Abstract
Background
Heavier tobacco smoking among people with schizophrenia (SCZ) has been suggested to reflect self-medication of cognitive deficits. The idea that cognitive-enhancing effects of nicotine are a primary motivator of tobacco consumption in SCZ and that abstinence would deprive SCZ of such beneficial effects may explain hesitation among providers to pursue smoking cessation in SCZ. This study tested predictions of the cognitive self-medication hypothesis.
Methods
In three counterbalanced sessions, 17 SCZ and 17 healthy control subjects (HCS), all smokers, were tested under ad libitum smoking, or 3.5 hours after abstaining and receiving a nicotine (14 mg/24 hrs) or placebo patch.
Results
Attention task performance was improved by transdermal nicotine relative to placebo, with intermediate performance by ad libitum smoking. These effects were of similar size in SCZ and HCS, and did not reflect remediation of functions disproportionately impaired in SCZ. Although more SCZ reported that the need to concentrate influenced their smoking, this was not reflected by these patients' actual behavior. Self-reported ability to concentrate changed with nicotine status in HCS but not SCZ, suggesting insensitivity of SCZ to nicotine-derived performance benefits. Nicotine plasma concentrations after ad libitum smoking were not associated with performance benefits but instead with the propensity to experience nicotine withdrawal upon abstinence. This association was seen selectively in SCZ, suggesting a possible reason for heavier smoking.
Conclusions
These findings suggest that subjective or objective attentional benefits are unlikely the primary driving force of tobacco consumption in SCZ and should not discourage providers from supporting quit attempts.
Keywords: schizophrenia, nicotine, smoking, self-medication, cognitive deficits, withdrawal
Introduction
The prevalence of tobacco smoking among people with schizophrenia (SCZ) is 3-5 times higher than in the general population, higher even when compared with many other psychiatric diagnoses(1-3). SCZ also tend to smoke more heavily and have more difficulty quitting(3-5). Self-medication theories hypothesize that SCZ smoke more heavily because the ingested nicotine alleviates schizophrenia symptoms or medication side-effects. SCZ display attentional, short-term memory and executive control deficits that limit everyday functioning(6;7). The possibility that SCZ self-medicate such deficits is supported by findings that nicotine and other nicotinic acetylcholine receptor (nAChR) agonists enhance sensory, alerting, attentional and mnemonic processes in SCZ(8-21), mimicking effects in healthy subjects(22) and laboratory animals(23;24). Improved attention is a particularly robust finding(22;25;26) and may underlie effects on higher functions. In SCZ, attention appears to be particularly impaired when requiring top-down control(27-29) or being distributed broadly(30;31). The cognitive self-medication hypothesis gained momentum with findings that certain information processing deficits in SCZ may reflect nAChR hypofunction(1;32-37). Overlap between functions impaired in schizophrenia and enhanced by nicotine has been suggested based on reports of larger performance-enhancing effects of nicotine in SCZ than healthy control subjects (HCS)(8;12-14;20;21;38;39).
The NIMH Tobacco Use and Cessation in Psychiatric Disorders Workgroup voiced concerns that a narrow focus on self-medication hypotheses as the cause for high smoking rates in psychiatric populations “may result in insufficient attention to other plausible explanations and discourage efforts in mental health treatment settings to promote tobacco cessation”(40), p.1693). Despite significant morbidity and mortality from smoking in SCZ(40-42), there is indication that health care providers often assign low priority to nicotine dependence treatment in the mentally ill(43-45) and in particular SCZ(45). The perception that SCZ smoke to achieve better cognitive functioning may explain hesitation to treat tobacco dependence aggressively.
To date, only indirect evidence supports the cognitive self-medication hypothesis. While more pronounced benefits from nicotine were reported in SCZ than HCS on diverse cognitive measures, it is unclear whether these effects motivate SCZ to smoke and account for higher smoking prevalence and severity. Specific predictions can be derived from the self-medication hypothesis. Performance improvement should be more pronounced in SCZ than HCS, and more pronounced on functions impaired in SCZ. Tobacco smoking should be a similarly effective means of achieving such improvement as experimenter-administered nicotine. SCZ should smoke with the purpose of achieving these improvements. The subjective ability to concentrate should be enhanced acutely with smoking or other forms of nicotine administration. Finally, if performance improvement was a major force driving the heavier smoking in SCZ, one may expect heavier smoking to be associated with greater benefits. The present study was designed to test these predictions, focusing on the attention domain.
Methods and Materials
Participants
Seventeen patients meeting Diagnostic and Statistical Manual of Mental Disorders-IV(46) criteria for schizophrenia (N=5 paranoid, 9 undifferentiated, 2 residual) or schizoaffective disorder (N=1), and 17 HCS, all smokers of ≥5 cigarettes or cigarillos/day on ≥5 days/week, completed the study. Three SCZ were treated with first-generation and 13 with second-generation antipsychotics; one was stably unmedicated. Eight SCZ additionally received antidepressant and 6 anxiolytic medication. Groups were matched demographically but differed in IQ (Table 1). Participants provided informed consent for a protocol approved by the University of Maryland Baltimore Institutional Review Board. For more detail, see Supplement 1.
