Abstract
Taxchinin A, with a 11(15→1)-abeo-taxane skeleton, is a major, but inactive taxoid contained in leaves of Taxus chinensis. In our design of dual-functional antitumor abeo-taxane derivatives, two fragments from antitumor agents with different molecular targets (the N-acyl-3′-phenylisoserine side chain from the antimitotic agent paclitaxel and an α,β-unsaturated carbonyl system from NF-κB inhibitors) were incorporated into the scaffold of taxchinin A. The resulting compounds displayed broad inhibitory effects against proliferation of tumor cell lines, with notable selectivity towards colon cancer, melanoma and renal cancer, when evaluated in the NCI-60 human tumor cell line screening panel. Based on the NCI-60 assay data, structure-activity relationship (SAR) correlations were elucidated. Mechanistic studies indicated that this new compound type can both destabilize microtubules and inhibit NF-κB activation, thereby inducing tumor cell apoptosis. This first report of the dual-functional taxoid-core compounds thus provides new opportunities for future drug development based on natural taxoid scaffolds.
1. INTRODUCTION
Cancer is a second leading cause of death in the USA. Natural products are excellent sources of novel chemical entities and have been widely applied as leads and privileged scaffolds for elaboration into efficacious anticancer drugs.1 Compared to single drug therapies, simultaneous effects on several targets in a related biological network often result in a superior therapeutic profile to control complex diseases including cancers.2 However, application of multi-drug combination therapy frequently leads to complex pharmacokinetic/pharmacodynamic relationships and undesired drug-drug interactions. As an alternative solution, development of appropriate single drugs to modulate multiple targets is gaining increasing interest. Among these efforts, one rational approach is to integrate corresponding structural requirements from selective ligands into a single molecule to produce new ligands that can span multiple targets.3
Paclitaxel (PXL) (Figure 1, Taxol, 1a) and its semisynthetic analogue docetaxel (1b) have become important anticancer drugs over the past two decades. Both agents induce tubulin polymerization into microtubules and stabilize the resulting microtubules, which causes cell cycle arrest at the G2/M phase, resulting in cell apoptosis.4 Studies on structure-activity relationship (SAR) correlations of PXL analogues have revealed that the N-acyl-3′-phenylisoserine side chain attached to the taxoid core at C-13 is a key pharmacophoric element.5 In order to find more effective derivatives and starting materials for semisynthesis, extensive phytochemical investigations of different yew trees have identified approximately 380 taxoids with normal skeletons. Moreover, 139 taxoids with an 11(15→1) abeo-taxane skeleton have been discovered primarily from Taxus chinensis, T. yunnanensis, and T. wallichiana.6 However, few advances have been made in structural modification of this taxoid type, as well as corresponding studies on biological activities.7 We isolated taxchinin A (Figure 1, 2), a major taxoid with the abeo-taxane skeleton from leaves of T. chinensis, and accumulated it in a relatively large amount (up to 50 g). Unfortunately, taxchinin A is totally inactive against tumor cell line proliferation. In subsequent studies, we found that analogues containing taxchinin A as the core template, but possessing an exocyclic unsaturated ketone in ring C exhibited satisfactory cytotoxicity.8 To date, the mechanisms associated with the inhibitory effects of these compounds against cancer cell proliferation have not yet been disclosed.
Figure 1.

Structures of paclitaxel, docetaxel, taxchinin A and parthenolide
Figure 2.
Design strategy for new dual-functional abeo-taxoid derivatives
NF-κB is a key nuclear transcription factor for tumor progression9 and drug resistance.10 It is often constitutively activated in human cancers. Therefore, suppression of NF-κB activation may have benefits in enhancing the efficacy of anti-cancer drugs, and overcoming drug resistance.11 Several NF-κB inhibitors have been employed as sensitizers in established cancer therapy over recent years. Increasing evidence has indicated that a combination of PXL with an NF-κB inhibitor, such as parthenolide (3, Figure 1) or curcumin, could augment the therapeutic efficacy in various cancer models.12 Moreover, the powerful NF-κB inhibitor parthenolide and its derivatives have also been studied alone in clinical trials to treat cancer, and various studies have revealed that the α,β-unsaturated carbonyl moiety is an important structural requirement for activity.13
In order to further explore the possible therapeutic application of inactive taxchinin A and to develop potential anticancer compounds with multiple antitumor mechanisms, we proposed to integrate the N-acyl-3′-phenylisoserine side chain (required for microtubule binding of PXL, Figure 1) and α,β-unsaturated carbonyl unit (required for NF-κB inhibition of parthenolide, Figure 1) into the skeleton of taxchinin A. We postulated that insertion of these two drug functionalities into the abeo-taxane scaffold of taxchinin A might produce new unique dual-functional derivatives with potent antitumor activity (Figure 2).
According to the above proposal, four new abeo-taxoids were synthesized and screened against the NCI-60 cell line assay. The newly synthesized compounds exhibited a unique antitumor spectrum. Notably, further mechanistic studies supported our above hypothesis that the new taxchinin A derivatives could disrupt the microtubule equilibrium and suppress NF-κB activation simultaneously. To the best of our knowledge, this is the first report of dual functional taxoids with both microtubule destabilizing and NF-κB inhibitory activities. Also, it is a new successful example for conversion of easily available inactive taxoids into potent anticancer compounds using rational medicinal chemistry design.
2. RESULTS AND DISCUSSION
2.1 Synthesis of abeo-Taxoids
Syntheses of compounds 9–14 and 16–17 are shown in Scheme 1. Compound 4 was prepared from taxchinin A (2) in 85% overall yield in two steps using the optimized procedure we reported previously.8 Initially, OH-13 of 2 was protected selectively by treatment with tert-butyldimethylchlorosilane (TBDMSCl) in imidazole and DMF. Later, the yield was increased to 90% by lowering the reaction temperature to −40 °C. Subsequent pyridinium chlorochromate (PCC) oxidation of the allylic alcohol (OH-5) provided α,β-unsaturated ketone 4. Treatment of TBS-protected ether 4 with HF-pyridine in pyridine afforded alcohol 5 in 87% yield. Use of tetra-n-butylammonium fluoride (TBAF) as the deprotection reagent resulted in the production of compound 6 (90% yield), in which a new double bond was formed between C-6 and C-7 by elimination of acetic acid. Parallel acylation of the deprotected hydroxyl (OH-13) of compound 6 with β-lactams 7 and 8 afforded the products 9 and 10, respectively. Acylation of compound 5 with 7 gave 11, together with 9. Compounds 12 and 10 were produced when β-lactam 8 was employed as the acylation reagent. Compound 13 was synthesized by introducing the N-acyl-3′-phenylisoserine side chain at the C-5 position of 2. Subsequent oxidation of the remaining hydroxyl (OH-13) of 13 afforded compound 14. Moreover, by using taxyunnansin A (15), another natural abeo-taxoid isolated from leaves of T. chinensis, rather than 2, compounds 16 and 17, which are abeo-paclitaxel and abeo-docetaxel derivatives, respectively, were also synthesized, according to the procedures we reported previously.14
Scheme 1.
