Abstract
The Extracellular signal-Regulated Kinases 1 and 2 (ERKs 1/2) are known to participate in regulating transcription in response to moderate depolarization (synaptic stimulation), but how the same active enzyme can differentially regulate distinct transcriptional programs induced with abnormal depolarization (high potassium) is unknown. We hypothesized that ERK1 or 2 accomplishes this differential nuclear response through close association with other proteins in stable complexes. In support of this hypothesis, we have found that immunoreactivity for an apparent high molecular weight phospho-ERK1-containing complex increased in response to synaptic stimulation, but decreased in response to high potassium; p-ERK immunoreactivity at 44/42 kDa increased in both cases. Evidence supporting the conclusion that the band of interest contained ERK1 in a complex, as opposed to it being an unrelated protein crossreacting with antibodies against p-ERK, is that ERK1 (p44 MAPK) and 14-3-3 were electro-eluted from the 160 kDa band cut from a gel. We also found the nuclear complexes to be exceptionally durable, suggesting a role for the crosslinking enzyme, transglutaminase, in its stabilization. In addition, we found other components of the ERK pathway, including MEK, ERK2, p90RSK, and Elk-1, migrating at higher-than-expected weights in brain nuclei. These results describe a novel stable complex of ERK1 in neuronal nuclei that responds differentially to synaptic and depolarizing stimulation, and thus may be capable of mediating gene transcription in a way distinct from the monomeric protein.
Keywords: Long-term potentiation, transcription, translation, CREB, Elk-1
Introduction
The Extracellular signal-Regulated Kinases 1 and 2 (ERKs 1/2; p44/p42 mitogen activated protein kinases, MAPKs) in neurons respond to a wide variety of stimuli including glutamate receptor activation (Bading et al. 1991), depolarization with potassium (Baron et al. 1996), long-term potentiation (LTP) and long-term depression (LTD)-inducing synaptic activity (Dudek et al. 2001; English et al. 1996; Thiels et al. 2002), and growth factors such as BDNF (Segal et al. 1996). As ERKs are thought to participate in regulating cellular transcriptional responses (Waltereit et al. 2001), how the active enzyme might differentially regulate transcriptional programs induced with synaptic activity (moderately depolarizing) as distinct from potassium (severely depolarizing) stimulation is unknown; ERK is activated in both situations. In one case, ERK would be activated with normal synaptic activity in the brain, possibly during learning (Blum et al. 1999), and in the other only under pathological conditions such as migraine aura, which is now thought to be due to a spreading-depression like phenomenon (Sanchez-del-Rio et al. 2004). Other calcium-activated enzymes and transcription factor phosphorylation events might play a role in distinguishing the two cases, but they too are typically activated with both potassium and synaptic stimulation (Bito et al. 1996). Determining how enzymes that are activated in both cases, such as ERK, distinguish the two stimulation conditions is thus critical to our understanding of activity-regulated transcription. Studying the enzymes in nuclear compartments could shed some light on the issue.
A little-discussed, but frequently made observation in neurobiology is that after synaptic stimulation, antibodies to phospho-ERK stain neuronal nuclei in addition to cytoplasm in dendrites and somata (Patterson et al. 2001), yet most antibodies to the total ERK (types 1 or 2), typically fail to stain the nuclei, even after stimulation. Because phosphorylated ERK has been shown to translocate to the nucleus in response to a variety of cell-stimuli in non-neuronal cells (Chen et al. 1992), the interpretation has been that neuronal activity similarly stimulates ERK translocation to the nucleus. However, translocation of ERK upon activation does not necessarily occur in every case; in PC12 cells both NGF and EGF activate ERK, but only NGF induces ERK to translocate to the nucleus (Traverse et al. 1992). Experiments using GFP-ERK have thus far been inconclusive in showing that ERK translocates to the nucleus with neuronal stimulation (Rosenblum et al. 2002), however, and these types of studies are complicated by the need for co-expression of a cytoplasmic retention protein to prevent nuclear localization under baseline conditions (MEK, (Horgan et al. 2003); MKP-3, (Karlsson et al. 2004)). It is also curious that such a robust translocation cannot be detected with conventional antibody methods, particularly because such translocation can be demonstrated easily with other types of stimulation (NGF, (Sano et al. 1995; Traverse et al. 1992); serum (Chen et al. 1992)) or with proteins such as p90rsk (Zhao et al. 1995). In other contexts we might generally propose that the ERK antigen-epitopes were simply inaccessible to the antibodies, possibly due to ERK being bound up in a protein complex, thus explaining the lack of nuclear staining. This explanation has led us to hypothesize that a pool of ERK might exist in neuronal nuclei in complexes in both the control and stimulated cases. Supporting the idea that ERK can exist in stable complexes in neuronal nuclei, we have found that immunoblots of nuclear extracts from rat forebrain or hippocampal CA1 mini-slices show not only the expected bands of 42 (ERK2) and 44-kDa (ERK1), but also a novel high molecular weight species of approximately 160-kDa immunoreactive for phospho-ERK1/2 (p-ERK).
That complexes of ERKs exist in neurons is not without precedent; scaffolding is an important method of ERK pathway regulation in yeast and in mammalian cells (Edmunds et al. 2004; Kolch 2005). Here we describe the first case where the complexed enzyme can be regulated independently from its uncomplexed form according to the type of neuronal depolarization. Further, because this complex is always localized in nuclei, it is well positioned to provide a mechanism by which different types of neuronal stimulation regulate alternative complements of gene transcription.