Table 1.
| People with Schizophrenia (mean ± stdev) | Healthy Control Subjects (mean ± stdev) | Statistic | P-value | |
|---|---|---|---|---|
| Age | 43.1 ± 10.5 (range 22-53) | 40.2 ±11.1 (range 25-52) | t(32)=.76 | P>0.4 |
| Male: Female | 14 : 3 | 14 : 3 | χ2 =0 | P=1 |
| African American : Caucasian | 5 : 12 | 8 : 9 | χ2 =1.12 | P=0.29 |
| Education (years) | 12.1 ± 2.1 | 12.5 ± 1.2 | t(32)=.70 | P>0.4 |
| Number of cigarettes/day | 17.9 ± 8.4 | 16.6 ± 9.7 | t(32)=.41 | P>0.6 |
| Years of Smoking | 21.9 ± 11.3 | 21.8 ± 11.4 | t(32)=.05 | P>0.9 |
| Number of quit attempts | 3.7 ± 3.4 | 3.8 ± 7.6 | t(32)=.06 | P>0.9 |
| Age start smoking | 17.8 ± 4.8 | 15.4 ± 3.4 | t(32)=1.64 | P=0.11 |
| Age smoking regularly | 19.5 ± 4.3 | 18.8 ± 5.7 | t(32)=.41 | P>0.6 |
| FTNDa total score | 4.6 ± 2.1 | 5.6 ± 1.8 | t(32)=1.43 | P=0.16 |
| Arrival CO (ppm)b | 29.5 ± 19.8 | 20.6 ± 6.8 | t(32)=1.76 | P=0.09 |
| Estimated IQc | 97.9 ± 12.2 | 111.1 ± 14.0 | t(32)=2.93 | P<0.01 |
| Brief Psychiatric Rating Scale | 36.6 ± 8.0 (range 24-52) | |||
| Scale for the Assessment of Negative Symptoms | 37.9 ± 13.7 (range 15-64) |
Fagerstrom Test for Nicotine Dependence
Average over all three test sessions
estimated by the vocabulary and matrix reasoning subscales of the Wechsler Abbreviated Scale of Intelligence(47)
Procedure
The study involved one training and three test sessions. During training, participants received instructions and practiced full versions of the attention tasks. They completed intelligence testing(47), the trait version of the State-Trait Anxiety Inventory (STAI(48); Supplement 1, Figure S2), the Fagerstrom Test for Nicotine Dependence (FTND(49)), questions related to nicotine use history, and a reasons-for-smoking questionnaire.
During test sessions, participants received in counterbalanced order (A) a transdermal nicotine patch (14 mg/24 hrs; Nicoderm CQ®, GlaxoSmithKline), (B) a placebo patch, or (C) could smoke as many of their cigarettes as they wished (ad libitum smoking). Participants and investigators were blind with regard to patch condition (see Supplement 1).
Participants were instructed to smoke as usual prior to study sessions. Upon arrival, a breath carbon monoxide (CO) reading (Micro Smokerlyzer) was taken. Participants completed the 8-item Minnesota Nicotine Withdrawal Scale (MNWS(50)), and a list of bidirectional scales sensitive to tobacco deprivation-induced mood changes(51); reported in Supplement 1). In the ad libitum smoking session, the attention tasks followed. In the two patch sessions, the patch was applied, participants abstained from smoking and spent a 3.5-hour absorption period watching TV or reading. After completing withdrawal questionnaires again, they performed the attention tasks. Participants could take short breaks during all testing sessions, but only in the ad libitum condition was smoking allowed. After task completion, participants again completed withdrawal questionnaires and the STAI state version (Supplement 1; Figure S2). Immediately after, a 5-mL venous blood sample was collected. Samples were centrifuged and plasma stored at −20° C until analysis. Nicotine, cotinine, trans-3′-hydroxycotinine and norcotinine were measured by liquid chromatography-tandem mass spectrometry (details in Supplement 1). Only nicotine and cotinine are reported here.
Cognitive tasks
In each session, participants completed two computerized tasks challenging aspects of attention impaired in SCZ.
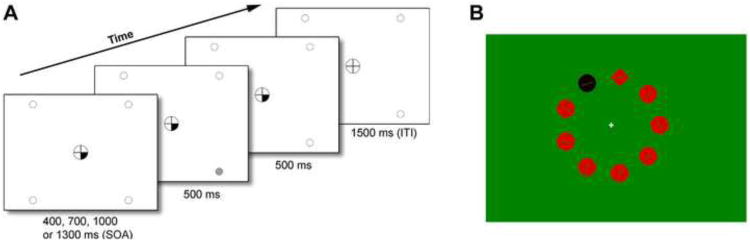
The Spatial Attentional Resource Allocation Task (SARAT) is a stimulus detection task manipulating the size of the attentional focus(52). Participants fixated a central circle throughout (trials with eye movements were eliminated). First, a central cue was presented consisting of one, two, or four quarters of the fixation circle turning black (Figure 1A). After a 400-1300-ms stimulus-onset-asynchrony, a 500-ms peripheral target (high or low contrast) appeared in one of the corners of the screen, to which participants responded by button-press. The cue indicated the likely target quadrant(s). Fewer cued locations enable more precise prediction of the target location and a narrower attentional focus(52). Increasing the number of cued locations increases spatial uncertainty and the need to monitor broadly, which was shown to potentiate impairment of SCZ(30). The cue provided invalid information on 20% of trials with one or two cued locations (not analyzed). The cue was not followed by a target on 9.7% of trials, presented unpredictably, to discourage anticipatory responding (not analyzed). There were 336 valid, 56 invalid and 42 cue-only trials, tested over 14 blocks interspersed by rest periods. Trial types were randomized. Task completion took one hour.
Figure 1.
(A) Components of a trial in the Spatial Attentional Resource Allocation Task. Onset of a central cue preceded target onset by a variable stimulus onset asynchrony (SOA) of 400, 700, 1000 or 1300 ms. The target was presented for 500 ms in the continuing presence of the cue, which remained on display until 500 ms after target offset. Only screen background was then presented for a 1500-ms intertrial interval (ITI) of. One, two, or all four target locations could be cued at the same time, thus varying the predictability of the target. (B) A stimulus display example during the Singleton Detection Task. The task was to indicate whether the line inside the shape singleton was vertical or horizontal. A black color singleton distractor was present on half of the trials.