Reagents and conditions. (a) TBDMSCl, DMF, −40°C; (b) PCC, CH2Cl2 (c) TBAF, THF, rt; (d) HF/CH3CN (95/5); (e) (i) NaHMDS, 7 or 8, THF, −78°C; (ii) 0.5 N HCl, THF, 0°C to rt.
2.2 Antitumor Activity and SAR Study
The newly synthesized derivatives 9–14 and 16–17 were evaluated for cytotoxicity against the NCI-60 cell line panel using standard protocols.15 Initially, these compounds were tested against the growth of 60 human tumor cell lines, including leukemia, non-small cell lung, colon, central nervous system (CNS), melanoma, ovarian, renal, prostate and breast cancers, at a single dose of 10 μM. At this concentration, compounds 14 and 16 were found to be totally inactive. The six remaining compounds (9–13, 17), which exhibited more than 50% growth inhibition at 10 μM, were further evaluated to determine their GI50 (50% growth inhibition) values, and the results are summarized in Table 1 and Supporting Information Figure S1.
Table 1.
GI50 values from NCI-60 human tumor cell line screening[a]
| GI50 (μM) | 9 | 10 | 11 | 12 | 13 | 17 | 1a[d] | 3[d] |
|---|---|---|---|---|---|---|---|---|
| MOLT-4 | 2.48 | 3.78 | 0.65 | ND[c] | 83.30 | 42.66 | 0.032 | 15.85 |
| NCI-H322M | 4.69 | 24.60 | 18.50 | 18.80 | NA | 50.12 | 0.050 | 19.95 |
| HCT-116 | 0.77 | 1.87 | 1.28 | 1.12 | 45.20 | 16.22 | 0.010 | 10.00 |
| SNB-19 | 15.30 | NA[b] | 33.70 | 27.5 | NA | 33.11 | 0.040 | 63.10 |
| LOXI MVI | 1.57 | 2.20 | 1.86 | 1.70 | NA | 21.38 | 0.016 | 7.94 |
| OVCAR-3 | 1.33 | 3.19 | 1.72 | 1.69 | 28.4 | 15.49 | 0.020 | 19.95 |
| RXF393 | 0.52 | 2.08 | 1.29 | 1.33 | 26.60 | 12.59 | 0.050 | 12.59 |
| DU-145 | 1.66 | 6.05 | 1.59 | 1.89 | 72.40 | 21.40 | 0.032 | ND |
| T-47D | 1.70 | 4.78 | 1.86 | 1.88 | NA | 38.90 | 0.010 | ND |
| Mean GI50 | 2.04 | 8.32 | 2.75 | 2.88 | 70.79 | 27.54 | 0.035 | 17.90 |
Data obtained from NCI-60 screening. MOLT-4, leukemia cell line; NCI-H322M, non-small-cell lung cancer cell line; HCT-116, colorectal carcinoma; SNB-19, CNS tumor cell lines; LOXI MVI, melanoma; OVCAR-3, ovarian cancer cell line; RXF393, renal cancer cell line; DU-145, prostate cancer cell line; T-47D, breast cancer cell line.
NA indicates GI50 value > 100 μM.
ND indicates not determined.
Data can be accessed from the compound name at the following web site: http://dtp.nci.nih.gov/docs/cancer/searches/standard_agent.html
Four designed compounds (9–12) exhibited broad inhibitory effects against tumor cell proliferation. Although they were largely less potent than PXL, they showed rather high potency in comparison with parthenolide (3) and their nontoxic parent taxchinin A (2). Moreover, based on the mean graph obtained for all cell lines at the GI50 level, these compounds also exhibited unique activity patterns (Figure S1, Supporting Information). In general, among the nine types of human tumor cell lines used in the screening, the colon, melanoma and renal tumor cell lines were particularly sensitive to this compound series, while the cell lines from non-small-cell lung cancer and central nervous system tumor were relatively resistant. The most potent derivative 9 preferentially inhibited the proliferation of the colorectal carcinoma cell line HCT-116 and the renal tumor cell line RXF393, with GI50 values of 0.77 and 0.52 μM, respectively. In addition, compounds 10 and 11 were also potent against the growth of leukemia cell lines. Compound 11 strongly inhibited the proliferation of the leukemia cell line MOLT-4 (GI50 0.65 μM).
Preliminary SAR conclusions were proposed on the basis of the above results (Figure 3). Compounds 9–12, which contain an exocyclic unsaturated ketone moiety, showed wide ranging inhibitory effects against the tested tumor cell lines, while compounds 13–14 and 16–17, without an exocyclic unsaturated ketone moiety, were inactive. In compounds 12 and 14, the positions of the two important functionalities, the N-acyl-3′-phenylisoserine side chain and α,β-unsaturated carbonyl moiety, are interchanged. Compound 12, with the phenylisoserine side chain at the C-13 position (A-ring) and an exocyclic unsaturated ketone moiety in the C-ring, exhibited high inhibitory potency (mean GI50 2.88 μM), while compound 14, with the phenylisoserine side chain at the C-5 position (C-ring) and an endocyclic unsaturated ketone moiety in the A-ring, was totally inactive. Compounds 11 and 12 exhibited good activity with mean GI50 values of 2.75 and 2.88 μM, whereas compounds 16 and 17, with an oxetane ring on ring C, rather than the exocyclic α,β-unsaturated carbonyl system at the same position found in 11 and 12, respectively, were inactive or weakly active. These results strongly suggested that an exocyclic α,β-unsaturated ketone moiety located in the C-ring is an essential functionality for activity. Moreover, a phenylisoserine moiety on C-13 (A-ring) is also important for activity, and minor changes of this functionality could greatly impact the activity. Among the tested compounds, analogue 9 with a C-13 N-benzoyl-3′-phenylisoserine side chain and a double bond between C-6 and C-7 exhibited the highest potency (mean GI50 2.04 μM). The parallel compound 10, in which a N-t-butoxycarbonyl moiety has replaced the N-benzoyl group of 9, showed four-fold lower activity. Interestingly, this potency difference was not apparent between compounds 11 and 12, which have a saturated bond between C-6 and C-7 with an acetoxy group on C-7. Further analysis showed that, when a C-13 N-benzoyl-3′-phenylisoserine side chain was present (11 vs 9), introduction of a double bond between C-6 and C-7 did not greatly affect the activity based on mean GI50 values. However, in the compounds with a C-13 N-t-butoxycarbonyl-3′-phenylisoserine (12 vs 10), the presence of the double bond in the latter compound resulted in decreased activity.
Figure 3.
