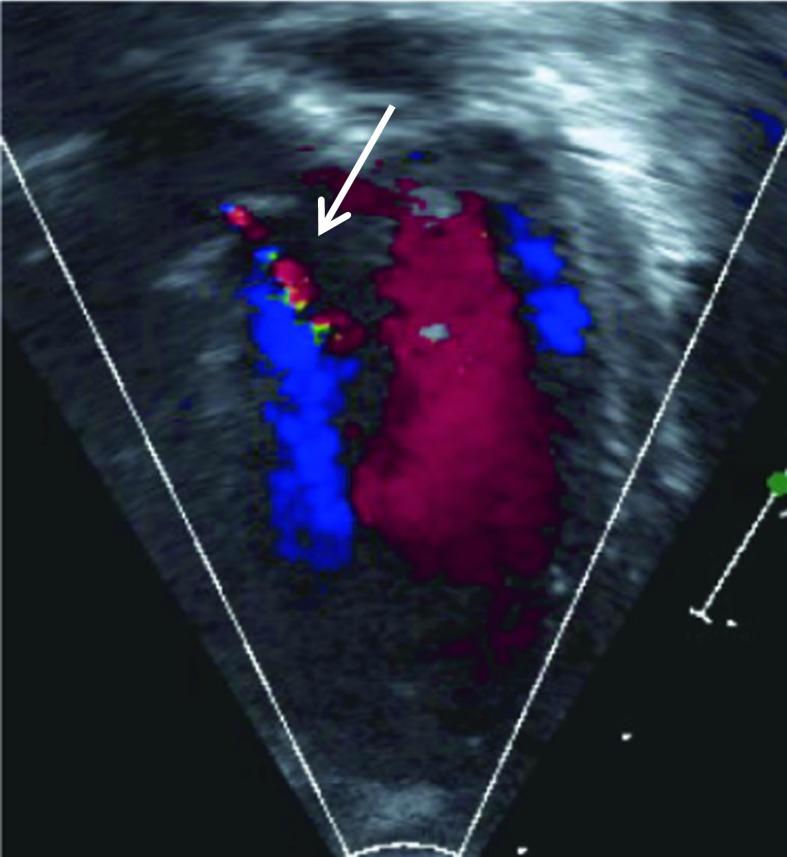
Fig. 4.
Color Doppler image from left ventricle showing trace aortic regurgitation in 178 day old infant with severe untreated MPS I. Note narrow central jet (arrow) extending into left ventricular outflow tract only
There were five infants who were ≤180 days of age at first echo (Table 4). The mean Z-score for IVSd was significantly less for these infants (p = 0.0215) when compared to infants >180 days of age, but there was no other significant difference between these two groups for any other parameters (LVID, LVPWd, aortic sinus diameter, or left ventricular function). There was no significant difference in the incidence of Z-scores more than 2 standard deviations greater than normal nor was there a significant difference in the incidence of significant mitral regurgitation between the two groups (data not shown).
Discussion
Previously, little information was available regarding the cardiac status of infants with severe untreated MPS I. In this report, we describe a lower incidence of ventricular hypertrophy and both a lower incidence and a milder expression of cardiac valve dysfunction than previously reported in older children with untreated MPS I (Leal et al. 2010; Wippermann et al. 1995). Comparison of the incidence of ventricular hypertrophy between infants and older children is best made with Leal’s study, since it also uses Z-scores. Left ventricular hypertrophy occurred in 2/13 (15 %) infants from our study as compared to 5/6 (83 %) in older children (Table 5). Unfortunately, Leal’s study does not differentiate between severe and attenuated MPS I, so it may not be representative for either the incidence of hypertrophy or valve findings in patients with a clear severe phenotype. The incidence of cardiac valve findings in Wippermann’s study of older children with severe MPS I is a good comparison to our infant study since color Doppler was used in all of his echocardiograms. While increased mitral valve thickness occurred universally in both our infants and Wippermann’s older children, only 3/13 (23 %) of our infants had mitral regurgitation compared to 10/12 (82 %) of his older children. Mitral regurgitation was only mild or mild/moderate in our three infants, but was moderate or severe in the majority of his older children. Mitral stenosis did not occur in our infants but was seen in 2/12 (17 %) of this older cohort. While increased aortic valve thickness occurred commonly in both our infants and Wippermann’s older children, significant aortic valve regurgitation or stenosis did not occur in our infants but was present in 4/12 (25 %) of the older children.
Table 5.
