Abstract
Traumatic brain injury (TBI) promotes neural stem/progenitor cell (NSC) proliferation in the adult hippocampus; however, it remains inconclusive whether proliferation of these cells results in newly generated mature neurons, leading to increased neurogenesis. When we traced the fates of proliferating cells labeled with bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU) we found the number of BrdU-positive cells increased in the hippocampus of TBI mice compared to the sham control. However, double immunostaining to distinguish their cell types showed that most of these cells were glia, and that only a small subpopulation is newborn granular neurons. There was no significant difference with respect to neurogenesis in the adult hippocampus between the injured and the control mice. These results indicate that TBI promotes cell proliferation including astrocyte activation and NSC proliferation. Nevertheless, the majority of the BrdU-positive cells are glia. The neurogenesis is not increased by TBI. These data suggest that TBI activates through promotion of NSC proliferation an innate repair and/or plasticity mechanism in the brain. However, additional intervention is required to increase neurogenesis for successfully repairing the damaged brain following TBI.
Keywords: Traumatic brain injury, Neurogenesis, Neural stem/progenitor cells, Gliogenesis, Hippocampal dentate gyrus, Subgranular zone
Introduction
Traumatic brain injury (TBI) is a serious public health problem in the United States (Cicerone, et al., 2005, McCarthy, et al., 2005, Prigatano, 2005, Salmond and Sahakian, 2005, Stiles, et al., 2005, Vakil, 2005). It represents a complex injury with a broad spectrum of symptoms and disabilities. Effective treatment options are nonexistent. Recent research has identified neural stem/progenitor cells (NSCs) in the adult mammalian hippocampus that can support neurogenesis throughout life, as demonstrated in rodents and primates, including humans (Cameron and McKay, 2001, Eriksson, et al., 1998, Eriksson, et al., 1998, Kornack and Rakic, 1999, Kuhn, et al., 1996, Leuner, et al., 2007). Currently the consensus among researchers in the field is that throughout adulthood, NSCs in the subgranular zone (SGZ) of the hippocampal dentate gyrus (HDG) continuously generate new neurons (Kempermann and Gage, 2000, Ming and Song, 2005) and develop into mature granular neurons (Ming and Song, 2005, Shapiro and Ribak, 2005, Zhao, et al., 2006). The pool of NSCs is a potential resource for repairing the damaged hippocampus following TBI.
Bromodeoxyuridine (5-bromo-2-deoxyuridine; BrdU) labeling experiments have suggested that TBI promotes cell proliferation in the adult hippocampus (Braun, et al., 2002, Chirumamilla, et al., 2002, Dash, et al., 2001, Kernie, et al., 2001, Ramaswamy, et al., 2005, Rice, et al., 2003, Rola, et al., 2006, Sun, et al., 2005, Sun, et al., 2007, Yoshimura, et al., 2003). There are distinct classes of NSCs in the adult HDG, including quiescent neural progenitors (QNPs), which carry stem cell properties, and their progeny, amplifying neural progenitors (ANPs) (Bull and Bartlett, 2005, Encinas and Enikolopov, 2008, Encinas, et al., 2006, Encinas, et al., 2008, Filippov, et al., 2003, Mignone, et al., 2004, Seaberg and van der Kooy, 2002, Seri and Garcia-Verdugo, 2001). We found that moderate TBI promotes proliferation of QNPs in the adult hippocampus (Gao, et al., 2009).
Although TBI promotes NSCs proliferation, the effect of TBI on neurogenesis is still controversial. There are conflicting reports about neurogenesis in the HDG. According to some studies neurogenesis decreases after TBI (Braun, et al., 2002, Rola, et al., 2006), whereas others have reported that it remains unchanged (Chirumamilla, et al., 2002, Rice, et al., 2003), or that it increases (Sun, et al., 2005, Sun, et al., 2007). The experimental procedure of these studies was to inject animals daily with BrdU consecutively for 7 days(i.p. once per day). This procedure assessed the cumulative effect of TBI on cell proliferation. It did not address what are those proliferating cells, when cell proliferation gets started, how long the promoted proliferation lasts, how many of those proliferating cells survive, and what kind of cell they differentiate into. Furthermore, besides its action on NSCs, TBI is known to induce proliferation of reactive astrocytes (Floyd and Lyeth, 2007, Sandhir, et al., 2008). Since there is large number of reactive glial cells labeled by BrdU as well, it is possible to mistake gliogenesis for neurogenesis following TBI. To address these questions, here we traced the fate of those proliferating cells following TBI in the combination of BrdU labeling, multiple cell-type specific markers, transgenic mouse, and microscopy with 3-dimensional reconstruction.
Materials and Methods
Animal Care
Male C57 BL/6 mice (Jackson Laboratories) were group-housed and kept in a 12/12-hour light/dark cycle with free to access to food and water ad libitum. The nestin-EGFP transgenic mice (C57/BL6) were kindly provided by Dr. Enikolopov at Cold Spring Harbor Laboratories and described previously (Mignone et al., 2004). The animals were used in experiments at an age of 8 weeks. All procedures were performed under protocols approved by Indiana University’s Animal Care and Use Committee.
Controlled Cortical Impact Traumatic Brain Injury
Eight-week-old mice were subjected to moderate, controlled cortical impact injury (CCI) or sham surgery as previously described (Gao and Chen, 2008, Gao, et al., 2008, Hall, et al., 2004, Hall, et al., 2005, Saatman, et al., 2006, Sullivan, et al., 1999, Sullivan, et al., 1999). Briefly, the mice were anesthetized and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA) prior to TBI. Using sterile procedures, the skin was retracted and a 4 mm craniotomy centered between the lambda and bregma sutures was performed. A point was identified midway between the lambda and bregma sutures and laterally midway between the central suture and the temporalis muscle. The skullcap was carefully removed without disruption of the underlying dura. Prior to injury induction, the tip of the impactor was angled and kept perpendicular to the exposed cortical surface. The mouse CCI model uses an electromagnetic impactor that allows one to alter the severity of the injury by controlling contact velocity and the level of cortical deformation independently. In the experiments for this study, the contact velocity was set at 3.0 m/sec and deformation at 1.0 mm. These settings will result in an injury of moderate severity. Following injury induction, a 4 mm wide disk made of dental cement (Dentsply Trubyte, Johnson and Johnson, Arlington, TX) was placed over the craniotomy site, adhered to the skull using cyanoacrylate, and allowed to dry prior to suturing of the wound. During surgery and recovery, the core body temperature of the animals was maintained at 36–37°C using a heating pad. Sham (noninjured) animals were subjected to craniotomy, but did not receive a CCI injury.
Pulse-Labeling of the Proliferating Cells Following TBI
The mice were subjected to moderate TBI or sham surgery as described, followed by administration of BrdU immediately after (time 0), and at 20 hours, 44 hours, 68 hours, and 1 week following TBI (5 mice for each time point; BrdU: 100 mg/kg in saline, i.p.; Sigma, St. Louis, MO). The mice were perfused 4 hours after BrdU injection to assess proliferation of NSCs and activated astrocytes.