The Singleton Detection Task was performed second. It manipulates the need to ignore salient distractors, a function impaired in SCZ(27;28). Circular search arrays were composed of nine shape stimuli, each 3.40° from a central fixation cross (Figure 1B). Red stimuli were presented against a green background. The red and green were isoluminant, determined by a flicker fusion procedure prior to the task. The target was a shape singleton, i.e. circle among diamonds or diamond among circles. Each stimulus contained a line. The task was to press one of two buttons indicating whether the line in the shape singleton was vertical or horizontal (50% chance). On half the trials, all stimuli were red. On the other half, one of the non-target items was a black color and luminance singleton serving as a distractor. Participants were informed that the color singleton was never the target. Each array remained on display until response. A 500-ms blank interval preceded the next array. There were 576 trials in total with short breaks every 36 trials. The entire task procedure took ∼45 min.
For greater detail, see Supplement 1.
Data analysis
Most dependent variables were analyzed by ANOVA including Group (SCZ, HCS) as a between-subject factor and Drug (placebo patch, nicotine patch, ad libitum smoking) as a within-subject factor. Additional within-subject factors compared within-session measurement time points or task conditions.
Results
Smoking variables (Table 1)
SCZ and HCS did not differ in years of smoking, cigarettes/day, age of starting smoking or smoking regularly, number of quit attempts, or FTND scores. The trend toward lower FTND scores in SCZ was driven by items affected by group home living (details in Supplement 1), suggested to lead to underestimation of dependence severity in SCZ(53). When two of these items with particularly low factor loadings in SCZ(53) were excluded, FTND scores were identical in SCZ (4.0 ±1.66) and HCS (4.0 ±1.46). Expired CO levels upon arrival trended to be higher in SCZ. However, there were no main effects or interaction in ANOVA with Group and Drug as factors.
Participants were asked to check those of five items that determined how much they smoked during a day (“my mood”, “how much money I have”, “if I have to be in places where I can't smoke”, “if I have to do things where I have to concentrate”, “if I am with other people who smoke”). Figure S1 shows the percentage of SCZ and HCS endorsing each item. A significant difference occurred for being with other smokers (χ2 =5.10, P<0.03), suggesting that fewer SCZ smoke socially. A trend difference was seen for having to concentrate (χ2 =3.11, P<0.078), which was endorsed by 9/17 SCZ and 4/17 HCS.
Nicotine plasma concentrations
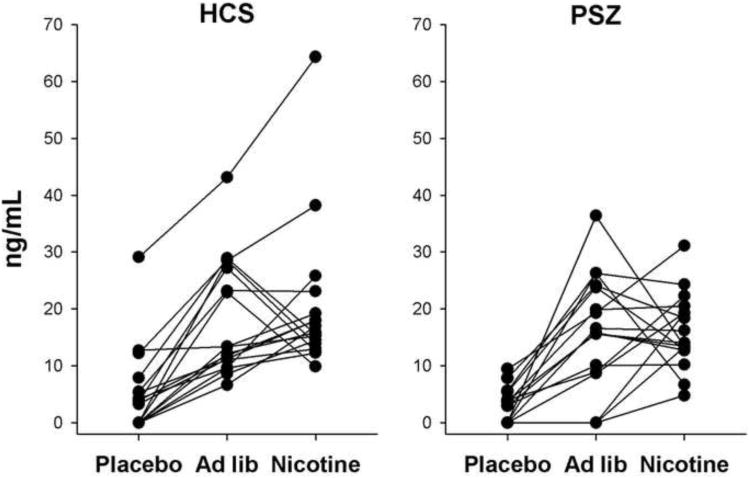
Nicotine concentrations within drug conditions differed widely between participants (Figure 2). Overall, however, a main effect of Drug [F(2,64)=140, P<0.001] in two-factor ANOVA confirmed higher concentrations in the ad libitum smoking [t(33)=;8.81, P<0.001] and nicotine patch condition [t(33)=;10.9, P<0.001] as compared with placebo. The ad libitum smoking and nicotine patch conditions did not differ [P>0.5]. There was no main effect of Group [P>0.4] and no Group by Drug interaction [P>0.6], indicating that nicotine concentrations did not differ between SCZ and HCS. The same pattern was observed with cotinine.
Figure 2.
Plasma nicotine concentrations of healthy control subjects (HCS) and people with schizophrenia (SCZ) at the end of cognitive test sessions per group and drug condition.
Nicotine Withdrawal
Figure 3A shows MNWS scores. On patch days, withdrawal was measured upon arrival, prior to, and after the attention tasks. In the ad libitum smoking session, there were only two ratings because testing commenced shortly after arrival. Arrival scores did not differ between sessions; there was no main effect of Drug (P>0.4) or Drug × Group interaction (P>0.7) in 2-factor ANOVA. However, SCZ overall had higher scores than HCS [main effect of Group: F(1,31)=14.7, P<0.001].
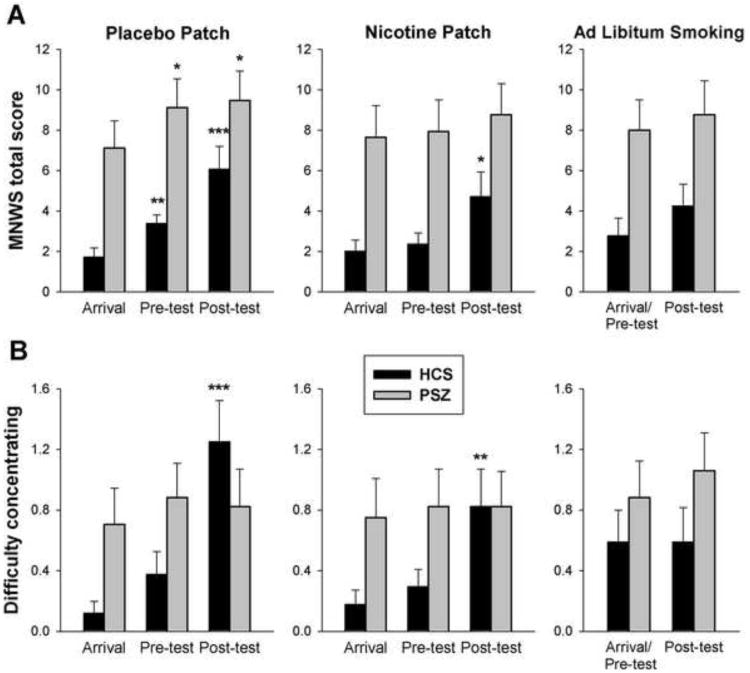
Figure 3.