Comparison of infants with severe, untreated MPS I cardiac data with published studies of older cohorts
| Current study | Leal et al. 2010 | Wippermann et al. 1995 | |
|---|---|---|---|
| Mean age (years) | 0.6 | 6.8 | 3.8 |
|
Incidence Z-score > 2 SD wall thickness |
2/13 (15 %) | 5/6 (83 %) | Not reported |
| Mitral valve | |||
| Incidence thickened mitral valve | 13/13 (100 %) | 5/6 (83 %) | 12/12 (100 %) |
| Incidence of mitral regurgitation None Trace Mild Moderate Severe |
4/13 (31 %) 6/13 (46 %) 3/13 (23 %) 0/13 (0 %) 0/13 (0 %) |
1/6 (17 %) 0/6 (0 %) 5/6 (83 %) 0/6 (0 %) 0/6 (0 %) |
2/12 (17 %) 0/12 (0 %) 4/12 (33 %) 4/12 (33 %) 2/12 (17 %) |
| Aortic valve | |||
| Incidence thickened aortic valve | 10/13 (77 %) | 5/6 (83 %) | 6/12 (50 %) |
| Incidence aortic regurgitation None Trace Mild Moderate Severe |
8/13 (62 %) 5/13 (38 %) 0/13 (0 %) 0/13 (0 %) 0/13 (0 %) |
3/6 (50 %) 0/6 (0 %) 3/6 (50 %) 0/6 (0 %) 0/6 (0 %) |
8/12 (67 %) 0/12 (0 %) 3/12 (25 %) 1/12 (8 %) 0/12 (0 %) |
Although cardiac pathology appeared milder, clinical expression of MPS was already evident in infancy as supported by the presence of increased mitral valve thickness in all infants (Braunlin et al. 2011). In addition, significant mitral valve regurgitation was present in 23 % of these infants and was seen as early as 25 days of life. Although our numbers are small, there did not appear to be a relationship between valve regurgitation and early or late infancy, gender, or mutation. It is unknown at present whether early intervention can arrest or reverse the pathological process within these very young cardiac valves.
While the mean left ventricular chamber dimensions and left ventricular wall thicknesses were all within 2 SD of normal, there were outliers (infants with Z-scores >2 SD normal). Two infants had wall thickness Z-scores >2 SD of normal and three others had Z-scores for left ventricular chamber dimension >2 SD of normal. Similarly, mean shortening fraction was normal in all infants although the measured ejection fraction in a single infant (Case #7) was moderately depressed. These parameters are known to respond favorably to either ERT or HCT (Braunlin et al. 2003, 2006; Wiseman et al. 2012) and this was true as well for Case 7 whose ejection fraction normalized after a period of ERT. Surprisingly, dilation of aortic sinus diameter to >2 SD of normal occurred in 5/13 (38 %) infants within the first year of life. The arrest or reversibility of this finding with early treatment is unknown.
The presence of significant coronary artery involvement is an important feature of severe MPS I even in infancy (Brosius and Roberts 1981; van den Broek et al. 2011) but has not been investigated in this, or other, reported series. It has been shown that critical coronary artery disease can occur very early in life in severe MPS I and may be a limiting factor even in the early treatment of this syndrome (van de Broek et al. 2011).
The incidence and severity of ventricular hypertrophy, depressed function, and valve pathology in MPS has been the subject of many reports over the past 35 years (Dangel 1998; Schieken et al. 1975; Gross et al. 1988; Fesslova et al. 2009; Mohan et al. 2002). It is important to recognize that during this time echo technology has evolved from the initial grainy M-mode images seen in 1975 to the high-resolution two-dimensional images of today. In addition, both spectral and color Doppler were developed, with color Doppler being the more recent technology. The presence of cardiac valve stenosis or regurgitation can be difficult to assess by spectral Doppler, but both are easily identifiable by color Doppler. Thus, when reporting the incidence of valve dysfunction in MPS, studies such as Wippermann’s utilizing color Doppler for all individuals within the study are likely the most accurate. Similarly, the use of Z-scores to assess the difference of a particular value from normal has been a relatively recent addition to the complete pediatric cardiac echo report (Lai et al. 2006) enabling an assessment not only of the presence of an abnormality but also its extent. Studies such as Leal’s use these metrics to permit a more accurate comparison of abnormalities of chamber dimensions and wall thicknesses between studies from various institutions.
For the younger patients with severe MPS I included in this study, the incidence of pathology was less than that of older patients and the severity milder, but it was not absent. The outcome of cardiac valve pathology when treatment with ERT and HCT is begun early in life is currently unknown. The absence of cardiac valve disease after ERT was started at 5 months of age and continued for 5 years as described in the infant reported by Gabrielli et al., while encouraging, may be related in part to the fact that attenuated, rather than severe, MPS I was present in this child. In severe MPS I, there may be some infants with early-onset valve regurgitation or rapidly progressing coronary disease, in whom even early treatment may be too late; however, it would seem reasonable to think that intervention within the first year of life, facilitated by newborn screening, will improve long-term outcomes in severe MPS I for most infants.