Determining the Fate of the Proliferating Neural Stem/Progenitor Cells Following TBI
Eight-week-old male C57/BL6 mice were subjected to moderate TBI or sham surgery as described. The sham and the injured mice (5 animals for each group) were given bromodeoxyuridine (BrdU) injections once per day for 7 days (50 mg/kg in saline, i.p.; Sigma, St. Louis, MO). Four weeks after the final BrdU injection the brains were fixed to evaluate the differentiation of BrdU-labeled cells in the hippocampus.
Tissue Processing
The animals were deeply anesthetized and then perfused transcardially with cold saline, followed by a fixative containing 4% paraformaldehyde (PFA) in PBS. The brains were removed, post-fixed overnight in PFA, and cryoprotected for 48 hours in 30% sucrose. Serial 30 μm thick coronal sections were cut using a cryostat (Leica CM 1950), and stored at −20°C. The sections were then processed for immunohistochemical analysis.
Immunohistochemistry
Every sixth section (180 μm interval), covering the distance of the hippocampus, was processed for immunohistochemical analysis. Free-floating sections were washed twice in PBS, incubated in 2N HCl for 1 hour at room temperature, and then soaked in 0.1 M borate buffer for 10 minutes (pH 8.4). After washing with PBS (3 times), the sections were incubated in blocking solution (0.1% Triton X-100, 1% bovine serum albumin, and 5% normal goat serum in PBS) for 1 hour at room temperature, followed by overnight incubation with primary antibody at 4°C. The sections were washed again with PBS (3 times), and incubated at room temperature for 2 hours with the secondary antibody. After treatment (2 minutes) with DAPI (4′,6-diamidino-2-phenylindole), the sections were washed with PBS (3 times), and mounted using Fluorescent Mount G. Primary antibodies and their final concentrations were as follows: anti-BrdU (1:400, rat, Accurate Chemical and Scientific), anti-NeuN (1:1000, mouse, Millipore), anti-nestin (1:1000; rabbit; Covance, Berkeley, CA), anti-GFAP (1:100, rabbit, Sigma), anti-Iba-1 (1:200, goat, Abcam), anti-EGFP (1:1000, rabbit, Invitrogen), anti-S100 (1:200, rabbit, Sigma). Secondary antibodies from Jackson ImmunoResearch Laboratories, Inc. were applied in a dilution of 1:1000.
Cell Counting
Immunohistochemistry was performed simultaneously on sections to detect the target cells. Series of every sixth section (30 μm thickness, 180 μm apart) through each hippocampus were processed. The cell density was determined through a blinded quantitative histological analysis. The profile count method was used. Every single BrdU-positive cell (even the partial of BrdU-positive nuclei at the border of section), or BrdU and specific cell marker (NeuN for mature neurons and GFAP for astrocytes) double-labeled cell in the different subregion of the dentate gyrus (including the molecular layer [ML], the granule cell layer [GCL], the subgranular zone [SGZ], and the hilus) in the multiplanes throughout the entire 30 μm section, was counted under a fluorescent microscope using the 40× objective through a whole series of sections.
The double-labeled cell was determined as follows. We used BrdU as an indicator. When the BrdU-positive cell showed up, we switched to the channel matching the cell specific marker. If the target cell also had been marked, we considered it as double-labeled cell. The total number of quantified cells was justified by correction (Coggeshall and Lekan, 1996). The contours of the dentate gyrus area and each subregion were created, and the volume was measured using Bioquant software (Nashville, TN). BrdU-positive or double-labeled cells were expressed as average number/mm3 (n=5 for each group).
Microscopy
The sections were analyzed using an inverted microscopy system (Zeiss Axiovert 200 M) combined with apotome and interfaced with a digital camera (Zeiss Axio Cam MRc5) controlled by a computer. Images were captured using apotome in software (AxioVision, v4.8) and assembled and labeled in Photoshop 7.0 (Adobe Systems).
Statistical Analysis
The collected data were analyzed using Student’s t-test with significance set at p<0.05.
Results
Moderate TBI Transiently Promotes Cell Proliferation in the Hippocampal Dentate Gyrus
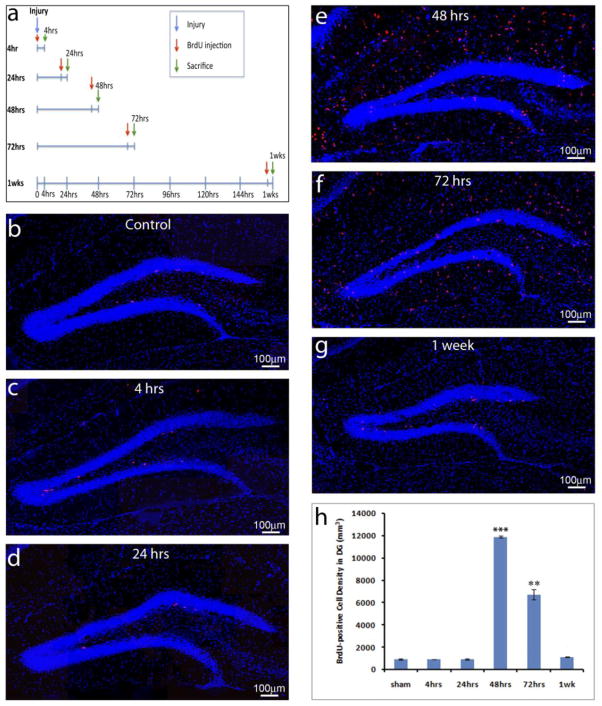
Analysis of the cumulative effect of TBI on cell proliferation 1 week after injury induction showed that TBI significantly promotes cell proliferation in the HDG (Braun, et al., 2002, Chirumamilla, et al., 2002, Dash, et al., 2001, Kernie, et al., 2001, Ramaswamy, et al., 2005, Rice, et al., 2003, Rola, et al., 2006, Sun, et al., 2005, Sun, et al., 2007, Yoshimura, et al., 2003). However, the temporal profile and long-term phenotypic fate of proliferating hippocampal cells are not well investigated. To address these questions, we assess cell proliferation at different days following TBI using a pulse-labeling with BrdU. As shown in Figure 1a, BrdU was injected either right after injury or, 1, 2, 3, or 7 days after injury. The mice were sacrificed 4 hours after BrdU injection (Figure 1a). This study allowed us to assess the proliferating cells within a 4-hour time window at different days after injury using immunostaining with anti-BrdU antibody. In the sham-controls, there was only a low level of cell proliferation labeled by BrdU in the HDG (927 ± 33/mm3, n=5) (Figure 1b, h). There was no significant increase in the density of BrdU-positive cells right after injury (928 ± 12/mm3, p=0.96, n=5) (Figure 1c, h) and 24 hours after injury (942 ±61/mm3, p=0.84, n=5) (Figure 1d, h). The density of BrdU-positive cells in the HDG increased dramatically at 48 hours (11882 ± 99/mm3, p<0.001, n=5) (Figure 1e, h) following TBI. At the 72-hour time point post-TBI the density of BrdU-positive cells was still high (6723 ± 448/mm3, p<0.005, n=5) (Figure 1f, h) but almost 50% lower than the peak at 48 hours. The density of BrdU-positive cells returned to near pre-injury baseline levels 7 days after surgery (1123 ±19/mm3, p=0.056, n=5) (Figure 1g, h). These pulse-labeling experiments indicate that TBI transiently but significantly promotes cell proliferation in the adult HDG.
