Abstract
Background
Barrett’s esophagus is a premalignant condition that is a risk factor for the development of esophageal adenocarcinoma, a disease whose incidence is rapidly increasing. Because aspirin and other nonsteroidal antiinflammatory drugs, such as celecoxib, may decrease the risk of developing esophageal cancer, we investigated the effect of long-term administration of celecoxib in patients with Barrett’s esophagus with dysplasia.
Methods
Chemoprevention for Barrett’s Esophagus Trial (CBET) is a phase IIb multicenter randomized placebo-controlled trial of celecoxib in patients with Barrett’s esophagus and low- or high-grade dysplasia. Patients were randomly assigned to treatment with 200 mg of celecoxib or placebo, both administered orally twice daily, and then stratified by grade of dysplasia. The primary outcome was the change from baseline to 48 weeks of treatment in the proportion of biopsy samples with dysplasia between the celecoxib and placebo arms. Secondary and tertiary outcomes included evaluation of changes in histology and expression levels of relevant biomarkers. All statistical tests were two-sided.
Results
From April 1, 2000, through June 30, 2003, 222 patients were registered into CBET, and 100 of them with low- or high-grade Barrett’s dysplasia were randomly assigned to treatment (49 to celecoxib and 51 to placebo). After 48 weeks of treatment, no difference was observed in the median change in the proportion of biopsy samples with dysplasia or cancer between treatment groups in either the low-grade (median change with celecoxib = − 0.09, interquartile range [IQR] = − 0.32 to 0.14 and with placebo = − 0.07, IQR = − 0.26 to 0.12; P = .64) or high-grade (median change with celecoxib = 0.12, IQR = − 0.31 to 0.55, and with placebo = 0.02, IQR = − 0.24 to 0.28; P = .88) stratum. No statistically significant differences in total surface area of the Barrett’s esophagus; in prostaglandin levels; in cyclooxygenase-1/2 mRNA levels; or in methylation of tumor suppressor genes p16, adenomatous polyposis coli, and E-cadherin were found with celecoxib compared with placebo.
Conclusions
Administration of 200 mg of celecoxib twice daily for 48 weeks of treatment does not appear to prevent progression of Barrett’s dysplasia to cancer.
Barrett’s esophagus is a premalignant condition in which normal squamous epithelium of the esophagus is replaced by specialized columnar mucosa. It occurs as a result of chronic gastroesophageal reflux and is associated with an increased risk of developing esophageal adenocarcinoma. The incidence of esophageal adenocarcinoma in the United States is rapidly increasing (1–4). The 5-year survival rate after surgical resection of esophageal cancer is approximately 24% (5). Although clinical trials evaluating potential new agents and new approaches in the treatment of esophageal adenocarcinoma are underway, this disease remains associated with high morbidity and mortality.
Strategies to prevent or reverse esophageal tumorigenesis include antireflux surgery, aggressive medical management of acid secretion, and ablation of premalignant tissue (6–8). With the exception of Barrett’s mucosal ablation with photodynamic therapy for patients with high-grade dysplasia, most strategies have not been effective (9). Photodynamic therapy can ablate high-grade dysplasia and substantially decrease the incidence of adenocarcinoma, but it is expensive and associated with adverse reactions, including prolonged photosensitivity, chest pain, and esophageal stricture (9). Hence, there is an urgent need for newer agents and/or methods to decrease the risk of progression from dysplasia to this deadly cancer.
Several epidemiologic studies (10–13) have found that, among patients at risk for esophageal cancer, treatment with aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) is associated with a decreased risk of esophageal cancer. One potential mechanism for chemoprevention is inhibition of cyclooxygenase (COX), an enzyme that is crucial to the synthesis of prostaglandins (PGs) from arachidonic acid (14). Esophageal tumorigenesis has been associated with overexpression of the inducible COX isoform COX-2 (15). Treatment with a COX-2 inhibitor led to a reduction in esophageal adenocarcinomas in an animal model of Barrett’s esophagus (16). We report the results of Chemoprevention for Barrett’s Esophagus Trial (CBET), a phase IIb randomized, parallel treatment, placebo-controlled, double-masked multicenter trial evaluating the long-term administration of celecoxib, a selective COX-2 inhibitor, in Barrett’s esophagus patients with lowor high-grade dysplasia.
Patients and Methods
Study Population
Patients with established diagnoses of Barrett’s esophagus and dysplasia and with specific information on the location (level) of the highest grade of dysplasia from a biopsy examination were eligible for this study. Other requirements included age of 18 years or older, an Eastern Cooperative Oncology Group performance status of less than 2, a serum creatinine level of 1.5 times or less the upper limit of normal, a serum glutamic-oxaloacetic transaminase/serum glutamate pyruvate transaminase of less than 1.5 times the upper limit of normal, and adequate bone marrow function (i.e., hemoglobin ≥ 9 g/dL, platelet count > 125 000 cells per µL, and white blood cell count > 3000 cells per µL). Patients were required to abstain from chronic use of NSAIDs or other COX-2 inhibitors while on study, except for the use of low cardioprotective doses (<100 mg/day) of aspirin for up to 30 days after they were randomly assigned to treatment. Patients were required to abstain from regular corticosteroid use by any route of administration.
Exclusion criteria included current use of anticoagulants, previous surgery to the esophagus or stomach within 3 months before random assignment, presence of reflux esophagitis of grades 2–4, history of active inflammatory bowel disease, history or confirmed diagnosis of invasive esophageal carcinoma, history of complete mucosal resection or ablation for Barrett’s esophagus by any technique, and diagnosis of esophageal, gastric, or duodenal ulcers that were at least 1 cm in diameter within 30 days before random assignment to treatment. All patients signed informed consent forms that were approved by each clinic’s Institutional Review Board at the time of screening and registration into CBET. When the patient was eligible for random assignment to treatment, he or she reaffirmed his or her consent at that visit.
Study Design
Details of the study design have been published elsewhere (17). In brief, patients were stratified by clinic and grade of dysplasia that was determined at baseline endoscopy. Patients who did not have a pathologically confirmed diagnosis of low- or high-grade dysplasia were not eligible but, when warranted, were reconsidered for study eligibility at their next regular follow-up visit. When the diagnosis of dysplasia was confirmed, patients were randomly assigned to treatment with 200 mg of celecoxib orally twice a day or placebo orally twice a day (in 1 : 1 treatment assignment ratio). Duration of treatment was at least 48 weeks and at the most 2 years. The primary endpoint was the change from baseline to 48 weeks of treatment in the proportion of biopsy samples with dysplasia between the celecoxib and placebo arms. Celecoxib and placebo pills, both identical in appearance, were provided by Pfizer (New York, NY).
Upper Endoscopic Procedure
Expert study gastroenterologists performed baseline and follow-up endoscopic examinations at 3-month intervals for patients with high-grade dysplasia or 6-month intervals for patients with lowgrade dysplasia. A rigorous and detailed endoscopic procedure was performed and documented by use of video-recording equipment, digital photography, and data forms (18). At each centimeter along the length of the Barrett’s esophagus, still photographs were obtained beginning at the gastroesophageal junction and ending at the most proximal level of the columnar mucosa (for quantitative endoscopy measurements). The length and type (i.e., circumferential, tongues, or islands of columnar epithelium) of the Barrett’s mucosa were noted. The presence of reflux esophagitis was graded according to the Savary–Miller classification system (19). Biopsy specimens were obtained with large-particle, “jumbo” biopsy forceps (Olympus America, Melville, NY) to optimize mucosal sample size for pathologic interpretation (18). Biopsy specimens were first obtained from a visible lesion within the Barrett’s esophagus, such as a nodule, ulcer, or plaque, and then four-quadrant biopsy specimens were systematically obtained at least every 1–2 cm in the appropriate areas of the Barrett’s esophagus, depending on the highest grade of dysplasia. At a previously confirmed area of dysplasia as identified by the distance from the incisors down to the area of known dysplasia in the esophagus, additional biopsy samples were obtained and snap frozen in liquid nitrogen for correlative biomarker studies (18). Similarly, if there was another previously identified area of dysplasia or suspicious area at a different level in the esophagus, additional biopsy samples were obtained for pathologic examination and biomarker studies. The additional biopsy specimens were obtained from visible lesions or at the area immediately adjacent to the sample obtained for pathologic examination. Biopsy specimens were also obtained from the distal esophagus and stomach to assess for active Helicobacter pylori infection. Biopsy specimens were placed in formalin or immediately snap frozen at the time of the endoscopy examination and then sent to and stored at the central specimen repository. Treatment of patients who were positive for H. pylori was left to the discretion of the gastroenterologist. The treatment regimen used was recorded as concomitant medications.
Histologic Evaluation
Initial eligibility determination and stratification for treatment assignment was based on the histology reading from the central pathologist. However, because of concerns regarding intra- and interobserver variability, eligibility determination and stratification for treatment assignment were later modified to require the agreement of two pathologists. The first histology reading was done centrally at the Department of Pathology, Johns Hopkins School of Medicine. The second histology reading of the same specimens was done at the patient’s local institution. If there was discordance in the two readings, then a third histology reading was obtained from another CBET institution. The final histologic diagnosis was based on consensus agreement among the CBET pathologists.
The study pathologists who performed the readings (E. Montgomery, G. Chejfec, G. Cortina, H. Rotterdam, L. J. Burgart, S. Hackett, T. Wu, J. Wasman) were all experts in the field of Barrett’s esophagus. Moreover, the pathologists were masked to each other’s diagnoses.
Study Follow-up
Information about concomitant medications, laboratory studies, treatment compliance, and adverse events was obtained at the follow-up visits scheduled every 12 weeks for all patients on study. Patients with low-grade dysplasia at baseline underwent an upper endoscopy examination every 6 months. Patients with high-grade dysplasia at baseline underwent an upper endoscopy examination every 3 months. Adverse events were graded in accordance with the Common Toxicity Criteria of the National Cancer Institute (version 2.0). (http://ctep.info.nih.gov/reporting/ctc_archive.html).
