Abstract
Recent evidence shows that traumatic brain injury (TBI) regulates proliferation of neural stem/progenitor cells in the dentate gyrus (DG) of adult hippocampus. There are distinct classes of neural stem/progenitor cells in the adult DG, including quiescent neural progenitors (QNPs), which carry stem cell properties, and their progeny, amplifying neural progenitors (ANPs). The response of each class of progenitors to TBI is not clear. We here used a transgenic reporter Nestin-GFP mouse line, in which QNP and ANP cells are easily visualized and quantified, to determine the targets of the TBI in the DG. We examined changes in proliferation of QNPs and ANPs in the acute phase following TBI and found that QNPs were induced by TBI insult to enter the cell cycle whereas proliferation of ANPs was not significantly affected. These results indicate that different subtypes of neural stem/progenitor cells respond differently to TBI insult. Stem cell activation by the TBI may reflect the induction of innate repair and plasticity mechanisms by the injured brain.
Keywords: Traumatic brain injury, Neural stem/progenitor, Proliferation, Transgenic mice
Introduction
TBI is the leading cause of death in children and young adults (Cicerone et al., 2005; McCarthy et al., 2005; Prigatano, 2005; Salmond and Sahakian, 2005; Stiles et al., 2005; Vakil, 2005). It is particularly worth noting that nearly 25,000 soldiers has been diagnosed with TBI (Bhattacharjee, 2008). Learning and memory impairment is one of the most significant residual deficits following TBI and is among the most frequent complaints of patients and their relatives (Hamm et al., 1992; Prigatano, 2005; Salmond and Sahakian, 2005; Scheff et al., 1997). The common neuropathological changes in the hippocampus after human closed-head injury and in experimental animal models of TBI suggest that the hippocampus is particularly vulnerable to the consequences of TBI (Ariza et al., 2006; Bonislawski et al., 2007; DeRidder et al., 2006; Hall et al., 2005; Isoniemi et al., 2006; Pullela et al., 2006; Saatman et al., 2006; Tran et al., 2006). Disturbances in the hippocampal function (Bonislawski et al., 2007; Pullela et al., 2006; Statler, 2006; Tasker, 2006) may play a leading role in TBI-related pathologies as the hippocampus is implicated in higher cognitive function (Pullela et al., 2006; Yavuz et al., 2006; Zhang et al., 2007) and is frequently associated with post-traumatic seizure generation (Gupta and Gupta, 2006; Pitkanen and McIntosh, 2006).
Hippocampus is one of the regions of the adult brain that can support neurogenesis throughout life, as demonstrated in rodents and primates, including humans (Cameron and McKay, 2001; Eriksson et al., 1998; Kornack and Rakic, 1999; Kuhn, et al., 1996; Leuner et al., 2007). New neurons are continuously generated from neural stem/progenitor cells in the subgranular zone (SGZ) of the dentate gyrus (DG) (Kempermann and Gage, 2000; Ming and Song, 2005). After exiting the cell cycle, the newborn cells migrate locally into the granule cell layer (GCL) and develop into mature granular neurons (Ming and Song, 2005; Shapiro and Ribak, 2005; Zhao et al., 2006). This pool of neural stem/progenitor cells is a potential resource for repairing the damaged hippocampus following TBI.
Neural stem/progenitor cells in the DG can be categorized into at least 2 subtypes based on their morphologies, protein marker expression, and pattern of division (Bull and Bartlett, 2005; Encinas and Enikolopov, 2008; Encinas et al., 2006, 2008, Filippov et al., 2003; Mignone et al., 2004, Seaberg and van der Kooy, 2002; Seri and Garcia-Verdugo, 2001). Radial glia-like progenitors have the soma and the nuclei residing in the SGZ, and an apical process crossing the granule cell layer and terminating with elaborated arbors of fine leaf-like processes in the molecular layer (ML). These cells express nestin, glial fibrillary acid protein (GFAP), vimentin, and brain lipid-binding protein (BLBP), but not the markers of mature neurons (e.g., NeuN). Only a small fraction (~1%) of these cells can be labeled with a nucleotide analog 5-bromo-2-deoxyuridine (BrdU) after a short pulse, indicating that they are normally quiescent; hence they are designated as quiescent neural progenitors (QNPs) (Encinas et al., 2006; Mignone et al., 2004). Under basal condition, QNPs play the role of stem cells; they undergo asymmetric divisions to generate small round or oval cells, that lack the radial process and express BLBP and low levels of nestin, but not GFAP, vimentin, or NeuN. These progeny cells undergo a series of symmetric divisions and can be labeled with BrdU with high frequency; they are described as amplifying neural progenitors (ANPs) (Encinas et al., 2006; Mignone et al., 2004). After exiting the cell cycle, these cells differentiate into granule neurons of the DG.