Average (±SEM) scores on the Minnesota Nicotine Withdrawal Scale (MNWS) of 17 healthy control subjects (HCS) and 17 people with schizophrenia (SCZ). The scales were administered upon arrival, after the absorption period (pre-test), and after cognitive testing (post-test). In the ad libitum smoking condition, arrival and pre-test was identical. Figure 3A shows total MNWS scores, Figure 3B the “difficulty concentrating” subitem.
Withdrawal within patch sessions was analyzed by 3-factor ANOVA with Group, Drug (nicotine, placebo) and Time (arrival, pre-test, post-test) as factors. Scores increased over time [Time main effect: F(2,60)=11.6, P<0.001], more so during placebo than nicotine patch sessions [Drug × Time interaction: F(2,60)=4.12, P<0.03]. In SCZ, scores were overall higher, but changes with time were blunted as compared with HCS [Group × Time: F(2,60)=3.61, P<0.04]. A significant increase from arrival was, however, still seen under placebo, and neither the Group × Drug (P>0.5) nor the Group × Drug × Time interaction (P>0.7) was significant. When exploring the items driving the Group × Time interaction, only “Difficulty Concentrating” was significant [F(2,60)=8.18, P<0.001]. Figure 3B shows large pre- to post-test increases in HCS, particularly under placebo. In contrast, “Difficulty Concentrating” in SCZ differed neither between drug conditions nor between within-session time points and appeared completely unaffected by nicotine status.
MNWS scores during ad libitum smoking resembled the nicotine patch condition (Figure 3A). Pre- and post-test scores (excluding arrival ratings in patch sessions) were compared between all three drug conditions by three-factor ANOVA. A main effect of Drug [F(2,62)=3.14, P=0.05] was due to a difference between placebo and transdermal nicotine [t(32)=2.31, P<0.03, paired t-test] with a trend difference also between placebo and ad libitum smoking (P=0.056), but not between transdermal nicotine and ad libitum smoking (P>0.9). There were main effects of Time (P<0.01) and Group (P<0.01), but no other significant effects. To test the prediction that smoking enhances SCZ's subjective ability to concentrate, we compared end-of-session “Difficulty Concentrating” between placebo and ad libitum smoking. There was a reduction in HCS [t(15)=2.32, P<0.04] but not SCZ [t(16)=1.07, P=0.3; Figure 3B].
Affect-related withdrawal scales were largely consistent with the above pattern (Supplement 1; Figure S3).
Behavioral Performance
SARAT
Performance was analyzed by four-factor ANOVA with Group, Drug, Number of Cued Locations (1,2,4), and Target Contrast (high, low) as factors.
Reaction time (RT) depended on task conditions as reported previously(30;52); see Supplement 1. Importantly, the slowing with greater spatial uncertainty was more pronounced for SCZ than HCS [Group × Number of Cued Locations: F(2,64)=4.16, P=0.02; Figure S4], replicating previous findings that SCZ are particularly impaired at distributing attention broadly(30).
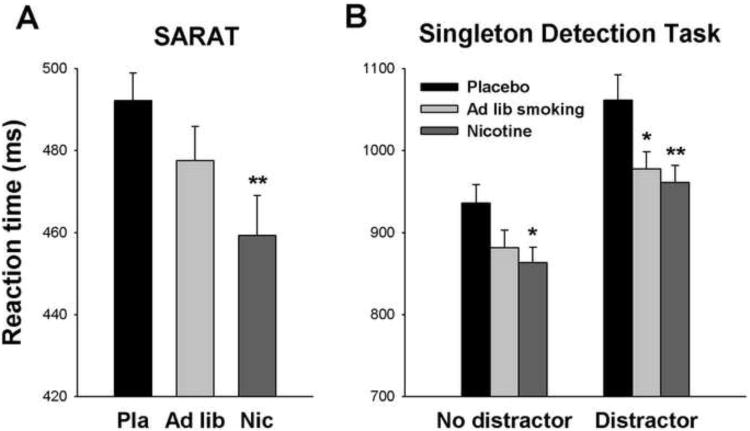
A main effect of Drug [F(2,64)=5.70, P<0.005] was largely due to RT reduction with transdermal nicotine relative to placebo (Figure 4A). Effect sizes of this reduction were similar between groups: Cohen's d=0.49 in SCZ, and d=0.46 in HCS. Ad libitum smoking produced intermediate performance not significantly different from either transdermal nicotine or placebo. Effects of Drug did not interact with Group (P>0.7), Number of Cued Locations (P=0.16) or Target Contrast (P>0.9), and no three- or four-way interaction was significant (Drug × Group × Cue: P=0.39), indicating that nicotine did not reverse specific impairment in SCZ.
Figure 4.
Average reaction time in each of the three drug conditions. A: Reaction time in the Spatial Attentional Resource Allocation Task (SARAT) of 34 subjects, including 17 healthy controls and 17 people with schizophrenia. B: Reaction time in the Singleton Detection Task of 30 participants, including 17 healthy controls and 13 people with schizophrenia, in the presence and absence of a color singleton distractor. Error bars reflect the SEM adjusted to remove between-subject variability in average reaction time across drug conditions(66;67). * P<0.05, ** P<0.01, paired t-test comparison with placebo condition. Please note the difference in scales adopted in panels A and B.
Omission errors were low (2.5% overall). Both nicotine and ad libitum smoking reduced omissions, but the main effect of Drug failed significance [F(2,64)=2.96, P=0.059], probably due to floor effects. Task condition effects are reported in Supplement 1.