Figure 1. TBI insults promote NSC proliferation in the adult hippocampus.
(a) The schematic shows pulse labeling of proliferating cells in the hippocampus at different days following traumatic brain injury (TBI). (b–g) Immunostaining with an anti- BrdU antibody (red) to identify proliferating cells in the hippocampus of sham control (b) or TBI mice at different time points after surgery (c–g). Nuclei are stained with DAPI (blue) to show the structure of the dental gyrus. (h) Quantification of BrdU-positive cells in the hippocampal dentate gyrus following TBI. (*** p<0.0001; ** p<0.001, n=5 for each group).
TBI significantly promotes gliogenesis while it only transiently increase NSC proliferation in the hippocampus
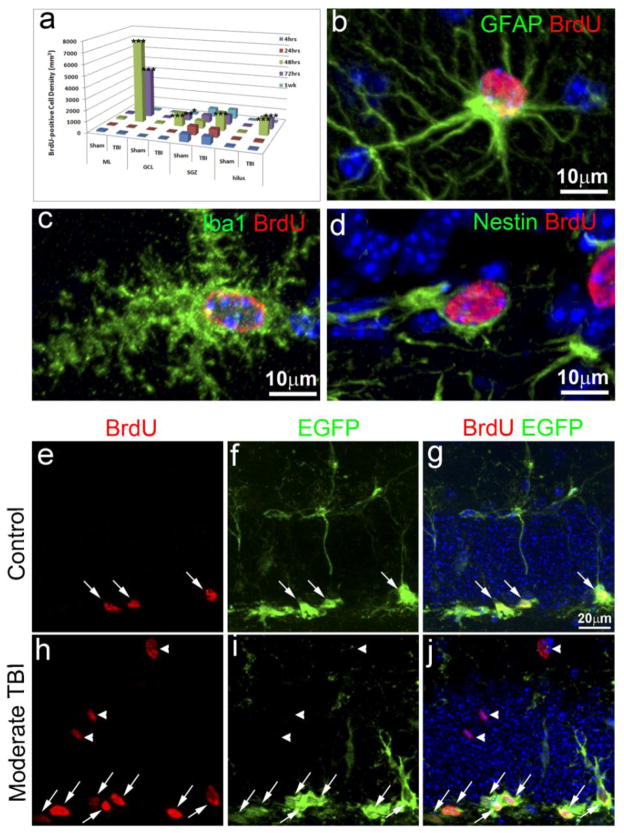
TBI not only promotes NSC proliferation, it also activates glia to enter the cell cycle (Laird, et al., 2008). BrdU labels cells in proliferating, it does not distinguish whether a proliferating cell is a NSC or a reactive glial cell. However, NSCs and reactive glial cells distribute at different locations in the hippocampus and express different cellular markers. These characteristics provide approaches to distinguish proliferating NSCs from reacting glial cells after injury. NSCs reside in the SGZ while gliogenesis mainly occurs in the other regions, such as the molecular layer (ML) and the hilus in the HDG (Myer, et al., 2006). In order to distinguish proliferating NSCs from reactive glia, we first analyzed where the proliferating cells were located. In the HDG of the sham control, the majority (74%) of the BrdU-positive cells were located in the SGZ, there was a very small number of BrdU-positive cells in other regions, including ML, GCL, and the hilus (Figure 2a). In the HDG of the TBI mice, no significant differences were observed in the number and distribution of the BrdU-positive cells in the HDG up to the 24 hours after injury. Nonetheless, at 48 hours and 72 hours following TBI (Figure 2a), the numbers of BrdU-positive cells were not only dramatically increased (Figure 1e, f and h), they were no longer restricted to the SGZ. Instead they were distributed over the entire hippocampus and the BrdU-positive cells were mainly found in the ML and the hilus of the HDG. The density of the BrdU-positive cells in the SGZ increased only transiently and showed a prominent peak increased at 48 hours after surgery (1623 ± 49/mm3, 2.3 times higher than the sham control 700 ± 32.8/mm3, p<0.001, n=5 for each group) (Figure 2a). The number of the BrdU-positive cells in the SGZ only represented 13.7% of the total number of BrdU-positive cells in the HDG. Nonetheless, there was a very dramatic increase of the number of BrdU-positive cells in the ML (7475 ± 77/mm3, n=5), GCL (1212 ± 85/mm3) and the hilus (1572 ± 49/mm3, n=5) 48 hours post-injury (Figure 2a), representing 62.9%, 10.2% and 13.2%, respectively, of the total number of BrdU-positive cells in the HDG at this particular time point after TBI. The number of BrdU-positive cells remained very high in the ML (4378 ± 362/mm3), GCL (558 ± 22/mm3) and the hilus (938 ± 77/mm3) in mice 72 hours post-injury (Figure 2a), representing 65.2%, 8.3% and 20.1%, respectively, of the total number of BrdU-positive cells in the HDG for this time point. However, these results show that TBI promotes significant cell proliferation in the adult hippocampal dentate gyrus (HDG), mainly in the ML, GCL and hilus, while cell proliferation in the SGZ is only transient with a peak at around 48 hours after injury. With respect to the proliferating cells in the different regions of the HDG 48 hours after TBI by immunostaining with different cell-specific markers, further analysis found that most of the BrdU-positive cells in the ML either colocalize with the astrocyte marker GFAP or with the microglia marker, Iba-1 (Figure 2b, c). Only the BrdU-positive cells in the SGZ colocalized with Nestin, which is a NSC marker (Figure 2d). To further assess the BrdU-positive cells in the SGZ, we took advantage of a nestin-EGFP transgenic mouse, in which EGFP driven by nestin promoter is expressed in the NSCs in the SGZ. In the sham operated mice, 48 hours after surgery, there were a total of 780 ± 59 BrdU-positive cells in the entire ipsilateral SGZ of the hippocampus. Of these BrdU-positive cells, 98.5% co-labeled with EGFP (Figure 2e–g, white arrows). While as in the TBI injured mice, there are a total of 1742 ± 122 BrdU-positive cells in the entire ipsilateral SGZ of the hippocampus, among them 97% of the BrdU-positive cells colocalized with EGFP (Figure 2h–j, white arrows), indicating that most of the BrdU-positive cells in the SGZ are NSCs either in the sham operated mice or in the TBI-injured mice. In contrast, the BrdU-positive cells in the GCL did not colocalize with EGFP (white arrowheads, Figure 2h–j). Most of the BrdU-positive cells in the GCL, ML and hilus colocalizwed with Iba I, a cell type specific marker for microglial at this time point after injury (Data not shown). Together, these results suggest that TBI significantly promotes gliogenesis in the ML and hilus, while it only transiently increases NSC proliferation in the SGZ with a peak at 48 hours after injury.
