Abstract
Cobalamin (Vitamin B12) plays an essential role both in the conversion of methylmalonyl-CoA to succinyl-CoA and in the synthesis of methionine (Met) from homocysteine (Hcy). Elevations of total homocysteine (tHcy), Met, methylmalonic acid (MMA), and 2-methylcitric acid (2MCA) are indicative of disorders in these related pathways, and can clinically present as methylmalonic acidemia, cobalamin defects or deficiency, propionic acidemia, homocystinuria, and hypermethioninemia. We have developed a fast, sensitive, and simple method for the simultaneous detection of plasma tHcy, MMA, Met, and 2MCA using liquid chromatography mass spectrometry (LC/MS/MS). All analytes were directly determined without the need of derivatization. Both positive and negative modes were used to achieve the best sensitivity and specificity. The two stereo isomers of 2MCA (2S, 3S) and (2R, 3S) were successfully separated and were designated as 2MCA1 and 2MCA2. The assays were linear up to a concentration of 800 μMol/l for tHcy, 2,000 μMol/l for Met, 80 μMol/l for MMA, 40 μMol/l for 2MCA1, and 40 μMol/l for 2MCA2 (80 μMol/l for total 2MCA), respectively. The recovery was between 84.42 % and 120.05 %. The intra-assay coefficient of variations (CVs) ranged from 2.1 % to 6.9 % (n = 20), and the inter-assay CVs ranged from 2.7 % to 11.6 % (n = 20). Reference intervals were established and verified (n = 125). A total of 15 patients with variable disorders in related pathway were successfully confirmed. The assay can be performed either in diagnostic laboratories or as second-tier, follow-up test in newborn screening laboratories.
A fast, sensitive, and simple LC/MS/MS method was developed successfully for the simultaneous detection of plasma total homocysteine, methylmalonic acid, methionine, and 2-methylcitric acid for diagnosis of disorders in related pathways.
Introduction
Cobalamin (Vitamin B12) plays an essential role both in the conversion of methylmalonyl-CoA to succinyl-CoA, and in the synthesis of methionine (Met) from homocysteine (Hcy) (Fig. 1). Two enzymatic reactions in mammalian cells require cobalamin as a cofactor. Adenosylcobalamin is the cofactor for methylmalonyl-CoA-mutase (MUT, EC 5.4.99.2), and methylcobalamin is a cofactor for methionine synthase (MTR, EC 1.16.1.18). Disorders of intracellular cobalamin metabolism may impair the function of either or both enzymes.
Fig. 1.
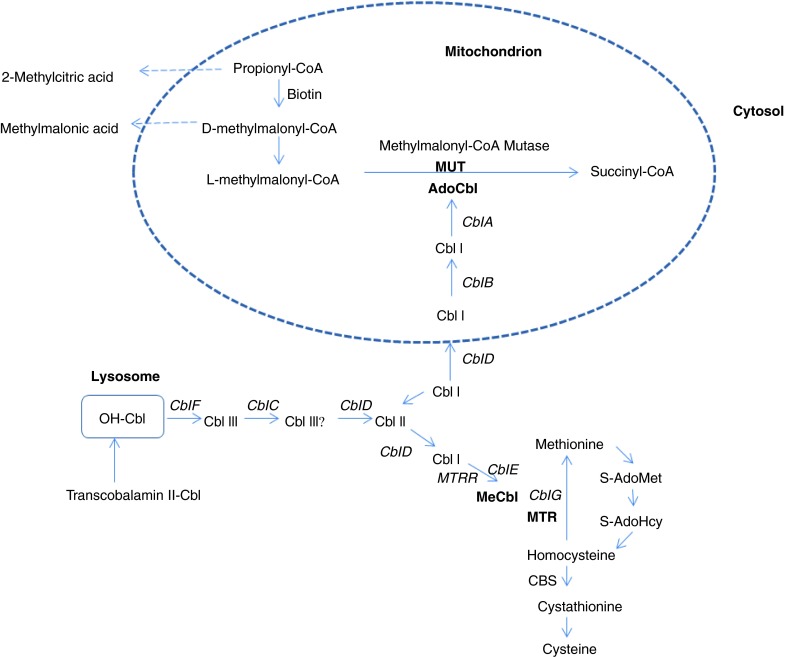
Metabolic pathways involving Cobalamin (Cbl) for MMA, tHcy, and Met. The enzyme propionyl-CoA carboxylase converts propionyl-CoA into D-methylmalonyl-CoA, which is biotin dependent, and then is racemized into l-methylmalonyl-CoA and isomerized into succinyl-CoA, a Krebs cycle intermediate. Adenosylcobalamin (AdoCbl) is the cofactor of the methylmalonyl-CoA mutase (MUT) reaction; methylcobalamin (MeCbl) is the cofactor of the methionine synthase (MTR) reaction. Abbreviations used: Cbl cobalamin, OH-Cbl hydroxycobalamin, AdoCbl adenosylcobalamin, MeCbl methylcobalamin, MTR methionine synthase, MTRR methionine synthase reductase, CBS cystathionine β synthase, S-AdoMet S-adenosylmethionine, S-AdoHcy S-adenosylhomocysteine
Acquired cobalamin deficiencies are very common worldwide (Obeid 2012), especially in elderly subjects (Andrès et al. 2004). Vegetarians are prone to develop cobalamin deficiencies, which often go unrecognized because of subtle clinical manifestations. Asymptomatic newborns with nutritional cobalamin deficiencies secondary to maternal cobalamin deficiencies have also been reported (Campbell 2005; Lemieux et al. 1992). Cobalamin levels have also been assessed in infants, children, and adolescents (Monsen and Ueland 2003). Cobalamin deficiency in infancy and childhood may cause irreversible neurologic damage (Requejo et al. 1997, Rasmussen et al. 2001, Garewal et al.1988). The concentration of tHcy in serum and plasma is increased in both folate and cobalamin deficiencies. MMA is another sensitive and specific marker of cobalamin function. These functional measurements are therefore useful in the identification of subclinical cobalamin states and reflect early changes in cobalamin status. tHcy is also elevated in pathologic states such as renal failure, thyroid dysfunction, and cardiovascular diseases (Monsen and Ueland 2003). Elevation of MMA and 2MCA can also be detected in end-stage renal failure patients (Henning et al. 1999).