Singleton Detection Task
Due to color blindness, four SCZ could not perform this task, resulting in N=13. Performance was analyzed by 3-factor ANOVA with Group, Drug and Distractor (present,absent) as factors.
Accuracy was high (97.5% overall). The main effect of Drug was significant [F(2,56)=6.87, P=0.002], with both transdermal nicotine and ad libitum smoking significantly enhancing accuracy relative to the placebo patch. No other effects were significant.
RT (median across trials; responses >5 s were excluded) was slower in SCZ than HCS [Group main effect: F(1,28)=9.62, P<0.005], and slower with than without a distractor [Distractor: F(1,28)=33.5, P<0.001]. There was a main effect of Drug [F(2,56)=4.33, P<0.02]. Transdermal nicotine significantly reduced RT relative to placebo. Intermediate performance with ad libitum smoking did not significantly differ from the nicotine or placebo patch condition. There was a trend for the distractor effect to be larger in SCZ than HCS (Distractor × Group: P=0.094). However, there were no interactions involving Drug (Drug × Distractor: P=0.18; Drug × Group: P>0.7; Drug × Group × Distractor: P>0.4).
To test whether effects of nicotine would manifest more specificity when limiting distractor trials to those most challenging top-down attentional control, we repeated ANOVA including only the two closer distractor distances that produced the largest distractor effects (Supplement 1; Figure S5). There were again main effects of Group, Drug, and Distractor, but Drug now interacted with Distractor [F(2,56)=3.19, P<0.05], reflecting larger RT reduction with transdermal nicotine and ad libitum smoking in the presence than absence of a distractor (Figure 4B). However, there were no interactions involving Group (Distractor × Group P=0.25; Drug × Group P>0.8; Drug × Group × Distractor P>0.9], indicating that despite specificity of the drug effect, this occurred in both groups and did not reflect remediation of deficits in SCZ.
The Need to Concentrate as Reason for Smoking
Participants had completed the attention tasks at least once prior and were aware of the need to concentrate for two hours in testing sessions. Thus, we expected the 9 SCZ who had endorsed that their smoking amount depended on their need to concentrate to smoke more in the ad libitum condition than the remaining 8 SCZ. However, subgroups differed neither in nicotine plasma concentrations [t(15)=1.36, P=0.19; analyses of the increase relative to placebo yielded the same pattern; Supplement 1], nor in end-of-session MNWS self-ratings of difficulty concentrating [t(15)=1.74, P=0.1], nor in SARAT RT benefit relative to placebo [t(15)=1.46, P=0.16]. The trends actually reflected less smoking, greater difficulty concentrating, and less RT benefit in the ad libitum condition in SCZ endorsing this item, drawing into question the accuracy of their self-reports. Groups did not differ on smoking severity or demographics, except that SCZ endorsing this item had fewer years of education [t(15)=2.36, P<0.04] and lower IQ [P=0.066].
Correlations
If performance benefits drove the heavier tobacco consumption in SCZ, with or without awareness, then higher levels of smoking-derived nicotine should predict greater smoking-induced performance benefits. This was tested by correlating nicotine plasma concentrations in the ad libitum smoking condition with SARAT RT reductions relative to placebo. A positive correlation would indicate that more smoking was associated with greater benefits. There was no such association in SCZ [R=-0.24, P>0.3; Pearson correlation] or HCS [R=-0.32, P>0.2]. The trends even suggest that lower nicotine concentrations were associated with greater improvement.
If, by contrast, nicotine withdrawal was a determinant of smoking, then the propensity to experience withdrawal in the absence of nicotine should predict smoking-derived nicotine intake. This was tested by correlating participants' MNWS increase from arrival to the end of the placebo session with nicotine concentrations in the ad libitum smoking session. A positive correlation was found for SCZ (R=0.68, P=0.003) but not HCS (R=-0.29, P=0.28); Figure 5. The correlations differed between groups (z=2.99, P<0.003), even after removing one outlier HCS (z=2.3, P<0.03). To test whether smoking-derived nicotine reduced withdrawal, nicotine concentrations in the ad libitum session were correlated with the decrease in end-of-session MNWS scores relative to placebo. A positive correlation would suggest that more smoking was associated with greater withdrawal reduction. This prediction was confirmed for SCZ [R=0.51, P<0.04] but not HCS (R=-0.31, P>0.24; difference between correlations: z=2.33, P<0.02; after removing outlier: z=1.91, P=0.056). Partial correlations controlling for IQ yielded almost identical results.
Figure 5.
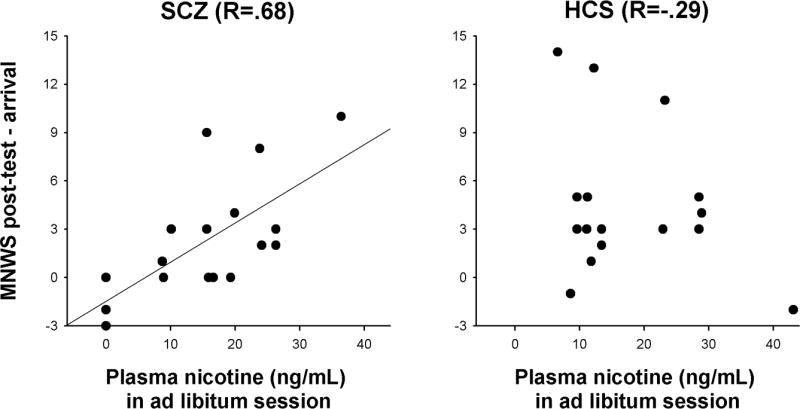
Pearson correlations in people with schizophrenia (SCZ) and healthy control subjects (HCS) between the increase in Minnesota Nicotine Withdrawal Scale (MNWS) scores over the course of the placebo session and nicotine plasma concentrations in the ad libitum smoking session. A significant association between abstinence-induced withdrawal and smoking-derived nicotine intake was seen in SCZ but not HCS.