Figure 2. Spatial and temporal distribution of the proliferating cells and their fate in the hippocampus one week after TBI.
(a) Quantification of the proliferating cells in the hippocampus after traumatic brain injury (TBI) and their spatial and temporal distribution (n=5). * p<0.05; ** p<0.001; *** p<0.0001. (b) Double immunostaining with anti-BrdU (red) and anti-GFAP (green) antibodies to identify proliferating astrocytes in the injured hippocampus. (c) Double immunostaining with anti-BrdU (red) and Iba1 (green) antibodies to identify proliferating microglia in the injured hippocampus. (d) Double immunostaining with anti-BrdU (red) and anti-nestin (green) antibodies to identify proliferating neural stem/progenitor cells (NSCs) in the injured hippocampus. (e) Immunostaining with anti-BrdU (red) to identify proliferating cells in the hippocampal dentate gyrus (HDG) of nestin-EGFP transgenic mice after sham treatment. (f) Immunostaining with anti-EGFP (green) to identify EGFP expressing NSCs in the HDG of nestin-EGFP transgenic mice after sham treatment. (g). Merged image of (e) and (f). The BrdU and EGFP double-positive cells are pointed out by white arrows. (h) Immunostaining with anti-BrdU (red) to identify proliferating cells in the hippocampal dentate gyrus of nestin-EGFP transgenic mice after TBI. (i) Immunostaining with anti-EGFP (green) to identify EGFP expressing NSCs in the HDG of nestin-EGFP transgenic mice after TBI. (j). Merged image of (h) and (i). The BrdU and EGFP double-positive cells are pointed out by white arrows. The BrdU+ positive cells without expressing EGFP are pointed out by white arrowheads.
Most of the surviving BrdU-positive cells were located in non-neurogenic areas in the hippocampus following TBI
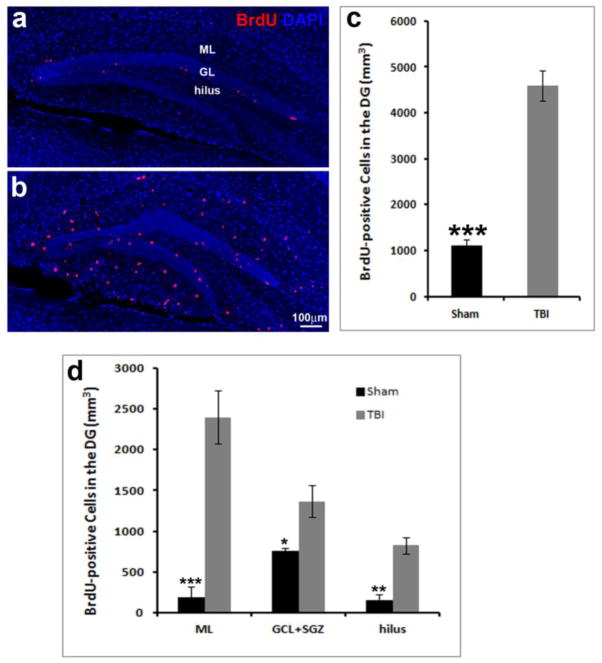
Eight-week-old male mice were subjected to moderate CCI injury or sham treatment as described (Gao and Chen, 2009, Gao, et al., 2008, Gao, et al., 2009). Subsequently the animals were injected with BrdU (50mg/kg) in saline consecutively for 7 days following CCI injury (i.p. once per day). Since it takes about 1 month for the newborn neurons to develop into mature neurons, the mice were kept for 28 days after the last injection with BrdU. After 1 month they were perfused transcardially with 4% paraformaldehyde, followed by removal of the brains for analysis. BrdU-labeled cells in the HDG were visualized by immunostaining with anti-BrdU antibody, which visualized a significant number of surviving BrdU-positive cells in the dentate gyrus (Figure 3a, b). Quantification showed 1104 ± 127/mm3 BrdU-positive cells in the HDG of the sham mice, while 4592 ± 329/mm3 BrdU-positive cells were counted in the HDG of the injured mice (Figure 3c). These results indicated that the number of BrdU-positive cells in the HDG of the injured mice was significantly higher than in the control mice (p<0.0001, n=5 for each group) 5 weeks following TBI.
Figure 3. Spatial and temporal distribution of the proliferated cells.
5 weeks after TBI.
Immunostaining with an anti-BrdU (red) antibody to identify proliferated cells in the hippocampus of the sham control (a) or TBI mice (b) 5 weeks after surgery. Nuclei are stained with DAPI (blue) to show the hippocampal dentate gyrus (HDG) structure. (c) Quantification of BrdU-positive cells in the HDG following TBI. (d) Distribution of BrdU-positive cells in the HDG following TBI. GL: granule cell layer. ML: molecular layer. (*** p<0.0001; ** p<0.001; * p<0.01, n=5 for each group).
We further determined the distribution of the surviving BrdU-positive cells in the different hippocampal subregions, namely the ML, GCL, SGZ, and the hilus. In the control mice most of the BrdU-positive cells were located in the inner one-third of the GCL, while the BrdU-positive cells on the ipsilateral side of the CCI-injured mice were scattered over the entire hippocampus. As shown in Figure 3d, in the control mice, we found 193 ± 29/mm3, 762 ± 77/mm3, and 149 ± 40/mm3 of the surviving BrdU-positive cells in the ML, GCL/SGZ, and the hilus, respectively. These numbers represent 17.5%, 69%, and 13.5% of the total number of BrdU-positive cells in the ML, GCL/SGZ, and the hilus, respectively. In the control mice 69% of the surviving BrdU-positive cells were located in the neurogenic area, including the GCL/SGZ, and only a small percentage of the surviving BrdU-positive cells were located in the non-neurogenic area, including the ML and hilus. In contrast, in the HDG of the injured mice, we found 2401 ± 196/mm3, 1365 ± 101/mm3, and 827 ± 50/mm3 of the surviving BrdU-positive cells were in the ML, GCL/SGZ, the hilus, respectively. These numbers correspond to 52.3%, 29.7%, and 18% of the total number of BrdU-positive cells in the ML, GCL/SGZ, and hilus, respectively. Most of the surviving BrdU-positive cells were located in non-neurogenic areas including the ML and the hilus (70.3%). Only 29.7% of the surviving BrdU-positive cells were located in the neurogenic area, including the GCL/SGZ. These results suggest that 5 weeks after surgery the number of BrdU-positive cells in the hippocampus is still significantly higher in the HDG of the CCI-injured mice than the sham mice. In the sham-control mice most of the surviving BrdU-positive cells were located in the GCL/SGZ, where the newborn neurons reside. In contrast, in the TBI-mice, the majority of the surviving BrdU-positive cells were located in the molecular layer, which does not undergo neurogenesis. These results suggest that the surviving BrdU-positive cells in the sham group might be newborn neurons, while most of the surviving BrdU-positive cells in the TBI group are likely reactive astrocytes.