SAR of taxoid-based derivatives
2.3 Apoptosis of the HCT-116 Cells Induced by Compounds 11 and 12
We then explored the mechanisms of action of these new abeo-taxane derivatives. Initially, compounds 11 and 12 were examined for apoptosis-induction ability. We used the HCT-116 cell line due to its sensitivity to this compound series. Compounds 11 and 12 showed antiproliferative activity against HCT-116 cells with GI50 values of 1.28 and 1.12 μM, respectively (Table 1). Apoptosis in HCT-116 cells was induced by treatment with compound 11 or 12 in a dose-dependent manner. Apoptotic cell number increased to 8.54% and 20.36% when the cells were treated with compound 11 at 1 and 2 μM, and to 4.93% and 19.87% for compound 12 at 1 and 2 μM, respectively (Figure 4). These results indicated that the anti-proliferative activity of 11 and 12 against HCT-116 cells might result from their ability to induce apoptosis.
Figure 4.
Compounds 11 and 12 induce apoptosis of HCT-116 cells. Cells were treated with 11 or 12 for 24 h. Treatment with 11 and 12 increased the early apoptotic (Annexin V +/PI -, lower right quadrant) and late apoptotic (Annexin V +/PI +, upper right quadrant) cells.
2.4 Tubulin Polymerization Assay
Because the new derivatives have the unique abeo-taxane core as well as a phenylisoserine side chain at C-13, we also tested them for direct effects on tubulin polymerization in a cell-free system, in comparison with PXL and abeo-docetaxel derivative 17. Compounds 11 and 12 interfered with microtubule assembly quite differently from PXL or 17 (Figure 5A). In accordance with previous reports,9,12,13 PXL induced tubulin polymerization and 17 exhibited a similar effect. Surprisingly, compounds 11 and 12 displayed microtubule destabilizing potency comparable to Ca2+-induced depolymerization. This finding is unusual, because various known derivatives of PXL (including abeo-paclitaxel) have commonly been found to display microtubule stabilization activity.5,7a,7c,7e To the best of our knowledge, our finding is the first report of taxoid-core derivatives that clearly inhibit tubulin polymerization. The above results imply that these two compounds should interact directly with tubulin and/or microtubules in a different manner from that of PXL and 17, even though the only structural difference between compounds 17 and 12 is the replacement of the oxetane ring in 17 with an exocyclic unsaturated carbonyl functionality in 12. This minor structural alteration led to opposite effects on tubulin polymerization. In other words, these two compounds could serve as a pair of “molecular switches” (12 for destabilization, 17 for stabilization of microtubules). In addition, we suppose that the unsaturated carbonyl unit in 12 could either react in a Michael-type addition with a thiol group on microtubules or induce a conformational change that might affect the binding mode of 12 to microtubules and, thus, produce a different effect on tubulin polymerization. Overall, such findings should be helpful in understanding how taxoids and their derivatives interact with microtubules.
Figure 5.
Effects of compounds on microtubule assembly. (A) Effects of compounds on tubulin polymerization in a cell-free system. Tubulin polymerization was detected in a fluorescence based cell-free system in the presence of DMSO (control), 500 μM CaCl2, 3 μM paclitaxel, 15 μM 11, 15 μM 12, or 30 μM 17. Tubulin polymerization was measured by fluorescence intensity at 420 nm emission and 340 nm excitation. Data are representative of three independent experiments performed in triplicate. (B) Effects of compounds on microtubule dynamics in PC-3 cells. PC-3 cells were cultured and treated with DMSO (control), 2 μM compounds 11, or 5 μM compound 12 for 24 h. Cells were stained with monoclonal antibody to α-tubulin and DAPI for DNA. The insert (upper panel) shows a typical image of metaphase cell with spindles. Cells with abnormal spindles and microtubules were detected among the cells treated with compound 11 or 12. Although both compounds gave similar defects on tubulin dynamics, significant effects were not seen in cells treated with 2 μM 12. Additional images are available in Supporting Information Figure S2. Bar, 25 μm.
When PC-3 prostate cancer cells were treated with 11 and 12, an immunofluorescence staining assay showed morphological changes in the microtubules (Figure 5B). Combretastatin A4 (CA4) and PXL were also evaluated as positive controls (Figure S2, Supporting Information). PXL induced over-polymerized microtubules in a bundled sheet-like pattern, while CA4, which binds to the colchicine-binding site, led to complete depolymerization of the microtubular network. Tubulin immunofluorescence staining of 11- or 12-treated mitotic cells indicated that these cells failed to assemble a typical mitotic spindle, while microtubules in interphase cells were not significantly disrupted. This phenotype was clearly different from that of CA4- or PXL-treated cells. In addition, 11- or 12-treated mitotic cells contained condensed chromatin, which failed to align at the metaphase plate, and were confirmed as prometaphase by staining with 4′,6-diamidino-2-phenylindole (DAPI). These observations demonstrated that 11 and 12 induce cell cycle arrest at prometaphase, which agrees with accumulation at G2/M phase as analyzed by flow cytometry.
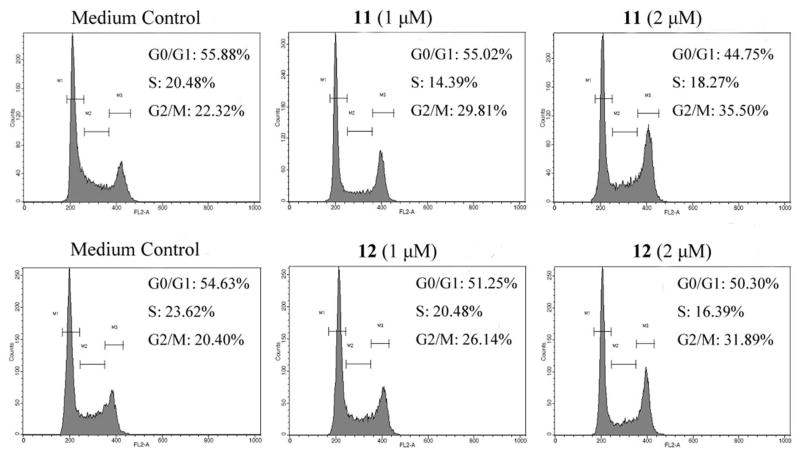
Cell cycle arrest in the G2/M phase is a characteristic effect of many antimitotic agents that disrupt microtubule assembly.16 To obtain further evidence that the new derivatives function by tubulin-interacting mechanisms, HCT-116 cells were treated with 11 and 12 for 12 h and evaluated by a PI staining-based fluorescence-activated cell sorting analysis. As postulated, compounds 11 and 12 caused dose-dependent accumulation of G2/M cells, accompanied with a reduction of G0/G1 cells (Figure 6). After treatment with compounds 11 and 12 at 2 μM, 36.98% and 33.31%, respectively, of cell numbers were accumulated in G2/M. The percentages of cells in G0/G1 were about 55.88% and 54.63% in the untreated cells, but gradually decreased to about 44.75% and 50.30% after treatment with compounds 11 and 12, respectively. Taken together, these results indicate that 11 and 12 target tubulin directly at a different binding site from colchicine or PXL, and disrupt microtubule dynamics, which subsequently induces cell cycle arrest at prometaphase.