There are a number of inherited defects that cause cobalamin deficiency or defects in the pathways of cobalamin absorption, transport, metabolism, or utilization (Fig. 1), such as congenital pernicious anemia (intrinsic factor deficiency, OMIM 261000) and Imerslund-Gräsbeck syndrome (OMIM 261100); transcobalamin II deficiency (OMIM 275350) and methylmalonic acidemia (OMIM 251000) due to methylmalonyl-CoA mutase (EC 5.4.99.2) deficiency (mut0, mut-); and intracellular cobalamin metabolism, identified by complementation class as Cbl C (OMIM 277400), Cbl D (OMIM 277410), including Cbl D variant 1 and variant 2, Cbl F (OMIM 277380), Cbl E (OMIM 236270), and Cbl G (OMIM 250940) (Obeid 2012).
Disorders that can cause elevated MMA alone are methylmalonyl-CoA mutase deficiencies (mut0, mut-), and adenosyl-Cbl-specific deficiencies, such as Cbl A, Cbl B, and Cbl D-variant 2 deficiencies. Combined methyl-Cbl and adenosyl-Cbl deficiencies, such as Cbl C, Cbl D, and Cbl F can cause elevations of both MMA and tHcy. Imerslund-Gräsbeck syndrome and transcobalamin II deficiency can also cause elevations of both MMA and tHcy. Disorders causing primarily hyperhomocysteinemia are classic homocystinuria (OMIM 606664) (cystathionine beta-synthase deficiency, CBS, EC. 4.2.1.22), and methyl-Cbl-specific deficiencies, such as Cbl D-variant 1, Cbl E, and Cbl G, as well as methylenetetrahydrofolate reductase (MTHFR, EC 1.5.1.20) deficiency (OMIM 236250).
Hyperhomocysteinemia can also result from folate deficiency, Vitamin B12 deficiency, or mutations in enzymes in remethylation enzymes. Elevation of tHcy is also a known risk factor for premature cardiovascular disease.
At present, several genetic conditions leading to abnormal methionine elevations are known. They are methionine adenosyltransferase (MAT, EC 2.5.1.6) I/III deficiency (OMIM 250850), homocystinuria due to cystathionine beta-synthase deficiency, deficiencies of glycine N-methyltransferase (GNMT, EC 2.1.1.20), (OMIM #614300), deficiencies of S-adenosylhomocysteine hydrolase (AHCY, EC 3.3.1.1) (OMIM 613752), and deficiencies of citrin (OMIM 605814, OMIM 603471) (Mudd 2011). Hypermethioninemia is also suggestive of mitochondrial dysfunction and liver diseases (Mudd 2011).
Several inborn errors of methylmalonic acid, methionine, and cobalamin metabolism are included in the supplemental newborn screening program recommended by ACMG (American College of Medical Genetics). These conditions rely on abnormal levels of propionylcarnitine (C3-acylcarnitine) and/or methionine. Unfortunately, these markers lack both disease specificity and poor diagnostic sensitivity (Turgeon et al. 2010; Ueland et al. 1993), and can sometimes lead to false-positive results. Therefore, measurement of specific markers such as tHcy, MMA, and 2MCA are more desirable. Marca G et al. (2007) reported a rapid second-tier test for measurement of 3-OH-propionic and MMA in dried blood spots to reduce the false-positive rate for propionylcarnitine. Turgeon et al. (2010) also reported a second-tier assay to determine tHcy, MMA, and 2MCA in dried blood spots by LC/MS/MS.
Many methods have been described for measurement of plasma MMA and/or tHcy by LC/MS/MS and GC/MS. Blom et al. (2007) reported an assay for MMA without derivatization by LC/MS/MS. Tomaiuolo et al. (2006) reported a method for determination of plasma tHcy by isotope dilution and electrospray-tandem mass spectrometry. Windelberg et al. (2005) reported an automated assay for the determination of MMA, tHcy, and related amino acids by GC/MS. Kushnir et al. (2001) reported an assay of dicarboxylic acid including MMA by tandem mass spectrometry. A simultaneous determination of total homocysteine, MMA, and possible Met using LC/MS/MS without derivatization was described by Hempen et al. (2008).
We therefore have developed a simple, fast LC/MS/MS assay for the simultaneous determination of tHcy, Met, MMA, and 2MCA in plasma or serum. Furthermore, all analytes are directly measurable without the need for an additional derivatization step, and both positive and negative ion modes were used to achieve the best sensitivity and specificity. This method consolidates all four analytes thus allowing for the diagnosis of disorders in related pathways. In addition, this test can also be used as a second-tier or confirmatory test for positive newborn screening.
Materials and Methods
Chemicals such as DL-D8-homocystine and D3-MMA were obtained from Cambridge Isotope Laboratories. TCEP-HCL was purchased from Thermo Scientific. DL-homocysteine, L-Met, and MMA were purchased from Sigma-Aldrich. 2MCA was purchased from CDN Isotope Laboratories, Canada. Other reagents, such as HPLC grade water, formic acid-optima LC/MS, and methanol-optima LC/MS were purchased from Fisher Scientific.