Moderate TBI Promotes Gliogenesis in the Hippocampal Dentate Gyrus
TBI is known to induce proliferation of reactive astrocytes as well as stem/progenitor cells (Floyd and Lyeth, 2007, Sandhir, et al., 2008). To distinguish between the 2 processes we analyzed the cell types of the surviving BrdU-positive cells by double immunostaining. Brain sections collected 28 days after BrdU injection were immunostained with anti-BrdU antibody and the antibody against GFAP, an astrocyte marker. The stained sections were evaluated under a Zeiss microscope. We observed a dramatic increase in the number of GFAP-positive cells in the HDG of the injured mice compared to the control mice (Figure 4). This result was confirmed by imaging of 3-dimesional reconstruction using a Zeiss microscope equipped with an apotome (Figure 4. c and f). The GFAP-positive cells were mainly located in the non-neurogenic regions, such as the ML and the hilus (Figure 4). A very large portion of the BrdU-positive cells in the ML (Figure 4) and the hilus (data not shown) colocalized with GFAP in cells that showed typical stellate-shaped astrocyte morphology. The results of the next studies, shown in Figure 5 and Figure 6, confirmed the increase of astrocytes in the ML and the hilus. These data indicate a significant increase in gliogenesis in the HDG following TBI.
Figure 4. Astrocyte activation in the hippocampal dentate gyrus following TBI.
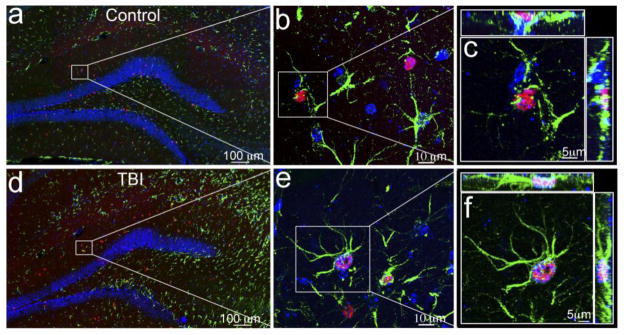
Double immunostaining with anti-BrdU (red) and anti-GFAP (green) antibodies to identify the proliferating astrocytes in the hippocampus of the sham (a–c) or traumatic brain injury (TBI) mice (d–f). The nuclei are stained with DAPI (blue) to show the hippocampal dentate gyrus (HDG) structure. (a, d) Proliferating astrocytes in the hippocampus of the sham or TBI mice at low magnification. BrdU-positive cells appear in red and GFAP-positive cells in green. (b, e) BrdU-positive astrocytes (indicated by the white box) in the molecular layer of sham or TBI mice at high magnification. (c, f) Fluorescent images with 3-dimensional reconstruction to confirm the proliferating astrocytes (indicated by a white box) in the molecular layer of sham or TBI mice.
Figure 5. Gliogenesis in the adult hippocampus following TBI.
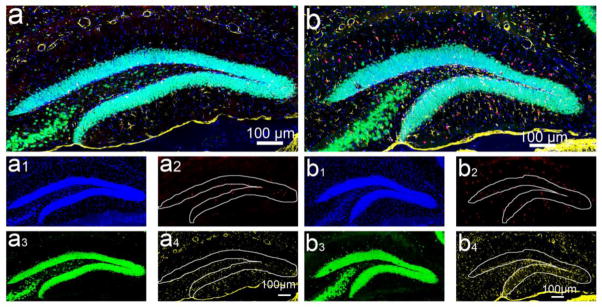
Immunostaining with anti-BrdU (red), anti-NeuN (green), and anti-GFAP (yellow) antibodies to identify gliogenesis and neurogenesis in the hippocampus of sham (a) and TBI mice (b) 5 weeks after injury. (a1–a4) are the separated channels of (a). (b1–b4) are the separated channels of (b). Nuclei are stained with DAPI (blue) to show the HDG structure.
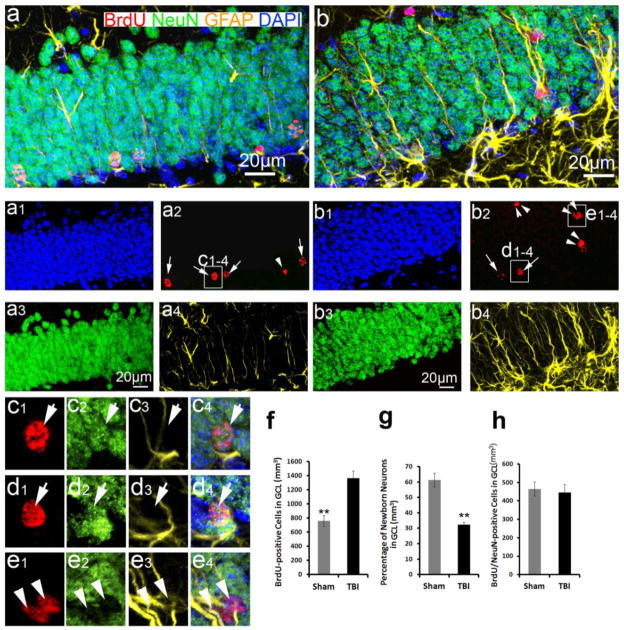
Figure 6. Neurogenesis in the hippocampal dentate gyrus following TBI.
(a, b) Enlarged images to show the granular cell layers in the hippocampus of sham control (a) and traumatic brain injury (TBI) mice (b) in Figure 5. Nuclei are stained with DAPI (blue) to show the granule cell layer (GCL) structure. (a1–a4) are the separated channels of (a). (b1–b4) are the separated channels of (b). The nuclei in cells that are round and have a diameter of more than 10 mm are pointed out by white arrows; oval- or spindle-shaped cells with a diameter of 10 mm or less are indicated by white arrowheads. (c1–c4) Enlarged images to confirm BrdU and NeuN double positive newborn neurons in the inner GCL of the control hippocampus. (d1–d4). Enlarged images to confirm BrdU and NeuN double positive newborn neurons in the inner GCL of the injured hippocampus. (e1–e4) Enlarged images to confirm BrdU and GFAP double positive astrocytes in the GCL of the injured hippocampus. (f) Quantification of the surviving BrdU-positive cells in the GCL of the hippocampus following TBI. (g) Quantification of neuronal differentiation in the hippocampal dentate gyrus following TBI. (h) Quantification of newborn neurons in the hippocampus following TBI. (** p<0.001, n=5 for each group).