All patients and study investigators remained masked until data collection was completed, unless the information was required for appropriate medical treatment. Study treatment could be terminated for several reasons, including development of a grade 3 or higher adverse event that, in the opinion of the study physician, was related to celecoxib treatment; development of adenocarcinoma of the esophagus or other invasive cancers; patient request; or other chronic NSAID use for longer than 2 weeks during study. Study follow-up continued even for patients whose treatment was discontinued.
Study Monitoring
A Treatment Effects Monitoring Committee (TEMC) was formed with independent experts in the fields of oncology, gastroenterology, pathology, and biostatistics to monitor the treatment effects of CBET. The TEMC was responsible for monitoring adverse events and for making recommendations to study sponsors regarding continuation of the study. The TEMC was not masked.
Quantitative Endoscopy
The surface area of Barrett’s esophagus was measured with quantitative endoscopy by use of an enhanced computer image analysis system, as described (20, 21). The system (US Patent 7,011,625) transformed photographs of Barrett’s esophagus into two-dimensional maps, and the surface area of Barrett’s esophagus was calculated from the reconstructed images. This enhanced computer image analysis system is not yet commercially available, but it is in development through Millennium Marketing Group (Overland Park, KS). Digitized images taken during the upper endoscopy procedure were used to calculate surface area of lesions. The expert study investigator (A. O. Shar) analyzing all digitized images was masked to the patient’s treatment assignment. Standard procedure guidelines for imaging were used by all study gastroenterologists. Videoendoscopic images of the Barrett’s mucosa were obtained every 1 cm from the gastroesophageal junction to the squamocolumnar junction with the lumen centered. Quantitative endoscopy was performed at baseline, 6 months, 1 year, and then yearly, for up to 3 years. All images digitization and area calculation took place at the Quantitative Endoscopy Center.
Assay for Cyclooxygenase-1 and -2 mRNA
Jumbo biopsy specimens obtained with a 9-mm open-span biopsy forceps obtained during baseline and 6-month follow-up endoscopic examinations from the first 40 patients were snap frozen and immediately sent to one expert study investigator (A. J. Dannenberg) for evaluation of COX-1 and -2 mRNA. Quantitative reverse transcription–polymerase chain reaction (PCR) of COX-1 and -2 mRNA was as previously described (22). Briefly, total RNA was isolated with RNeasy Mini kits from Qiagen (Santa Clarita, CA). Reverse transcription was performed with 5 µg of total RNA per 100-µL reaction mixture. Each PCR was carried out in 25 µL of a reaction mixture, containing 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, all four deoxynucleotide triphosphates (each at 0.2 mM), 2.5 U of AmpliTaq DNA polymerase, and 400 nM primers for COX-2 or −1 (COX-2 sense primer 5'-GGTCTGGTGCCTGGTCTGATGATG-3', COX-2 antisense primer 5'-GTCCTTTCAAGGAGAATGGTGC-3';COX-1 sense primer 5'-TGCCCAGCTCCTGGCCCGCCGCTT-3'; COX-1 antisense primer 5'-GTGCATCAACACAGGCGCCTCTTC-3'). Ten-microliter aliquots of the reverse-transcribed cDNA samples and various known amounts of COX-2 or −1 mimic (between 0.001 and 0.05 pg), adjusted to the abundance of the target cDNA, were added to the reaction mixture and coamplified for 35 cycles of denaturation at 94°C for 20 seconds, annealing at 65°C for 20 seconds, and extension at 72°C for 60 seconds, followed by a final extension at 72°C for 10 minutes. PCR products (25 µL) were then separated by electrophoresis on 1% agarose gels and visualized by ethidium bromide staining. A computer densitometer (Chem Doc; Bio-Rad Laboratories, Hercules, CA) was used to quantify the density of the bands.
Assay for Prostaglandins
Jumbo biopsy specimens obtained with a 9-mm open-span biopsy forceps obtained at baseline, 6 months, 12 months, and yearly up to 24 months were snap frozen and sent to one expert study investigator (V. W. Yang) for evaluation of prostaglandins. Prostaglandins PGD2, PGE2, PGF2α, thromboxane B2, and 6-keto-PGF1α were measured by gas chromatography–mass spectrometry as described elsewhere (23). Briefly, specimens were thawed on ice and manually homogenized in a glass microhomogenizer in 50 µL of a solution containing 138 mM NaCl, 5 mM KCl, 4 mM NaHCO3, 5.6 mM d -glucose, 0.3 mM Na2 HPO4, and 0.3 mM KH2PO4 containing 1 mM CaCl2 and then transferred to a microcentrifuge tube. An additional 60 µL of the same solution was used to rinse the homogenizer, and this rinse solution was added to the initial homogenate. The combined solution was then sonicated for 20 seconds with a Fisher Scientific Model 550 Sonic Dismembrator with a microtip probe. After sonication, tissue debris was removed by microcentrifugation at 12 000 g for 15 seconds. Ten microliters of the supernatant was removed for determination of protein. The remaining supernatant was divided into 25- µL aliquots, which were then incubated at 37°C for 30 minutes in the absence or presence of 10 µM arachidonic acid (Sigma, St. Louis, MO). The addition of arachidonic acid to the specimens has been shown to stimulate the in vitro synthesis of prostaglandins and hence further increase the sensitivity of detection (23). After this incubation, 25 µL of deuterated prostaglandin standards and 125 µL of acetone were added to each reaction mixture, and the combined solution was dried under a steady stream of nitrogen gas. As soon as the specimen was dried, 25 µL of 2% O-methoxylamine in pyridine was added to each sample. The samples were stored at −20°C until prostagland ins were quantified by gas chromatography–mass spectrometry. Prostaglandin levels were determined with a Fijnnagan MATSSQ771 gas chromatograph–mass spectrometer (Fijnnagan MAT, San Jose, CA). The level of each prostaglandin was determined from deuterated prostaglandin internal standards that were included in each reaction. All prostaglandin levels were normalized to the amount of protein in the sample.
Methylation Assay for p16, Adenomatous Polyposis Coli, and E-cadherin Genes
Formalin-fixed slides obtained at baseline and 12 months were sent to an expert study investigator for evaluation (J. G. Herman). Methylation status of genes for tumor suppressor p16, adenomatous polyposis coli (APC), and E-cadherin were measured by use of a nested PCR, as described previously (24). Samples were deparaffinized with xylene, and then DNA was extracted by proteinase K digestion overnight, followed by DNA phenol -chloroform extraction and ethanol extraction as described (24). Genomic DNA was diluted in 50 µL of water. Sodium bisulfite converts unmethylated cytosine to uracil when DNA is denatured, but methylated cytosines are resistant. Bisulfite treatment was carried out for 16 hours at 50°C as previously described (24). DNA samples were then purified with the Wizard DNA CleanUp System (Promega, Madison, WI) and desulfonated with NaOH. Samples were ethanol precipitated and resuspended in 20 µL of water. The bisulfite-modified DNA was subjected to a first-stage, multiplex PCR incorporating external primer sets for p16, APC, and CDH1. Each multiplex PCR was carried out in a total volume of 25 µL containing 0.5 U Jump Start Red Taq DNA polymerase (Sigma, St Louis, MO), 10 pmol of each external primer, and 4 µL of bisulfite-modified DNA. The external primer sequences used for the multiplex PCR are described elsewhere (24). PCR conditions included an initial denaturation at 95 °C for 5 minutes followed by amplification for 35 cycles (95 °C for 30 seconds, 56 °C for 30 seconds, and 72 °C for 30 seconds), and a final elongation at 72 °C for 5 minutes. Methylation-specific PCR was performed as previously described (24). Multiplex PCR products were diluted 1:1000, and 4 µL of this dilution was added to a second-stage PCR mixture with internal primers to discriminate methylated from unmethylated templates as described (24). Methylation-specific PCR products were analyzed on 6% polyacrylamide gels.
Statistical Analysis
The primary outcome was the change from baseline to 48 weeks of treatment in the proportion of biopsy samples exhibiting dysplasia between the two treatment arms. The secondary outcomes included changes from baseline to 48 weeks of treatment in the following parameters: the highest grade of dysplasia, the extent of high-grade dysplasia, the extent of low-grade dysplasia, and the surface area affected by Barrett’s esophagus (as measured by quantitative endoscopy). The tertiary outcomes included changes from baseline to 48 weeks of treatment in the levels of the following biomarkers: COX-1 and -2 mRNA; prostaglandins PGD2, PGE2, PGF2α, thromboxane B2, and 6-keto-PGF1α; and methylation of tumor suppressor genes p16, APC, and E-cadherin. These markers were selected because of their association with the COX pathway and/or the biology of Barrett’s esophagus.
The study was designed with a sample size of 200 patients to provide 90% power to detect a difference of 0.5 standard deviation in change from baseline in the proportion of biopsy samples exhibiting dysplasia. Because of slower than anticipated accrual, target enrollment was adjusted to 124 patients to provide 80% power. Randomization was accomplished by use of a documented generation scheme that provided a reproducible order of assignments with an expected yield of 1:1 randomization. All analyses among the randomized groups were based on the intention-to-treat principle. Because patients contributed various numbers of biopsy samples each of which could be dysplastic, binomial regression with robust variance estimation to account for withinpatient clustering was used to assess treatment group differences in proportion of biopsy samples with dysplasia or cancer at 48 weeks, after adjusting for proportion of biopsy samples with dysplasia at baseline (25). Treatment group differences in changes after 48 weeks of treatment in both highest grade of pathology and number of biopsy samples with dysplasia or cancer were obtained by use of ordered logistic regression (26). For all other outcomes, treatment group differences were assessed by use of the Wilcoxon rank sum test for continuous outcomes or Fisher’s exact test for categorical outcomes (27). All P values were from twosided tests and were nominal. Analyses were performed with SAS version 8.0 (28).