Discussion
This study tested predictions of the cognitive self-medication account for more prevalent and heavier tobacco smoking among SCZ. The prediction that SCZ derive greater cognitive improvement from nicotine than HCS, in particular on functions most impaired, is based on the idea that impairments resulted from nAChR hypofunction. In our attention paradigms, the effects of nicotine or ad libitum smoking did not differ between groups. We replicated disproportionately large impairment of SCZ spreading attention broadly(30), but the effects of nicotine or smoking were not more pronounced under these conditions. We identified a trend-impairment for SCZ to ignore salient distractors, but although effects of nicotine and smoking were more pronounced when these distractors were most disruptive, this was equally true for SCZ and HCS, yielding no evidence of larger benefits in SCZ or specific remediation of deficits. While there is always a possibility that critical interactions were missed due to lack of power, the complete absence of trends towards any interaction involving Group and Drug suggests that attention-enhancing effects of nicotine, although robust in SCZ, did not specifically ameliorate schizophrenia-related impairment.
The above was surprising in view of previous reports of larger effects of nicotine in SCZ. The functions showing this interaction differed between studies, ranging from information processing, sustained and selective attention and response inhibition to short-term memory and delayed recognition(8;12-14;20;21;38;39). With the exception of neurophysiological information processing indices, it is not always clear whether these improvements were specific to areas of disproportionate impairment in SCZ, and whether larger effects may have resulted from a lower baseline. Citation bias may also play a role; the effects of nicotine on the majority of tested performance indices did not differ between SCZ and HCS. Another factor differentiating the current study is that previous reports did not always match groups on smoking severity, and the majority tested smokers after at least overnight withdrawal. Thus, larger nicotine effects may in some cases reflect more pronounced withdrawal-induced deficits in SCZ.
If performance benefits are a significant driving force of tobacco smoking in SCZ, we would expect them to be similarly robust with smoking as with experimenter-administered nicotine. We found that, across groups, smoking appeared to be a less effective means of achieving performance improvement than transdermal nicotine, despite similar end-of-session nicotine plasma concentrations. The pharmacokinetics associated with the delivery devices may explain this. Cigarette-derived nicotine is marked by rapid peaks but is also rapidly metabolized(54;55), and it is unlikely that nicotine concentrations optimal for performance improvement were present throughout the testing period. By contrast, protracted release of nicotine from the patch ensures more sustained and stable concentrations(56-58).
When asked, more SCZ than HCS endorsed that how much they smoke depended on their need to concentrate. This could be seen as direct support for cognitive self-medication. However, further analyses comparing nicotine intake in the ad libitum smoking session indicated that these self-reports did not reflect actual behavior. Despite the evident need to concentrate, SCZ indicating that they adjusted their smoking based on this need actually tended to have lower smoking-derived nicotine concentrations and less subjective and objective improvement in their ability to concentrate. Furthermore, in the full SCZ sample, self-reported difficulty concentrating did not vary with drug condition or time-since-withdrawal, as in HCS. SCZ's subjective difficulty concentrating was higher overall but appeared completely insensitive to their current nicotine status. SCZ appeared to have no awareness of acute beneficial effects of nicotine on their ability to concentrate, despite performance benefits comparable to HCS.
Subjective state generally was less influenced by nicotine or smoking status in SCZ than HCS. Breath CO levels upon arrival trended to be higher in SCZ, but SCZ had higher MNWS scores at all times including arrival. MNWS ratings in SCZ did not depend on current nicotine deprivation status as in HCS, with similar trends also on the affect-related withdrawal scales and STAI (Supplement 1). A likely explanation is that higher trait negative affect(59) and other symptoms associated with schizophrenia overshadowed the perhaps more subtle changes related to acute nicotine status. This suggests that nicotine or smoking may not significantly modulate disease-related negative affectivity, including subjective difficulty concentrating.
Performance-enhancing effects could reinforce tobacco consumption independent of awareness. However, if these effects were a main reason behind the heavier smoking in SCZ, then one would expect greater smoking-derived nicotine intake to be associated with greater performance improvement. This prediction was not confirmed. If anything, more smoking was associated with smaller performance benefits, consistent with an inverted U-shaped dose-response function(23;60) on which most participants had exceeded nicotine concentrations optimal for attentional benefits. This is difficult to reconcile with the hypothesis that such benefits account for heavier smoking in SCZ and suggests that other factors drive smoking amount. Indeed, smoking-derived nicotine intake in SCZ, but not HCS, was robustly associated with their propensity to experience nicotine withdrawal upon abstinence, and with acute withdrawal reduction relative to placebo. Thus, the one factor identified that could explain heavier tobacco consumption among SCZ was not related to cognitive effects of nicotine but reflected more classical dependence aspects. Overall, our results suggest that despite clearly demonstrable improvements in attentional performance, these benefits do not appear to be a primary motivator of tobacco smoking in schizophrenia.
Study limitations include a relatively modest sample size, and the fact that the ad libitum smoking session was not blinded and differed in timing from the patch sessions. However, it is difficult to explain smaller performance benefits by ad libitum smoking relative to placebo with expectation effects or the lack of an absorption period-equivalent. Our cognitive performance indices were restricted to attentional functions. This ensured robust effects of nicotine and tested the most likely substrate of cognitive self-medication. Larger-scale studies should also probe mnemonic and executive control functions, but bearing in mind that effects may be secondary to effects on attention. We did not assess other “classical” aspects of dependence that may discriminate groups, such as positive reinforcement(61) or conditioned cue responses. Our study targeted a set of predictions derived from the cognitive self-medication hypothesis, and the complete absence of support is informative despite these limitations.
