Abstract
Background
Acute Traumatic Coagulopathy (ATC) occurs after severe injury and shock and is associated with increased bleeding, morbidity and mortality. The effects of ATC and hemostatic resuscitation on outcome are not well-explored. The PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study provided a unique opportunity to characterize coagulation and the effects of resuscitation on ATC after severe trauma.
Methods
Blood samples were collected upon arrival on a subset of PROMMTT patients. Plasma clotting factor levels were prospectively assayed for coagulation factors. These data were analyzed with comprehensive PROMMTT clinical data.
Results
There were 1198 patients with laboratory results of whom 41.6% were coagulopathic. Using International Normalized Ratio (INR)≥1.3, 41.6% (448) of patients were coagulopathic while 20.5% (214) were coagulopathic using partial thromboplastin time (PTT)≥35. Coagulopathy was primarily associated with a combination of an ISS>15 and a BD<−6 (P<.05). Regression modeling for INR-based coagulopathy shows that pre-hospital crystalloid (odds ratio (OR)=1.05), Injury Severity Score (ISS, OR=1.03), Glasgow Coma Scale (OR=0.93), heart rate (OR=1.08), systolic blood pressure (OR=0.96), base deficit (BD, OR=0.92) and temperature (OR=0.84) were significant predictors of coagulopathy (all P<.03). A subset of 165 patients had blood samples collected and coagulation factor analysis performed. Elevated ISS and BD were associated with elevation of aPC and depletion of factors (all P<.05). Reductions in factors I, II, V, VIII and an increase in aPC drive ATC (all p<.04). Similar results were found for PTT-defined coagulopathy.
Conclusions
ATC is associated with depletion of factors I, II, V, VII, VIII, IX and X and is driven by the activation of the protein C system. These data provide additional mechanistic understanding of the drivers of coagulation abnormalities after injury. Further understanding of the drivers of ATC and the effects of resuscitation can guide factor guided resuscitation and correction of coagulopathy after injury.
Keywords: PROMMTT, Coagulation, Bleeding, Trauma, Injury
Background
Trauma remains the leading cause of death between the ages of 1 and 44(1). Bleeding is the primary cause of these deaths, (2, 3) and coagulopathy contributes significantly to this hemorrhage and poor outcomes(4). While traditionally believed to be a result of iatrogenic causes (dilution, hypothermia and acidosis), the entity of an endogenous acute traumatic coagulopathy (ATC) which occurs prior to and independent of iatrogenic reasons is now understood and accepted to be the primary cause of perturbed coagulation after injury(5, 6). Several groups have reported on the prelevance of ATC and described its impact on patient outcome(7–11).
Despite these reports, little work has prospectively examined the clinical and demographic causes of ATC. Indeed while its presence in various cohorts has been detailed, the specific clinical drivers of ATC and the proteins that functionally mediate the effects of ATC have not been sufficiently examined. In addition many of the studies reporting on traumatic coagulopathy suffer from retrospectively-collected and incomplete data as well as non-purposed sample collection. The initial descriptive studies by Brohi and Macleod reported on retrospective data from a convenience cohort of injured civilian trauma patients and soldiers(12, 13). This retrospective look at demographic correlations with clinically measured prothrombin time (PT) and partial thromboplastin time (PTT) is confounded by uncertain temporal data and variability in sample procurement and measurement. The PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study provided a unique opportunity for controlled real-time prospective sample and data acquisition coupled with codified outcomes. A primary aim of the original study was to utilize these data to assess the clinical drivers of coagulation abnormalities and the effects of resuscitation on coagulopathy and outcome after severe trauma. Hence we report here on the clinical predictors and drivers of ATC.
Methods
The PROMMTT methodology has been extensively described elsewhere(14). Briefly all trauma patients meeting criteria for highest level trauma activation at 10 major level 1 trauma centers were enrolled into the PROMMTT study from July 2009 to October 2010. Patients were excluded if they were pregnant, prisoners, or under 16 years old. Patients were additionally excluded if they were transferred from outside hospitals, had more than 5 minutes of cardiopulmonary resuscitation prior to or during the first 30 minutes of hospitalization, or did not receive at least one unit of red blood cells (RBCs) within six hours after admission. The Committee on Human Research at the University of California San Francisco as well as Institutional Review Boards from each of the other nine centers and the United States Army Human Research Protections Office approved the protocol. Comprehensive demographic, injury and real-time resuscitation and transfusion data were collected by 24/7 research assistants.
Patients were followed until hospital discharge or death. For mortality analysis, patients surviving to hospital discharge were assumed to still be alive. Secondary outcome measures were also recorded for 28-day ventilator-free days, acute lung injury (American-European consensus conference definition)(15), and blood transfusions required in the first 24 hours. Multiple organ failure (MOF) was calculated using the Denver MOF score.
Data analysis was performed by the investigators. Normal-quantile plots were used to test for normal distribution. Relationships between injury quartiles and quartiles of activated protein C and continuous variables were tested with the Kruskall-Wallis test followed by a non-parametric test for trend. Correlation was assessed by Spearman correlation coefficients. Regression analysis was used to examine the relationship between injury, the coagulation system and outcomes. A p-value of ≤ 0.05 was chosen to represent statistical significance.
Results
1245 patients were prospectively enrolled from 10 trauma centers. These patients represent a severely injured (Injury Severity Score, ISS, 26.2 ± 15.3) relatively young (age 40.8 ± 18.7) cohort. The penetrating injury rate was 35.3% with an overall mortality rate of 21.4%. Patient characteristics of the overall clinical coagulopathy cohort are detailed in Table 1. The median PT of the cohort was 15.0 (13.4–17.3) and the median PTT was 27.6 (24.1–33.0). Using an a priori definition (International Normalized Ratio, INR, ≥1.3), 41.6% (N=448) were coagulopathic on arrival to the emergency department (ED). Demographics by INR-defined coagulopathy are detailed in Table 2. By partial thromboplastin time (PTT) criteria (PTT ≥ 35), 20.5% (N=214) were coagulopathic on ED arrival. Patient demographics by PTT-defined coagulopathy are also detailed in Table 2. Coagulopathic patients were younger and more severely injured, have more traumatic brain injury and more severe shock. In addition these patients with ATC have greater transfusion requirements, more ventilator days, a higher preponderance of multiorgan failure and a significantly higher unadjusted in-hospital mortality (32.6% vs 12.5%, p ≤0.001) (Table 2).
Table 1.