Results
Demographic Characteristics
From April 1, 2000, through June 30, 2003, a total of 222 patients were registered into CBET. Of the 222, 100 patients were randomly assigned to treatment (49 to celecoxib and 51 to placebo). Reasons for ineligibility at registration included discovery of esophageal cancer in 15 patients, lack of dysplasia in 83 patients, prescribed treatment in seven patients, abnormal laboratory values in four patients, and various other reasons among 13 patients. Of the remaining 100 patients, 18 patients did not have evaluable data either because of death, dropout, or missing data. The patients who were randomly assigned into CBET were primarily white, non-Hispanic males with a median age of 67 years. Baseline demographic characteristics were similar in the two groups, except for smoking history—73% of patients in the celecoxib arm were never smokers compared with 55% of those in the placebo arm (P = .01), and approximately 40% in the placebo arm were former smokers compared with 14% in the celecoxib arm (Table 1).
Table 1.
Characteristics of participants at baseline*
| Characteristic | Celecoxib (n = 49) | Placebo (n =51) | Total (n = 100) | P value† |
|---|---|---|---|---|
| Demographic | ||||
| Median age, y (IQR) | 68 (49 to 87) | 66 (50 to 82) | 67 (49 to 85) | .49 |
| Male, No. (%) | 45 (91.8) | 42 (82.4) | 87 (87.0) | .24 |
| White, non-Hispanic, No. (%] | 47 (95.9) | 48 (94.1) | 95 (95.0) | 1.00 |
| Smoking history, No. (%) | ||||
| Current | 6(12.2) | 3 (5.9) | 9 (9.0) | .01 |
| Never | 36 (73.5) | 28 (54.9) | 64 (64.0) | |
| Former | 7 (14.3) | 20 (39.2) | 27 (27.0) | |
| Body mass index, No(%)‡ | ||||
| Normal (18.5–24.9 kg/m2) | 11 (23.4) | 8 (15.7) | 19 (19.4) | .67 |
| Overweight (25.0–29.9 kg/m2] | 20 (42.6) | 23 (45.1) | 43 (43.9) | |
| Obese (>30.0 kg/m2) | 16(34.0) | 20 (39.2) | 36 (36.7) | |
| ECOG performance | ||||
| No. fully active (%) | 45 (91.8) | 49(96.1) | 94 (94.0) | .43 |
| Concomitant medications§, No. (%) | ||||
| Proton pump inhibitors | 43 (87.8) | 48 (94.1) | 91 (91.0) | .31 |
| Histamine H2 receptor antagonists | 2 (4.1) | 3 (5.9) | 5 (5.0) | 1.00 |
| Aspirin | 15(30.6) | 18 (35.3) | 33 (33.0) | .67 |
| Grade of reflux esophagitis, No. (%) | ||||
| 0 | 48 (98.0) | 50 (98.0) | 98 (98.0) | 1.00 |
| ≥1 | 1 (2.0) | 1 (2.0) | 2 (2.0) | |
| Type of Barrett’s esophagus, No. (%) | ||||
| Circumferential | 37 (75.5) | 34 (66.7) | 71 (71.0) | .38 |
| Tongue | 29 (59.2) | 33 (64.7) | 62 (62.0) | .68 |
| Island | 20 (40.8) | 15 (29.4) | 35 (35.0) | .30 |
| Short segment | 6(12.2) | 10 (19.6) | 16 (16.0) | .42 |
| Median proximal level of | 32 (24 to 40) | 32 (22 to 42) | 32 (24 to 40) | .64 |
| Barrett’s esophagus, cm (IQR) | ||||
| Grade of dysplasia, No. (%) | ||||
| High | 19(38.8) | 17 (33.3) | 36 (36.0) | .68 |
| Low | 30(61.2) | 34 (66.7) | 64 (64.0) | |
| Helicobacter pylori status | ||||
| No. positive (%) | 1 (2.0) | 1 (2.0) | 2 (2.0) | 1.00 |
QR = interquartile range; ECOG = Eastern Cooperative Oncology Group.
P values were calculated with a two-sided Fisher’s exact test for categorical variables or a two-sided Wilcoxon rank sum test for continuous variables.
Data were missing for two patients in the celecoxib group.
Proton pump inhibitors included were lansoprazole, omeprazole, rabeprazole, pantoprazole, and esomeprazole. Histamine H2 receptor antagonists were nizatidine, famotidine, cimetidine, and ranitidine.
Approximately 80% of patients were overweight (body mass index = 25.0–29.9 kg/m2) or obese (body mass index > 30.0 kg/m2). Ninety-four percent of patients had excellent performance status. H. pylori was rare in this patient population (only one patient in each treatment arm was positive for H. pylori). Approximately 90% of patients were taking proton pump inhibitors to inhibit acid reflux, and 33% of patients were taking cardioprotective doses of aspirin of less than 100 mg/day. Ninety-eight percent of patients had no evidence of reflux esophagitis.
The type of Barrett’s esophagus was primarily circumferential with presence of tongue-like extensions of columnar mucosa from the gastroesophageal junction in 62 patients. Fewer than 20% of patients had short-segment Barrett’s esophagus, defined as an area sufficient for biopsy procedures to be followed without resulting in complete resection of the dysplasia. The median proximal level of Barrett’s esophagus was 32 cm (interquartile range [IQR] = 24 to 40 cm). Sixty-four (64%) of the 100 patients were stratified into the low-grade stratum, and 36 (36%) were stratified into the high-grade stratum. There were no differences between treatment arms in the number of biopsy samples per patient at baseline or at 48 weeks. The median number of biopsy samples per patient at baseline was 20 (range = 3–58) for the celecoxib arm and was 20 (range = 5–47) for the placebo arm (P=.71). At 48 weeks, these values were 17 (range = 2–37) for the celecoxib arm and 16 (range = 1–43) for the placebo arm (P =.89).
Primary Outcome
The primary outcome was the change from baseline to 48 weeks (the active treatment period) in the proportion of biopsy samples exhibiting dysplasia between celecoxib (0.19, IQR = − 0.09 to 0.47) and placebo (0.15, IQR = 0.03 to 0.27) arms. At baseline, the median proportion of biopsy samples was the same regardless of treatment assignment or grade of dysplasia. The median change after 48 weeks of treatment in the proportion of biopsy samples with dysplasia in the celecoxib arm was −0.08 (IQR = −0.39 to 0.24) and in the placebo arm was −0.06 (IQR = −0.34 to 0.22) (P=.84) (Table 2). In the high-grade stratum, the median change in the celecoxib arm was 0.12 (IQR = − 0.31 to 0.55) and in the placebo arm was 0.02 (IQR = − 0.24 to 0.28) (P=.88). In the low-grade stratum, the median change in the celecoxib arm was −0.09 (IQR = −0.32 to 0.14) and in the placebo arm was −0.07 (IQR = −0.26 to 0.12) (P =.64). Changes were calculated by subtracting the baseline value from the value at 48 weeks. Six patients with high-grade dysplasia (three assigned to celecoxib and three assigned to placebo) had missing data at their 48-week visit but had been diagnosed with esophageal adenocarcinoma before this visit (i.e., informative missing data). Missing data for these patients were imputed with data from their most recent follow-up visit before the 48-week visit. For the change in highest grade of pathology, these six patients were imputed into the increase grade category. The effects of celecoxib and placebo on the proportion of biopsy samples with dysplasia were essentially identical and null.
Table 2.
