Abstract
Assembly of nanoscale materials from nanoparticle (NP) building blocks relies on our understanding of multiple nanoscale forces acting between NPs. These forces may compete with each other and yield distinct stimuli-responsive self-assembled nanostructures. Here, we report structural transitions between linear chains and globular assemblies of charged, polymer-stabilized gold NPs, which are governed by the competition of repulsive electrostatic forces and attractive poor solvency/hydrophobic forces. We propose a simple quantitative model and show that these transitions can be controlled by the quality of solvent, addition of a salt, and variation of the molecular weight of the polymer ligands.
Ensembles of inorganic nanoparticles (NPs) show collective electronic, optical and magnetic characteristics that originate from the coupling of size- and shape-dependent properties of individual NPs.1,2 A cost-efficient approach to the fabrication of nanoscale materials utilizes the self-assembly of NP building blocks and yields a broad range of nanostructures - from small clusters to large three-dimensional (3D) lattices.3,4 Control over the size and shape of these assemblies relies on our understanding of the nanoscale forces acting between NPs,5 e.g., dipole-dipole interactions,6 electrostatic forces,7 hydrogen bonding,8 hydrophobic,9 coordination10 and bio-specific forces.11 Attractive and repulsive interactions between NPs may change in magnitude and compete, depending on environmental factors.
Generation of different types of nanostructures from the same type of NPs offers a route to the fabrication of stimuli-responsive nanomaterials. Typically, this strategy relies on regio-specific attachment of distinct ligands to NP surface. For example, gold and semiconductor nanorods capped with different molecules along their long side and at the tips behave as amphiphilic molecules and organize in a variety of structures, when the quality of the solvent is selectively reduced for a particular ligand type.4,12 Site-specific attachment of distinct ligands to the NP surface is achieved due to their preferential binding to a particular crystalline facet,13 which may be challenging for small, close-to-spherical NPs and/or the use of macromolecular ligands. A more beneficial approach to distinct self-assembled nanostructures would rely on stimulus-dependent competition of nanoscale forces.
Here we report this approach by controlling structural transitions between two distinct types of nanostructures: linear and 3D assemblies of charged, polymer-stabilized gold NPs. The transitions were governed by the competition of attractive hydrophobic/poor solvency forces and repulsive electrostatic forces, and were achieved by varying the composition and ionic strength of the solvent or the molecular weight of the polymer ligands.
Small, spherical 23 nm-diameter gold NPs stabilized with cetyltrimethylammonium bromide were subjected to ligand exchange with thiol-terminated polystyrene (PS) molecules, as described elsewhere.14 The number average molecular weights of the PS ligands used in the present work were 5 × 103, 1.2 × 104, 2 × 104, 3 × 104 and 5 × 104 g/mol (later in the text referred to as PS-5K, PS-12K, PS-20K, PS-30K and PS-50K, respectively). Following ligand exchange, the NPs were redispersed in N,N-dimethylformamide (DMF). A solution of NPs in DMF was colloidally stable for at least, 5 months.
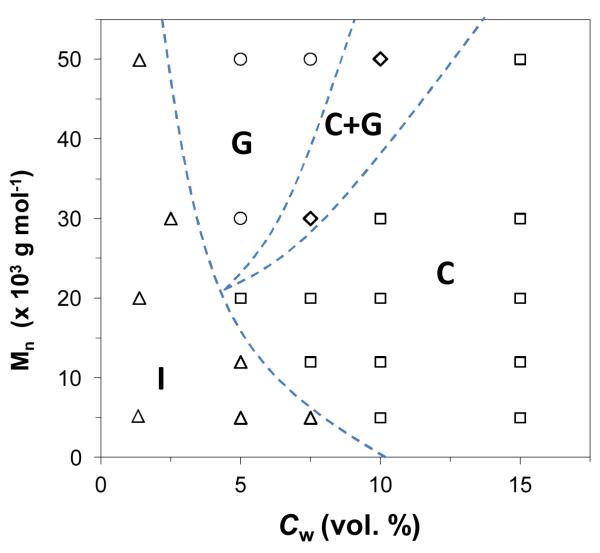
The self-assembly of the NPs was induced by adding various amounts of water to the solution of NPs in DMF, thereby reducing the quality of solvent for the PS ligands.15 To screen unfavorable interactions between the solvent and the PS molecules, the NPs associated in various types of structures. We observed three types of species formed in the colloidal solution when the content of water, Cw, in the DMF/ water mixture and the molecular weight, Mn, of the PS ligands were changed, namely, individual NPs, spherical 3D NP clusters (globules), and linear NP chains with occasional branches (Figure 1). For a particular range of CW and Mn, coexistence of chains and globules of NPs was observed. The self-assembly of the NPs was “mapped” by a phase-like diagram in the Mn-Cw parameter space. The diagram in Figure 2 shows the transitions between individual NPs (I), globules (G) and NP chains (C), as well as a narrow G + C range. No self-assembly occurred for the NPs stabilized with low-molecular weight PS and/or in the low Cw range. The ability of NPs to form globules vs. chains was determined by the interplay of Mn and Cw. At high Mn (≥ 30,000) and low Cw the formation of globules was favored, whereas for higher content of water (dependent on Mn), NP chains were the dominant species. The transition G → C was not sharp and occurred via a regime, in which both of the species were present.