Moderate TBI Does Not Increase Neurogenesis in the Hippocampal Dentate Gyrus
We proceeded to determine the cell types of the surviving BrdU-positive cells by triple immunostaining with anti-BrdU, anti-GFAP, and anti-NeuN antibodies. NeuN is a marker for mature neurons. The results of these experiments showed a significant increase in the number of BrdU-positive cells in the HDG in the injured mice. These cells were mainly distributed in the ML and the hilus (Figure 5), which confirms our previous results (Figure 3d). We selected 1-in-6 series of brain sections containing hippocampus to evaluate the BrdU-positive cells in the GCL, which is known to be neurogenenic. As shown in Figure 3d, we found a total of 762 ± 77/mm3 surviving BrdU-positive cells in the entire GCL of the control mice, while the entire GCL of the CCI-injured animals in comparison had 1365 ± 101/mm3 (Figure 6f). This difference is statistically significant (p<0.001, n=5 for each group), suggesting that more BrdU-positive cells survive in the GCL of the CCI-injured mice than in the GCL of the control animals. An analysis of the morphologies of the BrdU-positive nuclei in the GCL under high magnification or by fluorescent imaging found that, in the control mice, the BrdU-positive cells were mainly located in the inner one-third of the GCL, where the newborn neurons are located. The newborn neurons can be identified morphologically by their round shape and size of more than 10 μm in diameter (Figure 6a, pointed out by arrows). In contrast, in the GCL of the injured mice, the BrdU-positive cells were distributed over the entire GCL, and exhibited 2 very different nuclear morphologies. One type was of a round shape and more than 10 μm in diameter (Figure 6b, pointed out by arrows), while the other one was an oval or spindle shape with a diameter of less than 10 μm (Figure 6b, pointed out by arrowheads). The round nuclei colocalized with NeuN, but not with GFAP, indicating they were newborn granular neurons (Figure 6c, d). In contrast, the spindle-shaped nuclei did not colocalize with NeuN, but colocalized with GFAP (Figure 6e), indicating they were astrocytes. It is interesting that the appearance of some of the GFAP+/BrdU+ cells in the GCL do not take on the classic stellate astrocyte appearance but appear more similar to type-1 nestin expressing stem/progenitor cells extending long GFAP processes through the GCL (figure 6b). When we distinguished the type-1 nestin expressing NSCs from reactive astrocytes using a nestin-transgenic mouse in which EGFP only expressed in the type-1 NSCs but not in the astrocytes. The results showed that most of the GFAP-positive processes in the granular cell layer extending from SGZ and hilus are from GFAP-positive astrocytes, not from nestin+/GFAP+ type-1 neural stem/progenitor cells, only a few of them are from nestin+/GFAP+ type-1 neural stem/progenitor cells (Supplemental Figure 1). These results confirmed that there is a significant increase of gliogenesis in the GCL following TBI. We further quantified the cell types of BrdU-positive cells using 1-in-6 series of sections containing hippocampus. The results showed that 61 ± 5% of the BrdU-positive nuclei in the GCL of the control mice were newborn granular neurons, while only 33 ± 3% of the BrdU-positive nuclei in the GCL of the CCI-injured mice represented newborn neurons (Figure 6g). In the GCL of the control mice, the majority of the BrdU-labeled cells (465 ± 38/mm3) were co-labeled with NeuN (Figure 6h). They represented 43% of the total BrdU- positive cells in the entire HDG of the sham mice. Though a significant number of BrdU-positive cells was located in the GCL of the CCI-injured mice at a higher density than in the sham mice, only a small portion (445 ± 45/mm3) of these BrdU-positive cells was colabeled with NeuN (Figure 6h). These co-labeled cells represented just 9 % of the total BrdU-positive cells in the entire HDG of the injured mice. These results indicate that there is no significant difference in the number of newborn neurons between the sham and the injured mice (P=0.75, n=5 for each group) at 28 days after TBI. To assess whether this is partially because TBI delayed neuron maturation, we examined BrdU-positive cell with immunostaining using an antibody against doublecortin protein (Dcx). At 28 days after injury the BrdU cells no longer expressed Dcx (Supplemental Figure 2). This result did not support that TBI delayed the maturation of newborn neurons in the adult hippocampus. These studies indicated that TBI promotes the proliferation of NSCs without increasing neurogenesis.
Discussion
Traumatic brain injury (TBI) promotes cell proliferation in the adult hippocampus, suggesting that the brain has innate repair and/or plasticity mechanisms. However, when we assessed the distribution of these proliferating cells in the hippocampus, and traced their fate following TBI, we found that the proliferation of NSCs is only transiently increased, the majority of the proliferating and surviving BrdU-positive cells appeared to be astrocytes, and neurogenesis in the adult hippocampus did not significantly increase following TBI. In order to increase neurogenesis following TBI, and to repair the damage, additional events are required.
Recent reports that describe the use of bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU) labeling indicate that TBI increases proliferation of NSCs in the hippocampus (Braun, et al., 2002, Chirumamilla, et al., 2002, Dash, et al., 2001, Kernie, et al., 2001, Ramaswamy, et al., 2005, Rice, et al., 2003, Rola, et al., 2006, Sun, et al., 2005, Sun, et al., 2007, Yoshimura, et al., 2003). However, at this point these results are still controversial. Other reports have found that after TBI neurogenesis in the HDG decreases (Braun, et al., 2002, Rola, et al., 2006), increases (Sun, et al., 2005, Sun, et al., 2007), or remains unchanged (Chirumamilla, et al., 2002, Rice, et al., 2003). One reason for these conflicting results might be that they were obtained using different injury models, and that they differ in the severity level of the inflicted injury and in the technical difficulties encountered when assessing neurogenesis. Following TBI, the BrdU-labeled cells in the HDG could either be proliferating NSCs, newborn astrocytes from proliferating NSCs, or reactive astrocytes. Since there is a large number of reactive astrocytes in the dentate gyrus, with many of them proximally located to the newborn neurons in the subgranular zone, an analysis of NSC proliferation and neurogenesis in the HDG following TBI is complicated, in particular, when such an analysis is solely based on BrdU labeling. Because of the close proximity, the BrdU-labeled nucleus of the reactive glia at the border between the hilus and SGZ can be mistaken for the nucleus of a neuron and can be incorrectly identified as a newly generated neuron. Thus, one option to study neurogenesis following TBI could be to use triple immunostaining and a combination of an anti-BrdU antibody and antibodies against other cell-type-specific markers. This approach would allow one to distinguish the newborn neurons and the proliferating NSCs from the glia.