TBI has been shown to affect cell proliferation in the hippocampus. However, the results were not consistent between different reports. The results varied from promoting cell proliferation (Chirumamilla et al., 2002; Dash et al., 2001; Kernie et al., 2001; Sun et al., 2005; Yu et al., 2008), only promoting cell proliferation in certain time points (Rice et al., 2003), or decreasing cell proliferation (Rola et al., 2006) in the hippocampus following TBI. Furthermore, the classes of progenitors affected by the TBI are not known. Determining cell proliferation and the subclass of proliferating neural stem cell/progenitor cells in the hippocampus following TBI will help to understand the mechanisms of the stem/progenitors response to the TBI and may suggest potential therapeutic strategies to enhance neurogenesis for replacing the damaged neurons in the hippocampus and improving post-traumatic neurological recovery. Here we show that QNPs are induced to enter the cell cycle soon after TBI insult, while division of the ANPs is not significantly affected. Our results suggest that QNPs are extraordinarily sensitive toTBI insult and TBI may activate division of normally quiescent QNPs to compensate for the loss of mature hippocampal neurons.
Materials and methods
Animal care
Mice were housed according to the principles outlined in “Guidelines for Care and Use of Experimental Animals”. All procedures were approved by University of Kentucky and Indiana University IACUC. The Nestin-GFP transgenic mice (Gao and Chen, 2008; Mignone et al., 2004) were backcrossed to C57/BL6 for at least 10 generations.
Traumatic brain injury with controlled cortical impact
Mice (C57/BL6 or Nestin-GFP transgenic male mice, n = 5–6 in each group) of 6–8 weeks age were subjected to moderate controlled cortical impact (CCI) injury or sham surgery as previously described (Gao and Chen, 2008; Gao et al., 2008, 2009; Hall et al., 2005; Saatman et al., 2006; Sullivan et al., 1999). Briefly, the mice were anesthetized with 2.5%–4% isoflurane and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA) prior to TBI. Using sterile procedures, the skin was retracted and a 4 mm craniotomy centered between the lambda and bregma sutures was performed. A point was identified midway between the lambda and bregma sutures and midway between the central suture and the temporalis muscle laterally. The skullcap was carefully removed without disruption of the underlying dura. Prior to the injury, the head of the animal was angled on a medial to lateral plane so that the impacting tip was perpendicular to the exposed cortical surface. This was accomplished by rotating the entire stereotaxic frame in the transverse plane while leaving the nose bar at 5.0. The mouse CCI injury model uses a pneumatic impactor with which the experimenter can independently control the contact velocity and the level of cortical deformation, thus altering the severity of the injury. In these experiments, the contact velocity was set at 3.5 m/s and the amount of deformation was set at 0.5 mm, which results in an injury of moderate severity. Following injury, a 4 mm disk made from dental cement (Dentsply Trubyte) and Surgicel (Johnson and Johnson, Arlington, TX) was laid over the craniotomy site and adhered to the skull using cyanoacrylate and allowed to dry before the wound was stapled closed. During all surgical procedures and recovery, the core body temperature of the animals was maintained at 36–37 °C using a heating pad and a Hova-Bator incubator (37 °C, model 1583, Randall Burkey Co.). Sham (non-injured) animals received craniotomy, but no CCI injury.
Labeling the proliferating neural stem/progenitor cells following TBI
After moderate TBI or sham surgery, C57/BL6 or Nestin-GFP transgenic male mice received injection of BrdU once a day for one week following TBI (50 μg/g in 0.9% saline, i.p., Sigma, St. Louis, MO), or were pulse-labeled with single injection of BrdU at 4 or 72 h following TBI. The mice were perfused 24 h after the last BrdU injection to assess the status of BrdU-labeled cells.
Tissue processing
Animals were deeply anesthetized with an overdose of sodium pentobarbital and then perfused transcardially with cold 0.9% saline, followed by a fixative containing 4% paraformaldehyde (PFA) in PBS. The brains were removed and post-fixed in PFA overnight, and then cryoprotected with 30% sucrose for 48 h. Serial coronal sections (30 μm thick) were cut using a cryostat (MICOROM Microm HM 500 M) and stored at –20 °C. The sections were then processed for immunohistochemical analysis (Gao et al., 2008, 2009).