Changes in grade and extent of dysplasia after 48 weeks of treatment*
| Low-grade stratum |
High-grade stratum |
Combined |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Change | Celecoxib | Placebo | P† | Celecoxib | Placebo | P† | Celecoxib | Placebo | P† |
| Biopsy sample‡ | |||||||||
| Median proportion of biopsy samples with dysplasia at baseline (lQR) |
0.14 (−0.04 to 0.32) | 0.14(0.03 to 0.25) | 12 | 0.34 (0.02 to 0.66) | 0.26 (−0.03 to 0.55) | .99 | 0.19 (−0.09 to 0.47) | 0.15(0.03 to 0.27) | 18 |
| Median change in proportion of biopsy samples with dysplasia or cancer (lQR) |
−0.09 (−0.32 to 0.14) | −0.07 (−0.27 to 0.13) | .64 | 0.12 (−0.31 to 0.55) | 0.02 (−0.24 to 0.28) | .88 | −0.08 (−0.39 to 0.23) | −0.06 (−0.35 to 0.23) | .84 |
| Change in highest grade of pathology | |||||||||
| % patients with decrease (95% Cl) | 48.1 (28.7 to 68.1) | 51.8 (31.9 to 71.3) | .82 | 31.2 (11.0 to 58.7) | 16.7 (2.1 to 48.4) | .42 | 41.9 (27.0 to 57.9) | 41.0(25.6 to 57.9) | .89 |
| % patients with no change (95% CI) | 40.7 (22.4 to 61.2) | 37.0 (19.4 to 57.6) | 50.0 (24.7 to 75.3) | 58.3 (27.7 to 84.8) | 44.2(29.1 to 60.1) | 43.6 (27.8 to 60.4) | |||
| % patients with increase (95% CI) | 11.1 (2.4 to 29.1) | 11.1 (2.4 to 29.1) | 18.8 (4.0 to 45.6) | 25.0 (5.5 to 57.2) | 14.0(5.3 to 27.9) | 15.4 (5.9 to 30.5) | |||
|
Median No. of biopsy samples with dysplasia at baseline (IQR) |
3 (0 to 6) | 2 (−2 to 6) | .93 | 6 (0 to 12) | 4 (−4 to 12) | .36 | 4 (−2 to 10) | 3 (0 to 6) | .37 |
|
Change in No. of biopsy samples with dysplasia or cancer |
|||||||||
| % patients with decrease (95% CI) | 70.4 (49.8 to 86.2) | 70.4 (49.8 to 86.2) | .86 | 62.5 (35.4 to 84.8) | 50.0(21.1 to 78.9) | .41 | 67.4 (51.5 to 80.9) | 64.1 (47.2 to 78.8) | .81 |
| % patients with no change (95% CI) | 3.7 (0.1 to 19.0) | 11.1 (2.4 to 29.2) | 6.2 (0.2 to 30.2) | 0.0 (0.0 to 26.5) | 4.7 (0.6 to 15.8) | 7.7 (1.6 to 20.9) | |||
| % patients with increase (95% CI) | 25.9(11.1 to 46.3) | 18.5(6.3 to 38.1) | 31.2 (11.0 to 58.7) | 50.0(21.1 to 78.9) | 27.9(15.3 to 43.7) | 28.2 (15.0 to 44.9) | |||
| Quantitative endoscopy§ | |||||||||
| Median total surface area affected by Barrett’s esophagus at baseline, cm2 (IQR) |
24.4 (−1.11 to 59.9) | 17.6 (−18.0 to 53.2) | .85 | 28.2 (−7.0 to 63.4) | 15.6 (−14.9 to 46.1) | .61 | 26.5 (−6.8 to 59.8) | 15.6 (20.4 to 51.6) | .53 |
| Median change in total surface area affected by Barrett’s esophagus, cm2 (IQR) |
−2.2 (−10.6 to 6.2) | −0.6 (−8.5 to 7.3) | 17 | −3.8 (−13.7 to 6.1) | −0.6 (−5.2 to 4.0) | .34 | −2.5 (−12.4 to 7.4) | −0.6 (−8.5 to 7.3) | 12 |
Changes were calculated by subtracting the baseline value from the value at 48 weeks. Data for six patients (all in the high-grade stratum; three assigned to celecoxib and three assigned to placebo) who had missing data at their 48-week visit but were diagnosed with esophageal adenocarcinoma before their 48-week visit were imputed with information from their most recent follow-up visit before the 48-week visit For change in number of biopsy samples with dysplasia or cancer, data from these six patients were imputed to the patients increase category. Central reading or adjudicated reading if disagreement with local reading in highest grade of pathology was used; 28% of celecoxib specimens and 33% of placebo specimens had adjudicated readings at the 48-week visit. IQR = interquartile range; CI = confidence interval.
P values for proportion of biopsy samples with dysplasia at baseline were obtained by use of binomial regression with robust variance estimation due to within-patient clustering. P values for change in proportion of biopsy samples with dysplasia or cancer also used binomial regression with robust variance estimation modeling the follow-up value adjusted for the baseline value. P values for change in grade and change in number of samples with dysplasia or cancer were obtained by use of ordered logistic regression. P values for the number of biopsy samples with dysplasia at baseline, total surface area at baseline, and change in total surface area were obtained by use of the Wilcoxon rank sum test. All statistical tests were two-sided.
Number of participants: in the low-grade stratum, 27 from celecoxib arm and 27 from placebo arm; in high-grade stratum, 16 from celecoxib arm and 12 from placebo arm; in combined, 43 from celecoxib arm and 39 from placebo arm.
Number of participants: in low-grade stratum, 20 from the celecoxib arm and 22 from the placebo arm; in high-grade stratum, 11 from celecoxib arm and five from placebo arm; in combined, 31 from celecoxib arm and 27 from placebo arm.
Secondary Outcomes
Secondary outcome measures included change from baseline to 48 weeks of treatment in the following parameters: the highest grade of dysplasia, the extent of high-grade dysplasia, the extent of lowgrade dysplasia, and the surface area affected by Barrett’s esophagus (as measured by quantitative endoscopy). No change or a decrease in the highest grade of dysplasia after 48 weeks of treatment was observed in 37 of the 43 patients in the treatment arm (86%, 95% CI = 72% to 95%) and in 33 of the 39 patients in the placebo arm (84%, 95% CI = 69% to 94%) (Table 2). We observed a decrease in the highest grade of dysplasia in 13 of the 27 patients in the low-grade stratum of the celecoxib arm (48%, 95% CI = 29% to 68%); however, the changes were not statistically significantly different between the celecoxib and placebo arms (P = .82). Three of the 16 patients assigned to the celecoxib arm (19%, 95% CI = 4% to 46%) and three of the 12 patients assigned to the placebo arm (25%, 95% CI = 5% to 57%) in the high-grade stratum had an increase in the highest grade of dysplasia, but these results were not statistically significant (P = .42).
We also found no change after 48 weeks of treatment in the number of biopsy samples with dysplasia or cancer with respect to the treatment assignment (P = .81) (Table 2); however, the median number of biopsy samples with dysplasia at baseline was small (four in the celecoxib arm and three in the placebo arm). There were no differences between the treatment arms with respect to change in the number of biopsy samples with dysplasia or cancer, either overall or by stratum.
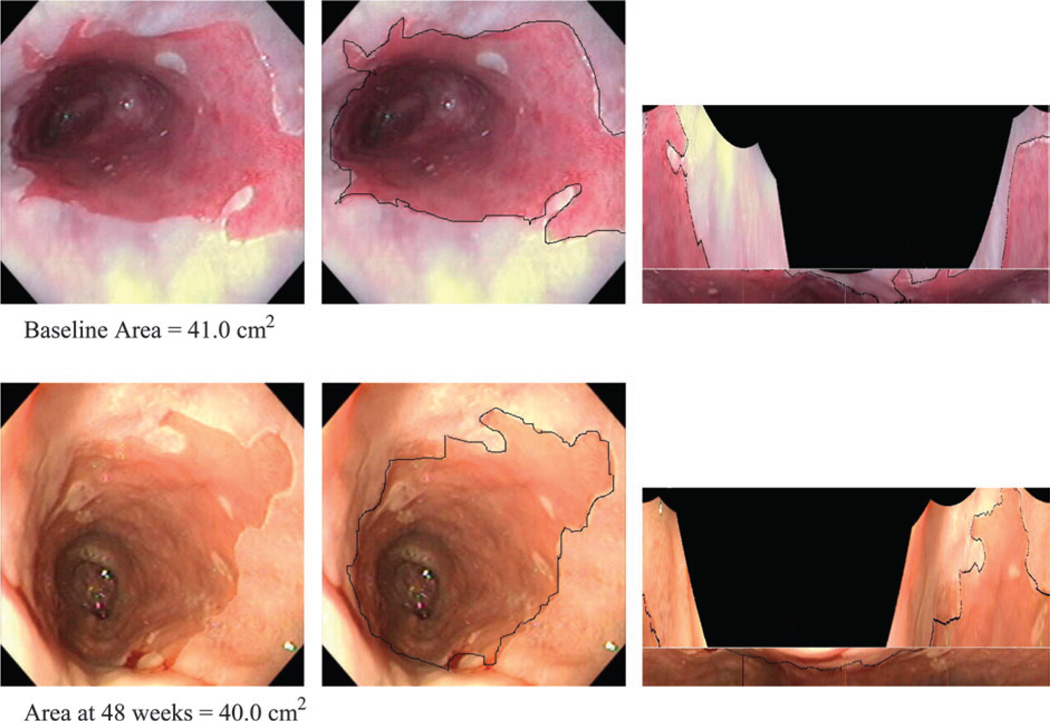
Total surface area affected by Barrett’s esophagus was measured by a quantitative endoscopic examination at baseline and 48 weeks of treatment (Table 2). No statistically significant differences in the median change of total surface area affected by Barrett’s esophagus were found after 48 weeks of treatment with respect to treatment assignment and grade of dysplasia (P = .12). Figure 1 illustrates an example of high-grade dysplasia as measured by quantitative endoscopy at baseline and 48 weeks.
Fig. 1.
Operations and transformations for quantitative endoscopy. Two series of three images are shown. The first row was taken at baseline, and the second row was taken at the 48-week follow-up. The baseline and 48-week follow-up images were of the same region in the esophagus. Left) Image of Barrett’s esophagus. Middle) The image with the structures to be measured outlined. Right) Transformed images of Barrett’s esophagus into two-dimensional maps allowed for quantitative measurement of the surface area of Barrett’s esophagus.
Tertiary Outcomes
The tertiary outcomes included changes from baseline to 48 weeks of treatment in the levels of the following biomarkers: COX-1 and -2 mRNA; prostaglandins PGD2, PGE2, PGF2α, thromboxane B2, and 6-keto-PGF1α; and methylation of tumor suppressor genes p16, APC, and E-cadherin. Paired tissue samples were available from 15 patients of the original 40 patients and were assayed for COX-1 and -2 mRNA levels (Table 3). At baseline, median levels of COX-2 mRNA were similar in samples from patients with high-grade dysplasia (16 patients) and in patients with low-grade dysplasia (36 patients); however, the number of samples in the high-grade dysplasia stratum was very small. No change in median level of COX-2 mRNA was observed after 24 weeks of treatment in the low-grade stratum (P =.08) or in the high-grade stratum (P =.75). Similar patterns were found for COX-1 mRNA.
Table 3.