Patient and coagulation characteristics of the PROMMTT cohort (N=1198/1245)
| Age | 40.8 ± 18.7 |
| % Male | 74.1% |
| Penetrating injury | 35.3% |
| Prehospital CPR | 1.9% |
| Prehospital intubation | 33.8% |
| Prehospital IVF | 750 (300–1500) |
| ISS | 26.2 ± 15.3 |
| AIS-head | 0 (0–3) |
| GCS | 14 (3–15) |
| ED heart rate | 105.9 ± 28.1 |
| ED systolic blood pressure | 108.1 ± 31.5 |
| ED temperature | 36.0 ± 1.1 |
| % MT | 23.9% |
| % to OR | 65.8% |
| Total hospital days | 12 (5–23) |
| ICU days | 5 (1–14) |
| Ventilator days | 1 (0–7) |
| % Multiorgan failure | 1.4% |
| Mortality (%) | 21.4% |
| INR | 1.2 (1.1–1.4) |
| PT | 15.0 (13.4–17.3) |
| PTT | 27.6 (24.1–33.0) |
| Fibrinogen | 229.5 ± 102.0 |
| Hemoglobin | 11.7 ± 2.3 |
| Platelet | 232.1 ± 83.3 |
| pH | 7.25 ± 0.14 |
| Base deficit | −7.0 ± 5.6 |
| Lactate | 4.2 (2.8–6.6) |
| % TEG run | 19.5% |
Table 2.
Patient characteristics by INR and PTT-based coagulopathy
| INR-based | PTT-based | |||||
|---|---|---|---|---|---|---|
| Coagulopathic | Non- coagulopathic |
P-value | Coagulopathic | Non- coagulopathic |
P-value | |
| N=488 | N=630 | N=214 | N=832 | |||
| Age | 38.6 ± 19.2 | 42.6 ± 18.0 | <0.001 | 40.4 ± 20.1 | 41.2 ± 18.3 | 0.62 |
| % Male | 75.2% | 71.9% | 0.24 | 72.0% | 73.9% | 0.60 |
| Penetrating injury | 30.8% | 32.7% | 0.55 | 21.5% | 34.3% | <0.001 |
| Prehospital CPR | 3.6% | 1.0% | 0.004 | 5.6% | 1.1% | <0.001 |
| Prehospital intubation | 50.1% | 24.8% | <0.001 | 62.0% | 29.0% | <0.001 |
| Prehospital IVF | 1000 (450–2000) | 500 (250–1000) | <0.001 | 1000 (425–2000) | 700 (300–1300) | <0.001 |
| ISS | 31.1 ± 15.5 | 23.9 ± 14.4 | <0.001 | 35.6 ± 16.6 | 24.6 ± 14.0 | <0.001 |
| AIS-head | 2 (0–4) | 0 (0–3) | <0.001 | 3 (0–5) | 0 (0–3) | <0.001 |
| GCS | 3 (3–14) | 15 (3–15) | <0.001 | 3 (3–7) | 14 (3–15) | <0.001 |
| ED heart rate | 111.7 ± 30.4 | 102.5 ± 25.9 | <0.001 | 110.5 ± 34.0 | 105.0 ± 26.2 | 0.03 |
| ED systolic BP | 104.3 ± 29.5 | 112.0 ± 31.8 | <0.001 | 101.5 ± 33.8 | 110.4 ±29.9 | <0.001 |
| ED temperature | 35.8 ± 1.2 | 36.1 ± 0.9 | <0.001 | 35.8 ± 1.1 | 36.0 ± 1.1 | 0.03 |
| % MT | 34.8% | 14.4% | <0.001 | 45.8% | 16.8% | <0.001 |
| % to OR | 62.6% | 64.3% | 0.61 | 58.7% | 64.7% | 0.11 |
| Total hospital days | 12 (3–26) | 12 (5–22) | 0.21 | 8 (1–28) | 13 (6–23) | <0.001 |
| ICU days | 6 (1–15) | 5 (2–13) | 0.70 | 5 (1–18) | 5 (2–14) | 0.60 |
| Ventilator days | 2 (0–9) | 1 (0–6) | 0.01 | 2 (0–10) | 1 (0–7) | <0.001 |
| % Multiorgan failure | 2.5% | 0.8% | 0.04 | 3.7% | 1.0% | 0.01 |
| Mortality (%) | 32.6% | 12.5% | <0.001 | 52.3% | 12.4% | <0.001 |
We next examined specific predictors of ATC by examining the relationship of ISS and Base Deficit (BD) on coagulopathy in the PROMMTT cohort based on previously published combination of injury severity and tissue hypoperfusion.(6, 8) Base deficit is inversely correlated with both prothrombin time (PT) (−0.26, p<.001) and PTT (−0.26, p<.001). Figure 1 shows a BD >−6 is associated with both prolonged PT (Figure 1A) and PTT (Figure 1B). A similar effect of ISS on INR and PTT is seen in Figure 1C and 1D. ISS is also correlated with PT (0.14, p<.001) and PTT-based coagulopathy (0.23, p<.001).
Figure 1.