The results by no means diminish the therapeutic potential of nAChR agonists for cognitive deficits in schizophrenia. Attention-enhancing effects of transdermal nicotine were robust in SCZ and HCS. nAChR agonists given via a delivery system with similar temporal stability may improve everyday functioning in SCZ, especially long-term when coupled with cognitive challenges inherent in daily life or induced by training interventions(62). Although attention-enhancing effects of nicotine are likely not the primary driving force of tobacco consumption in SCZ, these effects may have therapeutic benefits when optimally harvested.
The clinical implication of our findings is that smoking cessation would not deprive SCZ of an efficacious means of self-medicating attentional deficits but equates overcoming an addiction. Together with findings that smoking cessation does not exacerbate psychosis(63;64) and that chronic tobacco smoking has negative neurobiological effects that may contribute to cognitive impairment(65), this should help remove hesitation to motivate quit attempts among SCZ and initiate adequate treatments.
Supplementary Material
Acknowledgments
This project was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse, and NIDA Residential Research Support Services Contract N01DA-5-9909 (Kelly PI). We would like to thank Dr. Bernard Fischer for his help with medical clearances of study volunteers, Dr. Robert McMahon for statistical advice, and Sharon August and Leeka Hubzin for their help with subject recruitment. Thanks go to Dr. Ian Stolerman for helpful comments on this manuscript. We also thank all volunteers participating in this study.
Footnotes
Financial Disclosures: None of the authors reported any biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: Clinical phenomena and laboratory findings. American Journal of Psychiatry. 1998;155:1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- 2.Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness - A population-based prevalence study. Jama-Journal of the American Medical Association. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 3.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research. 2005;76:135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Williams JM, Gandhi KK, Lu SE, Kumar S, Steinberg ML, Cottler B, et al. Shorter interpuff interval is associated with higher nicotine intake in smokers with schizophrenia. Drug and Alcohol Dependence. 2011;118:313–319. doi: 10.1016/j.drugalcdep.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Souza MS, Markou A. Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology. 2012;62:1564–1573. doi: 10.1016/j.neuropharm.2011.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Depatie L, O'Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, et al. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- 9.Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- 10.Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, et al. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31:637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- 11.Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, et al. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- 12.Larrison-Faucher AL, Matorin AA, Sereno AB. Nicotine reduces antisaccade errors in task impaired schizophrenic subjects. Progress in NeuroPsychopharmacology & Biological Psychiatry. 2004;28:505–516. doi: 10.1016/j.pnpbp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Myers CS, Robles O, Kakoyannis AN, Sherr JD, Avila MT, Blaxton TA, et al. Nicotine improves delayed recognition in schizophrenic patients. Psychopharmacology. 2004;174:334–340. doi: 10.1007/s00213-003-1764-8. [DOI] [PubMed] [Google Scholar]
- 14.Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008;33:480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- 15.Jubelt LE, Barr RS, Goff DC, Logvinenko T, Weiss AP, Evins AE. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology. 2008;199:89–98. doi: 10.1007/s00213-008-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AhnAllen CG, Nestor PG, Shenton ME, McCarley RW, Niznikiewicz MA. Early nicotine withdrawal and transdermal nicotine effects on neurocognitive performance in schizophrenia. Schizophrenia Research. 2008;100:261–269. doi: 10.1016/j.schres.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. American Journal of Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, et al. Proof-of-concept trial of an alpha 7 nicotinic agonist in schizophrenia. Archives of General Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- 19.Woznica AA, Sacco KA, George TP. Prepulse inhibition deficits in schizophrenia are modified by smoking status. Schizophrenia Research. 2009;112:86–90. doi: 10.1016/j.schres.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 20.George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, et al. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002;26:75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 21.Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- 22.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hahn B, Sharples CGV, Wonnacott S, Shoaib M, Stolerman P. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003;44:1054–1067. doi: 10.1016/s0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 24.Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Molecular Neurobiology. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolerman IP, Mirza NR, Shoaib M. Nicotine Psychopharmacology -Addiction, Cognition and Neuroadaptation. Medicinal Research Reviews. 1995;15:47–72. doi: 10.1002/med.2610150105. [DOI] [PubMed] [Google Scholar]
- 26.Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Current Opinion in Pharmacology. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Hahn B, Robinson BM, Kaiser ST, Harvey AN, Beck VM, Leonard CJ, et al. Failure of Schizophrenia Patients to Overcome Salient Distractors During Working Memory Encoding. Biological Psychiatry. 2010;68:603–609. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oltmanns TF, Neale JM. Schizophrenic performance when distractors are present: attentional deficit or differential task difficulty? Journal of Abnormal Psychology. 1975;84:205–209. doi: 10.1037/h0076721. [DOI] [PubMed] [Google Scholar]
- 29.Luck SJ, Gold JM. The construct of attention in schizophrenia. Biological Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hahn B, Robinson BM, Harvey AN, Kaiser ST, Leonard CJ, Luck SJ, et al. Visuospatial Attention in Schizophrenia: Deficits in Broad Monitoring. Journal of Abnormal Psychology. 2012;121:119–128. doi: 10.1037/a0023938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elahipanah A, Christensen BK, Reingold EM. Visual search performance among persons with schizophrenia as a function of target eccentricity. Neuropsychology. 2010;24:192–198. doi: 10.1037/a0017523. [DOI] [PubMed] [Google Scholar]