Results
Differential response to synaptic and depolarizing (K+) stimulation
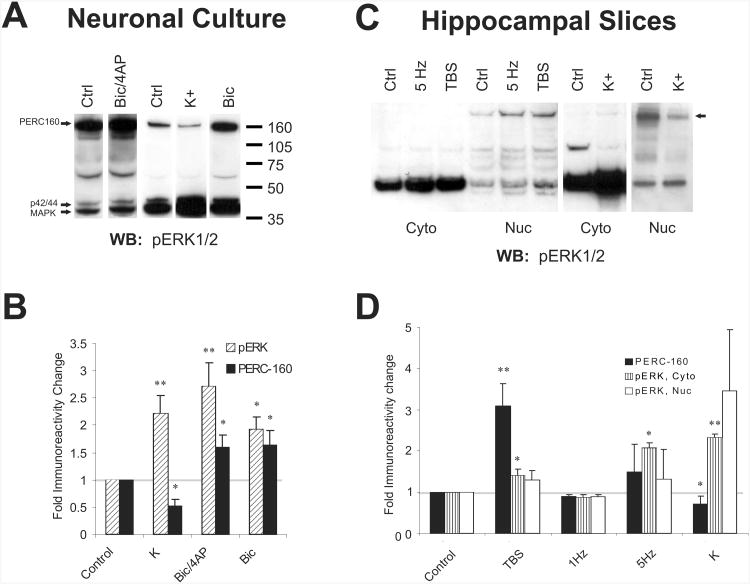
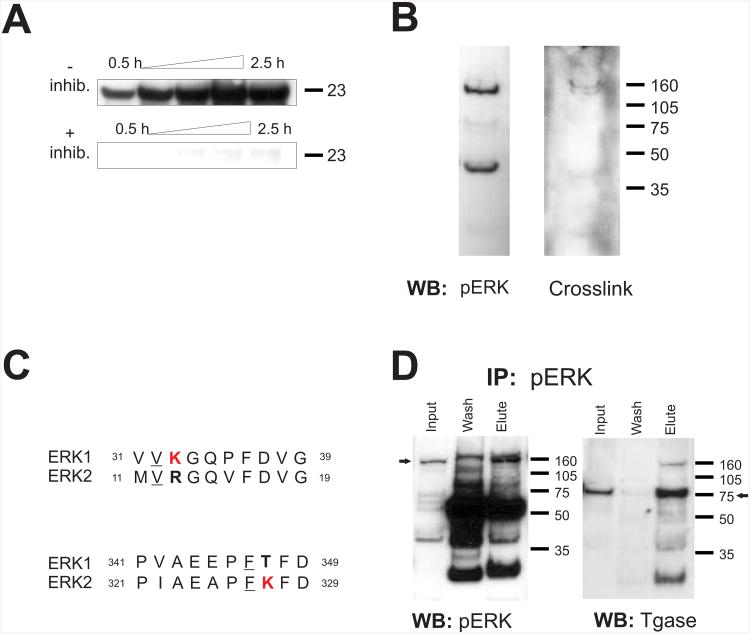
Gene transcription induced with neuronal activity in the form of action potentials and/or synaptic activity induces different genes than those induced with depolarization with high extracellular potassium, such as in the case of spreading depression during migraine aura (Choudhuri et al. 2002; Nedivi et al. 1993). ERK1/2, however, is phosphorylated and activated under both conditions equally well, as are many calcium- and calmodulin-dependent kinases that respond to neuronal activity (Bito et al. 1996). In order to test our hypothesis that ERK can exist in protein complexes able to respond differentially to the two situations, we purified nuclei from rat forebrain for analysis. Because the activated, dually phosphorylated form of ERK can be detected in nuclei histologically, and because antibodies raised against regions of ERK that are not phosphorylated typically fail to stain nuclei even under conditions known to result in ERK activation, we used antibodies raised against a dually phosphorylated peptide (Thr183 and Tyr185) (Promega) corresponding to the active enzyme to investigate whether high molecular weight entities could be observed on immunoblots of nuclear protein. In nuclei, but not cytosol cleared of nuclei, we observed a species of approximately 160 kDa, immunoreactive for phospho-ERK (p-ERK) in addition to the expected bands at 42 and 44 kDa, representing ERK2 and ERK1, respectively (forebrain not shown, but see Fig. 1A and throughout). A band estimated to migrate around 70 kDa, as well as some other intermediate bands, were visible in both cytosolic and nuclear fractions in approximately 75% of the western blots; the factors influencing the presence of these bands, however were not readily apparent. Interestingly, immunoreactivity for the “p-ERK reactive complex at 160 kDa” (PERC-160), like p-ERK, increased in neuronal cultures in response to synaptic activity enhanced with addition of the GABA antagonist bicuculline (with or without a potassium channel blocker, 4-amino pyridine (4AP)) to induce cell bursting within the network of neurons in culture (Hardingham et al. 2001)(Fig. 1A, B). Unlike p-ERK, however, PERC-160 appeared to be dephosphorylated in response to depolarization with potassium (Fig. 1A, B). Similar results were obtained in hippocampal mini-slices (Fig. 1C, D); as in culture, immunoreactivity for activated ERK at 42/44 kDa increased with both the synaptic stimulation (theta-burst stimulation) and the potassium depolarization, but the PERC-160 reactivity decreased in the potassium treatment. In some cases, such as is shown in Fig. 1C, the p-ERK immunoreactivity at 70 kDa decreased in the cytoplasm, suggesting a link or relationship with PERC-160. Neither the PERC-160 nor p-ERK reactivity were influenced when the same number of pulses was delivered at 1 Hz.
Fig. 1.
An apparent ERK-containing complex responds differentially to synaptic activity and potassium depolarization.
A. Representative western blots probed with anti-pERK1/2 antibodies. Samples were derived from cortical neuronal cultures that were treated for 5 minutes with 50 mM bicuculline, bicuculline with 75 mM 4-AP, or 60 mM potassium (K). ERK1 (p44), ERK2 (p42), and PERC160 are indicated with arrows.
B. Cumulative data for the fold change in pERK and PERC-160 immunoreactivity in samples derived cortical cultures that were treated as in A. Shown are means ± S.E.M.s (n=5, 9, and 8 for K, Bic/4AP, and Bic, respectively). * = significant with p<0.04, ** = significant with p<0.01 when compared with the unstimulated controls.
C. Representative anti-pERK1/2-probed western blots of nuclear and cytosolic fractions derived from CA1 mini-slices that were given theta-burst or 5 Hz synaptic stimulation or were treated with 60 mM potassium. Slices were harvested after 5 minutes.
D. Summary data for the fold change in pERK and PERC-160 immunoreactivity in nuclear and cytosolic samples derived from slices treated as in C (n=6, 2, 2, and 5 for TBS, 1 Hz, 5 Hz, and K, respectively). * = significant with p<0.04, ** = significant with p<0.01 when compared with the unstimulated controls.
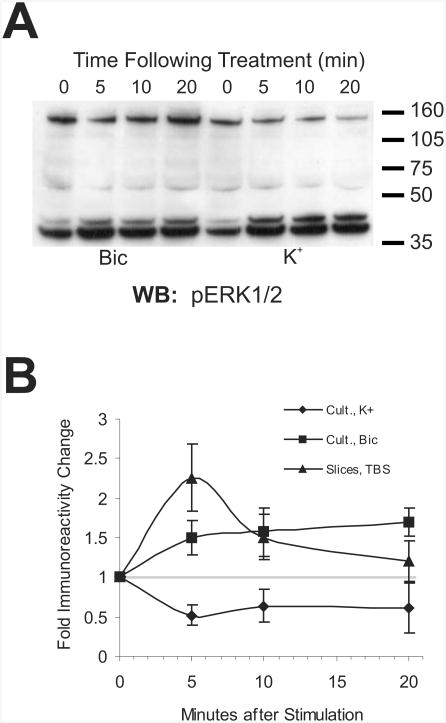
It could be that the increases in immunoreactivity for the PERC-160 band peaked early after potassium stimulation, and then subsided as the complex was dephosphorylated in the time prior to sample collection. This was not the case, however, as is shown in Fig. 2A and B: PERC-160 reactivity decreased immediately (5 minutes), and stayed down for at least 20 minutes. Immunoreactivity for PERC-160 increased during the same time frame when bicuculline was used to increase network activity in the cultures. For comparison, the increase in immunoreactivity in response to theta-burst stimulation in slices is also shown in Fig. 2B. In slices, PERC-160 immunoreactivity decreased rapidly after peaking at 5 minutes after stimulation, much like the immunostaining for p-ERK does in slices (Dudek et al. 2001). This indicates that constant synaptic activation is likely to be necessary for continued change in phosphorylation of PERC-160, and that abnormal depolarization leads to an apparent dephosphorylation.
Fig. 2.
Time course of putative complex phosphorylation/dephosphorylation.
A. Western blot probed with anti-pERK1/2 antibodies, demonstrating the change in PERC-160 immunoreactivity in samples from cortical cultures at 0, 5, 10 and 20 min following treatment with bicuculline or potassium.