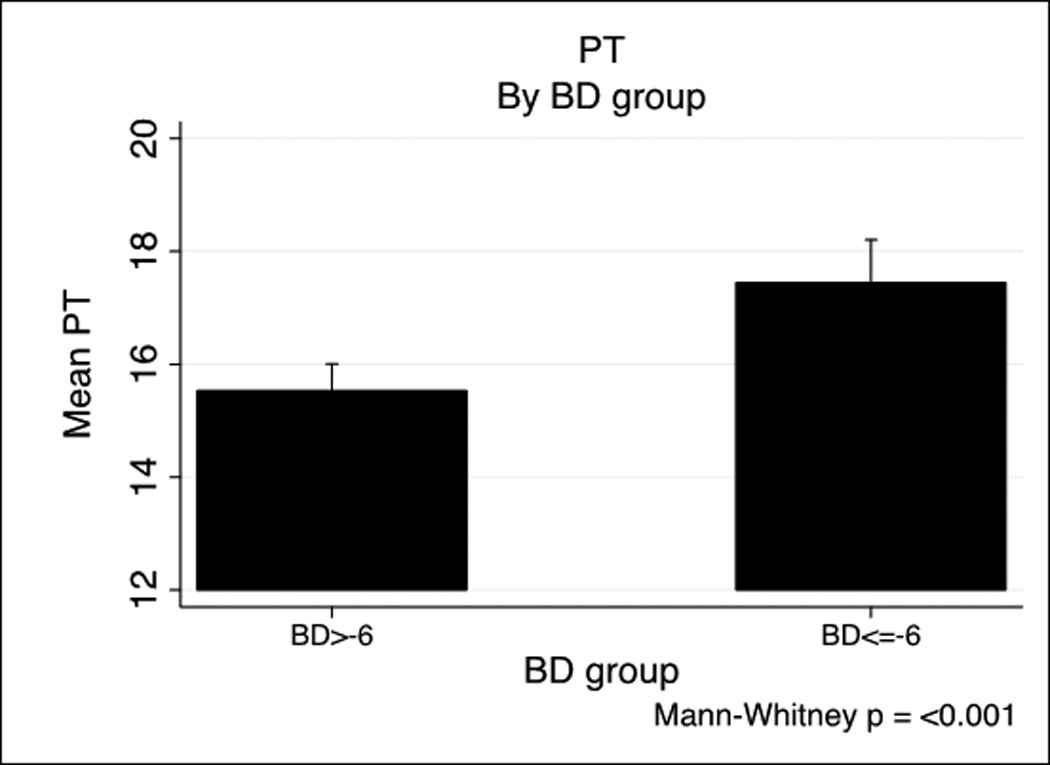
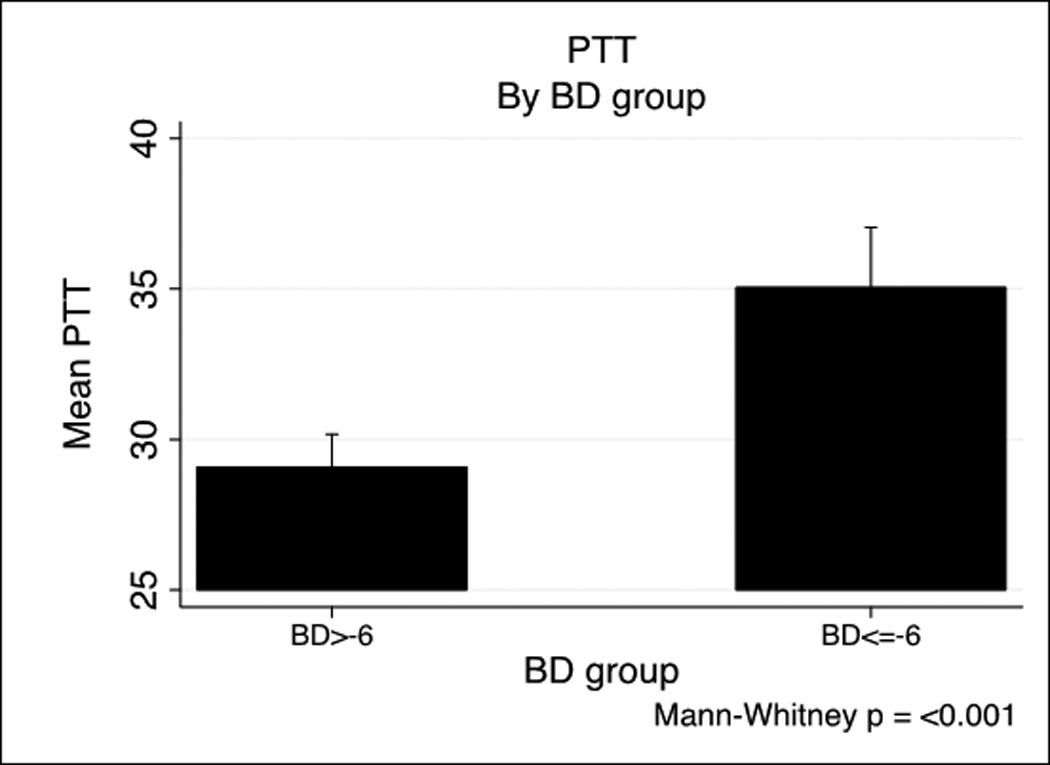
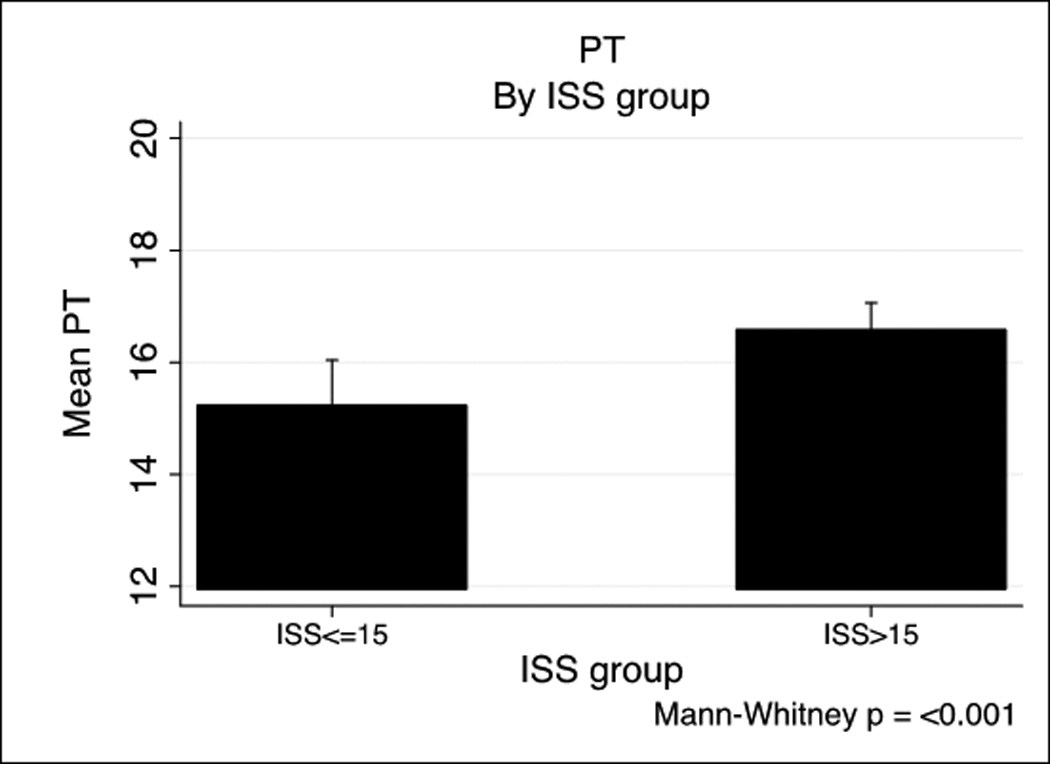
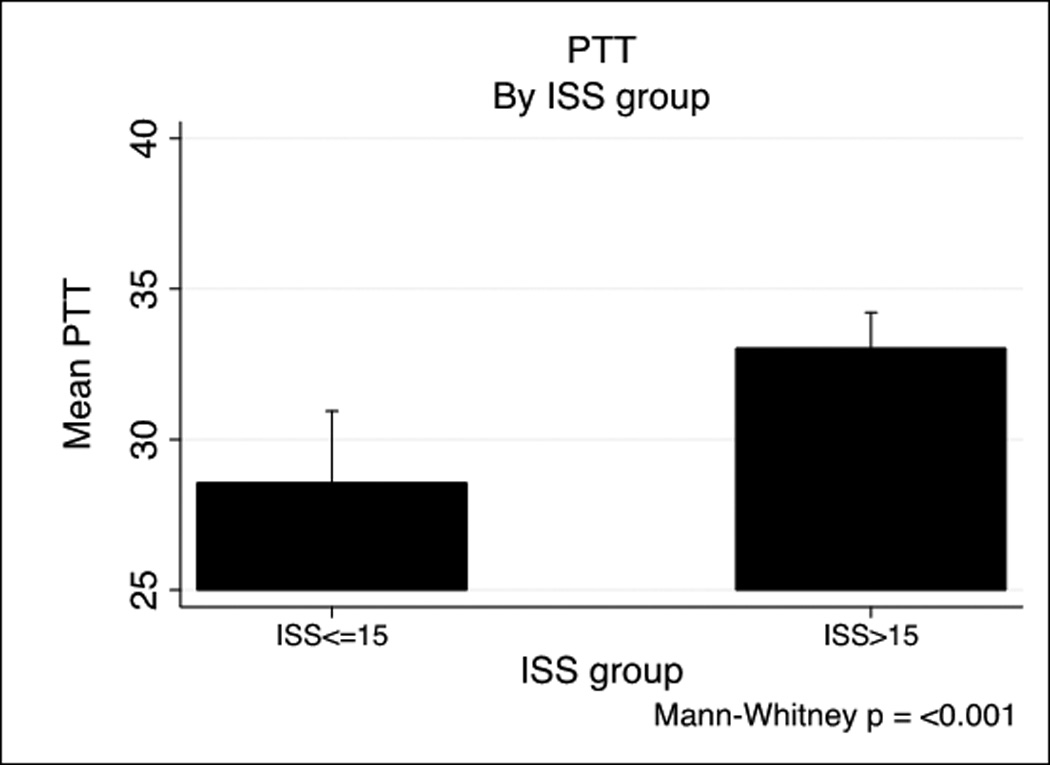
PT and PTT by base deficit and Injury Severity Score
We next examined the additive effects of shock and injury severity on coagulopathy(6, 16, 17). Figure 2 shows the combination of BD and ISS on PT (2A) and PTT (2B). To examine this association further, we next developed unadjusted and adjusted regression models for predictors of INR and PTT-based coagulopathy. The effector variables and relative