Figure 6.
Compound 11 and 12 induce cell cycle arrest at G2/M phase. HCT-116 cells were treated with 1 or 2 μM compounds 11 or 12 for 12 h. Cells were fixed, stained with propidium iodide (PI), and were assessed by flow cytometry. The percentages of cells in different DNA ploidy of 2N (G0/G1), between 2N and 4N (S) and 4N (G2/M) are indicated on the right of each cell cycle profile. Data shown are from one of three independent experiments.
2.5 Inhibition of NF-κB Activation
Having validated the microtubule-interfering effects of 11 and 12, we further evaluated their effects on the NF-κB signaling pathway. As we expected, compounds 11 and 12 suppressed TNF-α-induced NF-κB activation in a dose-dependent manner (Figure 7). Stimulation with TNF-α resulted in 6.5-fold increase in NF-κB luciferase activity over the control. TNF-α-induced NF-κB DNA binding activity was inhibited by 14%, 27% and 37% by compound 11 at 0.31, 0.62, and 1.25 μM, respectively; and by 9%, 12% and 28% by compound 12 at 0.62, 1.25, and 2.50 μM, respectively. We also examined the NF-κB inhibition of parthenolide (3) and abeo-docetaxel derivative 17 under the same conditions (Figure S3, Supporting Information). Parthenolide (3) exhibited a comparable NF-κB inhibitory effect to those of 11 and 12, while 17 showed no activity as expected. These results shown by the designed taxoids supported our initial hypothesis that an α,β-unsaturated ketone functionality is an essential structural requirement for inhibition of NF-κB. To the best of our knowledge, these new taxoid derivatives are the first to exhibit TNF-α-induced NF-κB inhibitory activity.
Figure 7.

Inhibitory effects of compounds 11 and 12 on TNF-α–induced NF-κB activation. HEK 293T cells transiently transfected with pNFκB-luc and pRL-TK vectors, were pretreated with DMSO, 0.31, 0.62, 1.25 μM compound 11 or 0.62, 1.25, 2.50 μM compound 12 for 1 h before TNF-α stimulation (25 ng/mL) for 18 h. The NF-κB reporter was assayed by measuring the luciferase activity as described under Materials and Methods. Data shown are from one of three independent experiments. *p<0.05, **p<0.01
3. CONCLUSION
In conclusion, we have demonstrated the first report of dual-functional taxoid derivatives exhibiting both microtubule destabilization and NF-κB inhibition. By incorporating essential functionalities from PXL and parthenolide into the skeleton of the inactive natural abeo-taxoid taxchinin A, we synthesized several new derivatives with notable selectivity against colon cancer, melanoma, and renal cancer. Mechanism of action studies revealed that these new compounds could destabilize microtubules and inhibit NF-κB activation, thereby inducing cell cycle arrest and apoptosis in tumor cells. This work provides a new strategy for future drug development based on natural taxoid scaffolds. Combination of the natural skeleton with appropriate pharmacophores has proved to be a valuable protocol for the discovery of new natural product-derived chemical entities with unique biological mechanisms. Further studies on these taxoid derivatives, including identification of the binding mode on tubulin/microtubules and the inhibitory step for suppressing NF-κB activation, are underway in our laboratories.
4. EXPERIMENTAL SECTION
Chemistry
All non-aqueous reactions were carried out under nitrogen atmosphere. Reagents were purchased from commercial sources and used as received. Dichloromethane and DMF were distilled from CaH2, and THF was distilled from Na prior to use. 1H and 13C NMR experiments were performed on a Bruker AM-400 or DRX-500 spectrometer at ambient temperature with acetone-d6 as the solvent. 2D NMR spectra were recorded on a Bruker DRX-500 NMR instrument. IR spectra were recorded on a Bio-Rad FTS-135 spectrometer with KBr pellets. UV spectra were obtained on a UV 2401 PC spectrometer. ESIMS and HRESIMS were taken on a VG Auto Spec-3000 or on a Finnigan MAT 90 instrument. Melting points (uncorrected) were determined on an XRC-1 micro melting point apparatus. Optical rotations were measured with a Horiba SEPA-300 polarimeter. Column chromatography was performed on silica gel (400μ, Qingdao Marine Chemical Inc. China). The purity of all tested compounds was determined by HPLC (Agilent Technologies 1200 Series) equipped with a C-18 bounded-phase column (ZORBAX, SB-C18, 4.6 mm× 250 mm). A gradient elution was performed with MeOH and water as a mobile phase and was monitored at 238 nm. All tested compounds were > 95% pure.
Synthesis of Compound 4.8
TBDMSCl (589 mg, 3.91 mmol) and imidazole (133.1 mg, 1.95 mmol) were added to a solution of 2 (600 mg, 0.98 mmol) in DMF (4.5 mL) at −40 °C. The reaction was stirred for 10 h at −40 °C until the starting material was consumed. The mixture was diluted with EtOAc and washed with water and brine. The organic layer was then dried (Na2SO4) and concentrated. The residue was purified by column chromatography (10% EtOAc in petroleum ether) to give 13-TBS-taxchinin A (672 mg, 94%).
The above product in CH2Cl2 (15 mL) was added to a solution of PCC (1.19 g, 5.54 mmol) in CH2Cl2 (15 mL). This solution was stirred for 8 h at rt. The reaction mixture was filtered and concentrated. The residue was purified by silica gel column chromatography (30% EtOAc in petroleum ether) to afford 4 (603 mg, 90%) as a white amorphous powder.
Synthesis of 5-Oxo-taxchinin A (5).8
HF (0.75 mL, 40% aq.) was added to a solution of 4 (75 mg, 0.10 mmol) in THF (14.25 mL). The reaction mixture was stirred for 2 h at room temperature (rt), and then diluted with EtOAc and treated with saturated aqueous sodium bicarbonate (10 mL). The organic layer was separated, washed with brine, dried (Na2SO4) and concentrated. The residue was purified by column chromatography (20% EtOAc in petroleum ether) to give 5 (55 mg, 87%) as colorless oil.
Synthesis of Compound 6.8
TBAF (0.76 mL, 0.76 mmol, 1M solution in THF) was added to a solution of 4 (500 mg, 0.69 mmol) in THF (20 mL). The reaction was stirred at rt and monitored by TLC until the starting material was consumed. The reaction mixture was diluted with EtOAc and washed with water and brine. The organic layer was dried (Na2SO4) and concentrated. The residue was purified by column chromatography (15% EtOAc in CHCl3) to give 6 (342 mg, 90%) as yellow oil.