B. Cumulative data for the change in PERC-160 immunoreactivity over time in samples derived from stimulated cortical cultures. Bic/4AP showed a trend very similar to that of Bicuculline alone. For comparison, data from CA1 mini-slices, stimulated with theta-bursts, are also shown (n=5, 4, and 7 for K, Bic, and TBS, respectively).
PERC-160 is an exceptionally stable complex
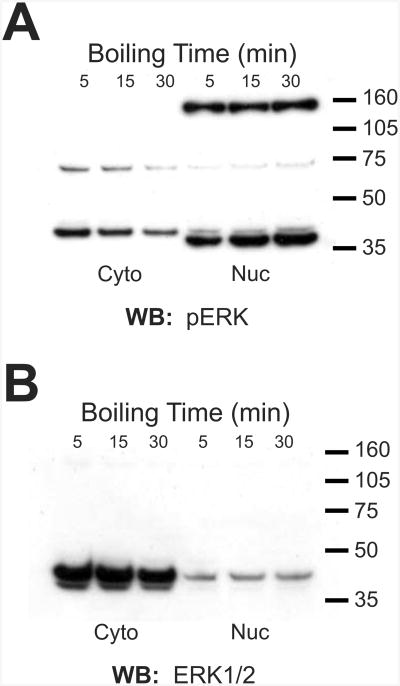
The observations presented thus far are from samples resolved under typical denaturing conditions during which samples were boiled in a standard SDS- and DTT- containing sample buffer. Therefore, if the band represents a complex, it is extremely stable. To determine whether PERC-160 is indeed a complex containing ERK, rather than a nuclear protein that cross-reacts with p-ERK antibodies and responds to neuronal activity, we sought to find conditions that may disrupt such an exceptionally stable complex. Samples were boiled for an extended amount of time, in an attempt to heat-denature the complex (Fig. 3 A, B). Boiling in SDS/DTT sample buffer for up to 30 minutes, however, did not reveal any decrease in the high band, nor did it consistently result in an increase in the bands representing ERK1/2 (Fig. 3B). In cytosolic fractions, the immunoreactivity appeared to actually decrease, which was likely the result of protein degradation. Similar results were obtained when samples were boiled in 9M Urea, 6M guanidine, or 10% SDS (not shown). This finding, on the surface, would indicate that PERC-160 is indeed a single protein, and not a complex containing ERK.
Fig. 3.
The putative complex is exceptionally stable.
A. Western blot of nuclear and cytosolic fractions from rat forebrain, probed with anti-pERK1/2 antibody. Samples in SDS-PAGE sample buffer were boiled for 5, 15, or 30 minutes.
B. Western blot of nuclear and cytosolic fractions from rat forebrain, probed with anti-ERK1/2 antibody. Samples in SDS-PAGE sample buffer were boiled for 5, 15, or 30 minutes. Similar findings were observed when samples were boiled with 6 M GdnHCl, 9 M urea, or 10% SDS.
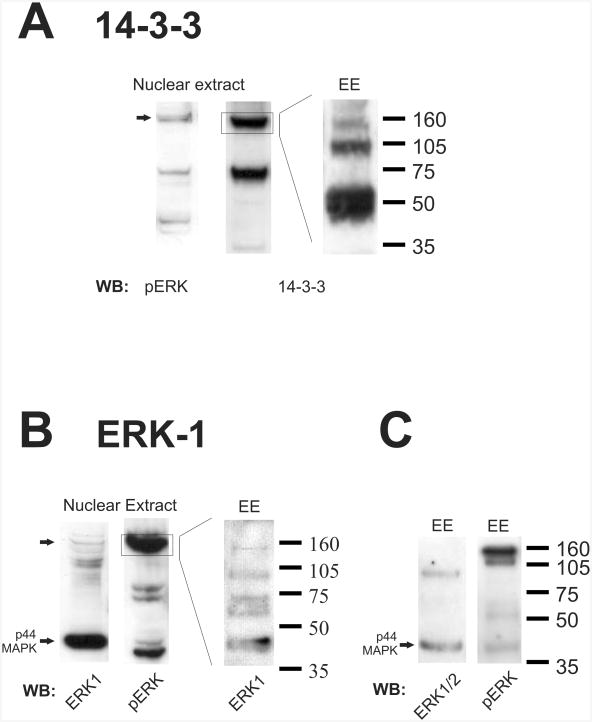
When probing immunoblots of neuronal nuclear preparations for known ERK-pathway interacting proteins, however, we found that an anti-14-3-3 antibody showed strong immunoreactivity at 160 kDa (Fig. 4A), as well as at 70 kDa. This observation was inconsistent with either a monomer or dimer of 14-3-3 that has a typical molecular weight of 25 kDa for most 14-3-3 isoforms. Supporting the hypothesis that the PERC-160 band contains 14-3-3 was the finding that 14-3-3-reactive bands could be generated by electro-eluting protein from PERC-160 when it was cut out of a gel (Fig. 4A). Additionally, the SDS and protein-containing eluant was concentrated in vacuo prior to gel electrophoreseis, resulting in saturating levels of SDS. This finding suggests that while normal sample preparations, and even some harsh denaturing conditions, do not break down PERC-160, near saturating concentrations of SDS can result in some disruption of this apparent complex.
Fig. 4.
14-3-3 and ERK1 are contained in the putative complex.
A. Material from forebrain nuclear extracts or electroeluted (EE) from the 160 kDa band. Western blots were probed with anti-14-3-3 antibody. Blot probed for pERK is shown for comparison.
B. Material from forebrain nuclear extracts or electroeluted (EE) from the 160 kDa band. Western blots were probed with an antibody specific for ERK1 (p44 MAPK). Blot probed for pERK is shown for comparison.
C. Western blots of electroeluted material probed with an anti-ERK antibody (recognizes both ERK1 and ERK2) or pERK. Note that the bulk of the pERK staining remains at 160 kDa.
Most antibodies to ERK1 or ERK2 did not recognize PERC-160. However, one must also recall that many antibodies to ERKs 1 and 2 do not recognize ERK in the nucleus histologically, even after activation when nuclear p-ERK is readily stained. A single ERK1 specific antibody did weakly recognize high molecular weight bands, one of which co-migrates with PERC-160 in preparations of neuronal nuclei (Fig. 4B). When proteins electro-eluted from the PERC-160 band were probed with this ERK1 antibody, several lower bands appeared, including one co-migrating with authentic ERK1 at 44 kDa, as stained with antibodies specific for ERK1 (Figs. 4B, C). An antibody against ERK (not specific for ERK1, but also recognizes ERK2) recognized an ERK1-sized band, but not the 160 kDa band present in the eluant, consistent with the idea that the reason most ERK antibodies do not recognize ERKs in neuronal nuclei is because they are bound up in complexes there. The majority of the PERC-160 band remained intact even after electro-elution and concentration with SDS, as seen when the eluant is probed with p-ERK. Note that a small amount of p-ERK reactivity is present in the eluant at 44 kDa (Fig. 4C). All of these lower bands (ERK1, p-ERK, 14-3-3) could only have come from the higher molecular weight entity, as only the 160 kDa region was cut from the gel for electroelution.