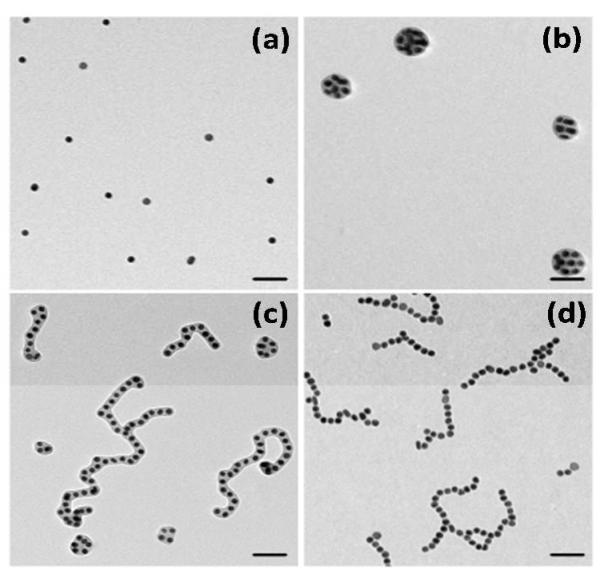
Figure 1.
Transmission electron microscopy (TEM) images of the representative NP species after adding of water to the colloidal NP solution in DMF. (a) Individual NPs stabilized with PS-5K at CW=5 vol. %. (b) Globules of NPs stabilized with PS-50K at CW=5 vol.%; (c) Mixture of chains and globules of NPs stabilized with PS-50K at CW=10 vol.%. (d) Chains of NPs stabilized with PS-5K at CW=15 vol.%. The scale bars are 100 nm. The self-assembly time tSA is 1 h.
Figure 2.
Phase-like diagram of the self-assembled structures of gold NPs, plotted in the Mn-Cw space. tSA= 1 h. A transition from self-assembled structures (globules or chains) to individual NPs was achieved with either sonication for 15 min, or heating of the system at 40 °C for 4h (see Supporting Information).
We carried out a detailed study of the NP chains and globules. The globular assemblies were similar to 3D clusters that were formed in tetrahydrofurane by PS-stabilized gold NPs.16 Similar to this earlier report, in our work, NP globules assembly in solution could be monitored by dynamic light scattering and could quenched by encapsulating the globules with a polystyrene-block-polyacrylic acid copolymer. The hydrodynamic diameter of the globules measured after self-assembly time, tSA, of 1 h in the DMF/water mixture was 102.5 ± 1.5 nm, in comparison with 32.8 ± 3.4 nm, measured for individual NPs in DMF solution (Figure 3a).
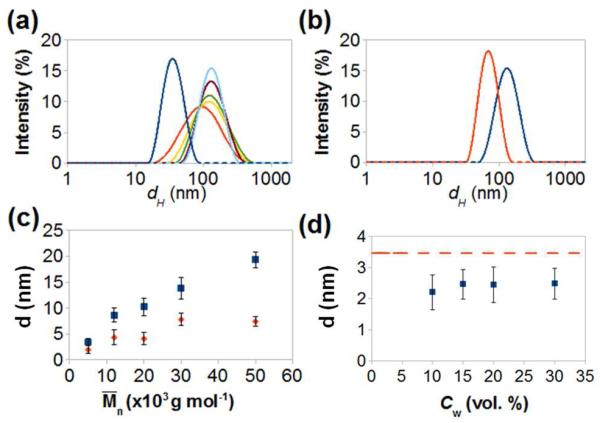
Figure 3.