Preparation of Calibrators and Controls
Stock solutions of Hcy, Met, MMA, and 2MCA were prepared in water at concentrations of 1.0, 0.2, 1.22 and 1.0 mg/ml, respectively. The combo internal standard solution including DL-D8-homocystine and D3-MMA was prepared at a concentration of 50 ng/100 μl. PSHM (plasma/serum/H2O mixture solution) was prepared in V:V:V=25:25:150. A standard stock solution was prepared by adding 1.351 ml of 1.0 mg/ml Hcy, 29.844 ml of 0.2 mg/ml Met, 0.097 ml of 1.22 mg/ml MMA, and 0.410 ml of 1.0 mg/ml 2MCA, and filled up to a volume of 50 ml with H2O. Working standard solutions were prepared by diluting 3:4, 1:2, 1:4, 1:20, and 1:40 from stock solutions with PSHM as calibrators. Three levels of controls were prepared by 1:4, 1:20, and 1:40 dilutions from the standard stock solution with PSHM. The three levels of controls were also used for the imprecision study.
Samples
Diagnosed patients’ plasma samples sent to the lab for follow up were used to evaluate this method.
Methods
100 μl aliquots of EDTA or heparinized plasma or serum samples were placed into 12 x 75 mm glass test tubes and 250 μl of HPLC grade water was added to each. 100 μl of combo IS was then added to each tube and mixed gently for 3 s. Then 50 μl of TCEP-HCL (1g/34.8 ml in water) was added and mixed gently for 3 s. The mixtures were vortexed twice for 15 s at a medium speed and incubated for 15 min each at room temperature. The solutions were transferred with plastic transfer pipette into Amicon Ultra 0.5 ml 10K Da and then centrifuged at 13,500 rpm for 10 min. The filtrates were transferred into HPLC vials, and 10 μl was injected into the instrument.
MS/MS Procedures
A triple-quadrupole MS/MS system (Applied Biosystem/MDS SCIEX API 4000 Qtrap) was coupled with a Shimadzu HPLC system and a Leap technologies auto sampler. All MS investigations were carried out with a turbo ion spray source operated in both positive mode and negative mode under multiple reaction monitoring (MRM) conditions. MRM transition of m/z 136.1/90.1 was monitored for tHcy, m/z 140.1/94.1 was monitored for D4-homocysteine (reduced form of D8-homocystine), and m/z 150.1/104.1 was monitored for Met, respectively. tHcy and Met were measured in positive ESI mode (run time 0–2.04 min) and quantified using D4-homocysteine. MRM transition of m/z 117.1/73.0 was monitored for MMA, m/z 204.9/125.0 for 2MCA1 and 2MCA2. MRM transition of m/z 120.1/76.0 was monitored for D3-MMA. MMA and 2MCA were measured in negative ESI mode (run time 2.05–6.0 min) and quantified using D3-MMA. 10 μl was injected into the LC column with the mobile phase flow of 0.7 ml/min. The LC separation was carried out on a reversed-phase C18 column (Phenomenex, Kinetex 2.6u C18 100A; 100 × 4.6 mm). Gradient elution of the analytes was achieved using a program with mobile phase A (aqueous 0.2% formic acid) and mobile phase B (0.2% formic acid in methanol) as follows: 3% B from 0 min to 4.4 min; 90% B from 4.5 min to 5 min; then back to 3% B from 5 min to 6 min.
Results
Linearity and Imprecision
Plasma standards of MMA, tHcy, Met, and 2MCA at five different concentrations of spiked analytes were used. They were as follows: tHCy: 150, 100, 50, 10, and 5 μMol/l; Met: 600, 400, 200, 40, and 20 μMol/l; MMA: 15, 10, 5, 1, and 0.5 μMol/l; total 2MCA: 30, 20, 10, 2, and 1 μMol/l. The results showed reproducible signals with a linear response (tHcy R2 = 0.9978; Met R2 = 0.9962; MMA R2 = 0.9992; 2MCA1R2 = 0.9973; 2MCA1R2 = 0.9976. n = 3 for each analyte). The determination is linear up to a concentration of 800 μMol/l for tHcy, 2,000 μMol/l for Met, 80 μMol/l for MMA, 40 μMol/l for 2MCA1, and 40 μMol/l for 2MCA2 (80 μMol/l for total 2MCA), respectively. If the amount is greater than the levels mentioned, the specimen is diluted accordingly and reanalyzed for an accurate calculation in the linear range. Intra-assay imprecision for tHcy, Met, MMA, and 2MCA1 and 2MCA2 was determined by assaying the same 20 replicates of 3 concentrations in 1 day (Table 1). Inter-assay imprecision was determined by assaying the same samples on 20 different days at 3 concentrations by 2 different technologists over a period of 2 months (Table 1).
Table 1.