Synthesis of Compound 9
Sodium hexamethyldisilazide (NaHMDS) in THF (0.06 mL, 0.12 mmol, 2M in THF) was added to a solution of 6 (55 mg, 0.10 mmol) and 7 (40.5 mg, 0.12 mmol) in anhydrous THF (5 mL) with stirring at −78 °C. After 30 min, the reaction was quenched with saturated aqueous NH4Cl and the mixture was extracted with EtOAc. The organic layer was washed with brine, dried over anhydrous Na2SO4, and concentrated. The residue was purified by column chromatography (25% EtOAc in petroleum ether) to give the product (96 mg) as colorless oil.
This intermediate was then dissolved in THF (35 mL) and treated with 0.5 N HCl (15 mL) at 0 °C. The reaction was allowed to warm to rt and stirring was continued for 4 h until completion. The mixture was then diluted with EtOAc. The organic phase was washed with saturated aqueous NaHCO3, brine, dried over Na2SO4 and concentrated. The residue was purified by column chromatography (20% EtOAc in petroleum ether) to give 9 (36 mg, 49%) as colorless oil: tR- HPLC: 11.16 min (97.8%); [α]10 D –29.9 (c 0.76, CH3OH); UV (CH3OH) λmax (log ε) 229.8 (4.12) nm; IR (KBr) νmax 3433, 2961, 2930, 2857, 1728, 1672, 1371, 1270, 1121, 1071, 1027, 713 cm−1; 1H NMR (acetone-d6, 500 MHz) δ 7.99 (d, J = 10.5 Hz, 1H), 7.97 (d, J = 7.5 Hz, 2H), 7.92 (d, J = 7.5 Hz, 2H), 7.63 (t, J = 7.0 Hz, 1H), 7.54-7.44 (overlap, 8H), 7.31 (t, J = 7.0 Hz, 2H), 7.25 (t, J = 7.5 Hz, 1H), 6.40 (d, J = 11.0 Hz, 1H), 6.13-6.08 (overlap 3H), 6.08 (d, J = 11.0 Hz, 1H), 5.60 (dd, J = 9.0, 4.5 Hz, 1H), 5.56 (t, J = 7.5Hz, 1H), 5.33 (s, 1H), 4.98 (d, J = 7.0 Hz, 1H), 4.65(t, J =4.5 Hz, 1H), 3.34 (d, J = 7.5 Hz 1H), 2.35-1.71 (m, 2H), 2.02 (s, 3H), 1.76 (s, 3H), 1.71 (s, 3H), 1.27 (s, 3H), 1.23 (s, 3H), 1.21 (s, 3H); 13C NMR (acetone-d6, 125 MHz) δ 189.7 s, 173.0 s, 171.8 s, 170.0 s, 167.3 s, 165.8 s, 154.8 d, 147.7 s, 143.4 s, 140.5 s, 138.4 s, 135.6 s, 134.3 d, 132.3 d, 130.6 s, 130.5 d, 129.9 d, 129.5 d, 129.2 d, 129.1 d, 128.5 d, 128.4 d, 128.3 d, 121.5 t, 80.7 d, 76.1 s, 75.4 d, 74.9 d, 70.2 d, 69.7 d, 68.7 s, 57.1 d, 48.6 d, 44.6 s, 37.9 t, 28.3 q, 27.5 q, 21.9 q, 21.7 q, 20.6 q, 12.1 q; HRMS (ESI, m/z) calcd for C47H49NO12Na [M+Na] +, 842.3152; found, 842.3159.
Synthesis of Compound 10
Similarly to the above procedure, coupling of 8 (48.6 mg, 0.14 mmol) and 6 (40 mg, 0.07 mmol) was performed using NaHMDS (0.04 mL, 0.09 mmol, 2M in THF), followed by removal of the C-2′ protecting groups with 0.5 N HCl (19 mL) to give 10 (26 mg, 44%) as colorless oil: tR- HPLC: 17.92 min (98.6%); [α]13 D –23.0 (c 0.31, CH3OH); UV (CH3OH) λmax (log ε) 230.6 (3.96) nm; IR (KBr) νmax 3434, 2960, 2928, 2874, 2855, 1726, 1627, 1466, 1458, 1369, 1271, 1123, 1073, 711 cm−1; 1H NMR (acetone-d6, 500 MHz) δ 8.00 (d, J = 7.2 Hz, 2H), 7.65 (t, J = 7.2 Hz, 1H), 7.52-7.47 (overlap, 3H), 7.39-7.25 (overlap, 5H), 6.44-6.37 (overlap, 2H), 6.20-6.10 (overlap, 3H), 6.07 (s, 1H), 5.64 (t, J = 7.2 Hz 1H), 5.37 (s 1H), 4.49(t, J = 4.8 Hz 1H), 3.38 (d, J = 8.1 Hz, 1H), 2.43-1.82 (m, 2H), 2.15 (s, 3H), 1.78 (s, 3H), 1.75 (3H, s), 1.39 (9H, s), 1.32 (s, 3H), 1.24 (q, 3H), 1.20 (q, 3H); 13C NMR (acetone-d6, 125 MHz) δ 189.6 s, 173.0 s, 171.9 s, 171.9 s, 170.1 s, 165.9 s, 154.9 d, 148.0 s, 143.7 s, 141.1 s, 138.4 s, 134.4 d, 130.8 s, 130.6 d, 129.9 d, 129.6 d, 129.1 d, 128.4 d, 128.2 d, 121.2 t, 80.6 d, 76.1 s, 75.5 d, 75.2 d, 70.5 d, 69.9 d, 68.9 s, 58.1 d, 55.6 s, 48.8 d, 44.8 s, 37.9 t, 28.7 q, 28.5 q, 27.6 q, 22.1 q, 22.1 q, 20.6 q, 12.2 q; HRMS (ESI, m/z) calcd for C45H53NO13Na [M+Na] +, 838.3414; found, 838.3412.
Conversion of Compound 5 into Compounds 11 and 9
Using the same procedure as for 9, compounds 7 (41.9 mg, 0.12 mmol) and 5 (63 mg, 0.10 mmol) were coupled with the use of NaHMDS (0.06 mL, 0.12 mmol, 2M in THF), followed by removal of the protecting groups at C-2′ with 0.5 N HCl (19 mL) to give 11 (20 mg, 22%) and 9 (23 mg, 27%).