effects (odds ratios, OR) can be seen in Table 3. When adjusted for ISS, Glasgow Coma Scale (GCS) and BD, each 100ml of pre-hospital crystalloid; ISS, GCS, heart rate (HR), systolic blood pressure (SBP), pH and BD were all predictors of INR-based coagulopathy. The predictors for PTT-based coagulopathy were identical except for HR which trended toward, but was non-significantly predictive (P =0.13).
Figure 2.
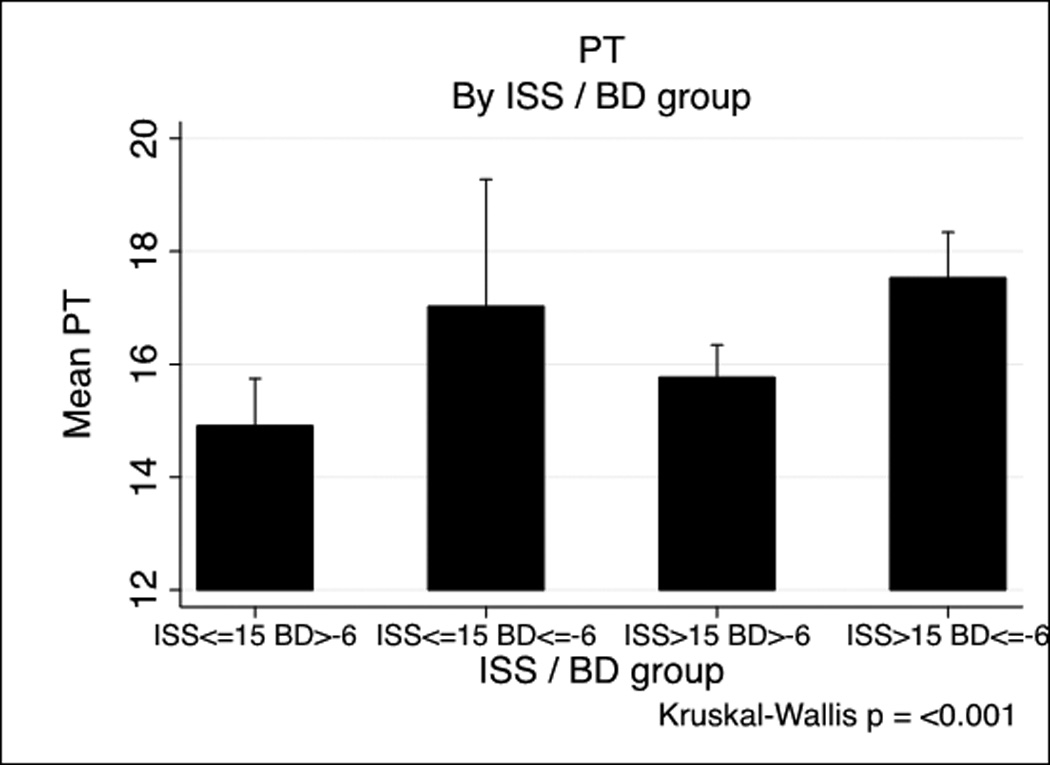
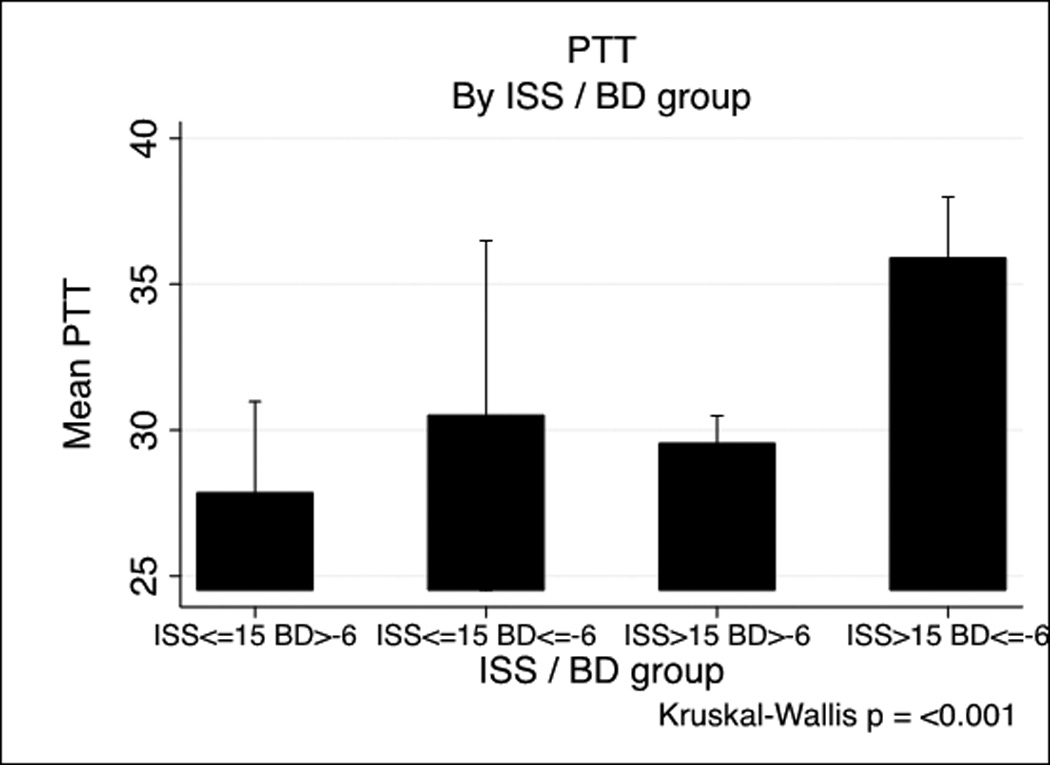
PT and PTT by Injury Severity Score/base deficit group
Table 3.