Transglutaminase may stabilize the PERC-160 complex
How might a complex of proteins remain so stable, even in the presence of harsh denaturing conditions? Transglutaminase is a calcium-activated enzyme that is capable of crosslinking proteins that can withstand even the harshest of denaturing conditions (Lorand et al. 2003). The resulting covalent bond, formed between a glutamine of one protein and a lysine of another, is equivalent to a peptide bond and thus cannot be disassembled without destruction of the protein or enzymatic reversal. To assay transglutaminase activity in nuclei, we used casein as an amine acceptor/glutamyl donor. Active transglutaminase enzyme attaches its substrate, biotinylated-cadaverine, to the exogenous casein target (Case et al. 2003). Consistent with previous studies (Lesort et al. 1998), we found significant transglutaminase activity in our nuclear preparations from brain tissue (Fig. 5A) that could be inhibited with cystamine (or GTPγS; data not shown). We found no evidence of transglutaminase activity in the assay in the absence of nuclear extract.
Fig. 5.
Transglutaminase may play a role in stabilizing the pERK-containing complex.
A. Time-course of the incorporation of biotinylated-pentylamine into casein (MW 23 kDa), indicating transglutaminase activity. Nuclear extract-treated samples in the absence or presence of cystamine (3 mM). Addition of GTPγS similarly inhibited activity (not shown). No activity was observed without nuclear extract.
B. Western blot of protein from forebrain nuclear extracts probed with an antibody specific for transglutaminase-catalyzed crosslinks. A western blot probed for pERK is shown for comparison.
C. Amino acids sequences of ERK1 and ERK2 showing lysines (K, bold) flanked with a valine (V) or phenylalanine (F) (underlined), indicating possible sites of transglutaminase-mediated crosslinking. ERK1 and ERK2 differ in these lysines.
D. Western blots of samples from forebrain nuclear extract immunoprecipitated with anti-pERK antibodies. Blots were probed with either anti-pERK, or anti- transglutaminase2.
Transglutaminase has a certain degree of specificity in that random proteins are not crosslinked in the presence of transglutaminase. First, the two candidate substrate proteins need to associate with each other to some extent. Second, upon association, the presumed glutamine substrate from one protein must be aligned very near the lysine of the other protein if the two proteins are to be crosslinked. This pairing between two transglutaminase-cross-linked proteins has been used to develop commercial antibodies that can be used to probe for substrates crosslinked by transglutaminase (but see (Johnson et al. 2004)). Fig. 5B shows that blots probed with the antibody show immunoreactivity at 160 kDa as well as at a slightly lower molecular weight. Interestingly, ERK1 and ERK2 protein sequences differ in lysines (K) flanked with the amino acids valine (V) or phenylalanine (F), which are considered favorable for the transglutaminase reaction (Fig. 5C) (Grootjans et al. 1995). ERK1 has one potential site of crosslinking near the N-terminal, missing from ERK2: 31..VVKGQPFDVG..40 (vs. 11..MVRGQVFDVG..20; ERK2), whereas ERK2 has one near the C-terminal missing from ERK1: 321..PIAEAPFKFD..330 (vs. 341..PVAEEPFTFD..350; ERK1). This suggests that ERK1 and ERK2 both could be substrates for transglutaminase, and that the two similar enzymes contain distinct sites of possible crosslinking or posttranslational modification.
To determine whether transglutaminase can associate with phosphorylated ERK, immunoprecipitations using antibodies against p-ERK were performed. Fig. 5D shows that transglutaminase can interact with p-ERK (or the PERC-160 complex) in that it can be co-precipitated with p-ERK. As an aside, 14-3-3 can be similarly co-immunoprecipitated with p-ERK (not shown). Due to the tendency of p-ERK and this PERC-160 protein to associate with beads in the absence of antibody, the reverse IP (immunoprecipitation of transglutaminase) was not meaningful.
With the knowledge that transglutaminase could be modifying the protein during the isolation of nuclear proteins, we performed several nuclear preparations (from rat forebrain) with cystamine present in the homogenization buffer and sucrose cushion. No apparent reduction in PERC-160 isolation was observed. Further evidence that transglutaminase already present in nuclei wasn't responsible for crosslinking during preparation was that lysing neurons in culture directly into sample buffer followed by immediate boiling still revealed the presence of PERC-160. Therefore, the complex was likely present in the nuclei prior to isolation and did not form as a result of disrupting the normal localization of transglutaminase.
Other members of the ERK pathway may similarly exist in nuclear complexes
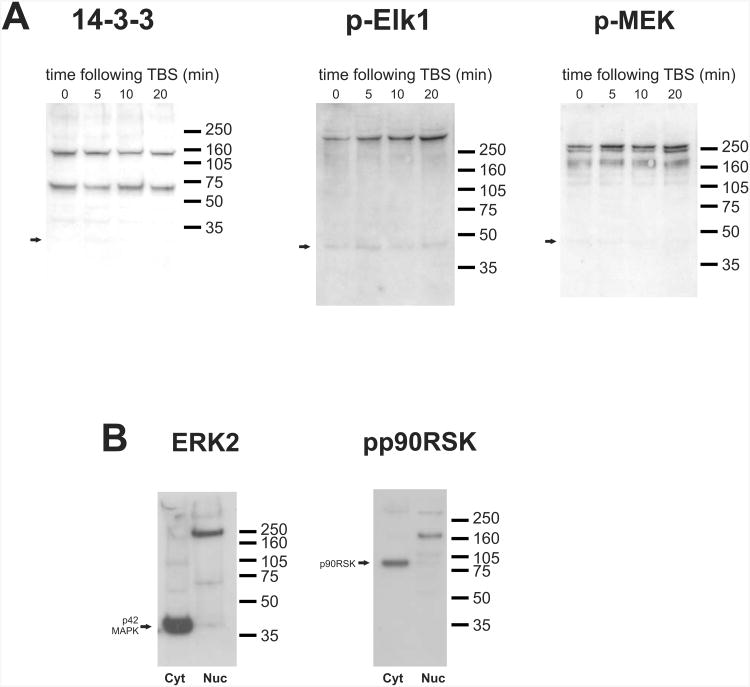
It is possible that ERK binds to 14-3-3 and other components of the pathway into a complex after activation and translocation to the nucleus. This scenario is difficult to assess, as the antibody that best recognizes the complex is one that recognizes ERK's phospho-epitope. Increases in immunoreactivity therefore, cannot distinguish between increases in phosphorylation and formation of the putative complex. One way to circumvent this problem and to test for formation of the complex is to monitor changes in the high molecular weight immunoreactivity to 14-3-3; if the complex is formed after stimulation, an increase in reactivity at 160 kDa should be observed after stimulation. We did not find this to be the case (Fig. 6A); no consistent change in 14-3-3 immunoreactivity at 160-(or 70-) kDa was observed after theta-burst stimulation, indicating that a preformed complex responds to synaptic stimulation with reversible phosphorylation, and is not formed as a result of stimulation over the time period investigated.
Fig. 6.
Other components of the ERK pathway migrate at high molecular weights in neuronal nuclei.
A. Western blots of nuclear fractions from hippocampal mini-slices 5, 10, and 20 min after theta burst stimulation probed with anti-14-3-3, anti-phospho-Elk1, or anti-phospho-MEK. Arrows represent the expected molecular weights.
B. Western blots of cytosolic and nuclear fractions of rat forebrain probed with an antibody specific for ERK2 (left) or phospho-p90rsk. Arrows indicate the expected molecular weights.