The location of the BrdU-positive cells in the hippocampus may provide supporting evidence for cell identity, as well. The HDG can be divided into 4 subregions: the ML, GCL, SGZ, and the hilus. Different subregions are composed of very different cell types. NSCs reside in the subgranular zone. Newborn neurons derived from the NSCs usually migrate into the GCL and are located in the inner granular cell layer close to the SGZ. Therefore, the BrdU-positive cell population in the GCL might include reactive astrocytes labeled by BrdU at the time of surgery, or they might be newborn neurons originating from the SGZ, which have migrated into the GCL. Typically, newborn granular neurons will neither migrate into the hilus nor the molecular layer. Thus, the BrdU-positive cells in these 2 subregions are likely not newborn granular neurons. A combination of immunostaining with a battery of antibodies, positional information, and high resolution imaging techniques should help us to assess gliogenesis and neurogenesis in the hippocampus following TBI.
When a combination of these techniques was used, we found that TBI significantly increased cell proliferation in the hippocampal dentate gyrus 48 to 72 hours after TBI. However, BrdU-positive cells were, for the most part, located in the ML and hilus, which are non-neurogenic. Cell proliferation in the SGZ, where the NSCs reside, was increased for a short period of time 48 hours after injury induction. These results suggest that most of the proliferating cells are not NSCs. Immunostaining with GFAP, and Iba1, further confirmed that the proliferating cells in the HDG are mostly reactive glia. TBI-enhanced NSC proliferation occurred only for a short period of time.
Further analysis showed that the number of BrdU-positive cells in the hippocampal dentate gyrus was significantly increased 5 weeks following TBI, and that the majority of these cells were reactivated astrocytes. Only a subpopulation of BrdU-positive cells in the hippocampal dentate gyrus represented newborn neurons. However, when we examined the fate of the proliferating NSCs 5 weeks after TBI by fluorescent microscopy followed by 3-dimensional reconstruction, we found no increase in neurogenesis in the hippocampus. On the contrary, neurogenesis was slightly decreased. These results indicate that moderate TBI enhances proliferation of NSCs without significantly increasing neurogenesis in the adult hippocampus. Other reports found an increase in neurogenesis after fluid percussion induced brain injury (Sun, et al., 2005) or hypoxic-ischemic brain injury (Miles and Kernie, 2008) in juvenile animals suggesting this may be age and injury model dependent. Our previous results, as well as the report by Rola et al. (Rola, et al., 2006), showed that most of the degenerating neurons in the inner granular neuron layer are immature newborn neurons. Further quantitative analysis demonstrated that the number of immature newborn neurons in the dentate gyrus is dramatically decreased in the ipsilateral hemisphere compared to the contralateral side (Gao, et al., 2008), and, therefore, might prevent neurogenesis from proliferating NSCs following TBI. TBI induces proliferation of NSCs in the HDG. However, neurogenesis is not increased, possibly due to the selective susceptibility of immature newborn neurons.
Our data suggest that TBI potentially activates an innate repair and/or plasticity mechanisms in the brain. Unfortunately, this process is not always successful. NSCs reside in a specialized microenvironment or niche (Wurmser, et al., 2004). Self-renewal and differentiation of NSCs is regulated by interactions between NSCs and the 3-dimensional local milieu or niche (Alvarez-Buylla and Lim, 2004, Doetsch, 2003, Fuchs, et al., 2004, Shen, et al., 2004, Tumbar, et al., 2004, Watt and Hogan, 2000, Wurmser, et al., 2004). The molecular mechanisms promoting NSC proliferation are still largely unknown. NSCs may respond to circulating molecules either released by the dying neurons or secreted from reactive glia following TBI. We are very interested in these molecules, and we would like to understand how NSCs are induced to enter the cell cycle after injury. Toward this goal, we have developed a method to isolate NSCs in situ to investigate the molecules that are modified by TBI (Gao and Chen, 2008). In order to successfully repair damage to the brain caused by TBI, additional events are required to increase not only proliferation of NSCs, but also to prevent newborn neurons from dying. Our recent data have shown that brain-derived neurotrophhic factor (BDNF) is involved in regulating newborn neuronal survival in the hippocampus following TBI (Gao and Chen, 2009). Characterizing the response of NSCs to TBI and understanding the molecular mechanisms that underlie the susceptibility of the newborn neurons might lead to novel therapeutic strategies that might serve as neuroprotective and neuroregenerative treatments. Thus, strategies that enhance neurogenesis are of particular interest based on their potential to replace the damaged neurons, as well as to improve post-traumatic neurological recovery.
Supplementary Material
(a–e). Double immunostaining with anti-GFAP and anti-EGFP were performed to identify GFAP-positive cells with their processes (a, red) and EGFP-positive cells with their processes (b, green) in the nestin-EGFP transgenic mice 28 days after sham treatment. (c) Staining with DAPI to show the nuclei in the hippocampal dentate gyrus (HDG). (e) An enlarged image from the white box in the (d). The processes from EGFP-positive neural stem/progenitor cells were also labeled by GFAP (white arrows). (d) An merged image of (a)–(c). (f–j). Double immunostaining with anti-GFAP and anti-EGFP were performed to identify GFAP-positive cells with their processes (f, red) and EGFP-positive cells with their processes (g, green) in the nestin-EGFP transgenic mice 28 days after TBI. (h) Staining with DAPI to show the nuclei in the HDG. (i) An merged image of (f)–(h). (j) An enlarged image from the white box in the (i). A few processes from EGFP-positive neural stem/progenitor cells were also labeled by GFAP (white arrows). Majority of the processes are only labeled by GFAP.
(a) Double immunostaining with anti-BrdU and anti-Dcx were performed to identify BrdU-positive cells and Dcx-positive newborn neurons in the hippocampal dentate gyrus (HDG) of control C57/BL6 mice. (b) Double immunostaining with anti-BrdU and anti-Dcx were performed to identify BrdU-positive cells and Dcx-positive newborn neurons in the HDG of traumatic brain injury (TBI) mice. Staining with DAPI to show the nuclei in blue.
Highlights.
TBI promotes cell proliferation in the adult hippocampus
Most of the proliferating cells in the hippocampus following TBI are reacting glia
Neural stem cell proliferation is transiently increased in the hippocampus following TBI.
Moderate TBI does not increase neurogenesis in the adult hippocampus.