Imprecision for tHcy, Met, MMA, and 2MCA (CV%)
| Concentration (μMol/l) | Intra-assay precision (n = 20) | Inter-assay precision (n = 20) | |
|---|---|---|---|
| tHcy | 50 | 4.9 % | 7.1 % |
| 10 | 4.4 % | 6.8 % | |
| 5 | 4.1 % | 6.5 % | |
| Met | 200 | 6.9 % | 11.6 % |
| 40 | 4.6 % | 10.9 % | |
| 20 | 3.5 % | 8.4 % | |
| MMA | 5 | 2.1 % | 2.7 % |
| 1 | 3.9 % | 4.4 % | |
| 0.5 | 4.9 % | 5.9 % | |
| 2MCA1 | 5 | 2.4 % | 4.1 % |
| 1 | 2.7 % | 6.0 % | |
| 0.5 | 7.5 % | 9.9 % | |
| 2MCA2 | 5 | 2.2 % | 9.5 % |
| 1 | 2.7 % | 10.3 % | |
| 0.5 | 4.5 % | 8.1 % |
Method Comparison
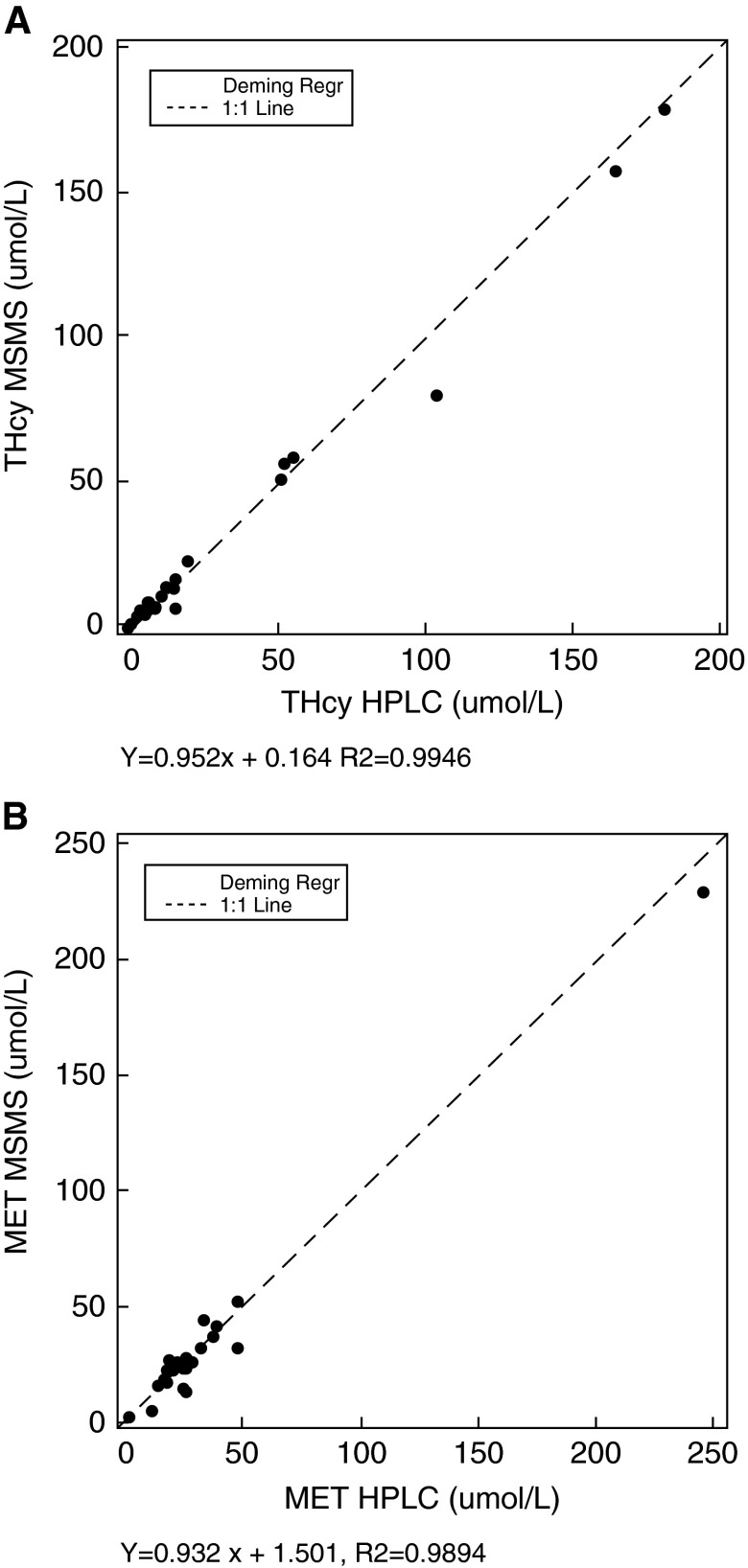
A correlation study was performed by determining tHcy (0.06–179 μMol/l) and Met (2.07–229 μMol/l) in 40 plasma samples by means of the new LC/MS/MS method in comparison with the HPLC assays routinely used in the lab. An X-Y plot showed excellent correlation between the two methods (Fig. 2).
Fig. 2.
Passing and Bablok regression lines for tHcy (A) and Met (B) in the correlation study of the LC/MS/MS method with the HPLC method (n = 40)
Recovery
Recovery was evaluated by the analysis of three different levels of tHcy, Met, MMA, and 2MCA diluted with PSHM (plasma/serum/H2O mixture solution) (Table 2). Recovery was defined as follows: final concentration – endogenous concentration/added concentration. The recoveries are shown in Table 2. Recovery study for MMA was also carried out by comparing the reference material (UTAK lab, Valencia, CA) at two levels, 0.2 and 1 μMol/l. The recoveries were 112 % and 105 %, respectively (n = 5).
Table 2.