When antibodies against other phosphorylated members of the ERK pathway were used, similar high-molecular weight immunoreactive species were observed, also only in nuclear preparations. Antibodies to a phosphorylated substrate of ERK, Elk-1, labeled a band higher than 250 kDa, which did intensify after theta-burst stimulation (Fig. 6A). Similarly, immunoreactivity for phosphorylated MEK, the upstream kinase that phosphorylates and activates ERK, is also increased after theta-burst stimulation at high molecular weights. In addition, another substrate of ERK, p90rsk, also migrated at a molecular weight higher than expected, at 160 kDa (Fig. 6B). Note that in the Elk-1, MEK, and p90rsk cases, the high-running bands are dominant in the nuclear fractions, while labeling at the expected molecular weights (62, 45, and 90 kDa respectively) is comparatively weak in nuclei. When antibodies against other regions of the molecules that do not label the phosphorylated forms were used, labeling was most typically found at the expected molecular weights. An exception was found with one ERK2-specific antibody, which labeled a 250 kDa band in nuclear fractions (Fig. 6B). Therefore, the putative complexes often appear to contain predominantly the phosphorylated, active forms of the proteins. Whether the complexes only become visible upon phosphorylation, as opposed to the proteins associating upon phosphorylation cannot be differentiated conclusively in our experiments.
Discussion
In neurons, the MAP-kinases ERK-1 and ERK-2 are activated under a variety of stimulation conditions that include depolarization, synaptic activation, and growth factors such as BDNF acting through Trk-receptors (Segal et al. 1996). The patterns of genes expression, however, for each of the various types of stimuli are likely very different (Choudhuri et al. 2002; Nedivi et al. 1993). It is possible, and indeed likely, that activation of additional kinases in stimulus-specific manners would result in the differential responses. Although this explanation may work well for distinguishing the different responses to depolarization versus growth factors or neuromodulators, for example, it is less clear how synaptically-induced depolarization is distinguished from depolarization due to hyperkalemic conditions; most calcium-stimulated enzymes are activated under both conditions (Bito et al. 1996). One way to accomplish a signal cut-off feature, to serve in cases of pathological depolarization, would be to have protein phosphatases closely associated with the active kinase. Associations between MAP-kinases, MAP-kinase-kinases, and appropriate MAP-kinase phosphatases have been well described in yeast (Martin et al. 2005), and rely on scaffolding molecules to bring the specific molecules together (Whitmarsh et al. 1998). Consistent with such an association with MAP-kinase phosphatases, our study shows that a complex-associated ERK1 can behave differently from its monomeric counterpart in that it can be dephosphorylated under conditions where the monomeric ERK is not. It remains unknown, however, which MAP-kinase phosphatase would be associated with PERC-160 and why it would be more active under conditions of potassium depolarization. Curiously, the dephosphorylation of CREB only occurs after NMDA receptor activation and not with potassium depolarization (Sala et al. 2000).
Scaffolding functional groups of enzymes together has been increasingly appreciated as a means by which enzymes can be regulated as groups (Ferrell 2000). In yeast, regulation of the scaffolding molecule itself can enhance or inhibit certain cellular responses depending on protein expression levels; one system demonstrating this behavior prominently features a MAP-kinase (Whitmarsh et al. 1998). Another function of scaffolding molecules is to localize the complex near its site of action, be it nuclear or cytoplasmic. In the case of PERC-160, the complex is clearly restricted to nuclei, and it is likely too big to exit through nuclear pores. Thus, crosslinking the proteins together could be a way the cell retains ERK in the nucleus without neosynthesis of nuclear retention proteins (Lenormand et al. 1998). We also find another p-ERK and 14-3-3 reactive band at 70 kDa in the cytosol, but it remains undetermined whether this represents an incomplete version of PERC-160, or whether it serves a unique cytosolic function. Importantly, scaffolding can serve to increase the speed of signaling, a critical feature if ERKs are to regulate genes such as arc, which can be detected as early as 2 minutes after stimulation (Guzowski et al. 1999; Waltereit et al. 2001). This association would certainly not be without trade-offs, however, in that scaffolding ERK to its nuclear substrates and/or upstream activators in such a 1:1:1 ratio would restrict any amplification processes (Kolch 2005) (Fig. 7). Nevertheless, scaffolding enzyme cascades, particularly those containing ERK, has been established as playing an important role in localizing and regulating enzyme function.
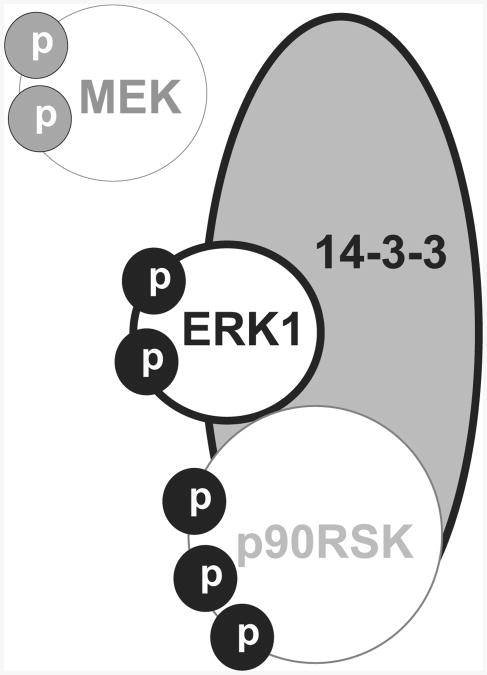
Fig. 7.
Proposed ERK-containing complexes in neuronal nuclei. ERK1 and 14-3-3 are contained in PERC-160, with phosphorylated MEK (p-MEK) and phosphorylated p90RSK possibly associated (not confirmed). Phospho-epitopes are most likely to be exposed, with the majority of the molecule(s) buried in the complex. Lighter-weight lines indicate speculated interactions.
14-3-3 is a small, acidic group of proteins that serves a diverse group of functions. This protein family can either positively modulate enzyme activity, in the cases of serotonin N-acetyltransferase (Obsil et al. 2001) and Protein Kinase C (Van Der Hoeven et al. 2000), or negatively modulate activity, such as with CaM-kinase kinase (Davare et al. 2004). Specifically in the ERK pathways, 14-3-3 does not impact the activity of MEK (Shimizu et al. 1994), but it has been reported to stimulate the enzyme upstream from MEK, Raf (Freed et al. 1994) (Irie et al. 1994) and associates with other upstream kinases in the pathway (Yamamori et al. 1995). Therefore 14-3-3 is not an unlikely candidate for association with ERK1 in a stable complex. Consistent with this is our finding that along with ERK1, 14-3-3 reactive bands can be electroeluted from PERC-160. The 14-3-3 could only have come from PERC-160 in that the high molecular weight complex was cut out of the gel, with the proteins subsequently removed from by electroelution.
The apparent presence of 14-3-3 in the 70 kDa band in both cytosolic and nuclear fractions could represent an incomplete version of the complex or one that is localizing phospho-ERK to non-nuclear targets in addition to nuclear ones. Because this 14-3-3- and pERK-immunoreactive band was observed inconsistently, however, no conclusions on its function can be made. It is possible that variations in conditions during nuclear purification could have resulted in its leakage from the nucleus. Further work will be required to characterize this possible clue to the composition PERC-160 complex.