Unadjusted and adjusted* predictors of INR and PTT-based coagulopathy
| INR-based coagulopathy | PTT-based coagulopathy | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| Odds ratio |
P-value | Odds ratio |
P-value | Odds ratio |
P-value | Odds ratio |
P-value | |
| Age | 0.988 | 0.002 | 0.992 | 0.15 | 0.998 | 0.52 | 1.004 | 0.54 |
| Male gender | 1.186 | 0.40 | 1.086 | 0.74 | 0.906 | 0.69 | 0.847 | 0.61 |
| Penetrating injury | 0.916 | 0.67 | 1.340 | 0.29 | 0.526 | 0.10 | 0.784 | 0.68 |
| Prehospital crystolloid | 1.050 | <0.001 | 1.044 | <0.001 | 1.033 | <0.001 | 1.017 | <0.001 |
| ISS | 1.033 | <0.001 | 1.025 | <0.001 | 1.046 | <0.001 | 1.028 | 0.004 |
| AIS-head | 1.174 | <0.001 | 0.982 | 0.77 | 1.344 | <0.001 | 1.027 | 0.57 |
| GCS | 0.896 | <0.001 | 0.934 | <0.001 | 0.841 | <0.001 | 0.886 | <0.001 |
| Heart rate | 1.127 | <0.001 | 1.079 | 0.002 | 1.073 | 0.001 | 1.035 | 0.13 |
| Systolic blood pressure | 0.921 | 0.002 | 0.946 | 0.04 | 0.906 | 0.01 | 0.921 | 0.02 |
| Temperature | 0.739 | <0.001 | 0.842 | 0.03 | 0.811 | 0.01 | 0.802 | 0.01 |
| pH | 0.604 | <0.001 | 0.774 | 0.01 | 0.595 | <0.001 | 0.735 | 0.01 |
| Base deficit | 0.903 | <0.001 | 0.924 | <0.001 | 0.898 | <0.001 | 0.911 | 0.01 |
Adjusted for Injury Severity Score (ISS), Glasgow Coma Scale (GCS), and base deficit
Having examined predictors for developing ATC, we next sought to determine the functional drivers associated with ATC. Table 4 shows factor levels by INR and PTT-based coagulopathy. In the INR-based coagulopathic group, all of the protease factor levels were reduced while aPC and PTT were significantly higher. Only d-Dimer was not significantly changed in the coagulopathic vs. non coagulopathic patients. A similar analysis for PTT-based coagulopathy was performed and all protease factors except for factor X were significantly lower while aPC, PT and d-Dimer were significantly higher (Table 4).
Table 4.
Factor levels by INR and PTT-based coagulopathy
| INR-based | PTT-based | |||||
|---|---|---|---|---|---|---|
| Coagulopathic | Non- coagulopathic |
P-value | Coagulopathic | Non- coagulopathic |
P-value | |
| N=69 | N=87 | N=32 | N=123 | |||
| PT | 18.5 (16.5–24.0) | 14.9 (13.8–16.6) | <0.001 | 21.0 (16.8–28.9) | 15.6 (14.2–18.0) | <0.001 |
| PTT | 32.6 (27.5–38.0) | 26.9 (23.4–30.5) | <0.001 | 38.2 (32.9–48.6) | 27.2 (23.9–31.6) | <0.001 |
| Fibrinogen | 279 ± 34 | 209 ± 11 | <0.001 | 213 ± 18 | 271 ± 30 | <0.001 |
| Factor II | 61.9 ± 24.0 | 74.5 ± 26.1 | 0.01 | 52.3 ± 26.0 | 72.9 ± 24.1 | 0.001 |
| Factor V | 35.1 ± 23.0 | 57.6 ± 30.4 | <0.001 | 24.8 ± 20.0 | 54.2 ± 28.7 | <0.001 |
| Factor VII | 74.0 ± 29.9 | 91.5 ± 37.6 | 0.01 | 67.7 ± 26.8 | 87.4 ± 36.0 | 0.01 |
| Factor VIII | 302.0 ± 237.4 | 405.6 ± 237.4 | 0.01 | 180.2 ± 143.7 | 407.2 ± 240.7 | <0.001 |
| Factor IX | 108.4 ± 78.1 | 125.4 ± 102.2 | 0.27 | 91.9 ± 48.6 | 124.6 ± 99.1 | 0.02 |
| Factor X | 60.3 ± 25.0 | 72.5 ± 32.6 | 0.02 | 57.0 ± 27.6 | 69.2 ± 29.9 | 0.07 |
| AT III | 70.7 ± 24.8 | 80.6 ± 27.0 | 0.01 | 68.6 ± 25.5 | 78.4 ± 26.3 | 0.06 |
| D-dimer | 4.0 (1.4–20.0) | 3.8 (0.0–14.8) | 0.25 | 12.8 (4.0–20.0) | 3.0 (0.0–15.5) | 0.02 |
| Protein C | 71.3 ± 24.9 | 87.9 ± 32.2 | <0.001 | 66.5 ± 28.6 | 84.3 ± 29.7 | 0.003 |
| aPC | 37.2 (13.5–64.6) | 8.1 (1.1–16.5) | <0.001 | 37.2 (17.5–64.6) | 6.9 (1.9–12.8) | 0.001 |
Finally we sought to determine the primary logistic drivers of INR/PT and PTT-based coagulopathy. Table 5 shows univariable and multivariable (adjusted for age, ISS, arrival GCS and BD) predictors of INR-based coagulopathy. When adjusted for injury severity and shock factors IIa, Va, VIIa VIIa IX, X, fibrinogen and protein C are predictors of INR≥1.3 (Table 5). Table 5 also shows a similar analysis for predictors of PTT≥35 based coagulopathy. Unlike the INR-based coagulopathy only factors IIa, Va, VIIIa, IXa, fibrinogen and aPC are significant predictors of PTT ≥35.