Changes in results from prostaglandin assays and COX-1 and -2 expression after 24 weeks of treatment*
| Lowgrade stratum |
High-grade stratum |
Combined |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | Celecoxib (n = 20) | Placebo (n = 16) | P† | Celecoxib (n = 8) | Placebo (n = 8) | P† | Celecoxib (n = 28) | Placebo (n = 24) | P† |
| PGE2, ng/mg of protein (IQR) | |||||||||
| −Arachidonic acid | |||||||||
| Median at baseline | 0.13 (−0.22 to 48) | 0.30 (−0.13 to 0.73) | .32 | 0.18 −0.89 to 1.25) | 0.04 (−0.81 to 0.89) | .49 | 0.14 (−0.28 to 0.56) | 0.23 (−0.23 to 0.69) | .81 |
| Median change | 0.00 (−0.26 to 0.26) | −0.06 (−0.40 to 0.28) | .37 | +0.15 −0.24 to 0.54) | +0.01 (−0.96 to 0.98) | .07 | +0.02 (−0.21 to 0.25) | −0.04 (−0.44 to 0.36) | 11 |
| +Arachidonic acid | |||||||||
| Median at baseline | 1.57 (−1.65 to 4.79) | 2.40 (−0.83 to 5.63) | .73 | 1.77 −1.47 to 5.01) | 1.61 (−1.50 to 4.72) | .53 | 1.57 (−1.68 to 4.82) | 1.75 (−1.47 to 4.97) | .96 |
| Median change | −0.11 (−2.38 to 2.16) | −0.06 (−4.22 to 4.10) | .95 | + 1.08 −1.83 to 3.99) | 0.00 (−3.72 to 3.72) | .09 | +0.08 (−2.53 to 2.69) | −0.01 (−3.53 to 3.51) | .39 |
| PGD2, ng/mg of protein (IQR) | |||||||||
| −Arachidonic acid | |||||||||
| Median at baseline | 0.09 (−0.13 to 0.31) | 0.22 (−0.16 to 0.60) | .26 | 0.180 to 0.36) | 0.08 (−0.4 to 0.20) | .12 | 0.12 (−0.09 to 0.33) | 0.13 (−0.18 to 0.44) | .79 |
| Median change | 0.00 (−0.16 to 0.16) | −0.06 (−0.35 to 0.23) | .34 | +0.08−0.24 to 0.40) | −0.00 (−0.26 to 0.26) | .21 | +0.02 (−0.19 to 0.23) | −0.03 (−0.28 to 0.22) | 14 |
| +Arachidonic acid | |||||||||
| Median at baseline | 0.93 (−0.64 to 2.50) | 1.46 (−2.11 to 5.03) | .36 | 1.25 −0.21 to 2.71) | 0.93 (−0.37 to 2.23) | .60 | 0.98 (−0.60 to 2.56) | 1.26 (−1.42 to 3.94) | .65 |
| Median change | −0.40 (−1.84 to 1.04) | −0.28 (−2.30 to 1.74) | .77 | + 1.09 −1.36 to 3.54) | −0.29 (−1.55 to 0.97) | .29 | −0.20 (−1.86 to 1.46) | −0.28 (−1.94 to 1.38) | .52 |
| PGF2α, ng/mg of protein (IQR) | |||||||||
| −Arachidonic acid | |||||||||
| Median at baseline | 0.21 (−0.20 to 0.62) | 0.17 (−0.68 to 1.02) | .86 | 0.15 −1.16 to 1.46) | 0.10 (−0.07 to 0.27) | .25 | 0.19 (−0.46 to 0.84) | 0.14 (−0.22 to 0.50) | .65 |
| Median change | +0.02 (−0.14 to 0.18) | −0.02 (−0.47 to 0.43) | .52 | +0.04 −0.29 to 0.37) | −0.02 (−0.40 to 0.36) | .83 | +0.02 (−0.14 to 0.18) | −0.02 (−0.28 to 0.22) | .57 |
| +Arachidonic acid | |||||||||
| Median at baseline | 1.63 (−1.37 to 4.63) | 3.16 (−2.80 to 9.12) | .31 | 1.63 −0.85 to 4.11) | 1.82 (−6.12 to 9.76) | 1.0 | 1.63 (−0.74 to 4.00) | 2.59 (−3.43 to 8.61) | .36 |
| Median change | −0.32 (−4.97 to 4.33) | −0.77 (−8.45 to 6.95) | .50 | +0.85 −2.65 to 4.35) | +0.10 (−2.28 to 2.48) | 0.67 | −0.14 (−4.19 to 3.91) | −0.48 (−4.44 to 3.48) | .53 |
|
Thromboxane B2, ng/mg of protein (IQR) |
|||||||||
| −Arachidonic acid | |||||||||
| Median at baseline | 0.06 (−0.02 to 0.14) | 0.09 (−0.02 to 0.20) | .23 | 0.05 −0.18 to 0.28) | 0.03 (−0.06 to 0.1 2) | .40 | 0.06 (0.03 to 0.1 5) | 0.08 (−0.01 to 0.17) | .67 |
| Median change | 0.00 (−0.06 to 0.06) | −0.02 (−0.14 to 0.10) | .75 | +0.02 −0.04 to 0.08) | −0.00 (−0.10 to 0.10) | .53 | +0.01 (−0.06 to 0.08) | −0.02 (−0.13 to 0.09) | .56 |
| +Arachidonic acid | |||||||||
| Median at baseline | 0.21 (−0.38 to 0.80) | 0.58 (−0.31 to 1.47) | .32 | 0.26 −0.30 to 0.82) | 0.28 (−0.25 to 0.81) | .75 | 0.24 (−0.34 to 0.82) | 0.51 (−0.11 to 1.13) | .58 |
| Median change | −0.07 (−0.27 to 0.13) | −0.04 (−0.74 to 0.66) | .92 | +0.23 −0.60 to 1.06) | +0.02 (−0.21 to 0.25) | .53 | −0.05 (−0.44 to 0.34) | −0.01 (−0.54 to 0.52) | .80 |
|
6−keto−F1α,ng/mg of protein (IQR) |
|||||||||
| −Arachidonic acid | |||||||||
| Median at baseline | 0.14(0 to 0.28) | 0.19 (0.05 to 0.33) | 15 | 0.22 0.11 to 0.33) | 0.13 (0 to 0.26) | .07 | 0.17 (0.02 to 0.32) | 0.17 (−0.03 to 0.31) | .81 |
| Median change | −0.03 (−0.15 to 0.09) | −0.10 (−0.29 to 0.09) | .46 | +0.08 −0.19 to 0.35) | −0.01 (−0.19 to 0.17) | .46 | −0.02 (−0.1 6 to 0.12) | −0.05 (−0.25 to 0.1 5) | .40 |
| +Arachidonic acid | |||||||||
| Median at baseline | 0.30 (−0.07 to 0.67) | 0.37 (−0.31 to 1.05) | .52 | 0.53 0.30 to 0.76) | 0.36 (−0.10 to 0.82) | .34 | 0.36 (−0.05 to 0.77) | 0.37 (−0.18 to 0.92) | .94 |
| Median change | −0.05 (−0.31 to 0.21) | −0.01 (−0.54 to 0.52) | .73 | +0.26 −1.23 to 1.75) | +0.02 (−1.00 to 1.04) | .34 | −0.01 (−0.35 to 0.33) | +0.02 (−0.78 to 0.82) | .87 |
| COX−1 mRNA,fg/µg*(IQR) | |||||||||
| Median at baseline | 14 (−3 to 31) | 20 (−1 to 41) | .56 | 10 −5 to 25) | 13 (3 to 23) | .80 | 10 (−7 to 27) | 16 (6 to 26) | .48 |
| Median change | 2 (−43 to 47) | 10 (−19 to 39) | 1.00 | 11 −1 to 23) | 2 (−8 to 12) | .33 | 11 (−22 to 44) | 4 (−13 to 21) | .41 |
| No. | 2 | 3 | 5 | 4 | 7 | 7 | |||
| COX-2 mRNA,fg/µg†(IQR) | |||||||||
| Median at baseline | 10 (3 to 17) | 47 (17 to 77) | .08 | 15 1 to 29) | 5 (1 to 9) | .34 | 13 (−1 to 27) | 12 (−17 to 41) | .64 |
| Median change | 5(−1 to 11) | −10 (−24 to 4) | .08 | 14 −13 to 41) | 10 (2 to 18) | .75 | 8 (−19 to 35) | 0 (−24 to 24) | .27 |
| No. | 2 | 3 | 5 | 5 | 7 | 8 | |||
Changes were calculated by subtracting the baseline value from the value at 24 weeks. Arachidonic acid was included in the prostaglandin assays to enhance the sensitivity of detection (23; see “Patients and Methods”). COX = cyclooxygenase; PG = prostaglandin; IQR = interquartile range.
Derived from two-sided Wilcoxon rank sum test.
Changes were calculated by subtracting the baseline value from the value at 24 weeks for 15 pairs in which the tissue biopsy sample that was used for COX expression analysis was from the area adjacent to the highest grade of dysplasia at both baseline and 24-week biopsy examinations.
No statistically significant difference was found in the levels of prostaglandins PGD2, PGE2, PGF2α, thromboxane B2, or 6-keto-PGF1α between the two treatment groups at baseline (Table 3) or in the median change of these levels after 6 months of treatment, regardless of treatment assignment and grade of dysplasia. At baseline, levels of PGD2, PGE2, PGF2α, and thromboxane B2 were lower in patients who were taking aspirin than in those who were not taking aspirin. However, we could identify no effect of confounding or modification associated with aspirin use at baseline on treatment group comparisons with respect to any change in the levels of prostaglandins in biopsy samples with dysplasia.
We examined promoter region methylation to determine whether these non–COX-2 pathway markers were affected during this trial. At baseline, methylation of p16, APC, and E-cadherin was frequent, and higher frequencies of DNA methylation were observed in high- versus low-grade dysplasia (Table 4). Paired baseline and posttreatment samples were available from 22 patients for comparison with methylation of all three genes. Among the 10 celecoxib-treated patients, six had no change in methylation of any of the three genes examined, two had a gain in methylation for one gene, and two had a loss of methylation in one gene. Among the 12 placebo-treated patients, six had no change in the methylation in any gene, three had a gain in methylation of one gene, and another three had a loss of methylation in one gene. Thus, no net change in methylation in either celecoxib or placebo group was observed, and overall 56 of the 66 paired methylation analyses were stable during the 48-week treatment. These results suggest that, although promoter region methylation of these genes may be a molecular marker of histologic progression, the difference in methylation between arms was not statistically significant.