For the most part, PERC-160 remains stable through a variety of harsh treatments including boiling in SDS, guanidine, or urea. It is curious, therefore, why immunoreactivity for phospho-ERK at its correct molecular weight (44/42 kDa) increases upon boiling of nuclear fractions, while decreasing slightly in cytosolic fractions (Fig. 3A). This was not apparent when the non-phospho-antibodies were used (Fig. 3B). The most likely explanation is that the apparent increase in phospho-ERK in the nuclear fraction is due to a small amount phospho-ERK (but not unphosphorylated ERK) falling apart from otherwise stable high molecular weight complexes. Interestingly, this was not accompanied by a decrease in the 160 kDa band, and so one must therefore postulate that the increase in phospho-p44/42 ERK is coming from either an unlabeled source, or else from PERC-160, with PERC-160 being replenished by some higher complex falling apart. Decreases in both the phospho- and non-phospho staining for p44/42 ERK in cytosolic fractions may be due to degradation of the protein under such harsh conditions.
How is it that this ERK1-containing complex is otherwise so stable? A possibility is that transglutaminase acts to crosslink the components of the complex, which makes the complex extremely resistant to denaturation. We show that transglutaminase associates with p-ERK and/or PERC-160 in that it co-immunoprecipitates with p-ERK. Further, an antibody against transglutaminase-mediated isopeptide crosslinks clearly recognizes the PERC-160 band in nuclear preparations. Moreover, one study looking at the distribution of such immunoreactivity for isopeptide crosslinks in brain found some structures in neuronal nuclei in hippocampus (Maggio et al. 2001). That transglutaminase can be active in nuclei in response to calcium has been described (Lesort et al. 1998), and transglutaminase associates with importin molecules (Peng et al. 1999), indicating a possible regulated nuclear function. To date, though, the only nuclear proteins demonstrated to be modified by transglutaminase in response to physiological stimulation are histones (starfish egg fertilization, (Nunomura et al. 2003)). Transglutaminase is activated under high calcium or low GTP conditions (Lorand et al. 2003), indicating that it may be activated during times of cellular stress, and nuclear localization of transglutaminase attenuates apoptosis (Milakovic et al. 2004). Thus it is unclear whether such a transglutaminase-stabilized complex would be formed in a physiological homeostatic role, or inadvertently in a pathological role. These results might have provided us with a mechanism for how the late phase of CREB phosphorylation is attributed to ERK activation without continued stimulation (Impey et al. 1998; Wu et al. 2001), but this explanation is unlikely because PERC-160 appears to be dephosphorylated with a time-course similar to that of monomeric ERK in slices. A similar complex containing ERK2 may instead be responsible.
What could be the purpose of having a complex stabilized so permanently? Protection from dephosphorylation or proteolysis is not likely, as PERC-160 is as sensitive to phosphatase and protease activity as p-ERK (data not shown). Also, because levels of the complex do not appear to form immediately after neuronal stimulation, it could be that transglutaminase-stabilized complexes form over much longer time frames; this may be one way that a cell could adjust its nuclear signaling to average neuronal activity over hours or days. Alternatively, the large complex may allow ERK and MEK to be primed for activity in a form too large for export out of the nucleus: MEK has a nuclear export sequence (Fukuda et al. 1996), and it is thought the unphosphorylated ERK must associate with MEK in order to leave the nucleus (Adachi et al. 2000). A preformed complex in the nucleus could also have the purpose of facilitating a rapid ERK signaling in response to action potentials by positioning it with its substrates like p90RSK (Adams et al. 2005; Zhao et al. 2005). As dimers of phosphorylated ERK1 have the highest levels of ERK activity (Philipova et al. 2005), the purpose of the complex may also be related specifically to maintain peak activity. Interestingly, stable complexes containing recombinant ERK1 dimers could be generated by incubation with extracts from sea urchin embryos but not from HeLa cells (Philipova et al. 2005). We propose that 14-3-3 and/or transglutaminase were present in the sea urchin extract.
ERK1 and ERK2 are very similar enzymes, but there is much to be learned about the different roles of the two; further research focusing on PERC-160 may aid in finding how they respond differentially to different types of neuronal stimuli.
Methods
Materials
Biotinylated-pentylamine was purchased from Pierce. All other chemicals were obtained from Sigma-Aldrich. Anti-ERK antibodies were obtained from Calbiochem; anti-p-ERK antibodies from Promega; anti-tubulin antibodies from Upstate Biotechnology; anti-transglutaminase II antibodies from Lab Vision Corporation; anti-isopeptide crosslink antibodies from CovalAb. All other antibodies were purchased from Cell Signaling Technology.
Acute slice preparation and electrophysiology
Hippocampal slices (350 μm thickness) were cut using a vibrating microtome and were trimmed to include only the CA1 region. The size of the resultant “mini-slice” was such that a single stimulating electrode placed in the center could generate a population spike out to the slice edge. Slices were prepared in ACSF containing 124 mM NaCl, 4 mM KCl, 1.25 mM NaH2PO4, 26 mM NaHCO2, 1 mM CaCl2, 3 mM MgCl2, 10 mM glucose, 2 mM kynurenic acid, and bubbled with 95/5% O2/CO2. Following trimming, mini-slices were maintained in an interface chamber (Haas top design) at 35° C with a 1mL/min perfusion of ACSF lacking kynurenic acid and with 2.5 mM CaCl2 and 1.5 mM MgCl2. Stimulation was carried out 2-4 hours after cutting using concentric bipolar stimulating electrodes (FHC). Pulse duration and intensity, 130 μsec at 60 μA, were chosen to produce population spikes across the majority of the mini-slice, resulting in an estimated 60 to 80 percent of the pyramidal neurons being activated based on phospho-ERK staining (Dudek et al. 2001). A theta-burst stimulation pattern (TBS; ten 100 Hz bursts of 4 pulses, at 5 Hz; delivered 3 times, 30 seconds apart), or 20 seconds at 5 Hz was used for the stimulation of the mini-slices, as both of these protocols are effective at stimulating ERK (Dudek et al. 2001). Alternatively, mini-slices were bathed in 60 mM KCl for 5 minutes after the disappearance of synaptic responses. Mini-slices were subsequently removed from the interface chamber with a brush, frozen in tubes on dry ice and stored at −80° C until nuclear extraction was carried out.
Primary Neuronal Cultures and Stimulation
Cortical neuron cultures were prepared from E18 rat embryos in a serum-free medium protocol as previously described (Brewer et al. 1993). Cultures were maintained for 21-30 days in vitro prior to experimentation in all cases. For stimulation, culture media was supplemented with 60 mM KCl or 50 μM bicuculline with or without 75 μM 4-aminopyridine for up to 20 min. A crude cytosolic fraction was prepared by removing the media and replacing it with lysis buffer (0.6 % Igepal CA-630, 10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT along with protease and phosphatase inhibitors). After removing the resultant solution (cytosol), a crude nuclear fraction was generated by scraping the remaining material into LDS-PAGE sample buffer. As the ERK complex was only observed in nuclear fractions, cells were harvested directly into sample buffer in some cases.