Abbreviations
- ASE
American Society of Echocardiography
- BSA
Body surface area
- ERT
Enzyme replacement therapy
- GAG
Glycosaminoglycan
- HCT
Hematopoietic cell transplantation
- IVSd
Interventricular septal thickness in diastole
- LVID
Left ventricular internal dimension in diastole
- LVIS
Left ventricular internal dimension in systole
- IVSd
Intraventricular septal wall thickness in diastole
- LVPWd
Left ventricular posterior wall thickness in diastole
- MPS I
Mucopolysaccharidosis Type I
- SD
Standard deviation
- SF
Shortening fraction
References to Electronic Database
MPS I, OMIM phenotype 607014, Locus MIM number 252800
Contributions of Individual Authors
Elizabeth Braunlin conceived the project, participated in the analysis of the data, provided the interpretation of the data, and is the guarantor of the study.
Luke Schroeder participated in the data collection and interpretation as well as critical revisions of the draft of the article.
Chester B. Whitley performed the mutation analysis for the infants and participated in the critical review of the manuscript.
James M. Berry performed the infant cardiac ultrasounds, provided interpretation of the data, and critical review of the manuscript.
Paul Orchard, Weston Miller, IV, and Jakub Tolar participated in the analysis and interpretation of data and critical revisions of the manuscript for intellectual content.
Guarantor
Dr. Elizabeth Braunlin will serve as the guarantor for the manuscript.
Competing Interest Statement
Drs. Elizabeth Braunlin, Paul Orchard, Jakub Tolar, and Chester B. Whitley have received funds for speaking, reimbursement for speaking and traveling to symposia from Genzyme (Orchard, Whitley) and Biomarin (all).
Dr. Braunlin is a consultant on a research study of MPS IV for Biomarin. Dr. Orchard receives research funding from Genzyme.
Dr. Whitley receives research funding from Genzyme and Biomarin.
Biomarin and Genzyme produce enzyme replacement therapy for lysosomal storage diseases. The conclusion of this study, earlier treatment is helpful, may increase the amount of enzyme these companies can sell.
Drs. Schroeder and Miller and Mr. James Berry have no financial interests to declare.
Details of Funding
The authors of this manuscript received no funding for any work involved in the writing of this manuscript.
Details of Ethics Approval
The Institutional Review Board at the University of Minnesota granted approval to access the patient information used in this study.
Patient Consent Statement
Patient consent was obtained prior to accessing any confidential patient information.
Footnotes
Competing interests: None declared
References
- Braunlin EA. Cardiac involvement in the MPS disorders. In: Moller JH, Hoffman JIE, editors. Pediatric cardiovascular medicine. Hoboken: Blackwell; 2012. pp. 982–991. [Google Scholar]
- Braunlin EA, Stauffer NR, Peters CH, et al. Usefulness of bone marrow transplantation in the Hurler syndrome. Am J Cardiol. 2003;92:882–886. doi: 10.1016/S0002-9149(03)00909-3. [DOI] [PubMed] [Google Scholar]
- Braunlin EA, Berry JM, Whitley CB. Cardiac findings after enzyme replacement therapy for mucopolysaccharidosis type I. Am J Cardiol. 2006;98:416–418. doi: 10.1016/j.amjcard.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Braunlin EA, Tolar J, Mackey-Bojack S, Masinde T, Krivit W, Schoen FJ. Clear cells in the atrioventricular valves of infants with severe human mucopolysaccharidosis (Hurler syndrome) are activated valvular interstitial cells. Cardiovasc Pathol. 2011;20:315–321. doi: 10.1016/j.carpath.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Brosius FC, Roberts WC. Coronary artery disease in the hurler syndrome: qualitative and quantitative analysis of the extent of coronary artery narrowing at necropsy in six children. Am J Cardiol. 1981;47:649–653. doi: 10.1016/0002-9149(81)90550-6. [DOI] [PubMed] [Google Scholar]
- Dangel JH. Cardiovascular changes in children with mucopolysaccharide storage diseases and related disorders – clinical and echocardiographic findings in 64 patients. Eur J Pediatr. 1998;57:534–538. doi: 10.1007/s004310050872. [DOI] [PubMed] [Google Scholar]