Table 4.
Methylation frequency at specific genes at baseline according to degree of dysplasia
| No. methylated/No in group (%) |
||
|---|---|---|
| Gene | Low grade | High grade |
| p16 | 18/26(66) | 14/15 (93) |
| Adenomatous polyposis col | 24/29 (83) | 18/19 (94) |
| E-cadherin | 9/26 (35) | 8/16 (50) |
Follow-up, Treatment Termination, and Adverse Events
Rigorous follow-up and adverse event monitoring were conducted throughout the study. The median follow-up was 2.0 years in both treatment arms (Table 5). The percent of scheduled follow-up visits completed was higher in the low-grade stratum than in the high-grade stratum. Reasons for decreased follow-up visits among the 36 patients in the high-grade stratum include diagnosis of esophageal cancer before the 48-week follow-up visit in six patients; patient requests in two; and diagnoses of deep venous thrombosis in one, acute pancreatitis in one, mitral valve prolapse in one, and vertigo in one.
Table 5.
Follow-up, adherence, and treatment termination *
| Low-grade stratum |
High-grade stratum |
Combined |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Cele (n = 30) | Plbo (n = 34) | P* | Cele (n = 19) | Plbo (n = 17) | P* | Cele (n = 49) | Plbo (n = 51) | P* |
| Follow-up | |||||||||
| Median time on study, y (range] |
2.1 (0.3–2.3) | 2.0 (0.0–2.4) | .75 | 1.4(0.2–2.3) | 1.3 (0.0–2.4) | .50 | 2.0 (0.2–2.3) | 2.0 (0.0–2.4) | .79 |
| % scheduled† follow-up visits completed |
83 | 81 | .68 | 59 | 54 | .48 | 74 | 72 | .94 |
| Treatment adherence | |||||||||
| Median observed/expected pill counts‡ where drug was dispensed at previous visit, % |
88 | 90 | .68 | 85 | 86 | .48 | 88 | 89 | .45 |
| % patients with treatment nterruption or early treatment termination |
33 | 38 | .68 | 42 | 59 | .32 | 37 | 45 | .40 |
| No. of patients unmasked before end of study |
0 | 0 | NC | 0 | 1§ | .47 | 0 | 1§ | .51 |
|
Reasons for termination of treatment |
.75 | .68 | .76 | ||||||
| No. of patients completed Study |
25 | 28 | 11 | 10 | 36 | 38 | |||
| No. of patients with esophageal cancer |
0 | 0 | 3 | 3 | 3 | 3 | |||
| No. of patients with esophagectomy due to persistent dysplasia |
0 | 0 | 2 | 0 | 2 | 0 | |||
| No. of patients who died | 0 | 2 | 0 | 0 | 0 | 2 | |||
| No. of patients with adverse event other than esophageal cancer or death |
3 | 3 | 2 | 1 | 5 | 4 | |||
| No. of patients requesting early termination |
1 | 1 | 1 | 1 | 2 | 2 | |||
| No. of patients with other reason∥ |
1 | 0 | 0 | 2 | 1 | 2 | |||
Cele = celecoxib; Plbo = placebo; NC = not calculable.
All statistical tests were two-sided Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical variables.
Adjusted for deaths. Completion defined as submission of Concomitant Medication form, Histology Report form, or Follow-up Medical History form at a visit.
Note that 149 (24%) of the 619 visits in which drug was dispensed were missing data for return of study drug.
This patient was hospitalized with acute pancreatitis.
Includes use of protocol-denied photodynamic therapy, comorbidity-requiring surgery, or allergic reaction to Bactrim.
Overall, no statistically significant difference in adverse events of grades 3 and 4 was observed between the two treatment arms (Table 6). Cardiovascular toxic effects of grades 3 and 4 were experienced by six patients in the placebo arm and three patients in the celecoxib arm. The toxic effects experienced by patients assigned to the celecoxib arm included hospitalization for inferior wall myocardial infarction after 16 months of treatment, urinary tract bleeding after 6 months of treatment, and development of shortness of breath and fluid retention after 4 months of treatment. Similar numbers of gastrointestinal toxic effects occurred in both treatment arms.
Table 6.
Reported serious or life-threatening coagulation adverse events *
| Celecoxib |
Placebo |
|||
|---|---|---|---|---|
| Adverse event category | Adverse event | Related | Adverse event | Related |
| Cardiovascular | Myocardial infarction Congestive heart failure Unstable angina |
Possibly Unlikely Possibly |
Chest pain Pulmonary embolism (death] Myocardial infarction (death] Deep vein thrombosis Transient ischemic attack Subarachnoid hemorrhage |
Possibly Not related Not related Not related Possibly Unlikely |
| Bleeding | Hematuria | Not related | Melena Bloody diarrhea Hematuria Hemoptysis |
Not related Unlikely Possibly Not related |
Data were submitted to the medical monitor at the National Cancer Institute. Only one patient reported each adverse effect.
Thirteen (27%, 95% CI = 15% to 41%) of the 49 patients in the celecoxib arm and 13 (25%, 95% CI = 14% to 40%) of the 51 patients in the placebo arm terminated treatment early. Reasons for early termination of treatment included development of esophageal cancer in six patients, death in two, adverse events other than death in nine, patient request in four, and other reasons in three. All six patients whose endoscopic biopsy specimen was diagnosed as cancer were in the high-grade dysplasia stratum (three in the celecoxib arm and three in the placebo arm). Among these six patients, one was treated with photodynamic therapy, one was treated with the yttrium aluminum garnet laser, and four patients underwent esophagectomy [two of the four patients were diagnosed with high-grade dysplasia, and two were diagnosed with esophageal cancer—one with TisN0Mx and the other with T2N0Mx stage cancer (29)]. Two patients in the placebo arm of the low-grade stratum died (one from postoperative myocardial infarction after popliteal artery aneurysm surgery and one from postoperative pulmonary embolism after lobectomy for lung cancer). Because both patients were in the placebo arm, their deaths were deemed unrelated to treatment.
Discussion
Administration of 200 mg of celecoxib or placebo twice daily for 48 weeks of treatment did not change the proportion of biopsy samples diagnosed with dysplasia or cancer among patients with Barrett’s dysplasia. During the first year of treatment 36 (44%) of the 82 patients experienced no change in their highest grade of dysplasia. Approximately 50% of patients in the low-grade stratum and 25% of patients in the high-grade stratum, regardless of treatment assignment, experienced a decrease in the highest grade of dysplasia. This decreased percentage of patients in the highest grade of dysplasia without additional treatment underscores the difficulties in tissue biopsy sampling, our lack of understanding of the natural history of Barrett’s esophagus, and the variability of histologic diagnosis (30–32).
This study has several limitations. The difficulty in tissue biopsy sampling can be demonstrated by the 122 CBET patients who were registered but were not randomly assigned to treatment. Most of these registered patients had a preregistration histologic diagnosis of dysplasia, but they did not have evidence of dysplasia at the time of their baseline endoscopy. Despite the intensive endoscopic biopsy protocol, the area that was used to establish the initial preregistration diagnosis of dysplasia may not have been sampled. Among the 122 registered but not included patients, 83 patients were diagnosed as indefinite for dysplasia. The intensive endoscopic biopsy protocol resulted in a new diagnosis of esophageal cancer for 15 registered patients. Tissue biopsy sampling, al though imperfect, is currently the best available method to assess the malignant potential of Barrett’s esophagus.
Another limitation is that natural reversion of dysplasia without any intervention was more likely to occur in patients with low-grade dysplasia than with high-grade dysplasia. More patients in the high-grade stratum in the placebo arm (25%) experienced an increase in their highest grade of dysplasia than patients in the low-grade stratum (11%). This observation further illustrates the clinical challenge posed for physicians in the management of patients with high-grade dysplasia.
A final limitation is that dysplasia grading is an imperfect predictor of cancer because of the low intra- and interobserver agreement among pathologists (31,32). The low degree of inter-observer agreement in diagnosing low-grade dysplasia may contribute to histologic inconsistency of dysplasia. This inconsistency may create the false appearance of histologic reversion. Although the interobserver agreement in diagnosing high-grade dysplasia was much higher in our previous study (32),it was not 100%. The CBET investigators sought to minimize this limitation by requiring a majority histologic diagnosis of dysplasia for entry into this study. This requirement increased the complexity of randomization process and had an impact on patient accrual, but it provided more confidence in the histologic diagnosis.
Whether celecoxib treatment would alter the portion of the esophagus (i.e., the length of esophagus) affected by Barrett’s esophagus was unknown. Because of the difficulty in using centimeter markings on the upper endoscope to appropriately determine length of Barrett’s esophagus, we measured the total surface area affected by Barrett’s esophagus by use of a quantitative endoscopic examination. The median total surface area affected by Barrett’s esophagus at baseline in patients with low- and high-grade dysplasia was similar. This result was expected because having a higher grade of dysplasia does not necessarily mean a larger total surface area of Barrett’s esophagus. Treatment with celecoxib for 48 weeks, compared with placebo, did not change the total sur face area affected by Barrett’s esophagus, regardless of grade.