Nuclear Extract Preparation
For the isolation of nuclei from the hippocampal mini-slices, a Nuclei PURE Prep kit from Sigma was utilized, with the following supplements added to all buffers: protease inhibitor cocktail III (Calbiochem), phosphatase inhibitor cocktail II (Calbiochem), 1 mM DTT and 3 mM cystamine. Using the included procedure as a guide, approximately 12 mini-slices were homogenized in 200 μL of the supplemented lysis buffer containing 0.6% Igepal CA-630. The homogenate was diluted with 270 μL of supplemented 1.6 M sucrose solution and layered upon 150 μL of the same sucrose solution and spun in a centrifuge at 41.5 k × g for 2 hours. The sucrose solutions were removed and centrifuged for 140 k × g for 1 hour with the supernatant reserved as the cytosolic fraction. The nuclei were resuspended in 100 μL nuclei storage buffer and centrifuged for 30 minutes at 4 k × g. The supernatant was discarded and the nuclei were resuspended in 33 μL deionized water, snap-frozen on dry ice, and stored at −80°C until analyzed by SDS-PAGE. Large-scale preparations were performed in a similar manner, but scaled-up accordingly, starting with forebrain and hippocampi from two rats.
Complex Isolation
For isolation of the PERC-160 complex prior to electroelution, 250 μL of resuspended nuclei from 2 brains were sonicated (20 one second pulses, amplitude 60) with a probe tip. Following nuclear disruption, the sample was brought to 20% saturation with ammonium sulfate and centrifuged for 15 minutes at 16 k × g. The pellet was discarded and the supernatant was adjusted to 50% saturation with ammonium sulfate prior to being spun in a centrifuge for 20 minutes at 6 k × g. The precipitate was subsequently diluted with 2.25 mL HEPES buffer (100 mM, pH 7.4), mixed with 100 mg of Reactive Brown 10 agarose (pre-equilibrated in the same buffer) and allowed to incubate with gentle end-over-end mixing for 2 hours at 4°C. A Handee spin column (Promega) was used to separate the protein-bound agarose from the binding solution. The resin was then washed twice with HEPES buffer and incubated with end-over-end mixing with 500 μL salt solution (2.5 M NaCl, 0.2 M NH4OH) for 1 hour to remove some proteins. Following this extraction, the protein-bound resin was separated from the salt solution, washed with HEPES buffer twice, and the protein of interest was eluted with LDS-PAGE sample buffer (Invitrogen). The resultant solution was diluted with 1.85 mL of acetate buffer (200 mM, pH 2.7), and dialyzed against the same buffer. Following dialysis, 100 mg pre-equilibrated PhosphoSelect Resin was added to the sample, and the slurry was allowed to incubate with gentle end-over-end mixing for 2 hours at 25°C. After incubation, the resin was washed twice with acetate buffer and resuspended in LDS sample buffer as before.
Electrophoretic Analysis
All electrophoresis materials (gels, buffers and membranes) were purchased from Invitrogen and used according to the manufacturer's instructions. For immunoblot analysis, PVDF membranes were utilized and developed with ECL Plus reagent (Amersham Bioscience). Protein loading was corrected for by immunoanalysis of tubulin. Quantification of visualized bands was accomplished using ImageQuant 5.0 software. All experiments were repeated at a minimum of one additional time with similar results observed. When comparing samples from different conditions, a two-tailed t-test was used to determine statistical significance.
Transglutaminase Assay
The transglutaminase assay was based on methods previously described (Case et al. 2003; Johnson et al. 1997). Briefly, a solution containing 50 mM HEPES, 0.5 M NaCl, 10 mM CaCl2, 1 mM DTT, 5 μM N,N-dimethylcasein, and 2 mM 5-(biotinamido)pentylamine, pH 7.4 was prepared and the reaction initiated with the addition of a 5% reaction volume of nuclear extract. This reaction proceeded at 25°C with aliquots removed every 30 min and frozen in liquid nitrogen. The inhibitory potential of cystamine, GTPγS or calcium chelators (EDTA and EGTA) on this reaction was determined by adding the chemicals to achieve final concentrations of 12 mM, 500 μM, or 5 mM, respectively, prior to the addition of the nuclear extract. Samples were analyzed for biotinylation of the N,N-dimethylcasein by probing an immunoblot with a horseradish peroxidase-streptavidin conjugate that was subsequently developed using ECL Plus.
Acknowledgments
We thank J. Paige Adams and Rachel Robinson for some of the Western blots and Negin Martin, Marc Sommer, and Mariel Birnbaumer for critical reading of the manuscript. This research was supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences.
References
- Adachi M, Fukuda M, Nishida E. Nuclear export of MAP kinase (ERK) involves a MAP kinase kinase (MEK)-dependent active transport mechanism. J Cell Biol. 2000;148:849–856. doi: 10.1083/jcb.148.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JP, Dudek SM. Late-phase long-term potentiation: Getting to the nucleus. Nat Rev Neurosci. 2005;6:737–743. doi: 10.1038/nrn1749. [DOI] [PubMed] [Google Scholar]
- Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Baron C, Benes C, Tan HV, Fagard R, Roisin MP. Potassium chloride pulse enhances mitogen-activated protein kinase activity in rat hippocampal slices. J Neurochem. 1996;66:1005–1010. doi: 10.1046/j.1471-4159.1996.66031005.x. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: A Ca2(+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Case A, Stein RL. Kinetic analysis of the action of tissue transglutaminase on peptide and protein substrates. Biochemistry. 2003;42:9466–9481. doi: 10.1021/bi030084z. [DOI] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of ERK- and RSK-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri R, Cui L, Yong C, Bowyer S, Klein RM, Welch KM, Berman NE. Cortical spreading depression and gene regulation: Relevance to migraine. Ann Neurol. 2002;51:499–506. doi: 10.1002/ana.10158. [DOI] [PubMed] [Google Scholar]
- Davare MA, Saneyoshi T, Guire ES, Nygaard SC, Soderling TR. Inhibition of calcium/calmodulin-dependent protein kinase kinase by protein 14-3-3. J Biol Chem. 2004;279:52191–52199. doi: 10.1074/jbc.M409873200. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Fields RD. Mitogen-activated protein kinase/extracellular signal-regulated kinase activation in somatodendritic compartments: Roles of action potentials, frequency, and mode of calcium entry. J Neurosci. 2001;21:RC122. doi: 10.1523/JNEUROSCI.21-02-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmunds JW, Mahadevan LC. MAP kinases as structural adaptors and enzymatic activators in transcriptional complexes. J Cell Science. 2004;117:3715–3723. doi: 10.1242/jcs.01346. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- Ferrell JEJ. What do scaffold proteins really do? Sci STKE. 2000;2000:PE1. doi: 10.1126/stke.2000.52.pe1. [DOI] [PubMed] [Google Scholar]
- Freed E, Symons M, Macdonald SG, McCormick F, Ruggieri R. Binding of 14-3-3 proteins to the protein kinase raf and effects on its activation. Science. 1994;265:1713–1716. doi: 10.1126/science.8085158. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Gotoh I, Gotoh Y, Nishida E. Cytoplasmic localization of MAP kinase kinase directed by its N-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export sequence. J Biol Chem. 1996;271:20024–20028. doi: 10.1074/jbc.271.33.20024. [DOI] [PubMed] [Google Scholar]