Immunohistochemistry
Series of every sixth section (180 μm apart) through each hippocampus were processed. Free-floating sections were washed twice in PBS, incubated in 2N HCl (30 min at 37 °C), and rinsed in 0.1 M borate buffer, pH 8.4 (10 min). Sections were incubated in blocking solution (0.1% Triton X-100, 1% bovine serum albumin, 5% normal goat serum in PBS) for 1 h at room temperature, followed by an overnight incubation with primary antibody at 4 °C. Sections were then washed with PBS for 3 times and incubated with the secondary antibody at room temperature for 2 h. After being treated with DAPI for 2 min, the sections were washed with PBS 3 times and mounted using Fluorescentmount G. Primary antibodies and their final concentrations were as follows: anti-BrdU antibody (1:400, rat, Accurate Chemical and Scientific) with one or two of antibodies raised against GFP (1:1000, rabbit, Millipore), NeuN (1:1000, mouse, Millipore), or GFAP (1:50, rabbit, Sigma). Secondary antibodies from Jackson ImmunoResearch Laboratories, Inc. were applied with dilution of 1:1000 (Gao and Chen, in press).
Microscopy and quantification
The sections were analyzed by light microscopy at a primary magnification of ×10–63 using an invert microscopy system (Zeiss, Axiovert 200 M equipped with Apotome) interfaced with a digital camera (Zeiss, Axio Cam MRc5) controlled by a computer. Images were captured with software (AxioVision, v4.0) and assembled and labeled in Photoshop 7.0 (Adobe Systems). BrdU-positive cell in the DG (including cells in the ML, GCL, SGZ, and hilus) were separately counted under a fluorescent microscope at ×40 magnification through whole series of sections. DG area contours were created, and the volumes were measured using BioQuant system (BioQuant image analysis corporation, USA). The thickness of the tissue section was determined by measuring the depth of “z”-axis under the microscope equipmented with Apotome. BrdU-positive cells were expressed as average number per mm3. In Nestin-GFP transgenic mice, QNPs exhibit triangular soma and extend an apical process that crosses the granular cell layer (GCL) and terminates with an arbor of fine processes in the molecular layer (ML), whereas ANPs show round or oval shape and very short processes. To avoid counting the cells that their cell bodies were cut, we excluded those cells within 5 μm of uppermost and 5 μm of lowermost focal planes. The collected data were analyzed by Student's t-test with significance set at p<0.05.
Results
Moderate traumatic brain injury significantly promotes cell proliferation in the hippocampal dentate gyrus
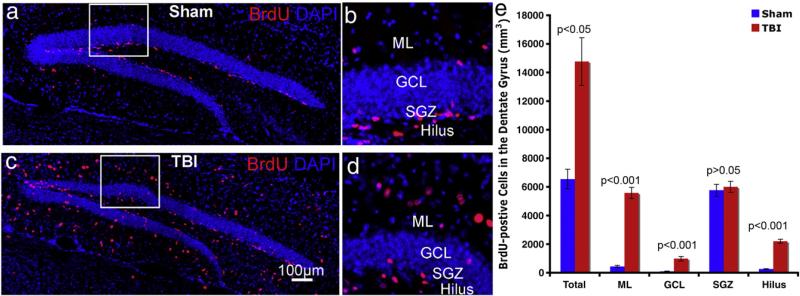
To determine the response of stem/progenitor cells in the adult hippocampus to a moderate TBI insult, we examined cell proliferation following TBI. Male mice (C57 BL/6, 6–8 weeks old) were subjected to sham treatment or to a moderate controlled cortical impact (CCI) injury to reproduce the histopathology observed clinically in humans that have suffered focal TBI. After the surgery, the animals received injections of BrdU (i.p., 50 μg/g body weight) once per day for one week. The next day after the last BrdU injection, the animals were perfused, brains were collected for analysis, and labeled cells in the DG were visualized by immunostaining with antibody against BrdU (Figs.1a, c). The number of BrdU-positive cells in the DG of CCI-injured mice (Fig. 1c) was markedly higher than in the DG of sham-treated mice (Figs. 1a–d). Quantification showed that there were 6548 (SD=687)/mm3 BrdU-positive cells in the DG of sham mice vs. 14769 (SD=1671) /mm3 BrdU-positive cells in the DG on the ipsilateral side of CCI-injured mice (Fig. 1e). These results indicated that, consistent with previous reports (Chirumamilla et al., 2002; Ramaswamy et al., 2005; Rice et al., 2003; Sun et al., 2005), TBI significantly increased cell proliferation (p <0.001) in the DG on the ipsilateral side of CCI-injured mice.