(a) Variation in the hydrodynamic diameter of the globules formed by NPs capped with PS-50K at CW =7.5 vol. %, measured at tSA of 0, 20, 30, 40, 50 and 60 min, shown with blue, red, yellow, green, violet, and light blue curves, respectively. (b) Hydrodynamic diameters of globules obtained from NPs stabilized with PS-50K at CW=5 vol. % (red curve) and at CW=7.5 vol.% (blue curve). (c) Variation in interparticle distance in the chains at CW = 15 vol. % (red diamonds) and in the close-packed arrays of NPs dried on the TEM grid at CW = 0 (blue squares), plotted as a function of Mn of PS ligands. (d) Variation in inter-particle spacing plotted vs. CW in the DMF/water mixture for the chains formed by NPs stabilized with PS-5K. The dashed red line shows the corresponding interparticle distance in close-packed arrays dried on the TEM grid at CW = 0.
With increasing concentration of water in the DMF/water mixture, the diameter of the globules (determined by analyzing TEM images) increased from ~72 nm (Cw=5 vol. %) to ~ 84 nm (Cw =7.5 vol. %). The corresponding change in the hydrodynamic radius of the globules (determined in dynamic light scattering experiments) is shown in Figure 3b.
Chains of NPs grew in a marked similarity to step-growth polymerization, with the average number of NPs in the chains increasing linearly with time, similar to our earlier work on the self-assembly of gold nanorods.17,18 The average distance, d, between the NPs in the chains increased with the molecular weight of PS ligands (Figure 3c), consistent with a larger polymer volume localized in the gap between the NPs. The same trend was observed for the dense NP arrays obtained by drying their solution in DMF on the TEM grid. For such arrays, the interparticle distance was consistently larger than that in the chains (Figure 3c), due to the more extended vitrified conformation of PS ligands in a good solvent. For 10≤Cw≤ 30 vol. %, interparticle distance did not significantly change (Figure 3d), in agreement with our earlier work.15
The structural transitions shown in Figure 2 were reversible. For example, for NPs stabilized with PS-50k, the C → I and G → I transformations occurred when the concentration of water in the system was reduced to ~2 vol. %. Importantly, the C→ I transition occurred via the globular state, as shown in the phase diagram in Figure 2.
The structural transitions between NP chains and globules were rationalized as follows. The resultant structure of NP assemblies was determined by the minimization of the total energy of the system, ΔEt, governed by the contributions of attraction forces (favoring a compact globular structure) and repulsion forces (favoring the formation of chains) as
| (1), |
In eq. 1, ΔEel is the change in energy of repulsive electrostatic interactions and ΔEh is the change in energy representing a combined effect of hydrophobic/poor solvency attraction forces (later in the text referred to as “hydrophobic forces”). We ignored attractive short-range van der Waals interactions, since over the entire range of inter-particle separations hydrophobic forces dominated. A weak contribution of van der Waals forces was also supported by the absence of association of PS-stabilized NPs at low content of water in the DMF/water mixture.
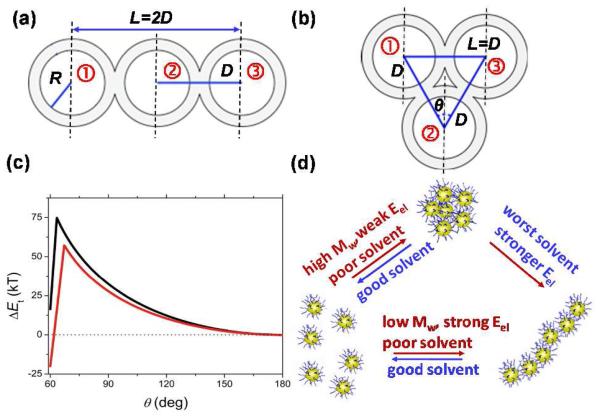
Figure 4a and b illustrates the configurations formed by three adjacent NPs in the chains and globules, respectively. The type of structure was determined by the angle θ between the lines connecting the centre of the second NP with the centers of adjacent 1-st and 3-d NPs. The value of θ changed from 60° (the globular shape) to 180° (the chain shape).
Figure 4.
(a,b) Schematics of three neighboring NPs in the chain (a) and in the globule (b). The inner and outer circles illustrate the metal Au cores and the PS shells, respectively. For notations see Supporting Information. (c) Change in the total energy of the system with angle θ between the lines connecting the centre of the second NP with the centers of the 1-st and 3-d NPs (shown in (b)). Red and black lines correspond to the DMF/water mixtures with Cw of 5 and 15 vol.%, respectively. (d) Schematic of the structural transitions in NP ensembles.