Analytical recovery of the assay
| Concentration, μMol/l | ||||
|---|---|---|---|---|
| Expected concentration | Endogenous mean (n=3) | Detected mean (n=5) | Recovery* (%) | |
| tHcy | 200 | 1.17 | 170.0 | 84.42 |
| 40 | 1.17 | 38.78 | 94.03 | |
| 4 | 1.17 | 4.88 | 92.75 | |
| Met | 400 | 4.50 | 478.0 | 118.38 |
| 80 | 4.50 | 96.60 | 115.13 | |
| 8 | 4.50 | 13.42 | 111.55 | |
| MMA | 20 | 0.04 | 23.76 | 118.61 |
| 4 | 0.04 | 4.84 | 120.05 | |
| 0.4 | 0.04 | 0.51 | 118.00 | |
| 2MCA1 | 20 | 0.10 | 18.00 | 89.49 |
| 4 | 0.10 | 3.78 | 91.95 | |
| 0.4 | 0.10 | 0.49 | 96.00 | |
| 2MCA2 | 20 | 0 | 18.90 | 94.50 |
| 4 | 0 | 3.53 | 88.25 | |
| 0.4 | 0 | 0.35 | 87.75 | |
*Recovery: Detected concentration - endogenous concentration/expected concentration%
Limit of Detection
The detection of limit was carried out by preparing and measuring the lowest standard solution that had been diluted with water by factors of 1 (undiluted), 2, 4, 8, and 14. The limit of detection for tHcy, Met, MMA, 2MCA1, and 2MCA2 was determined to be 0.3, 1.25, 0.03, 0.03, and 0.03 μMol/l, respectively.
Reference Intervals
Reference intervals were established and verified by analyzing 125 anonymous individual male and female plasma samples (ages 7 months to 20 years) with normal chemistry results, and which would otherwise be discarded. The concentration distribution in these specimens were calculated and evaluated with EP evaluator software. The histogram was obtained to ensure normal distribution. Outliers were excluded. A central 95 % interval was used as reference interval. The reference intervals are 2.47–12.51 μMol/l for tHcy, 6–53 μMol/l for Met, 0.05–0.29 μMol/l for MMA, and 0.02–0.26 μMol/l for 2MCA (total), respectively. The reference intervals were similar to those reported by others (Obeid 2012; Rasmussen et al. 2001; Allen et al. 1993).
Patients’ Results
Fifteen diagnosed patient specimens that were sent to the lab for follow up were analyzed to evaluate this method (Table 3). Two diagnosed classical homocystinuria patients showed elevations of tHcy and Met. Three methylmalonic acidemia patients showed significant elevations of MMA and 2MCA. Five diagnosed cobalamin C defect patients showed elevations of MMA, tHcy, and slight elevation of 2MCA. Three diagnosed propionic acidemia patients showed elevation of 2MCA with a normal MMA level. One patient with MTHFR showed elevation of tHcy with normal Met. This patient was receiving Met supplementation. One patient with Vitamin B12 deficiency showed elevation of MMA, but normal tHcy. Figure 3 depicts chromatograms from a patient with propionic acidemia (B), a normal individual (C), a patient with a Cbl C defect (D), and a standard solution (A) for comparison.