- 32.Adams CE, Stevens KE. Evidence for a role of nicotinic acetylcholine receptors in schizophrenia. Front Biosci. 2007;12:4755–4772. doi: 10.2741/2424. [DOI] [PubMed] [Google Scholar]
- 33.Freedman R, Coon H, MylesWorsley M, OrrUrtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, et al. Association of promoter variants in the alpha 7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Archives of General Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- 35.Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology. 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- 36.Wing VC, Wass CE, Soh DW, George TP. A review of neurobiological vulnerability factors and treatment implications for comorbid tobacco dependence in schizophrenia. Addiction Reviews. 2012;1248:89–106. doi: 10.1111/j.1749-6632.2011.06261.x. [DOI] [PubMed] [Google Scholar]
- 37.Petrovsky N, Quednow BB, Ettinger U, Schmechtig A, Mossner R, Collier DA, et al. Sensorimotor Gating is Associated with CHRNA3 Polymorphisms in Schizophrenia and Healthy Volunteers. Neuropsychopharmacology. 2010;35:1429–1439. doi: 10.1038/npp.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olincy A, Johnson LL, Ross RG. Differential effects of cigarette smoking on performance of a smooth pursuit and a saccadic eye movement task in schizophrenia. Psychiatry Research. 2003;117:223–236. doi: 10.1016/s0165-1781(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 39.George TP, Termine A, Sacco KA, Allen TM, Reutenauer E, Vessicchio JC, et al. A preliminary study of the effects of cigarette smoking on prepulse inhibition in schizophrenia: involvement of nicotinic receptor mechanisms. Schizophr Res. 2006;87:307–315. doi: 10.1016/j.schres.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 40.Ziedonis D, Hitsman B, Beckham J, Zvolensky M, Adler L, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine & Tobacco Research. 2008;10:1691–1715. doi: 10.1080/14622200802443569. [DOI] [PubMed] [Google Scholar]
- 41.Hennekens CH. Increasing global burden of cardiovascular disease in general populations and patients with schizophrenia. Journal of Clinical Psychiatry. 2007;68:4–7. [PubMed] [Google Scholar]
- 42.Shanmugam G, Bhutani S, Khan DA, Brown ES. Psychiatric considerations in pulmonary disease. Psychiatric Clinics of North America. 2007;30:761–+. doi: 10.1016/j.psc.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? American Journal of Psychiatry. 2003;160:2228–2230. doi: 10.1176/appi.ajp.160.12.2228. [DOI] [PubMed] [Google Scholar]
- 44.Montoya ID, Herbeck DM, Svikis DS, Pincus HA. Identification and treatment of patients with nicotine problems in routine clinical psychiatry practice. American Journal on Addictions. 2005;14:441–454. doi: 10.1080/10550490500247123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duffy SA, Kilbourne AM, Austin KL, Dalack GW, Woltmann EM, Waxmonsky J, et al. Risk of Smoking and Receipt of Cessation Services Among Veterans With Mental Disorders. Psychiatric Services. 2012;63:325–332. doi: 10.1176/appi.ps.201100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: 2000. [Google Scholar]
- 47.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 48.Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 49.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence - A Revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 50.Hughes JR. Tobacco withdrawal in self-quitters. J Consult Clin Psychol. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- 51.Parrott AC, Garnham NJ, Wesnes K, Pincock C. Cigarette smoking and abstinence: comparative effects upon cognitive task performance and mood state over 24 hours. Human Psychopharmacology. 1996;11:391–400. [Google Scholar]
- 52.Hahn B, Ross TJ, Stein EA. Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage. 2006;32:842–853. doi: 10.1016/j.neuroimage.2006.04.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinberg ML, Williams JM, Steinberg HR, Krejci JA, Ziedonis DM. Applicability of the Fagerstrom test for nicotine dependence in smokers with schizophrenia. Addictive Behaviors. 2005;30:49–59. doi: 10.1016/j.addbeh.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 54.Henningfield JE, Keenan RM. Nicotine Delivery Kinetics and Abuse Liability. Journal of Consulting and Clinical Psychology. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- 55.Williams JM, Gandhi KK, Lu SE, Kumar S, Shen JW, Foulds J, et al. Higher nicotine levels in schizophrenia compared with controls after smoking a single cigarette. Nicotine & Tobacco Research. 2010;12:855–859. doi: 10.1093/ntr/ntq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmer KJ, Buckley MM, Faulds D. Transdermal Nicotine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy as an aid to smoking cessation. Drugs. 1992;44:498–529. doi: 10.2165/00003495-199244030-00011. [DOI] [PubMed] [Google Scholar]
- 57.Gupta SK, Benowitz NL, Jacob P, III, Rolf CN, Gorsline J. Bioavailability and absorption kinetics of nicotine following application of a transdermal system. Br J Clin Pharmacol. 1993;36:221–227. doi: 10.1111/j.1365-2125.1993.tb04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fant RV, Henningfield JE, Shiffman S, Strahs KR, Reitberg DP. A pharmacokinetic crossover study to compare the absorption characteristics of three transdermal nicotine patches. Pharmacol Biochem Behav. 2000;67:479–482. doi: 10.1016/s0091-3057(00)00399-3. [DOI] [PubMed] [Google Scholar]
- 59.Horan WP, Blanchard JJ, Clark LA, Green MF. Affective traits in schizophrenia and schizotypy. Schizophrenia Bulletin. 2008;34:856–874. doi: 10.1093/schbul/sbn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task-demands. Psychopharmacology. 2002;162:129–137. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- 61.Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hahn B, Gold JM, Buchanan RW. The potential of nicotinic enhancement of cognitive remediation training in schizophrenia. Neuropharmacology. 2013;64:185–190. doi: 10.1016/j.neuropharm.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 63.Dalack GW, Becks L, Hill E, Pomerleau OF, Meador-Woodruff JH. Nicotine withdrawal and psychiatric symptoms in cigarette smokers with schizophrenia. Neuropsychopharmacology. 1999;21:195–202. doi: 10.1016/S0893-133X(98)00121-3. [DOI] [PubMed] [Google Scholar]
- 64.Weiner E, Ahmed S. Smoking cessation in schizophrenia. Current Psychiatry Reviews. 2013 in press. [Google Scholar]
- 65.Durazzo TC, Meyerhoff DJ, Jo Nixon S. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health. 2010;7:3760–3791. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson's method. Tutorials in Quantitative Methods for Psychology. 2007;1 [Google Scholar]
- 67.Morey RD. Confidence intervals from normalized data: a correction of Cousineau (2005) Tutorial in Quantitative Methods for Psychology. 2008;4:61–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.