Table 5.
Unadjusted and adjusted* factor predictors of INR and PTT-based coagulapathy
| INR-based coagulopathy | PTT-based coagulopathy | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
| Odds ratio |
P-value | Odds ratio |
P-value | Odds ratio |
P-value | Odds ratio |
P-value | |
| Fibrinogen | 0.983 | <0.001 | 0.981 | <0.001 | 0.978 | <0.001 | 0.981 | <0.001 |
| Factor II | 0.980 | <0.001 | 0.980 | <0.001 | 0.970 | <0.001 | 0.965 | 0.01 |
| Factor V | 0.970 | <0.001 | 0.973 | <0.001 | 0.953 | <0.001 | 0.961 | <0.001 |
| Factor VII | 0.985 | <0.001 | 0.981 | 0.001 | 0.983 | 0.002 | 0.980 | 0.30 |
| Factor VIII | 0.998 | <0.001 | 0.999 | 0.003 | 0.993 | <0.001 | 0.995 | <0.001 |
| Factor IX | 0.998 | <0.001 | 0.999 | <0.001 | 0.995 | <0.001 | 0.994 | 0.045 |
| Factor X | 0.985 | <0.001 | 0.981 | <0.001 | 0.987 | 0.04 | 0.984 | 0.13 |
| AT III | 0.985 | 0.02 | 0.992 | 0.22 | 0.987 | <0.001 | 1.003 | 0.85 |
| D-dimer | 1.028 | 0.09 | 1.009 | 0.33 | 1.071 | 0.23 | 1.058 | 0.21 |
| Protein C | 0.980 | <0.001 | 0.983 | 0.03 | 0.980 | 0.07 | 0.983 | 0.30 |
| aPC | 1.026 | 0.03 | 1.038 | 0.03 | 1.021 | 0.02 | 1.035 | 0.02 |
Adjusted for age, Injury Severity Score, arrival Glasgow Coma Scale and base deficit
Discussion
We present here analysis of both the clinical predictors and mechanistic drivers of acute traumatic coagulopathy. Utilizing the unique prospective data collection and blood sampling methodology of the PROMMTT study allows us to show that ATC occurs secondary to severe injury and shock and is mediated by a combination of protein C activation and clotting factor inactivation and depletion. Understanding of the clinical drivers and factor and protease milieu (both presented here) that comprise coagulation after trauma represent a crucial first step to comprehensively understanding and mediating the drivers and trajectory of coagulopathies after injury.
ATC is a newly recognized endogenous biological process, which occurs in approximately one third of severely injured patients(4). In the late 1970s with components in the blood bank and crystalloid on the shelf, the trauma community extrapolated new data and understanding of shock from luminaries such as Carrico and Shires and began to resuscitate with large volumes of oxygen-carrying capacity in the form of packed RBCs and large volumes of isotonic crystalloid in the name of restoring vascular flow(4). While data supports that this large volume crystalloid-based resuscitation regime was saving lives, there were unfortunate sequelae of this (then thought to be) necessary treatment. One of the primary effects of large volumes of cool diluting resuscitation fluid was coagulopathy and there is a vast literature exploring the effects of hypothermia, dilution and acidosis (with its concomitant reperfusion washout) on coagulation. Each of these (hypothermia, acidosis and dilution) is known to impair thrombin production and together they are now termed iatrogenic coagulopathy(4).
Until the initial discovery of and subsequent research into ATC, little attention was paid to early coagulopathy while hemostatic resuscitation goals were of secondary importance to large volume reversal of shock. Two discoveries within the past decade changed our understanding of coagulation after trauma and confirmed the importance of hemostatic resuscitation. In 2003 Brohi and Macleod concurrently published two papers describing ATC as an endogenous coagulation disturbance, which was separate and distinct from iatrogenic causes(12, 13). Subsequently other groups have confirmed the incidence of ATC and have conclusively shown that it is associated with poor outcomes. Our group has subsequently published evidence that ATC is primarily driven by activation of the protein C system(6, 18). Others have begun to characterize acquired and endogenous coagulation factor deficiencies after injury(19). Clinical work in this area has been taken into the basic science lab and clinical observation codified in both animal and in vitro investigations(8, 18, 20).
Coupled with this new biological understanding a second set of discoveries heightened interest in the benefits of hemostatic resuscitation. New data published in the early 2000s in both military and civilian cohorts suggested that attenuating inflammatory and coagulation perturbations after injury significantly reduced morbidity and mortality(2, 21). While this initial data indicated that plasma-based balanced ratio resuscitation was beneficial, critics noted that the data were retrospective and subject to potential survival bias(22–24). To address this issue, the PROMMTT study was funded to provide real-time prospectively-collected data on current resuscitation practices in order to elucidate the optimal plasma and platelet to RBC ratios to treat severe injury and shock. While eliminating many data collection issues that plagued other studies, PROMMTT also provided an ability to prospectively collect blood samples allowing survey of the coagulation milieu after trauma(14).
Our initial and primary finding is that ATC occurs among 42.7% using an INR cutoff and 20% using a PTT-based cutoff in the PROMMTT dataset (which represents a cohort of severely injured trauma patients receiving at least one unit of RBCs). Both of these cutoffs have been published previously and represent a tradeoff between sensitivity and specificity based on standard laboratory testing(7). Patients with ATC by either definition were more severely injured and suffered from a greater degree of shock, in keeping with previously published data(7, 17). Patients with ATC by either definition had longer hospital and ICU stays, more time on the ventilator, a higher burden of MOF, and significantly increased unadjusted in-hospital mortality. Examining the predictors of ATC more carefully reveals that PT/INR-based coagulopathy is driven by both injury severity and tissue hypoperfusion with shock being a stronger predictor of ATC. PTT-defined coagulopathy is similarly driven by injury and shock with more equal contributions between tissue injury and shock.