The change in energy of attraction upon NP association was calculated as
| (2), |
where ΔEst is the reduction in the surface energy when two NPs form contact, γ is the interfacial tension between the collapsed PS ligands and the DMF/water mixture, and ΔA is the change in area of this interface when two NPs associate.
The change in electrostatic energy for two associating NPs in the absence of added salt was calculated using the approximation developed for charged spherical NPs functionalized with polymer brushes19 as a logarithmic function of the surface-to-surface interparticle distance as
| (3), |
where R is the radius of the gold core of the NP; D is the center-to-center distance between the two neighboring PS-coated NPs; L is the center-to-center distance between the 1-st and the 3-rd PS-coated NPs (equal to 2D and D for θ of 180 and 60°, respectively), as shown in Figure 4a,b; k is the Boltzmann constant and T is the absolute temperature. In Eq. 3, lB is the Bjerrum length (=e2/εkT), the distance at which the total interaction potential of counter-ions is approximately equal to the thermal energy kT, where e is the elemental charge and ε is the dielectric constant of the solvent. The NP configuration at θ=180° was chosen as a reference. Thus eq. 3 is a function of the surface-to-surface separation between NPs with respect to the 180° configuration.
We evaluated the variation in ΔEt for the assembly of NPs stabilized with PS-50K in two solvent DMW/water mixtures at Cw of 5 and 15 vol.%, in which the NPs assembled into the globules and the chains, respectively. For calculations, we used R=11.5 nm, and ε of 40 and 49 20 (corresponding to lB of 1.14 and 1.4 nm, respectively), γ of 1.4 × 10−3 J/m2 to 1.9 × 10−3 J/m2, and d of 5 and 6.5 nm for Cw of 5 and 15 vol.%, respectively. The derivation of eq. 3 and details of the calculations are given in Supporting Information.
Figure 4c shows the variation in ΔEt for two solvent compositions. For Cw=5 vol.%, the NP configuration with a minimum in ΔEt at θ=60° was favored (corresponding to NP globules), due to the stronger contribution of the surface energy. For Cw=15 vol.%, the deeper minimum in ΔEt was achieved at θ = 180° (corresponding to NP chains), due to the larger contribution of electrostatic repulsion. Both predictions were in agreement with experimental results. We note that individually, both types of nanostructures have been reported for gold NPs, although in different systems. Chains were observed for charged gold NPs stabilized with low-molecular weight ligands,21,22 while the “non-directional” attraction between PS- or DNA-capped gold NPs led to the formation of 3D lattices.3
The schematic in Figure 4d summarizes the structural transitions in assemblies of gold NPs. Poor solvency conditions in conjunction with strong electrostatic repulsion yielded NP chains. Poor solvency conditions and weak electrostatic forces yielded globules. Improvement in the quality of solvent and reduction in Eel led to the C → G transition. When the quality of solvent was not sufficiently reduced, the NPs existed as individual species. Reduction in the quality of solvent did lead to G → I transitions, because of the kinetic trapping of globular structures (see Figure S6b).
We note that in the self-assembly experiments, the addition of water to the NP solution in DMF increased (although at a different rate) both Eh and Eel, due to the reduction in solvent quality for the PS ligands and the increase in solvent polarity, respectively. To validate the argument about competing Eh and Eel, we conducted NP self-assembly in the DMF/water mixture in the presence of NaCl. The addition of NaCl to the DMF/water mixture reduced the quality of solvent for the PS ligands23 and suppressed the electric double layer interactions of the NPs,24 thus favoring “non-directional” hydrophobic forces and the formation of globules. This trend is shown in Table 1: with increasing content of NaCl in the DMF/water mixture, C → G transitions took place, with an intermediate regime, in which both species were present.
Table 1.
Effect of the addition of NaCl and THF on NP assembly*
| CNaCl (mM) | φTHF/DMF | |||||
|---|---|---|---|---|---|---|
| 10 | 50 | 300 | 0.1 | 0.5 | 1 | |
| Structure | C | C+G | G | C+G | G | G |
NaCl and THF were added to the DMF/water solution of NPs stabilized with PS-50K at CW=15 vol. %.