Fig. 1.
TBI insult promotes cell proliferation in the adult hippocampus. (a, b) Immunostaining with antibody against BrdU (red) to identify proliferating cells in the hippocampus of sham-operated mice (a, b) or of mice after TBI surgery (c, d). Nuclei are stained with DAPI (blue) to show the DG structure. Quantification shows the distribution of proliferating cells in the different subregions of hippocampus. ML: molecular layer; GCL: granule cell layer. SGZ: subgranular zone.
Not only the number, but also the distribution of BrdU-positive cells in the subregions of DG was significantly different after TBI. The DG can be divided into four subregions, including ML, GCL, SGZ, and hilus (Figs. 1b, d). Different subregions are composed of different cell types. In the DG of mice with sham treatment, a majority of BrdU-positive cells were found in the SGZ (Figs. 1a, b), where neural stem/progenitor cells reside. By contrast, in the DG of mice with CCI injury, the BrdU-positive cells were not confined to the SGZ. Instead, they were scattered around the entire DG, including ML, GCL, and hilus (Figs. 1c, d). To further assess the distribution of BrdU-positive cells in the different subregions of the DG, we quantified these BrdU-positive cells in the ML, GCL, SGZ, and hilus individually. In sham-operated mice, there were 434 (SD=80)/mm3, 92 (SD=24)/mm3, 5764 (SD=413)/mm3, and 258 (SD=24)/mm3 BrdU-positive cells in the ML, GCL, SGZ, and hilus, respectively, representing 6.6%, 1.4%, 88.0%, and 3.9% of BrdU-positive cells (Fig. 1e). Thus, in the sham-operated group, the majority of BrdU-positive cells were located in the SGZ, the site of neural stem/progenitor cells. In the TBI group, there were 5581 (SD=390)/mm3, 984 (SD=159)/mm3, 6003 (SD=386)/mm3, 2202 (SD=139)/mm3 BrdU-positive cells in the ML, GCL, SGZ, and hilus, respectively (Fig. 1e); this represented 37.8%, 6.7%, 46.6%, and 14.9% of BrdU-positive cells, respectively. Thus, in the TBI-exposed brain, a large fraction of BrdU-labeled cells was located in the ML and hilus, in addition to the SGZ. Compared to the sham group, the number of BrdU-positive cells in the subregions of the DG on the ipsilateral side in CCI-exposed mice was 13.72 times (SD=4.52) higher for the ML, 13.18 times (SD=9.63) higher for the GCL, 1.04 times for the SGZ (SD=0.04), and 8.81 times (SD=2.52) higher for the hilus. These results indicate that TBI significantly increased the number of BrdU-positive cells in the ML, GCL, and hilus, but only slightly increased it in the SGZ (from 5764/mm3 to 6003/mm3; the increase was not statistically significant). Thus, these results show that TBI significantly increased cell proliferation in the adult DG; they also demonstrate that the majority of the labeled cells were located in the subregions outside the area of neural stem/progenitor cell residence, suggesting that after several days of labeling most of the BrdU-positive cells were represented not by neural stem/progenitor cells.