Regression analysis suggests that shock and tissue injury contribute significantly to INR-based coagulopathy with secondary contributions of well-defined iatrogenic causes including hypothermia and pre-hospital crystalloid. An interesting conundrum is teasing out the relative contributions of iatrogenic and endogenous causes of ATC. While pre-hospital crystalloid and temperature were predictors of coagulopathy, most patients in the PROMMTT dataset including those suffering from coagulopathy had relatively little pre-hospital crystalloid and non-clinically significant hypothermia suggesting that these ‘iatrogenic’ drivers in the regression analysis were more likely surrogates for more severely injured trauma patients. This is in keeping with other published work suggesting that ATC is driven primarily by a combination of tissue injury (evidenced by ISS) and shock (evidenced by BD). Unfortunately even in the granular PROMMTT data there is insufficient discriminatory data to tease out the relative contributions of endogenous and iatrogenic drivers. Because of this limitation, we do not suggest that the cutoffs for INR or PTT-defined coagulopathy should be used as a predictive score to activate a massive transfusion protocol. Rather, we suggest that any patient with significant injury and shock should be resuscitated with a plasma-based balanced hemostatic resuscitation protocol. There is good evidence to suggest that hemostatic resuscitation is beneficial independent of the coagulant function of the patient(25). Controlled resuscitation trials such as PROPPR, an ongoing NIH-funded multicenter study, are required to discern these differences.
Our second objective in the PROMMTT laboratory study was to examine the functional drivers of acute traumatic coagulopathy. When adjusted for age, ISS, GCS and BD, INR-defined coagulopathy was driven by reduced factor levels (IIa, Va, VIIa, VIIIa, IX and X) and fibrinogen coupled with activation of the protein C system (reduced protein C and increased aPC). PTT-based coagulopathy was characterized by reductions of factors (IIa, Va, VIIIa, IXa) fibrinogen and an increase in aPC. Similar to the predictors of ATC, reduced factor levels were driven by a combination of shock and injury. These data confirm single center data suggesting that ATC is mediated by activation of the protein C system(7, 8). Activated protein C is a serine protease, which once activated in a mechanism that involves thrombin, thrombomodulin and the endothelial protein C receptor, mediates its anticoagulant effects through direct proteolytic cleavage of factors Va and VIIIa(26). Our data show decreased Va and VIIIa levels which supports the aPC hypothesis that ATC is mediated through proteolytic inactivation of Va and VIIIa. Rizolli and colleagues have also published results indicating broad depletion of factor levels after injury suggestive of coagulopathy after trauma(19). Closer examination of our data suggests however that aPC cleaved factors V and VIII are most strongly affected by injury severity and shock and are most strongly inversely correlated with INR/PT and PTT. Indeed we believe that this is the case and there is direct mechanistic evidence to support this hypothesis(26, 27). It remains unclear whether reduced factor levels in general represent enhanced but non-pathologic functional protease activation toward fibrin production or whether reduced levels seen here and published by others is evidence of dilution or pathologic depletion.
Activated protein C mediates enhanced fibrinolyisis through actions on PAI-1 and t-PA. However fibrinolysis as evidenced by increased d-Dimer levels is not an adjusted predictor of ATC in these data. It is possible that enhanced fibrinolysis is not a primarily a driver of ATC as there are widely varying rates of fibrinolysis published in the literature or that d-Dimer is an insufficiently precise measure of ATC. Ideally examination of viscoelastic data would better to delineate fibrinolysis after trauma. Such investigation is planned and underway in the PROPPR study.
One last finding of interest is the association of reduced fibrinogen (Factor I) and ATC. This is a strong association, which has been previously suggested by others(28, 29). Mechanistically it may represent depletion of substrate, or reduced bioavailability(30). In either case as the unactivated substrate of final coagulation cascade its repletion through plasma based resuscitation or direct fibrinogen concentrate warrants significant further investigation.
There are several limitations to this study. First, we report only baseline laboratory results due to resource and IRB issues that precluded the collection of longitudinal samples. These issues have been resolved and prospective longitudinal sampling is part of the ongoing PROPPR study. Another limitation pertains to the cutoffs used to define ATC. While we utilized previously-published a priori cutoffs of INR≥1.3 and PTT≥35, these definitions remain an open analytical question. It is unclear if these cutoffs are appropriate for varying outcomes such as transfusion of blood products, functionally measured coagulopathy or mortality, and this question cannot be answered by these data.
How ATC relates to actual phenotypic bleeding also remains an open question. To address this requires standardization of the amount of bleeding and the cause of to determine if poor outcomes are caused by anatomic bleeding (large holes in vessels) or from microvascular or low pressure bleeding where coagulopathy might be contributory. Elucidation of this issue is currently underway by several groups. Lastly whether the poor outcomes associated with ATC and the benefits of balanced product resuscitation are mediated through the repair of perturbed clotting (thereby preventing bleeding and death) or from other inflammomodulatory effects remains a very important question. While bleeding is important there is building evidence suggesting that coagulopathy actually represents a perturbed inflammatory milieu and is associated with endotheial, epithelial and organ dysfunction. Hence whether hemostatic resuscitation prevents mortality by the attenuation of bleeding or the attenuating of an ‘endotheliopathy’ remains a crucial question which will only be addressed by a comprehensive characterization of coagulation and inflammation after injury coupled with animal and in vitro based studies.
In conclusion we present data here suggesting that ATC occurs after severe injury and shock. ATC is associated with factor reduction and reduced fibrinogen levels. Our data here are likely mediated by activation of protein C which inhibits factors Va and VIIIa and ATC is associated with significantly worse outcomes. The PROMMTT study represents a first step toward large multicenter characterization of coagulation after injury.
Acknowledgments
Funding/Support: This project was funded by the U.S. Army Medical Research and Materiel Command subcontract W81XWH-08-C-0712. Infrastructure for the Data Coordinating Center was supported by CTSA funds from NIH grant UL1 RR024148. Laboratory funding was provided by NIH GM-085689 (MJC)
Role of the Sponsor: The sponsors did not have any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit this manuscript for publication.
Dr Holcomb reported serving on the board for Tenaxis, the Regional Advisory Council for Trauma, and the National Trauma Institute; providing expert testimony for the Department of Justice; grants funded by the Haemonetics Corporation, and KCI USA, Inc. and consultant fees from the Winkenwerder Company. Dr Wade reported serving on the Science Board for Resuscitation Products, Inc. and the Advisory Board for Astrazeneca.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: No other disclosures were reported.
Disclaimer: The views and opinions expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Army Medical Department, Department of the Army, the Department of Defense, or the United States Government.
Previous Presentation of the Information Reported in the Manuscript: These data were presented at the PROMMTT Symposium held at the 71st Annual Meeting of the American Association for the Surgery of Trauma (AAST) on September 10–15, 2012 in Kauai, Hawaii.
Contributor Information
Matt Kutcher, Email: Matthew.Kutcher@ucsfmedctr.org.
Britt Redick, Email: redickb@sfghsurg.ucsf.edu.