Similarly, treatment with celecoxib for 24 weeks compared with placebo did not change the levels of COX-2 mRNA, PGD2, PGE2, PGF2α, thromboxane B2, or 6-keto-PGF1α, regardless of grade. This finding was unexpected. The samples obtained for biomarker studies were adjacent to tissue biopsies with histologic confirmation of dysplasia. However, the samples for biomarker studies themselves had no histologic confirmation of dysplasia. We recognize that Barrett’s esophagus is multifocal and patchy in nature. So, it is possible, although unlikely, that the samples used for these biomarker assays were not dysplastic tissue. Additional studies that evaluate COX-2 expression by immunohistochemistry in dysplastic tissues appear to be warranted.
Although we found no changes in the levels of COX-2 mRNA, we did anticipate differences in the levels of prostaglandin between the two treatment arms based on the proposed mechanism of action of COX-2 inhibitors. The lack of differences in prostaglandin levels regardless of treatment arm or dysplasia grade indicated that the celecoxib dose was probably inadequate. At the initiation of CBET, the 200-mg dose of celecoxib twice daily was selected because of concerns for safety and for tolerability in a trial of potentially long duration (up to 3 years). However, a 400-mg dose of celecoxib twice daily may be more biologically meaningful (33). As expected, tumor suppressor genes p16, APC, and E- cadherin were methylated in samples with low- or high-grade dysplasia. After the 48-week treatment with celecoxib or placebo, no statistically significant change in methylation levels from baseline in either group was found, which is not surprising given the lack of histologic changes. Methylation of these three genes, or of any other hypermethylated locus, should not be directly affected by COX-2 inhibition; therefore, we cannot rule out the possibility that these genes are potential markers of molecular changes underlying the histologic changes associated with dysplasia. We have previously observed that histologic progression is associated with underlying progression in the number of hypermethylated loci in squamous cell carcinoma of the esophagus (24). The higher rate of methylation of the genes for p16, APC, and E-cadherin in samples with high-grade dysplasia compared with samples with low-grade dysplasia, which we found in this study, support the possibility that hypermethylation of multiple loci may compliment histologic changes as a surrogate for disease progression and should be investigated in future studies.
The lack of a statistically significant difference between the treatment groups that we observed was not due primarily to inadequate recruitment. The study was powered at 80% for a total of 124 patients overall. Only 100 patients were randomly assigned to treatment, and only 82 (43 in the celecoxib arm and 39 in the placebo arm) of the 100 patients contributed data to the comparison for the primary outcome. But because the effect size was–2% (i.e., an 8% decrease in the combined celecoxib group and a 6% decrease in the combined placebo group in the proportion of biopsy samples with dysplasia or cancer at 48 weeks), the addition of 42 patients would most likely not result in a statistically significant difference between celecoxib and placebo groups. The result is essentially null, i.e., an effect size of 0. More subjects will not make such a result statistically significant.
The apparent inability of celecoxib, compared with placebo, to decrease the percentage of samples with dysplasia is probably not due to the patient population. The characteristics of CBET patients, as anticipated, were typical of patients with Barrett’s dysplasia: white males who were aged 60–70 years, who were overweight or obese, and who were taking antireflux medications (e.g., proton pump inhibitors or histamine H2-receptor antagonists). It is not unusual for patients with Barrett’s esophagus to be on such medications because it was probably their symptoms of gastroesophageal reflux disease that led them to undergo clinical evaluation for Barrett’s esophagus. Whether the antireflux medications could be associated, either positively or negatively, with dysplasia modification is unknown. Despite several studies, the association of antireflux medications with the reversion of low- or high-grade dysplasia in Barrett’s esophagus remains unclear (34–36).
Although celecoxib treatment was associated with increased cardiovascular toxic effects in another trial (37), we found that patients in the placebo arm experienced more cardiovascular toxic effects than those in the celecoxib arm, although the total number of patients with such toxic effects was small. Another potential toxic effect was gastrointestinal adverse events, but such events were low and similar in both treatment arms. Thus, daily treatment with celecoxib (400 mg) for up to 2 years, compared with placebo, was reasonably well tolerated with few serious adverse events.
Sixty-four (64%) of 100 patients randomly assigned to treatment into CBET had low-grade dysplasia. Before study entry, patients with high-grade dysplasia were offered the standard-of-care surgery and additional treatments, including photodynamic therapy and mucosal ablation. These patients were aware of other treatment options, refused surgery, and understood the risks associated with a placebo-controlled trial. Although patients with high-grade dysplasia had a 50% chance of being randomly assigned to the placebo arm, all patients with high-grade dysplasia remained under close surveillance, undergoing rigorous endoscopic biopsies every 3 months. Six patients in the high-grade stratum were diagnosed with esophageal adenocarcinoma through their endoscopic biopsy specimens within their first year on study (three in the celecoxib arm and three in the placebo arm). Of the four patients who underwent esophagectomy, two patients were diagnosed with high-grade dysplasia with no evidence of invasive adenocarcinoma, and despite the initial diagnosis of esophageal cancer on the endoscopic biopsy specimen, there was no evidence of invasive cancer in the postresection specimen. The six patients diagnosed with esophageal cancer had Barrett’s esophagus for periods of several months to 20 years. The variability in the clinical course of this disease underscores the management challenges posed by highgrade dysplasia, including the requirement for an upper endoscopy examination every 3 months, the lack of predictive markers to determine risk of disease progression, and the difficulties with sampling variation.
The lack of secondary chemoprevention with celecoxib in patients with Barrett’s dysplasia was disappointing. However, CBET is one of the few prospective chemoprevention trials in patients with Barrett’s dysplasia, and through it, we have gained valuable information about the disease process and the challenges of conducting such a study.
CONTEXT AND CAVEATS.
Prior knowledge
Aspirin and nonsteroidal anti-inflammatory drugs, such as cele-coxib, may decrease the risk of developing esophageal cancer.
Study design
Phase IIb multicenter randomized placebo-controlled trial of celecoxib in patients with Barrett’s esophagus and low- or high-grade dysplasia.
Contribution
Celecoxib (200 mg) taken twice daily for 48 weeks does not appear to prevent progression of Barrett’s dysplasia to cancer.
Implications
At low doses, celecoxib is not a good chemopreventive agent for esophageal cancer.
Limitations
The 200-mg dose of celecoxib twice daily may have been too low to have an effect. Intra- and interobserver agreement on grading of dyplasia was low, and the natural reversion of dysplasia without intervention was more likely to occur among patients with low-than with high-grade dysplasia.
Acknowledgments
This research was supported by grants N01-CN-85185 from the National Cancer Institute and NQ4-99-02-007 from Pfizer. The authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
Dr A. J. Dannenberg is currently conducting research sponsored by Pfizer, the manufacturer of celecoxib. Dr J. G Herman is a paid consultant for OncoMethylome Sciences.
Footnotes
Notes
Members of CBET Research Group include:
Clinical centers—Columbia Presbyterian Medical Center, New York City, NY: Charles J. Lightdale, MD (director); Kevin Bukowski, RN (Coordinator); Heidi Rotterdam, MD; Peter Green, MD; Ruben Garcia-Carasquillo, MD; Robert Bruce MacArthur, PharmD; Judith Ellis, RPh; Gina Steriti; Hines VA Hospital, Hines, IL: Stephen Sontag, MD (Director); Thomas Schnell, MD (Codirector); Susan O’Connell, RN (2000, Coordinator); Theresa Simon, RN (Coordinator); Gregorio Chejfec, MD; Jack Leya, MD; Sally Nicol, RN; Richard Brandstedt, RPh; Jean Seidel, Mariann Eichorst, HT; Johns Hopkins Hospital, Baltimore, MD: Marcia Irene Canto, MD, MHS (Director); Christine L. Smith, RN (Coordinator); Tsung-The Wu, MD PhD (2000); Elizabeth Montgomery, MD; William J. Ravich, MD; Thomas W. Kensler, MD; Laurie McClelland, RN; Hye Kim, RPh; Mari Robinette Deasel; Mayo Clinic, Rochester, MN: Kenneth K Wang, MD (Director); Lori S. Lutzke (Coordinator); Navtej S. Buttar, MD; Louis M. Wong Kee Song, MD; Julie R. Horihan; Sandra S. Showalter, RPh; Lawrence J. Burgart, MD; Thomas C. Smyrk, MD; Portland VA Medical Center, Pordand, OR: Douglas Faigel, MD (Director); Shane Fanning, BS (Coordinator); Anne Rader, MD; David Lieberman, MD; Mary Garard, RN; Vickie L. Vonderohe, RPh; Southern Arizona VA, Tucson, AZ: Richard E. Sampliner, MD (Director); Patricia Martinez, RN BSN (Coordinator); Ronnie Fass, MD; Sheri Crawford, RPh; R. Anil Prasad, MD; Aychut Bhattacharyya, MD; UCLA Center for the Health Sciences, Los Angeles, CA: Wilfred M. Weinstein, MD (Director); Sheri Wiggett, RN (Coordinator); Galen Cortina, MD; Andrew Ippoliti, MD; Joseph Pisegna, MD; Debbie Chung, PharmD; University Hospitals of Cleveland, Cleveland, OH: Amitabh Chak, MD (Director); Laurie Logan, RN (Coordinator); Gerard Isenberg, MD; Michael V Sivak, MD; Richard C.K. Wong, MD; Wendy Brock, RN; Beth Bednarchik, RN; Michael J. Banchy, MD; Jay Wasman, MD; Josseph E. Willis, MD.