- Grootjans JJ, Groenen PJ, de Jong WW. Substrate requirements for transglutaminases. Influence of the amino acid residue preceding the amine donor lysine in a native protein. J Biol Chem. 1995;270:22855–22858. doi: 10.1074/jbc.270.39.22855. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJL, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- Horgan AM, Stork PJ. Examining the mechanism of ERK nuclear translocation using green fluorescent protein. Exp Cell Res. 2003;285:208–220. doi: 10.1016/s0014-4827(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Irie K, Gotoh Y, Yashar BM, Errede B, Nishida E, Matsumoto K. Stimulatory effects of yeast and mammalian 14-3-3 proteins on the Raf protein kinase. Science. 1994;265:1716–1719. doi: 10.1126/science.8085159. [DOI] [PubMed] [Google Scholar]
- Johnson GV, Cox TM, Lockhart JP, Zinnerman MD, Miller ML, Powers RE. Transglutaminase activity is increased in Alzheimer's disease brain. Brain Res. 1997;751:323–329. doi: 10.1016/s0006-8993(96)01431-x. [DOI] [PubMed] [Google Scholar]
- Johnson GV, LeShoure RJ. Immunoblot analysis reveals that isopeptide antibodies do not specifically recognize the epsilon-(gamma-glutamyl)lysine bonds formed by transglutaminase activity. J Neurosci Meth. 2004;134:151–158. doi: 10.1016/j.jneumeth.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Mathers J, Dickinson RJ, Mandl M, Keyse SM. Both nuclear-cytoplasmic shuttling of the dual specificity phosphatase MKP-3 and its ability to anchor MAP kinase in the cytoplasm are mediated by a conserved nuclear export signal. J Biol Chem. 2004;279:41882–41891. doi: 10.1074/jbc.M406720200. [DOI] [PubMed] [Google Scholar]
- Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- Lenormand P, Brondello JM, Brunet A, Pouyssegur J. Growth factor-induced p42/p44 MAPK nuclear translocation and retention requires both MAPK activation and neosynthesis of nuclear anchoring proteins. J Cell Biol. 1998;142:625–633. doi: 10.1083/jcb.142.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesort M, Attanavanich K, Zhang J, Johnson GV. Distinct nuclear localization and activity of tissue transglutaminase. J Biol Chem. 1998;273:11991–11994. doi: 10.1074/jbc.273.20.11991. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: Crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Maggio N, Sellitti S, Capano CP, Papa M. Tissue-transglutaminase in rat and human brain: Light and electron immunocytochemical analysis and in situ hybridization study. Brain Res Bull. 2001;56:173–182. doi: 10.1016/s0361-9230(01)00649-9. [DOI] [PubMed] [Google Scholar]
- Martin H, Flandez M, Nombela C, Molina M. Protein phosphatases in MAPK signalling: We keep learning from yeast. Mol Microbiol. 2005;58:6–16. doi: 10.1111/j.1365-2958.2005.04822.x. [DOI] [PubMed] [Google Scholar]
- Milakovic T, Tucholski J, McCoy E, Johnson GVW. Intracellular localization and activity state of tissue transglutaminase differentially impacts cell death. J Biol Chem. 2004;279:8715–8722. doi: 10.1074/jbc.M308479200. [DOI] [PubMed] [Google Scholar]
- Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- Nunomura K, Kawakami S, Shimizu K, Hara T, Nakamura K, Terakawa Y, Yamasaki A, Ikegami S. In vivo cross-linking of nucleosomal histones catalyzed by nuclear transglutaminase in starfish sperm and its induction by egg jelly triggering the acrosome reaction. Eur J Biochem. 2003;270:3750–3759. doi: 10.1046/j.1432-1033.2003.03761.x. [DOI] [PubMed] [Google Scholar]
- Obsil T, Ghirlando R, Klein DC, Ganguly S, Dyda F. Crystal structure of the 14-3-3zeta:Serotonin N-acetyltransferase complex. A role for scaffolding in enzyme regulation. Cell. 2001;105:257–267. doi: 10.1016/s0092-8674(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Pittenger C, Morozov A, Martin KC, Scanlin H, Drake C, Kandel ER. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron. 2001;32:123–140. doi: 10.1016/s0896-6273(01)00443-3. [DOI] [PubMed] [Google Scholar]
- Peng X, Zhang Y, Zhang H, Graner S, Williams JF, Levitt ML, Lokshin A. Interaction of tissue transglutaminase with nuclear transport protein importin-alpha3. FEBS Lett. 1999;446:35–39. doi: 10.1016/s0014-5793(99)00018-6. [DOI] [PubMed] [Google Scholar]
- Philipova R, Whitaker M. Active ERK1 is dimerized in vivo: Biphosphodimers generate peak kinase activity and monophosphodimer maintain basal ERK1 activity. J Cell Science. 2005;118:5767–5776. doi: 10.1242/jcs.02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum K, Futter M, Voss K, Erent M, Skehel PA, French P, Obosi L, Jones MW, Bliss TV. The role of extracellular regulated kinases I/II in late-phase long-term potentiation. J Neurosci. 2002;22:5432–5411. doi: 10.1523/JNEUROSCI.22-13-05432.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;20:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-del-Rio M, Reuter U. Migraine aura: New information on underlying mechanisms. Curr Opin Neurol. 2004;17:289–293. doi: 10.1097/00019052-200406000-00009. [DOI] [PubMed] [Google Scholar]
- Sano M, Kohno M, Iwanaga M. The activation and nuclear translocation of extracellular signal-regulated kinases (ERK-1 and -2) appear not to be required for elongation of neurites in PC12d cells. Brain Res. 1995;688:213–218. doi: 10.1016/0006-8993(95)00558-8. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Kuroda S, Yamamori B, Matsuda S, Kaibuchi K, Yamauchi T, Isobe T, Irie K, Matsumoto K, Takai Y. Synergistic activation by Ras and 14-3-3 protein of a mitogen-activated protein kinase kinase kinase named Ras-dependent extracellular signal-regulated kinase kinase stimulator. J Biol Chem. 1994;269:22917–22920. [PubMed] [Google Scholar]
- Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E. Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of ELK-1. J Neurosci. 2002;22:2054–2062. doi: 10.1523/JNEUROSCI.22-06-02054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Hoeven PC, Van Der Wal JC, Ruurs P, Van Blitterswijk WJ. Protein kinase C activation by acidic proteins including 14-3-3. Biochem J. 2000;347:781–785. doi: 10.1042/0264-6021:3470781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D. Arg3.1/arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci. 2001;21:5484–5493. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem Sci. 1998;23:481–485. doi: 10.1016/s0968-0004(98)01309-7. [DOI] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Activity-dependent CREB phosphorylation: Convergence of a fast, sensitive calmodulin kinase pathway and a slow, less sensitive mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 2001;98:2808–2813. doi: 10.1073/pnas.051634198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori B, Kuroda S, Shimizu K, Fukui K, Ohtsuka T, Takai Y. Purification of a Ras-dependent mitogen-activated protein kinase kinase kinase from bovine brain cytosol and its identification as a complex of b-Raf and 14-3-3 proteins. J Biol Chem. 1995;270:11723–11726. doi: 10.1074/jbc.270.20.11723. [DOI] [PubMed] [Google Scholar]
- Zhao M, Adams JP, Dudek SM. Pattern-dependent role of NMDA receptors in action potential generation: Consequences on extracellular signal-regulated kinase activation. J Neurosci. 2005;25:7032–7039. doi: 10.1523/JNEUROSCI.1579-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Bjorbaek C, Weremowicz S, Morton CC, Moller DE. Rsk3 encodes a novel pp90rsk isoform with a unique N-terminal sequence: Growth factor-stimulated kinase function and nuclear translocation. Mol Cell Biol. 1995;15:4353–4363. doi: 10.1128/mcb.15.8.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]