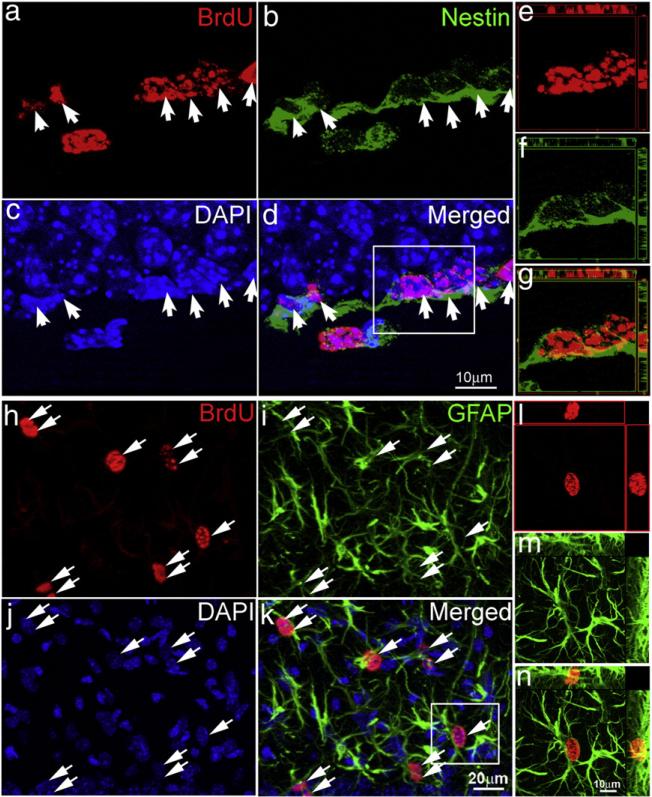
TBI is known to induce proliferation of reactive astrocytes, besides its action on stem/progenitor cells (Floyd and Lyeth, 2007; Sandhir et al., 2008). To examine the cell type of BrdU-labeled cells in the DG following TBI, we performed double immunostaining for BrdU and either for Nestin, a marker for neural stem/progenitor cells, or for GFAP, a marker for stem cells and reactive astrocytes. The results showed that BrdU-positive cells in the SGZ of both sham mice and TBI-exposed mice were co-labeled with Nestin (Figs. 2a–d, white arrows), suggesting that these cells correspond to neural stem/progenitor cells. To further confirm colocalization of BrdU with Nestin, cells were further imaged using confocal microscopy (white box in the panel d of Fig. 2). Three-dimensional reconstruction of the confocal images confirmed that the BrdU-positive cells expressed Nestin (Figs. 2e–g). However, we found that the majority of BrdU-positive cells in the ML (Figs. 2h–n), GCL and hilus were co-labeled with GFAP, suggesting that the BrdU-positive cells in these regions of the DG are reactive astrocytes (see Supplemental Fig. 1 for images with low magnification). We again applied confocal microscopy to confirm that those BrdU-positive cells in the ML, GCL, and hilus are reactive astrocytes (Figs. 2l–n). These results indicate that the BrdU-positive cells in the SGZ (whose number only slightly increased after the TBI) correspond to neural stem/progenitor cells and their immediate progeny, whereas BrdU-positive cells in the ML, GCL, and hilus, whose number significantly increased following TBI, correspond to reactive astrocytes.
Fig. 2.
Determining the cell types the proliferating cells in the hippocampus following moderate TBI. (a–g) Double immunostaining with antibody against BrdU (red) and Nestin (green) was performed to visualize the proliferating neural stem/progenitor cells in the SGZ of the hippocampus after TBI. (a) BrdU. (b) Nestin. (c) DAPI. (d) Merge of (a) to (c). White arrows indicate proliferating neural stem/progenitor cells. (e–g) Confocal microscopy was performed to verify the colocalization of BrdU with Nestin in the cells within the white box in panel (d). (h–n) Double immunostaining with antibody against BrdU (red) and GFAP (green) was performed to show the reactive astrocytes in the ML of the hippocampus after TBI. (h) BrdU. (i) GFAP. (j) DAPI. (k) Merge of (h) to (j). White arrows indicate the reactive astrocytes. (e–g) Confocal microscopy was performed to verify the colocalization of BrdU with GFAP in the cells within the white box in panel (k).
Determining the subtypes of proliferating neural stem/progenitor cells in the hippocampus following moderate traumatic brain injury
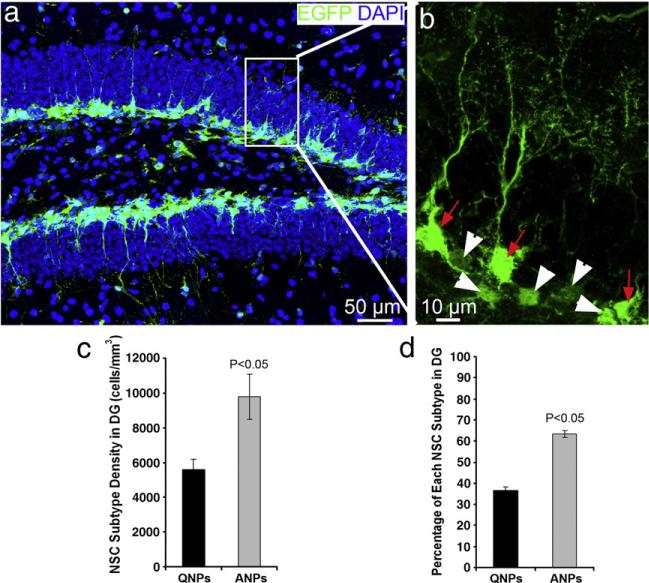