Compound 11: colorless oil
tR- HPLC: 11.30 min (97.3%); [α]10 D 37.5 (c 0.39, CH3OH); UV (CH3OH) λmax (log ε) 227.6 (4.14) nm; IR (KBr) νmax 3434, 2955, 2871, 1726, 1670, 1640, 1466, 1385, 1368, 1272, 1241, 1042, 990, 711 cm−1; 1H NMR (acetone-d6, 500 MHz) δ 8.02-8.00 (overlap, 3H), 7.97 (d, J = 7.5 Hz, 2H), 7.62 (t, J = 7 Hz, 1H),7.56-7.48 (overlap, 7H), 7.38 (t, J = 8.0 Hz, 2H), 7.29 (t, J = 7.5 Hz, 1H), 6.12 (brs, 1H), 5.85 (s, 1H), 5.80 (s, 1H), 5.77 (t, J = 7.5 Hz, 1H), 5.55 (s, 1H), 5.15 (brs, 1H), 5.24 (brs, 1H), 4.82 (s, 1H), 3.37 (d, J = 7.5 Hz, 1H), 2.61-2.54 (overlap, 2H), 2.34 (m, 1H), 2.05 (s, 3H), 1.29 (s, 3H), 1.14 (s, 3H), 1.96 (s, 3H), 1.92 (s, 3H), 2.34-1.74 (m, 4H), 0.96 (s, 3H). 13C NMR (acetone-d6, 125 MHz) δ 198.3 s, 173.2 s, 170.5 s, 170.4 s, 170.4 s, 167.4 s, 166.3 s, 147.7 s, 141.0 s, 141.0 s, 144.9 s, 135.7 s, 133.9 d, 132.4 d, 131.4 s, 130.6 d, 129.4 d, 129.3 d, 129.2 d, 128.8 t, 128.4 d, 128.2 d, 128.2 d, 82.2 d, 75.8 s, 75.4 d, 74.8 d, 71.0 d, 70.6 d, 70.1 d, 69.8 s, 56.5 d, 43.9 s, 42.9 d, 38.3 t, 39.4 t, 28.9 q, 28.2 q, 22.1 q, 21.0 q, 21.0 q, 13.1 q, 12.1 q; HRMS (ESI, m/z) calcd for C49H53NO14Na, 902.3363 [M+Na] +; found, 902.3359.
Conversion of Compound 5 into Compounds 12 and 10
Coupling of compounds 8 (32.8 mg, 0.10 mmol) and 5 (50 mg, 0.08 mmol) using NaHMDS (0.05 mL, 0.10 mmol, 2M in THF) was performed with the same procedure as for 9. Subsequent removal of the protecting groups at C-2′ with 0.5 N HCl (15 mL) gave 12 (18 mg, 25%) and 10 (23 mg, 34%).
Compound 12: colorless oil
tR- HPLC: 19.31 min (98.1%); [α]18 D 16.3 (c 0.24, CH3OH); UV (CH3OH) λmax (log ε) 228.4 (3.60) nm; IR (KBr) νmax 3433, 2960, 2925, 2874,2853, 1729, 1629, 1466, 1369, 1273, 1247, 1124, 1073, 745, 707 cm−1; 1H NMR (acetone-d6, 500 MHz) δ 8.03 (d, J = 8.0 Hz, 2H), 7.62 (t, J = 7.5 Hz, 1H), 7.50 (t, J = 7.5 Hz, 2H), 7.45 (d, J = 7.5 Hz, 2H), 7.36 (t, J = 7.5 Hz, 2H), 7.28 (t, J = 7.0 Hz, 1H), 6.40 (d, J = 10.0 Hz, 1H), 6.15 (br s, 1H), 5.92 (d, J = 7.5 Hz, 1H), 5.87-5.85 (overlap, 2H), 5.59 (s, 1H), 5.31 (d, J = 10.0 Hz, 1H), 5.24 (m, 1H), 4.53 (brs, 1H), 3.44 (d, J = 7.5 Hz, 1H), 2.57-1.92 (m, 4H), 2.05 (s, 3H), 1.96 (s, 3H), 1.93 (s, 3H), 1.90 (s, 3H), 1.45 (s, 9H), 1.29 (s, 3H), 1.19 (s, 3H), 1.00 (s, 3H); 13C NMR (acetone-d6, 125 MHz) δ 198.3 s, 173.1 s, 170.7 s, 170.5 s, 170.3 s, 167.7 s, 165.8 s, 147.9 s, 145.2 s, 142.5 s, 136.3 s, 133.9 d, 131.5 s, 130.7 d, 129.4 d, 129.2 d, 128.2 d, 128.0 d, 126.5 t, 82.2 d, 75.8 s, 75.0 d, 74.9 d, 71.1 d, 70.8 d, 70.2 d, 66.9 s, 57.5 d, 55.4 s, 44.0 s, 43.0 d, 39.5 t, 38.2 t, 29.1 q, 28.8 q, 28.3 q, 22.3 q, 21.1 q, 21.0 q, 13.2 q, 12.0 q; HRMS (ESI, m/z) calcd for C47H57NO15Na [M+Na] +, 898.3625; found, 898.3624.
Synthesis of Compound 13
Using the same procedure as for 9, coupling of compounds 7 (66 mg, 0.20 mmol) and 2 (100 mg, 0.16 mmol) was performed with NaHMDS (0.10 mL, 0.20 mmol). Removal of the C-2′ protecting groups with 0.5 N HCl (37 mL) gave 13 (73 mg, 51%) as colorless oil. tR- HPLC: 17.37 min (96.9%); [α]18 D –22.9 (c 0.37, CH3OH); UV (CH3OH) λmax (log ε) 228.4 (3.80) nm; IR (KBr) νmax 3430, 2958, 2926, 2872, 2855, 1732, 1465, 1374, 1376, 1270, 1252, 1221, 1173, 1122, 1072, 1029, 746, 711 cm−1; 1H NMR (acetone-d6, 500 MHz) δ 7.91 (d, J = 7.5 Hz, 2H), 7.64 (t, J = 7.2 Hz,1H), 7.51 (t, J = 7.5 Hz, 2H), 7.45 (d, J = 7.5 Hz, 2H), 7.34 (t, J = 7.0 Hz, 2H), 7.25 (t, J = 7.5 Hz, 1H), 6.63 (d, J = 10.5 Hz,1H), 6.55 (br s, 1H), 6.19 (d, J = 8.0 Hz 1H), 6.07 (d, J = 10.5 Hz, 1H), 5.55 (m, 1H), 5.34 (s, 1H), 4.96 (s, 1H), 4.72-4.69 (overlap, 3H), 3.41 (d, J = 8.0 Hz, 1H), 2.60-2.46 (m, 2H), 2.16 (s, 3H), 2.14-2.08 (m, 2H), 2.05 (s, 3H), 2.02 (s, 3H), 1.78 (s, 3H), 1.40 (s, 9H), 1.29 (s, 3H), 1.19 (s, 3H), 1.07 (s, 3H); 13C NMR (acetone-d6, 125 MHz) δ 172.1 s, 171.8 s, 170.4 s, 170.4 s, 170.0 s, 165.0 s, 153.8 s, 141.5 s, 141.5 s, 134.3 d, 134.1 s, 130.3 s, 130.3 d, 129.7 d, 129.1 d, 128.1 d, 128.0 d, 115.7 t, 77.7 d, 77.2 d, 77.0 d, 75.8 s, 75.6 d, 70.9, d, 69.6 d, 69.3 s, 68.4 d, 58.0 d, 55.6 s, 46.0 s, 44.0 d, 41.1 t, 35.6 t, 28.7 q, 28.3 q, 27.1 q, 22.1 q, 21.5 q, 20.9 q 14.0 q, 12.5 q; HRMS (ESI, m/z) calcd. for C47H59NO15Na [M+Na]+, 900.3782; found, 900.3787.