Table 3.
Diagnosed patients and elevations of abnormal compounds (μMol/l)
| Normal level | Hcy | Met | MMA | 2MCA |
|---|---|---|---|---|
| 2.5–12.5 | 6–53 | 0.05–0.29 | 0.02–0.26 | |
| Homocystinuria (n = 2) | 92.4–103 | 419–759 | NL* | NL |
| MMA (n = 3) | NL | NL | 439–944 | 8.8–32.9 |
| Cobalamin C defect (n = 5) | 17.4–114 | NL | 0.58–46.3 | 0.15–1.12 |
| PPA (n = 3) | NL | NL | NL | 9.21–34.7 |
| Cobalamin deficiency (n = 1) | NL | NL | 0.88 | NL |
| MTHFR (n = 1) | 117 | 29.3 | NL | NL |
*NL: Normal values
Fig. 3.
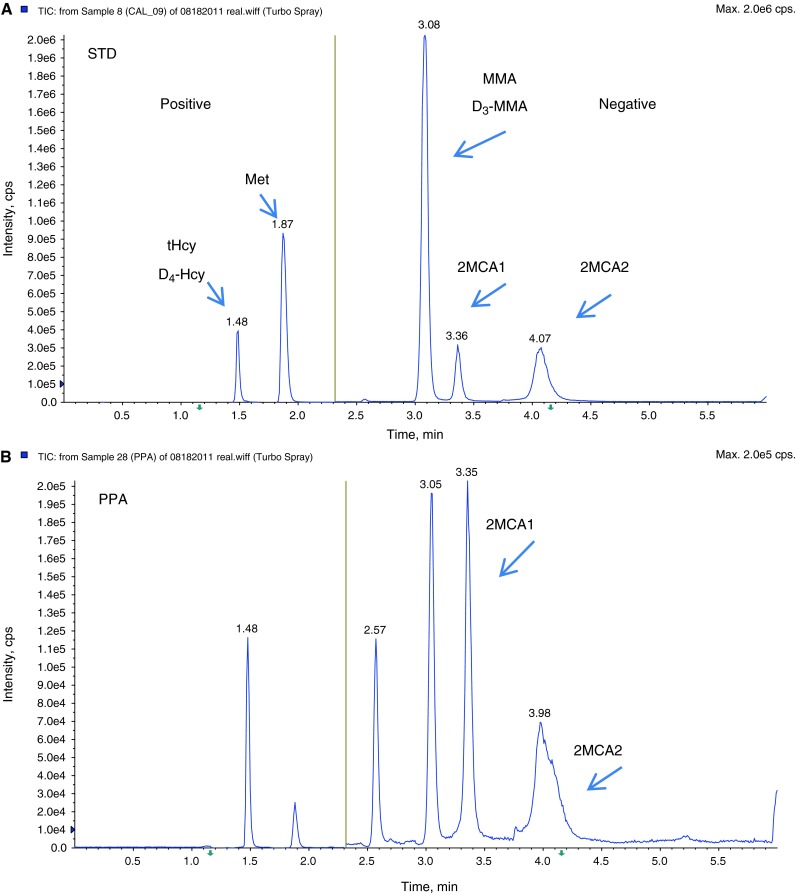
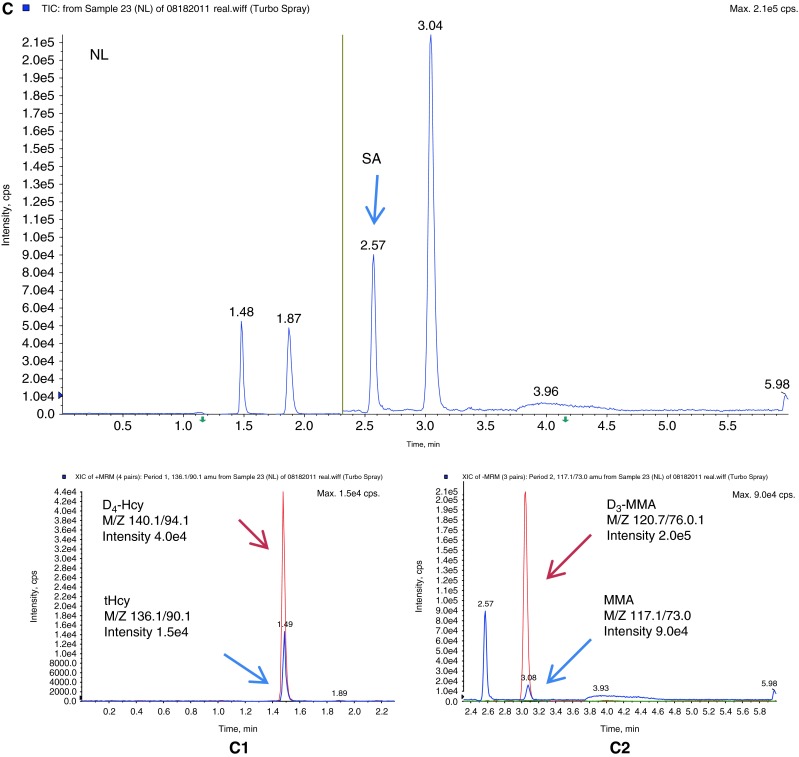
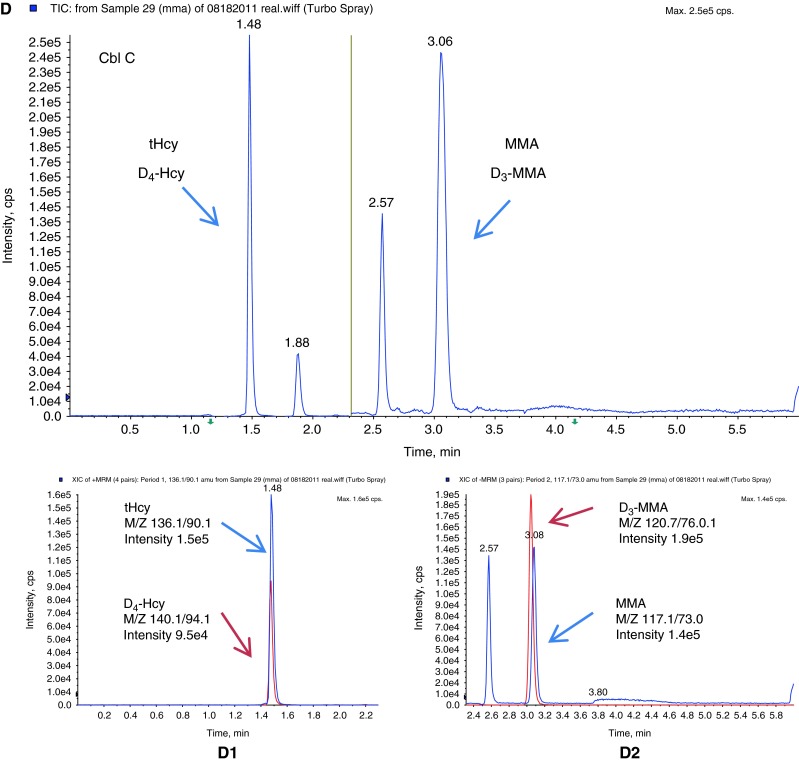
Multiple reaction monitoring chromatograms of (A) standard solution (STD); (B) a patient with propionic acidemia (PPA); (C) a normal individual (NL), and (C1) scan intensity of tHcy (blue) and internal standard D4-Hcy (red) in this normal individual, and (C2) scan intensity of MMA (blue) and internal standard, D3-MMA (red) in this normal individual; (D) a patient with cobalamin C defect (Cbl C), and (D1) scan intensity of tHcy (blue) and internal standard D4-Hcy (red) in this Cbl C defect patient, and (D2) scan intensity of MMA (blue) and internal standard D3-MMA (red) in this Cbl C defect patient. tHcy total homocysteine, D4-Hcy D4-homocysteine, MMA methylmalonic acid, D3-MMA D3-methylmalonic acid, 2MCA1 2-methylcitric acid 1, 2MCA2 2-methylcitric acid 2, SA succinic acid. The yellow line in the middle represents the switch from positive ESI mode to negative ESI mode. The run time is 6 min
Discussion
The primary objective of this study was to develop a simple, fast LC/MS/MS method for the simultaneous and quantitative determination of tHcy, Met, MMA, and 2MCA. The method was developed, validated, and successfully applied to the analysis of clinical samples. All four analytes are directly measurable without the need for an additional derivatization step. The simultaneous measurement is a significant improvement in the diagnosis of disorders in related pathways.