As described above, there are at least two types of neural stem/progenitor cells in the SGZ, QNPs and ANPs (Encinas et al., 2006). These cell types differ in their morphology and molecular markers and QNPs can give rise to ANPs. Since QNPs and ANPs may respond differently to the consequences of TBI insult, we sought to determine the changes in each of these classes of progenitors following TBI. The immunocytochemical approaches to stain neural stem/progenitor cells using antibodies to Nestin or GFAP visualize mainly the processes of neural stem/progenitor cells and make the accurate quantification of the progenitors problematic. To distinguish between the different classes of progenitors in vivo and to quantify the TBI-induced changes, we employed a line of transgenic mice bearing a transgene for the enhanced green fluorescent protein (GFP) driven by the regulatory elements of the Nestin gene which is normally expressed in the neural stem and progenitor cells (Nestin-GFP mice) (Mignone et al., 2004). In this transgenic reporter line, both QNPs and ANPs express Nestin-driven GFP, but they can be distinguished by their morphology and also by additional markers (Encinas and Enikolopov, 2008; Encinas et al., 2006, 2008). QNPs have triangular soma and extend an apical process that crosses the GCL and terminates with an arbor of fine processes in the ML (Fig. 3, arrows in red), whereas ANPs have round or oval shape and very short processes (Fig. 3, arrowheads in white). Quantification showed that there were 5594(SD =584)/mm3 QNPs and 9767 (SD=1294)/mm3 ANPs in the hippocampus (Fig. 3c). The density of the QNPs in the DG is significantly less than that of ANPs, suggesting that majority (~65%) of the Nestin-GFP positive neural stem/progenitor cells in the adult mouse hippocampus are represented by ANPs (Fig. 3d).
Fig. 3.
Quiescent neural progenitors (QNPs) and amplifying neural progenitors (ANPs) are distinguishable in the hippocampus of Nestin-EGFP transgenic mice. (a, b) EGFP immunostaining was performed to reveal the QNPs and ANPs in the adult hippocampus of Nestin-EGFP transgenic mice. QNPs are marked by white arrows, while the ANPs are marked by white arrowheads. (c, d). Quantification of QNPs and ANPs in the adult hippocampus of Nestin-EGFP transgenic mice.
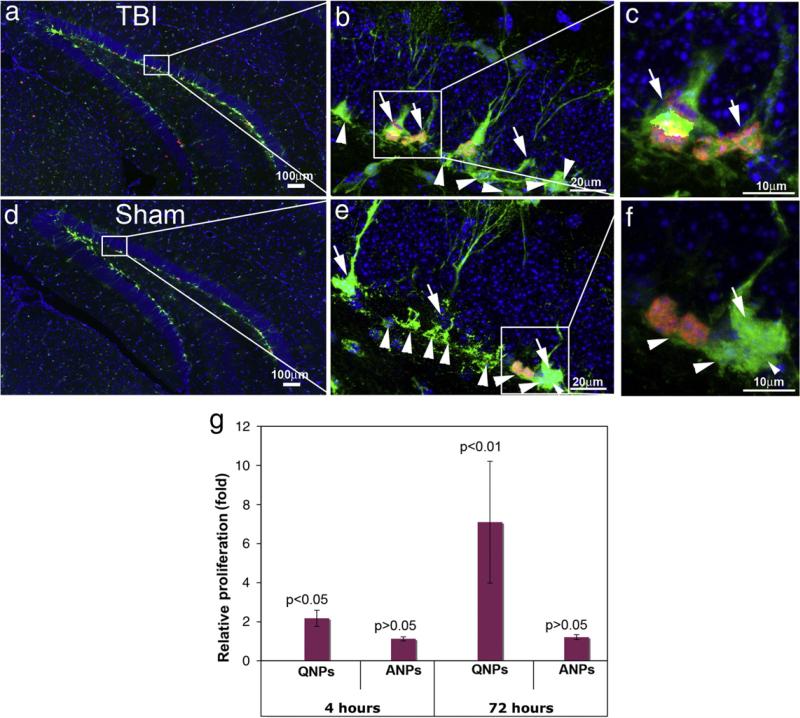
We then assessed proliferation of hippocampal stem and progenitor cells in the sham-treated mice and CCI-injured mice. Nestin-GFP transgenic mice at 8 weeks age were subjected to moderate CCI injury or sham surgery and received BrdU injection to label the proliferating QNPs and ANPs. Since QNPs can divide to give rise to ANPs we pulse-labeled the cells with BrdU following CCI injury or sham treatment in order to distinguish the proliferating QNPs from the proliferating ANPs. Animals received one injection of BrdU (i.p. 50 μg/g body weight) either 4 h or 72 h after the surgery. They were sacrificed 24 h following BrdU injection, and the brain sections were immunostained using antibodies against BrdU and GFP. The QNPs (white arrows) and ANPs (white arrowheads) in the SGZ can be distinguished both in the TBI-exposed mice (Figs. 4a–c) or sham-operated mice (Figs. 4d–f) based on GFP immunostaining. The proliferating QNPs and ANPs were double-labeled by antibodies to GFP (green) and BrdU (red) (Figs. 4a–f); thus, the