Acknowledgments
This work was supported by funding from the Indiana Spinal Cord & Brain Injury Research Grants (SCBI 200-12), the Ralph W. and Grace M. Showalter Research Award, Indiana University Biological Research Grant, NIH grants RR025761 and 1R21NS072631-01A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 2.Braun H, Schafer K, Hollt V. BetaIII tubulin-expressing neurons reveal enhanced neurogenesis in hippocampal and cortical structures after a contusion trauma in rats. J Neurotrauma. 2002;19:975–983. doi: 10.1089/089771502320317122. [DOI] [PubMed] [Google Scholar]
- 3.Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 5.Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- 6.Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, Ellmo W, Kalmar K, Giacino JT, Harley JP, Laatsch L, Morse PA, Catanese J. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil. 2005;86:1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Encinas JM, Enikolopov G. Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol. 2008;85:243–272. doi: 10.1016/S0091-679X(08)85011-X. [DOI] [PubMed] [Google Scholar]
- 11.Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Encinas JM, Vazquez ME, Switzer RC, Chamberland DW, Nick H, Levine HG, Scarpa PJ, Enikolopov G, Steindler DA. Quiescent adult neural stem cells are exceptionally sensitive to cosmic radiation. Exp Neurol. 2008;210:274–279. doi: 10.1016/j.expneurol.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 15.Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 16.Floyd CL, Lyeth BG. Astroglia: important mediators of traumatic brain injury. Prog Brain Res. 2007;161:61–79. doi: 10.1016/S0079-6123(06)61005-4. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Chen J. Direct isolation of neural stem cells in the adult hippocampus after traumatic brain injury. J Neurotrauma. 2008;25:985–995. doi: 10.1089/neu.2008.0460. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, Chen J. Conditional knockout of brain-derived neurotrophic factor in the hippocampus increases death of adult-born immature neurons following traumatic brain injury. J Neurotrauma. 2009 doi: 10.1089/neu.2008.0744. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, Deng-Bryant Y, Cho W, Carrico KM, Hall ED, Chen J. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J Neurosci Res. 2008 doi: 10.1002/jnr.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Deng-Bryant Y, Cho W, Carrico KM, Hall ED, Chen J. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J Neurosci Res. 2008;86:2258–2270. doi: 10.1002/jnr.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao X, Enikolopov G, Chen J. Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus. Exp Neurol. 2009;219:516–523. doi: 10.1016/j.expneurol.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- 24.Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- 25.Kempermann G, Gage FH. Neurogenesis in the adult hippocampus. Novartis Found Symp. 2000;231:220–235. discussion 235–241, 302–226. [PubMed] [Google Scholar]
- 26.Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001;66:317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- 27.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16:154–164. doi: 10.1159/000111560. [DOI] [PubMed] [Google Scholar]
- 30.Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci U S A. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy ML, MacKenzie EJ, Durbin DR, Aitken ME, Jaffe KM, Paidas CN, Slomine BS, Dorsch AM, Berk RA, Christensen JR, Ding R. The Pediatric Quality of Life Inventory: an evaluation of its reliability and validity for children with traumatic brain injury. Arch Phys Med Rehabil. 2005;86:1901–1909. doi: 10.1016/j.apmr.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 32.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 33.Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus. 2008;18:793–806. doi: 10.1002/hipo.20439. [DOI] [PubMed] [Google Scholar]
- 34.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 35.Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 36.Prigatano GP. Impaired self-awareness after moderately severe to severe traumatic brain injury. Acta Neurochir Suppl. 2005;93:39–42. doi: 10.1007/3-211-27577-0_5. [DOI] [PubMed] [Google Scholar]
- 37.Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 38.Rice AC, Khaldi A, Harvey HB, Salman NJ, White F, Fillmore H, Bullock MR. Proliferation and neuronal differentiation of mitotically active cells following traumatic brain injury. Exp Neurol. 2003;183:406–417. doi: 10.1016/s0014-4886(03)00241-3. [DOI] [PubMed] [Google Scholar]
- 39.Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp Neurol. 2006;202:189–199. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 40.Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma. 2006;23:1241–1253. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- 41.Salmond CH, Sahakian BJ. Cognitive outcome in traumatic brain injury survivors. Curr Opin Crit Care. 2005;11:111–116. doi: 10.1097/01.ccx.0000155358.31983.37. [DOI] [PubMed] [Google Scholar]
- 42.Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seri B, Garcia-Verdugo JM. Astrocytes Give Rise to New Neurons in the Adult Mammalian Hippocampus. The Journal of Neuroscience. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shapiro LA, Ribak CE. Integration of newly born dentate granule cells into adult brains: hypotheses based on normal and epileptic rodents. Brain Res Brain Res Rev. 2005;48:43–56. doi: 10.1016/j.brainresrev.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 47.Stiles J, Reilly J, Paul B, Moses P. Cognitive development following early brain injury: evidence for neural adaptation. Trends Cogn Sci. 2005;9:136–143. doi: 10.1016/j.tics.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan PG, Thompson MB, Scheff SW. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- 50.Sun D, Colello RJ, Daugherty WP, Kwon TH, McGinn MJ, Harvey HB, Bullock MR. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J Neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- 51.Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. J Clin Exp Neuropsychol. 2005;27:977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- 54.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 55.Wurmser AE, Palmer TD, Gage FH. Neuroscience. Cellular interactions in the stem cell niche. Science. 2004;304:1253–1255. doi: 10.1126/science.1099344. [DOI] [PubMed] [Google Scholar]
- 56.Yoshimura S, Teramoto T, Whalen MJ, Irizarry MC, Takagi Y, Qiu J, Harada J, Waeber C, Breakefield XO, Moskowitz MA. FGF-2 regulates neurogenesis and degeneration in the dentate gyrus after traumatic brain injury in mice. J Clin Invest. 2003;112:1202–1210. doi: 10.1172/JCI16618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a–e). Double immunostaining with anti-GFAP and anti-EGFP were performed to identify GFAP-positive cells with their processes (a, red) and EGFP-positive cells with their processes (b, green) in the nestin-EGFP transgenic mice 28 days after sham treatment. (c) Staining with DAPI to show the nuclei in the hippocampal dentate gyrus (HDG). (e) An enlarged image from the white box in the (d). The processes from EGFP-positive neural stem/progenitor cells were also labeled by GFAP (white arrows). (d) An merged image of (a)–(c). (f–j). Double immunostaining with anti-GFAP and anti-EGFP were performed to identify GFAP-positive cells with their processes (f, red) and EGFP-positive cells with their processes (g, green) in the nestin-EGFP transgenic mice 28 days after TBI. (h) Staining with DAPI to show the nuclei in the HDG. (i) An merged image of (f)–(h). (j) An enlarged image from the white box in the (i). A few processes from EGFP-positive neural stem/progenitor cells were also labeled by GFAP (white arrows). Majority of the processes are only labeled by GFAP.
(a) Double immunostaining with anti-BrdU and anti-Dcx were performed to identify BrdU-positive cells and Dcx-positive newborn neurons in the hippocampal dentate gyrus (HDG) of control C57/BL6 mice. (b) Double immunostaining with anti-BrdU and anti-Dcx were performed to identify BrdU-positive cells and Dcx-positive newborn neurons in the HDG of traumatic brain injury (TBI) mice. Staining with DAPI to show the nuclei in blue.