Analytes were measured in 6-min intervals between injections. tHcy (m/z 136/90.1) and Met (m/z 150.0/104.1) were quantified using D4-homocysteine (m/z 140.1/94.1) in positive mode (0–2.04 min). MMA (m/z 117.1/73.0) and two isomers of 2MCA (m/z 204.9/125.0) were measured in negative mode (2.05–6.0 min) and quantified using D3-MMA (120.1/76). Both negative and positive modes were used to achieve the best sensitivity and specificity. The chromatography is carried on by gradient program through Kinetex-C18 column. All five peaks were successfully separated. Succinic acid has the same transition with MMA (m/z 117.1/73.0), and it appeared in negative mode (2.57 min). Because there are two isomers of 2MCA, the sum of the two was reported.
The ACMG recommends newborn screening for propionic acid, Met, and Cbl metabolism in their panel of 29 conditions. The elevation of propionylcarnitine (C3-acylcarnitine) can indicate propionic acidemia (OMIM #606054), methylmalonic acidemia, or congenital cobalamin defect or deficiency. Increased C3-acylcarnitine and abnormally low concentrations of Met are also observed in newborns with Vitamin B12 deficiency (Turgeon et al. 2010). If C3-acylcarnitine is elevated in newborn screening, then multiple tests, such as urinary organic acids analysis, plasma tHcy, Met, and/or plasma MMA are needed for the differential diagnosis between methylmalonic acidemia, propionic acidemia and cobalamin defects and deficiencies. Our assay is able to differentiate between methylmalonic acidemia and propionic acidemia by simultaneous measurement of MMA and 2MCA. This assay can also detect tHcy, MMA, and Met simultaneously, which enables identification of patients with remethylation defects, such as Cbl D-Varl, Cbl E, Cbl G, and MTHFR deficiency, all of which are characterized by low Met and increased tHcy (Turgeon et al. 2010).
The reference ranges were verified using 125 plasma specimens from Children’s Hospital of Los Angeles patients with normal chemistry values. Additionally, the reference interval for 2MCA was established. To our knowledge, this is the first 2MCA reference interval using LC/MS/MS in plasma or serum specimens, which is very similar with that analyzed by GC/MS (Allen et al. 1993). Turgeon et al. (2010) reported a method to measure 2MCA in dried blood spots. We developed a LC/MS/MS method to detect 2MCA, along with MMA, which can provide additional diagnostic information for propionic acidemia, methylmalonic acidemia, and also can be used as a confirmatory test for elevated C3 as observed in newborn screening.
A total of 15 patients diagnosed with disorders in these pathways were reanalyzed when follow-up specimens were received, and all were successfully reconfirmed. One MTHFR patient showed elevated tHcy with a normal Met level. This patient had been receiving Met supplementation.
The assay described can be performed either in diagnostic laboratories or as second-tier, follow-up test in newborn screening laboratories. Our assay is characterized by simple sample preparation (without derivatization), rapid chromatography, high specificity, and small sample size. Simultaneous determination of MMA and tHcy in the same serum or plasma specimen is useful in the diagnosis of both cobalamin and folate deficiencies. The inclusion of methionine may give additional information and can also be diagnostic for methionine disorders. Simultaneous determination of 2MCA provides a differential diagnosis of methylmalonic acidemia versus propionic acidemia and is especially useful for follow-up of newborns screened with elevations of C3-acylcarnitine. A dried blood spot method is currently being evaluated in our laboratory.
Summary
Cobalamin (Vitamin B12) plays an essential role both in the conversion of methylmalonyl-CoA to succinyl-CoA and in the synthesis of methionine (Met) from homocysteine (Hcy). Elevations of total homocysteine (tHcy), Met, methylmalonic acid (MMA), and 2-methylcitric acid (2MCA) are indicative of disorders in these related pathways, and can clinically present as methylmalonic acidemia, cobalamin defects or deficiency, propionic acidemia, homocystinuria, and hypermethioninemia. We have developed a fast, sensitive, and simple method for the simultaneous detection of plasma tHcy, MMA, Met, and 2MCA using liquid chromatography mass spectrometry (LC/MS/MS). All analytes were directly determined without the need of derivatization. Both positive and negative modes were used to achieve the best sensitivity and specificity. The two stereo isomers of 2MCA (2S, 3S) and (2R, 3S) were successfully separated and were designated as 2MCA1 and 2MCA2. The assays were linear up to a concentration of 800 μMol/l for tHcy, 2,000 μMol/l for Met, 80 μMol/l for MMA, 40 μMol/l for 2MCA1, and 40 μMol/l for 2MCA2 (80 μMol/l for total 2MCA), respectively. The recovery was between 84.42 % and 120.05 %. The intra-assay coefficient of variations (CVs) ranged from 2.1 % to 6.9 % (n = 20), and the inter-assay CVs ranged from 2.7 % to 11.6 % (n = 20). Reference intervals were established and verified (n = 125). A total of 15 patients with variable disorders in related pathway were successfully confirmed. The assay can be performed either in diagnostic laboratories or as second-tier, follow-up test in newborn screening laboratories.
A fast, sensitive, and simple LC/MS/MS method was developed successfully for the simultaneous detection of plasma total homocysteine, methylmalonic acid, methionine, and 2-methylcitric acid for diagnosis of disorders in related pathways.
Acknowledgments
The authors would like to thank Dr. Larry Sweetman (Baylor Research Institute, Dallas, TX) for providing the 2MCA standard; Dr. Robert Allen (Division of Hematology, Department of Medicine, University of Colorado Health Sciences Center, Denver) for parallel testing of 2MCA on GC/MS; and the technologists in Biochemical Genetics and Special Chemistry, Department of Pathology and Laboratory Medicine, Children’s Hospital Los Angeles for analyzing controls and patient’s samples.