The competition between the hydrophobic and electrostatic forces was further demonstrated by adding tetrahydrofuran (THF) to the NP solution in the DMF/water mixture at THF/DMF volume ratios of 0.1≤φTHF/DMF≤1. Since the Flory-Huggins interaction parameters for PS-DMF and PS-THF solutions are 0.46 and 0.4, respectively,21,25,26 in the presence of THF, the quality of the solvent for the PS ligands was improved. Thus the hydrophobic interactions between the PS-coated NPs in the DMF/THF/water solution were weaker than in the DMF/water mixture. On the other hand, the addition of THF reduced the polarity of the mixed solvent (the dielectric constant of THF is 7.5 27), thereby suppressing electrostatic interactions between the NPs. With increasing ratio φTHF/DMF in the solution, the structure of NP assemblies changed from globules + chains (φTHF/DMF =0.1, ε = 42) to globules (φTHF/DMF=1, ε = 31) (Table 1).
To conclude, we have demonstrated reversible structural transitions in assemblies of polymer-coated gold NPs. We show that by tuning the delicate balance between the hydrophobic and electrostatic forces, assembly of NPs in two distinct configurations - 3D globules or linear chains - can be achieved. A particular nanostructure can be realized in several ways: by tuning solvent polarity, by adding a salt, and by varying the molecular weight of the polymer ligands. Since the formation of chain or globular assemblies was governed by the interaction of ligands, rather than affected by the nature of the gold core, we hypothesize that the reported behavior is general and similar transitions can be expected for other types of charged NPs coated with polymer ligands.
Supplementary Material
ACKNOWLEDGMENTS
EK, R.C., A.K., and H.T-A. thank NSERC Canada (Strategic Network Grant and Discovery grant) for financial support of this work. MR acknowledges support from the National Science Foundation under grants CHE-0911588, DMR-0907515, DMR-1121107, and DMR-1122483, the National Institutes of Health under 1-P5-HL107168, 1-P01-HL108808-01A1 and the Cystic Fibrosis Foundation. A.K. acknowledges Ontario Trillium Scholarship. R.C. thanks I. Gourevich for help with TEM imaging.
Footnotes
Supporting Information Available: Synthesis of gold NPs, description of self-assembly experiments, tests of the reversibility of the structural transitions, the description of interfacial tension measurements and calculations of the change of total energy of the system. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Nie Z, Petukhova A, Kumacheva E. Nat. Nanotech. 2010;5:15–25. doi: 10.1038/nnano.2009.453. [DOI] [PubMed] [Google Scholar]; (b) Wang L, Xu L, Kuang H, Xu C, Kotov NA. Acc. Chem. Res. 2012;45:1916–1926. doi: 10.1021/ar200305f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Romo-Herrera JM, Alvarez-Puebla RA, Liz-Marzán LM. Nanoscale. 2011;4:1304–1315. doi: 10.1039/c0nr00804d. [DOI] [PubMed] [Google Scholar]
- 2.(a) Gao Y, Tang Z. Small. 2011;7:2133–2146. doi: 10.1002/smll.201100474. [DOI] [PubMed] [Google Scholar]; (b) Carbone C, et al. Adv. Funct. Mater. 2011;21:1212–1228. [Google Scholar]; (c) Xu L, Kuang H, Xu C, Ma W, Wang L, Kotov NA. J. Am. Chem. Soc. 2012;134:1699–1709. doi: 10.1021/ja2088713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Nykypanchuk D, Maye MM, Van Der Lelie D, Gang O. Nature. 2008;451:549–552. doi: 10.1038/nature06560. [DOI] [PubMed] [Google Scholar]; (b) Park SY, Lytton-Jean AKR, Lee B, Weigand S, Schatz GC, Mirkin CA. Nature. 2008;451:553–556. doi: 10.1038/nature06508. [DOI] [PubMed] [Google Scholar]