Mary Nelson, Email: NelsonM@sfghsurg.ucsf.edu.
Mariah Call, Email: mariahcall10@gmail.com.
M Margaret Knudson, Email: pknudson@sfghsurg.ucsf.edu.
Martin A Schreiber, Email: schreibm@ohsu.edu.
Eileen M Bulger, Email: ebulger@u.washington.edu.
Peter Muskat, Email: muskatp@UCMAIL.UC.EDU.
Louis H Alarcon, Email: AlarconL@ccm.upmc.edu.
John G Myers, Email: myersjg@uthscsa.edu.
Mohammad H Rahbar, Email: mohammad.h.rahbar@uth.tmc.edu.
Karen J Brasel, Email: kbrasel@mcw.edu.
Herb A Phelan, Email: herb.phelan@utsouthwestern.edu.
Deborah J del Junco, Email: Deborah.j.deljunco@uth.tmc.edu.
Erin E Fox, Email: Erin.e.fox@uth.tmc.edu.
Charles E Wade, Email: Charles.E.Wade@uth.tmc.edu.
John B Holcomb, Email: John.Holcomb@uth.tmc.edu.
Bryan A Cotton, Email: bryan.a.cotton@uth.tmc.edu.
Nena Matijevic, Email: nevenka.matijevic-aleksic@uth.tmc.edu.
References Cited
- 1.Geneva: World Health Orginization; Injury Chart Book. [Google Scholar]
- 2.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46(5):685–686. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, et al. Causes of death in U.S. Special Operations Forces in the global war on terrorism: 2001–2004. Ann Surg. 2007;245(6):986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. The Surgical clinics of North America. 2012;92(4):877–891. doi: 10.1016/j.suc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Howard BM, Daley AT, Cohen MJ. Prohemostatic interventions in trauma: resuscitation-associated coagulopathy, acute traumatic coagulopathy, hemostatic resuscitation, and other hemostatic interventions. Seminars in thrombosis and hemostasis. 2012;38(3):250–258. doi: 10.1055/s-0032-1306435. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255(2):379–385. doi: 10.1097/SLA.0b013e318235d9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MJ, West M. Acute traumatic coagulopathy: from endogenous acute coagulopathy to systemic acquired coagulopathy and back. The Journal of trauma. 2011;70(5 Suppl):S47–S49. doi: 10.1097/TA.0b013e31821a5c24. [DOI] [PubMed] [Google Scholar]
- 8.Davenport R, Manson J, De'Ath H, Platton S, Coates A, Allard S, et al. Functional definition and characterization of acute traumatic coagulopathy. Critical care medicine. 2011;39(12):2652–2658. doi: 10.1097/CCM.0b013e3182281af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SJ, Lee SW, Han GS, Moon SW, Choi SH, Hong YS. Acute traumatic coagulopathy decreased actual survival rate when compared with predicted survival rate in severe trauma. Emergency medicine journal : EMJ. 2011 doi: 10.1136/emermed-2011-200630. [DOI] [PubMed] [Google Scholar]
- 10.Maegele M, Paffrath T, Bouillon B. Acute traumatic coagulopathy in severe injury: incidence, risk stratification, and treatment options. Deutsches Arzteblatt international. 2011;108(49):827–835. doi: 10.3238/arztebl.2011.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scalea TM. Hemostatic resuscitation for acute traumatic coagulopathy. Scandinavian journal of trauma, resuscitation and emergency medicine. 2011;19:2. doi: 10.1186/1757-7241-19-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 13.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 14.Rahbar MH, Fox EE, del Junco DJ, Cotton BA, Podbielski JM, Matijevic N, et al. Coordination and management of multicenter clinical studies in trauma: Experience from the PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) Study. Resuscitation. 2012;83(4):459–464. doi: 10.1016/j.resuscitation.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 16.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–1217. doi: 10.1097/TA.0b013e318169cd3c. discussion 7. [DOI] [PubMed] [Google Scholar]
- 17.Cohen MJ, Brohi K, Ganter MT, Manley GT, Mackersie RC, Pittet JF. Early coagulopathy after traumatic brain injury: the role of hypoperfusion and the protein C pathway. J Trauma. 2007;63(6):1254–1261. doi: 10.1097/TA.0b013e318156ee4c. discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 18.Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32(6):659–665. doi: 10.1097/SHK.0b013e3181a5a632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizoli SB, Scarpelini S, Callum J, Nascimento B, Mann KG, Pinto R, et al. Clotting factor deficiency in early trauma-associated coagulopathy. The Journal of trauma. 2011;71(5) Suppl 1:S427–S434. doi: 10.1097/TA.0b013e318232e5ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M, et al. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/beta-catenin signaling. Stem Cells Dev. 2011;20(1):89–101. doi: 10.1089/scd.2010.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62(2):307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 22.Borgman MA, Spinella PC, Holcomb JB, Blackbourne LH, Wade CE, Lefering R, et al. The effect of FFP:RBC ratio on morbidity and mortality in trauma patients based on transfusion prediction score. Vox Sang. 2011;101(1):44–54. doi: 10.1111/j.1423-0410.2011.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 24.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 25.Brown L, Call MS, MS K, Cohen MJ. A High FFP:PRBC Transfusion Ratio Decreases Mortality in All Massively Transfused Trauma Patients Regardless of Admission INR. J Trauma. 2011 doi: 10.1097/TA.0b013e318227f152. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esmon CT. The protein C pathway. Chest. 2003;124(3 Suppl):26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 27.Esmon CT. Inflammation and the activated protein C anticoagulant pathway. Semin Thromb Hemost. 2006;32(Suppl 1):49–60. doi: 10.1055/s-2006-939554. [DOI] [PubMed] [Google Scholar]
- 28.Sorensen B, Tang M, Larsen OH, Laursen PN, Fenger-Eriksen C, Rea CJ. The role of fibrinogen: a new paradigm in the treatment of coagulopathic bleeding. Thrombosis research. 2011;128(Suppl 1):S13–S16. doi: 10.1016/S0049-3848(12)70004-X. [DOI] [PubMed] [Google Scholar]
- 29.Stinger HK, Spinella PC, Perkins JG, Grathwohl KW, Salinas J, Martini WZ, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. The Journal of trauma. 2008;64(2 Suppl):S79–8S5. doi: 10.1097/TA.0b013e318160a57b. discussion S. [DOI] [PubMed] [Google Scholar]
- 30.Martini WZ, Holcomb JB. Acidosis and coagulopathy: the differential effects on fibrinogen synthesis and breakdown in pigs. Ann Surg. 2007;246(5):831–835. doi: 10.1097/SLA.0b013e3180cc2e94. [DOI] [PubMed] [Google Scholar]