Abbreviations
- 2MCA
2-methylcitric acid
- Cbl
Cobalamin
- GC/MS
Gas chromatography–mass spectrometry
- Hcy
Homocysteine
- HPLC
High performance liquid chromatography
- LC/MS/MS
Liquid chromatography-mass spectrometry
- Met
Methionine
- MMA
Methylmalonic acid
- MTHFR
Methylenetetrahydrofolate reductase
- SA
Succinic acid
- TCEP-HCL
Tris 2-carboxyethyl phosphine hydrochloride
- tHcy
Total homocysteine
Footnotes
Competing interests: None declared
References
- Allen R, Stabler SP, Savage DG, et al. Elevation of 2-methylcitric acid I and II levels in serum, urine, and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism. 1993;42(8):978–988. doi: 10.1016/0026-0495(93)90010-L. [DOI] [PubMed] [Google Scholar]
- Andrès E, Loukili NH, Noel E, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004;171:251–259. doi: 10.1503/cmaj.1031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom HJ, Rooij AV, Hogeveen M. A simple high-throughput method for the determination of plasma methylmalonic acid by liquid chromatography-tandem mass spectrometry. Clin Chem Lab Med: 2007;45(5):645–650. doi: 10.1515/CCLM.2007.117. [DOI] [PubMed] [Google Scholar]
- Campbell CD. Two newborns with nutritional vitamin B12 deficiency: challenges in newborn screening for vitamin B12 deficiency. Heamatologica. 2005;90(12):e119–e121. [PubMed] [Google Scholar]
- Garewal G, Narang A, Das KC. Infantile tremor syndrome: a vitamin B12 deficiency syndrome in infants. J Trop Pediatr. 1988;34:174–178. doi: 10.1093/tropej/34.4.174. [DOI] [PubMed] [Google Scholar]
- Hempen C, Wanschers H, Der Sluijs V, et al. A fast liquid chromatographic tandem mass spectrometric method for the simultaneous determination of total homocysteine and methylmalonic acid. Anan Bioanal Chem. 2008;391:263–270. doi: 10.1007/s00216-008-1953-8. [DOI] [PubMed] [Google Scholar]
- Henning BF, Riezler R, Tepel M, Langer K, et al. Evidence of altered homocysteine metabolism in chronic renal failure. Nephron. 1999;83:314–322. doi: 10.1159/000045423. [DOI] [PubMed] [Google Scholar]
- Kushnir MM, Komaromy-Hiller G, Shushan B, et al. Analysis of dicarboxylic acids by tandem mass spectrometry. High-throughput quantitative measurement of methylmalonic acid in serum, plasma, and urine. Clin Chem. 2001;47:1993–2002. [PubMed] [Google Scholar]
- Lemieux B, Ogier H, Lambert MA. Nutritional vitamin B12 deficiency: two cases detected by routine newborn urinary screening. Eur J Pediatr. 1992;151:218–20. doi: 10.1007/BF01954389. [DOI] [PubMed] [Google Scholar]
- Marca GL, Malvagia S, Pasquini E, et al. Rapid 2nd-tier test for measurement of 3-OH-propionic and methylmalonic acids on dried blood spots: Reducing the false-positive rate for propionylcarnitine during expanded newborn screening by liquid chromatography-tandem mass spectrometry. Clin Chem. 2007;53(7):1364–1369. doi: 10.1373/clinchem.2007.087775. [DOI] [PubMed] [Google Scholar]
- Monsen ALB, Ueland PM. Homocysteine and methylmalonic acid in diagnosis and risk assessment from infancy to adolescence. Am J Clin nutr. 2003;78:7–21. doi: 10.1093/ajcn/78.1.7. [DOI] [PubMed] [Google Scholar]
- Mudd SH. Hypermethioninemias of genetic and non-genetic origin: a review. Am J Med Genet Part C Semin Med Genet. 2011;157:3–32. doi: 10.1002/ajmg.c.30293. [DOI] [PubMed] [Google Scholar]
- Obeid RHW. Cobalamin deficiency. Subcell Biochem. 2012;56:301–22. doi: 10.1007/978-94-007-2199-9_16. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Fernhoff PM, Scanlon KS. Vitamin B12 deficiency in children and adolescents. J Pediatr. 2001;138:10–17. doi: 10.1067/mpd.2001.112160. [DOI] [PubMed] [Google Scholar]
- Requejo AM, Ortega RM, Navia B, et al. Folate and Vitamin B12 status in a group of preschool children. Int J Vitam Nutr Res. 1997;67:171–175. [PubMed] [Google Scholar]
- Tomaiuolo M, Vecchione G, Grandone E, et al. A new method for determination of plasma homocystine by isotope dilution and electrospray tandem mass spectrometry. J Chrom B. 2006;842:64–69. doi: 10.1016/j.jchromb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Turgeon C, Magera MJ, Cuthbert CD, et al. Determination of total homocysteine, methylmalonic acid, and 2-methylcitric acid in dried blood spots by tandem mass spectrometry. Clin Chem. 2010;56(11):1686–1695. doi: 10.1373/clinchem.2010.148957. [DOI] [PubMed] [Google Scholar]
- Ueland PM, Refsum H, Stabler SP, et al. Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem. 1993;39:1764–1779. [PubMed] [Google Scholar]
- Windelberg A, Arseth O, Kvalheim G, et al. Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylcholoformate derivatization and gas chromatography–mass spectrometry. Clin Chem. 2005;51:2103–2109. doi: 10.1373/clinchem.2005.053835. [DOI] [PubMed] [Google Scholar]