- Fesslova V, Corti P, Sersale G, et al. The natural course and the impact of therapies of cardiac involvement in the mucopolysaccharidoses. Cardiol Young. 2009;19:170–178. doi: 10.1017/S1047951109003576. [DOI] [PubMed] [Google Scholar]
- Gabrielli O, Clarke LA, Bruni S, Coppa GV. Enzyme-replacement therapy in a 5-month-old boy with attenuated presymptomatic MPS I: 5 year follow-up. Pediatrics. 2010;125:e183–187. doi: 10.1542/peds.2009-1728. [DOI] [PubMed] [Google Scholar]
- Gillingsrud EO, Krivit W, Summers CG. Ocular abnormalities in the mucopolysaccharidoses after bone marrow transplantation. Longer follow-up. Ophthalmology. 1998;105:1099–1105. doi: 10.1016/S0161-6420(98)96014-6. [DOI] [PubMed] [Google Scholar]
- Gross DM, Williams JC, Caprioli C, Dominquez B, Howell RR. Echocardiographic abnormalities in the mucopolysaccharide storage diseases. Am J Cardiol. 1988;61:170–176. doi: 10.1016/0002-9149(88)91325-2. [DOI] [PubMed] [Google Scholar]
- Hatle L, Angelsen B. Doppler ultrasound in cardiology: physical principles and clinical applications. 2. Philadelphia: Lea-Febiger; 1985. p. 93. [Google Scholar]
- http://graphpad.com
- http://parameterz.blogspot.com
- Lai WW, Geva T, Shirali GS. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19:1413–1430. doi: 10.1016/j.echo.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Leal GN, de Paula AC, Leone C, Kim CA. Echocardiographic study of paediatric patients with mucopolysaccharidosis. Cardiol Young. 2010;20:254–261. doi: 10.1017/S104795110999062X. [DOI] [PubMed] [Google Scholar]
- Lopez L, Colan SD, Frommelt PC, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- McGill JJ, Inwood AC, Corman DJ, et al. Enzyme replacement therapy for mucopolysaccharidosis VI from 8 weeks of age – a sibling control study. Clin Genet. 2010;77:492–498. doi: 10.1111/j.1399-0004.2009.01324.x. [DOI] [PubMed] [Google Scholar]
- Mohan UR, Hay AA, Cleary MA, Wraith JE, Patel RG. Cardiovascular changes in children with mucopolysaccharide disorders. Acta Paediatr. 2002;91:799–804. doi: 10.1111/j.1651-2227.2002.tb03330.x. [DOI] [PubMed] [Google Scholar]
- Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Valle D, Sly WS, editors. The metabolic basis of inherited diseases. 8. New York: McGraw-Hill; 2001. pp. 3421–3452. [Google Scholar]
- Schieken RM, Kerber RE, Ionasescu VV, Zellweger H. Cardiac manifestations of the mucopolysaccharidoses. Circulation. 1975;52:700–705. doi: 10.1161/01.CIR.52.4.700. [DOI] [PubMed] [Google Scholar]
- Sifuentes M, Doroshow R, Hoft R, et al. A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab. 2007;90:171–180. doi: 10.1016/j.ymgme.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Tylki-Szymanska A, Jurecka A, Zuber Z, Rozdzynska A, Marucha J, Czartorynska B. Enzyme replacement therapy for mucopolysaccharidosis II from 3 months of age: a 3-year follow-up. Acta Paediatr. 2012;101:e42–47. doi: 10.1111/j.1651-2227.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- Van den Broek L, Backx PC, Coolen H, et al. Fatal coronary artery disease in an infant with severe mucopolysaccharidosis type I. Pediatrics. 2011;127:e1343–1346. doi: 10.1542/peds.2009-2047. [DOI] [PubMed] [Google Scholar]
- Weisstein JS, Delgado E, Steinbach LS, Hart K, Packman S. Musculoskeletal manifestations of Hurler syndrome: long-term follow-up after bone marrow transplantation. J Pediatr Orthop. 2004;24:97–101. doi: 10.1097/01241398-200401000-00019. [DOI] [PubMed] [Google Scholar]
- Wippermann CF, Beck M, Schranz D, Huth R, Michel-Behnke I, Jungst B-K. Mitral and aortic regurgitation in 84 patients with mucopolysaccharidoses. Eur J Pediatr. 1995;154:98–101. doi: 10.1007/BF01991908. [DOI] [PubMed] [Google Scholar]
- Wiseman DH, Mercer J, Tylee K et al (2012) Management of mucopolysaccharidosis type IH (Hurler’s Syndrome) presenting in infancy with severe dilated cardiomyopathy: a single institution’s experience. J Inherit Metab Dis. doi:10.1007/s10545-012-9500-3 [DOI] [PubMed]