proliferation status of each progenitor class was assessed based on the morphologies of GFP-positive cells and BrdU incorporation. More than 9000 of QNPs and 11,000 of ANPs were counted, we found that in the sham group 14.99 (SD=0.52)% of the ANPs, but only 1.73 (SD=0.16)% of the QNPs were labeled with BrdU. This indicates that normally ANPs are active in proliferation and represent 89.7% of the proliferating neural stem/progenitor cells in the DG; in contrast, QNPs are relatively quiescent, representing only 10.3% of the proliferating neural stem/progenitor cells. However, 4 h after TBI, the percentage of proliferating QNPs was significantly increased from 1.73 (SD=0.16)% to 3.76 (SD=0.48)%, a 2.17 fold difference (Fig. 4g). In contrast, proliferation of ANPs was only slightly and non-significantly increased from 14.99 (SD=0.52)% to 16.77 (SD=0.39)%, a 1.12 fold difference (Fig. 4g). When brain sections were analyzed 72 h after the CCI injury, the number of BrdU-labeled QNPs was 7.09 times higher (p <0.01) in the TBI mice compared to the control animals. The number of BrdU-labeled ANPs was 1.20 times greater in the TBI mice than in the control animals but this difference was not statistically significant (Fig. 4g). These results suggest that different subtypes of neural stem/progenitor cells in the DG are differentially regulated by the TBI insult. In particular, our results demonstrate a significant increase in proliferation of normally quiescent QNPs in response to TBI.
Fig. 4.
Assessing the proliferation of QNPs and ANPs in the hippocampus following moderate TBI. Double immunostainings were performed to reveal the proliferating QNPs and ANPs in the adult hippocampus with antibodies against EGFP and BrdU. (a–c). Proliferation of QNPs and ANPs in the adult hippocampus of moderate CCI-injured mice. (d–f). Proliferation of QNPs and ANPs in the adult hippocampus of moderate CCI-injured mice. Nuclei are stained with DAPI (blue) to show the DG structure. QNPs are marked by white arrows, while the ANPs are marked by white arrowheads. (g) The proliferation rates of QNP and ANP were obtained in CCI-injured mice and control mice. Their relative proliferation rates in fold were calculated by comparing the proliferation rates in CCI-injured mice to control mice.
Discussion
TBI at moderate level of impact is common to a significant portion of TBI survivors, and causes significant social and economic burden. Despite the growing understanding that TBI results in neuropathological changes in the hippocampus that can cause neurological dysfunction, i.e., cognitive impairment (Chua and Ng, 2001; Dixon et al., 2003) and epilepsy (D'Ambrosio et al., 2005; Kelly, 2004; Pitkanen and McIntosh, 2006) there is still no effective treatment for these disorders. Recent evidence has identified neural stem/progenitor cells in the adult mammalian hippocampus that produce new neurons throughout life (Kempermann and Gage, 2000; Ming and Song, 2005). These neural stem/progenitor cells are a potential resource for repairing the damaged hippocampus following TBI.