Oxidation of Compound 13 to 14
PCC (78 mg, 0.36 mmol) in CH2Cl2 (8 mL) was added to a solution of 13 (53 mg, 0.06 mmol) in CH2Cl2 (10 mL). The reaction was stirred at rt and monitored by TLC until the starting material was consumed. The mixture was filtered and concentrated. The residue was purified by silica gel column chromatography (40% EtOAc in petroleum ether) to afford 14 (28 g, 53%) as colorless oil: tR-HPLC: 24.32 min (98.6%); [α]18 D 37.0 (c 0.55, CH3OH); UV (MeOH) λmax (log ε) 235.0 (3.97) nm; IR (KBr) νmax 3443, 2962, 2929, 2874, 2857, 1724, 1602, 1497, 1468, 1454, 1370, 1272, 1239, 1174, 1120, 1071, 1027, 981, 917, 747, 706 cm−1; 1H NMR (acetone-d6, 500 MHz) δ 8.01 (d, J = 7.0 Hz, 2H), 7.62 (t, J = 7.0 Hz, 1H), 7.50 (t, J = 7.5 Hz, 2H), 7.45 (d, J = 7.5 Hz, 2H), 7.34 (t, J = 6.5 Hz, 2H), 7.25 (t, J = 7.0 Hz, 1H), 6.37 (d, J = 9.0 Hz,1H), 6.26-6.12 (overlap, 2H), 5.70 (br s, 1H), 5.50 (s, 1H), 5.12 (br s, 1H), 5.03 (s, 1H), 4.96 (br s, 1H), 4.60 (br s, 1H), 3.06 (d, J = 9.0 Hz, 1H), 2.73-1.70 (m, 4H), 2.06 (s, 3H), 1.98 (s, 3H), 1.93 (s, 3H), 1.91 (s, 3H), 1.37 (s, 9H), 1.28 (s, 3H), 1.19 (s, 3H), 1.17 (s, 3H); 13C NMR (acetone-d6, 125 MHz) δ 206.8 s, 174.4 s, 171.7 s, 170.8 s, 170.4 s, 170.4 s, 166.7 s, 162.1 s, 148.4 s, 141.4 s, 133.9 d, 131.4 s, 130.6 d, 129.4 d, 129.1 d, 128.1 d, 128.0 d, 116.1 t, 76.1 s, 75.3 d, 74.9 d, 71.8 d, 70.8 d, 70.1, d, 69.2 d, 63.3 s, 57.5 d, 55.6 s, 46.4 d, 45.1 s, 44.6 t, 33.5 t, 29.1 q, 28.6 q, 27.3 q, 21.7 q, 21.0 q, 20.9 q, 15.2 q, 9.5 q; HRMS (ESI, m/z) calcd for C47H57NO15Na [M+Na] +, 898.3625; found, 898.3628.
Synthesis of Compounds 16 and 17
Compounds 16 and 17 were prepared from 15 following the procedures described in our previous paper.14
NCI-60 Human Tumor Cell Line Screening
Compounds were evaluated in the NCI’s human tumor 60-cell line screening, and data calculations were performed as described.15
Apoptosis Assay
HCT-116 cells were treated with increasing amounts of compound 11 or 12 for 24 h, washed with phosphate buffered saline (PBS) and after trypsinization, apoptosis was evaluated using annexin V-fluorescein 5-isothiocyanate (annexin V-FITC)/propidium iodine (PI) staining with an Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, San Jose, USA). Samples were analyzed by flow cytometry using FACSCalibur and CellQuest software (Becton Dickinson). Co-staining with Annexin V and PI allows classification of viable cells (Annexin V-negative, PI-negative) from early apoptotic cells (Annexin V-positive, PI-negative) and late apoptotic or necrotic cells (Annexin V-positive, PI-positive).
Tubulin Polymerization Assay
The effects of compounds on tubulin polymerization were determined by tubulin polymerization assay kit (Cytoskeleton, Denver, CO, USA). Standards and tested compounds were mixed with tubulin according to manufacturer’s instructions. Fluorescence was detected by a temperature regulated fluorimeter capable of reading at 410–460 nm in kinetic mode using excitation filters of 340–360 nm. The resulting polymerization curve is representative of the three phases of microtubules polymerization, namely nucleation, growth, and steady state equilibrium. Compounds or proteins that interact with tubulin will often alter one or more of the characteristic phases of polymerization.
Immunofluorescence Staining of Tubulin
PC-3 cells were grown on 8-well chamber slide (Lab-Tech) for 36 h prior to treatment with DMSO (control), 2 μM or 10 μM compound 11, 5 μM or 10 μM compound 12, 200 nM CA4, or 200 nM PXL for 24 h. Cells were fixed in 4% paraformaldehyde in PBS, and permeabilized with 0.5% Triton X-100 in PBS. Cells were labeled with mouse monoclonal antibody to α-tubulin (B5-1-2, Sigma) followed by FITC conjugated secondary antibody (Sigma).17 Nuclei were labeled with DAPI. Fluorescence labeled cells were observed using an Olympus BX61 fluorescence microscope, and images were captured by an ORCA-R2 digital camera (Hamamatsu Photonics) controlled by Volocity 5.5.2 software (Perkin Elmer Inc.) (Microscopy Service Laboratory, UNC-CH). Final images were prepared using Adobe Photoshop.
Cell Cycle Analysis
Cell cycle was evaluated by measurement of the DNA content by PI (St Louis, MO) staining. Briefly, HCT-116 cells were seeded in six-well plates at a density of 5×105 cells/well 24 h prior to treatment with compounds 11 or 12. After 12 h, cells and supernatants were collected together, centrifuged at 1000 rpm for 5 min and pelleted. The pellet was resuspended and fixed with 70% EtOH overnight at 4 °C. After being stained with PI, cells were subjected to flow cytometric cell cycle analysis. Experiments were repeated a minimum of two times.
Luciferase Reporter Assay
HEK 293T cells were transiently transfected with pNFκB-luc (Beyotime) and pRL-TK (Promega, Madison, WI) vectors using Lipofectamine 2000 transfection reagent (Invitrogen) for 12 h. The cells were then pre-incubated with different concentrations of compound for 1 h and were subsequently activated with TNF-α for 18 h. Transcriptional activity was determined by measuring the activities of firefly and Renilla reniformis luciferases in Luminoskan Ascent Microplate Luminometer (Thermo Scientific) using Dual-Luciferase Reporter Assay (Promega) according to the manufacturer’s instructions.
Supplementary Material
Acknowledgments
The authors thank the US National Cancer Institute for screening of 60 human tumor cell lines (NCI-60). This work was supported by the National Natural Science Foundation of China (81172941, 21032002, 20802083, and 90813004) and the Ministry of Science and Technology (2011CB915503, and 2009CB522303). Support was also due in part to NIH grant CA177584 from the National Cancer Institute awarded to K.H. Lee.