- 4.(a) Petukhova A, Greener J, Liu K, Nykypanchuk D, Nicolaÿ R, Matyjaszewski K, Kumacheva E. Small. 2012;8:731–737. doi: 10.1002/smll.201101297. [DOI] [PubMed] [Google Scholar]; (b) Zhao N, Liu K, Greener J, Nie Z, Kumacheva E. Nano Lett. 2009;8:3077–3081. doi: 10.1021/nl901567a. [DOI] [PubMed] [Google Scholar]; (c) Zhao N, Vickery J, Guerin G, Park JI, Winnik MA, Kumacheva E. Angew. Chem. Int. Ed. 2011;50:4606–4610. doi: 10.1002/anie.201004915. [DOI] [PubMed] [Google Scholar]; (d) Fava D, Nie Z, Winnik MA, Kumacheva E. Adv. Mater. 2008;20:4318–4322. [Google Scholar]
- 5.Min Y, Akbulut M, Kristiansen K, Golan Y, Israelachvili J. Nature Mater. 2008;7:527–538. doi: 10.1038/nmat2206. [DOI] [PubMed] [Google Scholar]
- 6.Thomas JR. J. Appl. Phys. 1966;37:2914–2915. [Google Scholar]
- 7.Kalsin AM, Fialkowski M, Paszewski M, Smoukov SK, Bishop KJM, Grzybowski BA. Science. 2006;312:420–424. doi: 10.1126/science.1125124. [DOI] [PubMed] [Google Scholar]
- 8.Mayer CR, Neveu S, Secheresse F, Cabuil V. J. Colloid Interface Sci. 2004;273:350–355. doi: 10.1016/j.jcis.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 9.Rasch MR, Rossinyol E, Hueso JL, Goodfellow BW, Arbiol J, Korgel BA. Nano Lett. 2010;10:3733–3739. doi: 10.1021/nl102387n. [DOI] [PubMed] [Google Scholar]
- 10.Si S, Raula M, Paira TK, Mandal TK. ChemPhysChem. 2008;9:1578–1584. doi: 10.1002/cphc.200800121. [DOI] [PubMed] [Google Scholar]
- 11.Caswell KK, Wilson JN, Bunz UHF, Murphy CJ. J. Am. Chem. Soc. 2003;125:13914–13915. doi: 10.1021/ja037969i. [DOI] [PubMed] [Google Scholar]
- 12.Figuerola A, et al. Adv. Mater. 2009;21:550–554. doi: 10.1002/adma.200801928. [DOI] [PubMed] [Google Scholar]
- 13.Murphy CJ, Sau TK, Gole AM, Orendorff CJ, Gao J, Gou L, Hunyadi SE, Li T. J. Phys. Chem. B. 2005;109:13857–13870. doi: 10.1021/jp0516846. [DOI] [PubMed] [Google Scholar]
- 14.Nie ZH, Fava D, Kumacheva E, Zou S, Walker GC, Rubin-stein M. Nat. Mater. 2007;6:609–614. doi: 10.1038/nmat1954. [DOI] [PubMed] [Google Scholar]
- 15.Brandrup J, Immergut EH, Grulke EA. Polymer Handbook. 4th Ed. Wiley and sons; New York: 1999. [Google Scholar]
- 16.Sánchez-Iglesias A, et al. ACS Nano. 2012;6:11059–11065. doi: 10.1021/nn3047605. [DOI] [PubMed] [Google Scholar]
- 17.Liu K, Nie Z, Zhao N, Li W, Rubinstein M, Kumacheva E. Science. 2010;329:197–200. doi: 10.1126/science.1189457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee A, Andrade GFS, Ahmed A, Souza ML, Coombs N, Tumarkin E, Liu K, Gordon R, Brolo AG, Kumacheva E. J. Am. Chem. Soc. 2011;133:7563–7570. doi: 10.1021/ja2015179. [DOI] [PubMed] [Google Scholar]
- 19.Zhulina EB, Boulakh AB, Borisov OV. Z. Phys. Chem. 2012;226:625–643. [Google Scholar]
- 20.Kumbharkhane AC, Puranik SM, Mehrotra SC. J. Solution Chem. 1993;22:219–229. [Google Scholar]
- 21.Zhang H, Wang D. Angew. Chem. Int. Ed. 2008;47:3984–3987. doi: 10.1002/anie.200705537. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Chen G, Zhao Y, Silber G, Wang Y, Xing S, Han Y, Chen H. Phys. Chem. Chem. Phys. 2010;12:11850–11860. doi: 10.1039/c0cp00127a. [DOI] [PubMed] [Google Scholar]
- 23.Liu K, Resetco C, Kumacheva E. Nanoscale. 2012;4:6574–6580. doi: 10.1039/c2nr31832f. [DOI] [PubMed] [Google Scholar]
- 24.Israelachvili JN. Intermolecular and Surface Forces. Acad. Press; San Diego, CA: 2011. [Google Scholar]
- 25.Wolf BA, Willms MM. Makromol. Chem. 1978;179:2265–2277. [Google Scholar]
- 26.Schulz GV, Baumann H. Makromol. Chem. 1968;114:122–138. [Google Scholar]
- 27.Franks F, editor. Water, A Comprehensive Treatise. Vol. 2. Plenum Press; New York: 1973. Ch. 7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.