Resource Centers—Central Blood Laboratory, Covance, Indianapolis, IN: Sue Hackett, Tonya Funk; Chair’s Office, The Johns Hopkins Cancer Center, Baltimore, MD: Arlene A. Forastiere, MD (Study Chair); Elisabeth I. Heath, MD (Study Cochair); Ed Proctor, BS; Stewart P. Craig, MS; Susan Louden; Coordinating Center, The Johns Hopkins Center for Clinical Trials, Baltimore, MD: Steven Piantadosi, MD, PhD (Director); Curtis Meinert, PhD (Codirector); Janet Holbrook, PhD (1999); Barbara Martin, PhD (2000); Aynur Ünalp-Arida, MD PhD; James Tonascia, PhD; Michele Donithan, MHS; Mark Van Natta, MHS; Saundra K. Krieger, MSW (1999–2001); Milana Isaacson; Linda Roberts; Kapreena Owens; Pat Belt; John Dodge; Michael Smith; Karen Collins; Helen Cromwell; Betty Collison; COX-1/ COX-2 analysis, Weill Medical College of Cornell University, New York, NY: Andrew J. Dannenberg, MD; Baoheng Du, MD; Drug Distribution Center, Searle/Pharmacia, Peapack, NJ: Gary Gordon, MD (1999–2000); Daniel R. Vlock, MD; Paula Locker, MS; Ann Jambois (1999–2000); Helena Conn; Shawn J. Calderon; Methylation analysis, The Johns Hopkins University, Baltimore, MD: James G Herman, MD; Department of Pathology, The University of Texas M. D. Anderson Cancer Center, Houston, TX: Stanley R. Hamilton, MD; Cheryl Willis; Monitoring, CCS Associates, Mountain View, CA: Caroline C. Sigman, PhD; Kate Guyton, PhD DABT; Donya Bagheri, MS DABT; Connee Northway; Project Office, National Cancer Institute, Bethesda, MD: Ernest Hawk, MD MPH; Jaye Viner, MD; Ellen Richmond, MS RN; Gary P. Topper; Linda Parreco, RN; Florence M. Mann; Prostaglandin analysis, The Johns Hopkins University, Baltimore, MD, Emory University, Atlanta, GA: Walter Hubbard, PhD; Deborah Geiman; Vincent W. Yang, MD PhD; Quantitative endoscopy, Robert Wood Johnson Foundation, Princeton, NJ: Albert O. Shar, PhD; Specimen banking, The Johns Hopkins Pathology, Baltimore, MD: Tsung-The Wu, MD PhD (2000); Elizabeth Montgomery, MD; Mari Robinette Deasel.
Committees—Executive Committee: Arlene A. Forastiere, MD; Gary Gordon, MD (1999–2000); Ernest Hawk, MD MPH; Elisabeth I. Heath, MD; Steven Piantadosi, MD PhD; Aynur Ünalp-Arida, MD PhD; Wilfred M. Weinstein, MD; TEMC: Voting Members—Joseph Aisner, MD (Chair); Joel K. Greenson, MD; Elizabeth H. Hammond, MD; David Harrington, MD (Vice-Chair); Stuart J. Spechler, MD; Nonvoting Members—Arlene A. Forastiere, MD; Ernest Hawk, MD MPH; Elisabeth I. Heath, MD; Curtis Meinert, PhD; Steven Piantadosi, MD PhD; Aynur Ünalp-Arida, MD, PhD.
References
- 1.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 2.Brown LM, Silverman DT, Pottern LM, Schoenberg JB, Greenberg RS, Swanson GM, et al. Adenocarcinoma of the esophagus and esophagogastric junction in white men in the United States: alcohol, tobacco, and socioeconomic factors. Cancer Causes Control. 1994;5:333–340. doi: 10.1007/BF01804984. [DOI] [PubMed] [Google Scholar]
- 3.Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett’s esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212–215. [PubMed] [Google Scholar]
- 4.Shaheen NJ, Crosby MA, Bozymski EM, Sandler RS. Is there publication bias in the reporting of cancer riskin Barrett’s esophagus? Gastroenterology. 2000;119:333–338. doi: 10.1053/gast.2000.9302. [DOI] [PubMed] [Google Scholar]
- 5.Ellis FH., Jr Standard resection for cancer of the esophagus and cardia. Surg Oncol Clin N Am. 1999;8:279–294. [PubMed] [Google Scholar]
- 6.Klaus A, Hinder RA. Indications for antireflux surgery in Barrett’s. Semin Laparosc Surg. 2001;8:234–239. [PubMed] [Google Scholar]
- 7.DeMeester TR. Management of adenocarcinoma arising in Barrett’s esophagus. Semin Thorac Cardiovasc Surg. 1997;9:290–301. [PubMed] [Google Scholar]
- 8.van den Boogert J, van Hillegersberg R, Siersema PD, de Bruin RW, Tilanus HW. Endoscopic ablation therapy for Barrett’s esophagus with high-grade dysplasia: a review. Am J Gastroenterol. 1999;94:1153–1160. doi: 10.1111/j.1572-0241.1999.01058.x. [DOI] [PubMed] [Google Scholar]
- 9.Overholt BF, Lightdale CJ, Wang KK, Canto MI, Burdick S, Haggitt RC, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–498. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 10.Thun MJ, Namboodiri MM, Calk EE, Flanders WD, Heath CW., Jr Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 11.Funkhouser EM, Sharp GB. Aspirin and reduced risk of esophageal carcinoma. Cancer. 1995;76:1116–1119. doi: 10.1002/1097-0142(19951001)76:7<1116::aid-cncr2820760703>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Farrow DC, Vaughan TL. Determinants of survival following the diagnosis of esophageal adenocarcinoma (United States) Cancer Causes Control. 1996;7:322–327. doi: 10.1007/BF00052937. [DOI] [PubMed] [Google Scholar]
- 13.Bateman DN, Colin-Jones D, Hartz S, Langman M, Logan RF, Mant J, et al. Mortality study of 18000 patients treated with omeprazole. Gut. 2003;52:942–946. doi: 10.1136/gut.52.7.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell. 2003;4:431–436. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 15.Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett’s esophagus and adenocarcinoma: ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487–496. doi: 10.1016/s0016-5085(00)70254-x. [DOI] [PubMed] [Google Scholar]
- 16.Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett’s esophagus. Gastroenterology. 2002;122:1101–1112. doi: 10.1053/gast.2002.32371. [DOI] [PubMed] [Google Scholar]
- 17.Heath EI, Canto MI, Wu TT, Piantadosi S, Hawk E, Unalp A, et al. Chemoprevention for Barrett’s esophagus trial Design and outcome measures. Dis Esophagus. 2003;16:177–186. doi: 10.1046/j.1442-2050.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 18.Levine DS, Haggitt RC, Blount PL, Rabinovitch PS, Rusch VW, Reid BJ. An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus. Gastroenterology. 1993;105:40–50. doi: 10.1016/0016-5085(93)90008-z. [DOI] [PubMed] [Google Scholar]
- 19.Savary M, Miller G. The esophagus Handbook and adas of endoscopy. Solothurn (Switzerland): Verlag Gassman. 1978:135–142. [Google Scholar]
- 20.Kim R, Baggott BB, Rose S, Shar AO, Mallory DL, Lasky SS, et al. Quantitative endoscopy: precise computerized measurement of metaplastic epithelial surface area in Barrett’s esophagus. Gastroenterology. 1995;108:360–366. doi: 10.1016/0016-5085(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 21.Kim R, Rose S, Shar AO, Weiner M, Reynolds JC. Extent of Barrett’s metaplasia: a prospective study of the serial change in area of Barrett’s measured by quantitative endoscopic imaging. Gastrointest Endosc. 1997;45:456–462. doi: 10.1016/s0016-5107(97)70173-1. [DOI] [PubMed] [Google Scholar]
- 22.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, et al. TCyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 23.Yang VW, Geiman DE, Hubbard WC, Spannhake EW, Hylind LM, Hamilton SR, et al. Tissue prostanoids as biomarkers for chemopreven-tion of colorectal neoplasia: correlation between prostanoid synthesis and clinical response in familial adenomatous polyposis. Prostaglandins Other Lipid Mediat. 2000;60(l-3):83–96. doi: 10.1016/s0090-6980(99)00054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo M, Ren J, House MG, Qi Y, Brock MV, Herman JG. Accumulation of promoter methylation suggests epigenetic progression in squamous cell carcinoma of the esophagus. Clin Cancer Res. 2006;12:4515–4522. doi: 10.1158/1078-0432.CCR-05-2858. [DOI] [PubMed] [Google Scholar]
- 25.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 26.Mccullagh P, Nelder JA. Generalized linear models. 2nd ed. London (U.K.): Chapman and Hall; 1989. [Google Scholar]
- 27.Altman D. Practical statistics for medical research. London (U.K.): Chapman and Hall; 1991. [Google Scholar]
- 28.SAS Institute Inc. SAS/STAT User’s Guide. Version 8. Cary (NC): SAS Institute; 1999. [Google Scholar]
- 29.Greene FL, Page DL, Fleming ID 6th ed, editors. AJCC Cancer Staging Manual. New York (NY: Springer Verlag; 2002. [Google Scholar]
- 30.Reid BJ, Haggitt RC, Rubin CE, Roth G, Surawicz CM, Van Belle G, et al. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery E, Goldblum JR, Greenson JK, Haber MM, Lamps LW, Lauwers GY, et al. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol. 2001;32:379–388. doi: 10.1053/hupa.2001.23511. [DOI] [PubMed] [Google Scholar]
- 32.Montgomery E, Bronner MP, Goldblum JR, Greenson JK, Haber MM, Hart J, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 33.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 34.Sharma P, Sampliner RE, Camargo E. Normalization of esophageal pH with high-dose proton pump inhibitor therapy does not result in regression of Barrett’s esophagus. Am J Gastroenterol. 1997;92:582–585. [PubMed] [Google Scholar]
- 35.Sampliner RE. Effect of up to 3 years of high-dose lansoprazole on Barrett’s esophagus. Am J Gastroenterol. 1994;89:1844–1848. [PubMed] [Google Scholar]
- 36.Hillman LC, Chiragakis L, Shadbolt B, Kaye GL, Clarke AC. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett’s oesophagus. Med J Aust. 2004;180:387–391. doi: 10.5694/j.1326-5377.2004.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 37.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]