ABBREBIATIONS USED
- CA4
combretastatin A4
- CNS
central nervous system
- DAPI
4′,6-diamidino-2-phenylindole
- FITC
fluorescein 5-isothiocyanate (FITC)
- GI50
(50% growth inhibition)
- NaHMDS
sodium hexamethyldisilazide; National Cancer Institute
- PBS
phosphate buffered saline
- PCC
pyridinium chlorochromate
- PI
propidium iodine
- PXL
paclitaxel
- SAR
structure-activity relationship
- TBAF
Tetra-n-butylammonium fluoride
- TBDMSCl
tert-butyldimethylchlorosilane
Footnotes
ASSOCIATED CONTENT
Supporting Information Available: Growth inhibitory activity of compounds 9-12 against a panel of 60 human tumor cell lines, effects of compounds 11, 12, PXL, and CA4 on microtubule dynamics in PC-3 cells and inhibitory effects of compounds 3 and 17 on TNF-α–induced NF-κB activation. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Newman DJ. Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem. 2008;51:2589–2599. doi: 10.1021/jm0704090. [DOI] [PubMed] [Google Scholar]; (b) Mann J. Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer. 2002;2:143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- 2.Petrelli A, Giordano S. From single- to multi-target drugs in cancer therapy: when a specificity becomes an advantage. Curr Med Chem. 2008;15:422–432. doi: 10.2174/092986708783503212. [DOI] [PubMed] [Google Scholar]
- 3.Morphy R, Rankovic Z. Designing multiple ligands – medicinal chemistry strategies and challenges. Curr Pharm Des. 2009;15:587–600. doi: 10.2174/138161209787315594. [DOI] [PubMed] [Google Scholar]
- 4.Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly invitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 5.Kingston DGI. Taxol, a molecule for all seasons. Chem Commun. 2001:867–880. [Google Scholar]
- 6.(a) Baloglu E, Kingston DGI. The taxane diterpenoids. J Nat Prod. 1999;62:1448–1472. doi: 10.1021/np990176i. [DOI] [PubMed] [Google Scholar]; (b) Shigemori H, Kobayashi J. Biological activity and chemistry of taxoids from the Japanese yew, Taxus cuspidata. J Nat Prod. 2004;67:245–256. doi: 10.1021/np030346y. [DOI] [PubMed] [Google Scholar]; (c) Li SH. Taxoids. In: Sun HD, Li SH, editors. In The Chemistry of Diterpenoids. 1. Chemical Industry Press; Beijing: 2011. pp. 278–366. [Google Scholar]
- 7.(a) Samaranayake G, Magri NF, Jitrangsri C, Kingston DGI. Modified taxols. 5. Reaction of taxol with electrophilic reagents and preparation of a rearranged taxol derivative with tubulin assembly activity. J Org Chem. 1991;56:5114–5119. [Google Scholar]; (b) Qin Y, Fang QC, Liang XT. Semisynthesis and biological evaluation of taxayuntin 13-[N-benzoyl-(2′R,3′S)-3′-phenylisoserinate] Chin Chem Lett. 1996;7:886–887. [Google Scholar]; (c) Chordia MD, Kingston DGI, Hamel E, Lin CM, Long BH, Fairchild CA, Johnston KA, Rose WC. Synthesis and biological activity of A-nor-paclitaxel analogues. Bioorg Med Chem. 1997;5:941–947. doi: 10.1016/s0968-0896(97)00035-7. [DOI] [PubMed] [Google Scholar]; (d) Tremblay S, Soucy C, Towers N, Gunning PJ, Breau L. Characterization of an abeo-taxane: brevifoliol and derivatives. J Nat Prod. 2004;67:838–845. doi: 10.1021/np0304565. [DOI] [PubMed] [Google Scholar]; (e) Tang SB, Yang C, Brodie P, Bane S, Ravindra R, Sharma S, Jiang Y, Snyder JP, Kingston DGI. Bridging converts a noncytotoxic nor-paclitaxel derivative to a cytotoxic analogue by constraining it to the T-taxol conformation. Org Lett. 2006;8:3983–3986. doi: 10.1021/ol061438s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Guo N, Lou LG, Cong YW, Peng LY, Zhao QS. Synthesis, cytotoxic activity, and SAR analysis of the derivatives of taxchinin A and brevifoliol. Bioorg Med Chem. 2008;16:4860–4871. doi: 10.1016/j.bmc.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karin M. Nuclear factor-[kappa]B in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 10.(a) Bentires-Alj M, Barbu V, Fillet M, Chariot A, Relic B, Jacobs N, Gielen J, Merville MP, Bours V. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003;22:90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]; (b) Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappa B. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen HM, Tergaonkar V. NF kappa B signaling in carcinogenesis and as a potential molecular target for cancer therapy. Apoptosis. 2009;14:348–363. doi: 10.1007/s10495-009-0315-0. [DOI] [PubMed] [Google Scholar]
- 12.(a) Gao ZW, Zhang DL, Guo CB. Paclitaxel efficacy is increased by parthenolide via nuclear factor-kappaB pathways in in vitro and in vivo human non-small cell lung cancer models. Curr Cancer Drug Tar. 2010;10:705–715. doi: 10.2174/156800910793605776. [DOI] [PubMed] [Google Scholar]; (b) Sohma I, Fujiwara Y, Sugita Y, Yoshioka A, Shirakawa M, Moon JH, Takiguchi S, Miyata H, Yamasaki M, Mori M, Doki Y. Parthenolide, an NF-kappaB inhibitor, suppresses tumor growth and enhances response to chemotherapy in gastric cancer. Cancer Genomics Proteomics. 2011;8:39–47. [PubMed] [Google Scholar]; (c) Ganta S, Amiji M. Coadministration of paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol Pharm. 2009;6:928–939. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- 13.Ghantous A, Gali-Muhtasib H, Vuorela H, Saliba NA, Darwiche N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov Today. 2010;15:668–678. doi: 10.1016/j.drudis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Zhang HB, Liu JK, Su J, Li Y, Yao ZJ, Zhao QS. Fragmentations of 13-oxo-taxyunnansin A and their application to preparation of abeo-paclitaxel and abeo-docetaxel analogues. Tetrahedron Lett. 2011;52:139–142. [Google Scholar]
- 15.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 16.Altmann KH, Gertsch J. Anticancer drugs from nature–natural products as a unique source of new microtubule-stabilizing agents. Nat Prod Rep. 2007;24:327–357. doi: 10.1039/b515619j. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa-Goto K, Wu PC, Lai CY, Hamel E, Zhu H, Zhang L, Kozaka T, Ohkoshi E, Goto M, Bastow KF, Lee KH. Antitumor agents. 284. New desmosdumotin B analogues with bicyclic B-ring as cytotoxic and antitubulin agents. J Med Chem. 2011;54:1244–1255. doi: 10.1021/jm1011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.