Several lines of evidence show that TBI regulated cell proliferation in the adult hippocampus. However, the results were not consistent between different reports. These results varied from promoting cell proliferation in the hippocampus (Chirumamilla et al., 2002), in the DG (Kernie et al., 2001; Sun et al., 2005) (Yu et al., 2008), or in the SGZ (Sun et al., 2005) (Yu et al., 2008); increasing cell proliferation in the SGZ only at certain time points following TBI (Rice et al., 2003); or decreasing proliferation in SGZ (Rola et al., 2006). The variation may partially due to the facts that different research groups used different injury models (fluid percussion or controlled cortical impact), different injury severities (from moderate to severe), different timing (from 6 h to 14 days following injury), and different labeling methods (BrdU, Ki67, or tritated thymidine (3H-htymidine). Furthermore, besides regulating proliferation of neural stem/progenitor cells in the SGZ, TBI also activates glial in the hippocampus including the region in the hilus closed to SGZ (Supplemental Fig. 1). There is large number of reactive glial in the hilus closed to the borderline between the hilus and SGZ. Although neural stem/progenitor cells reside at SGZ, not at the hilus (Gage, 2000), it is technically difficult to distinguish a BrdU-positive reactive astrocyte from a BrdU-positive neural stem/progenitor cell solely based on BrdU staining, if the BrdU-positive cells is located closed to the borderline between the hilus and SGZ. A second marker is required to distinguish them. In our study, we took the advantage of a line of transgenic mice in which neural stem/progenitor cells were labeled by GFP driven by Nestin promoter. It gives a clear separation between the hilus and the SGZ. Furthermore, we performed double immunostaining with second cell type specific markers in addition to BrdU labeling to distinguish GFAP-positive cells from Nestin-positive cells. This approach gives us the privilege to determine whether a BrdU-positive cell is a reactive glial or a proliferating neural stem/progenitor cell. Our result showed that the proliferation of neural stem/progenitor cells in the SGZ is only slightly increased following TBI, instead, large portion of the proliferating cells are located outside the SGZ where there is no neural stem/progenitor cells. Our results support a model that TBI only slightly increased neural stem/progenitor cell proliferation but significantly promote glial activation in the DG at the early acute phase following TBI.
There are at least two types of early neural progenitors in the DG of the adult hippocampus, including normally quiescent stem cells (QNPs) and their rapidly amplifying progeny (ANPs). Here we show that TBI selectively increases proliferation of QNPs in the adult hippocampus, suggesting that TBI may activate innate repair and/or plasticity mechanisms in the adult brain. A recent report used genetic labeling and genetic cell ablation to show that early Nestin-expressing progenitors (Nestin+/Dcx– cells) in the DG are induced by the TBI to enter the cell cycle and are required for the TBI-induced augmentation of the hippocampal neurogenesis (Yu et al., 2008). Note, however, that the Nestin+/Dcx– class encompasses at least two subclasses, the QNPs and their progeny, the ANPs (Encinas and Enikolopov, 2008; Encinas et al., 2006, 2008). The differential response of these subclasses to the TBI is not known. We took advantage of a reporter Nestin-GFP mouse line, in which QNPs and ANPs are easily distinguishable in vivo, to determine the changes in their division following the TBI. In our experiments, only 35% of Nestin-GFP-positive cells in the DG was represented by the QNPs, the remaining 65% corresponding to the ANPs (Fig. 3). Normally, only a fraction of these cells can be labeled by BrdU after a short pulse (Filippov et al., 2003; Kronenberg et al., 2003). After 24 h of pulse labeling with BrdU, 15% of ANPs in the SGZ are labeled; these labeled cells represented 89.7% of the proliferating cells in the SGZ. The fraction of labeled QNPs is much lower, corresponding to 1.5% of the total QNPs and reflecting the quiescent state of this progenitor subclass. TBI induces dramatic changes in the proliferative behavior of the early progenitors in the DG, such that the number of proliferating QNPs is significantly increased. It is 2.17 times higher in the TBI-exposed than in the sham-treated animals 4 h after the treatment and 7.09 times higher 72 h after the treatment. These results suggest that TBI may significantly increase proliferation of QNPs, perhaps inducing their asymmetric divisions. The TBI-induced changes in proliferation of ANPs are much less pronounced, with 1.12 and 1.20 fold increase at 4 and 72 h after the treatment, respectively. The differences in proliferation of ANPs are not great enough to show statistical significance. This result may suggest that the QNPs are extraordinarily sensitive to TBI insult and firstly induced to enter cell cycle in the acute phase following TBI. Meanwhile, in our current study, the mice received only single BrdU injection for 24 h at early acute phase following TBI, which only pulse-labeled a small percentage of proliferating cells in a very narrow time window. This approach is much more sensitive to detect the change in proliferation of quiescent cells in a small number, such as QNPs. It might be not sensitive enough to detect the changes in proliferation of active cells in much higher number, such as ANPs. It will be very interested to determine whether this wave of induced proliferation of QNPs is followed by increase of ANP proliferation at the latter stage.
The molecular mechanisms behind this phenomenon are unknown. It is possible that the selective response of the QNPs to TBI is related to their unusual morphology. The apical process of a QNPs ends with an extensive assembly of very fine leaf-like structures in the ML (Encinas et al., 2006; Mignone et al., 2004). These leaf-like extensions significantly increase the membrane surface of the distal part of the QNP and may potentially be highly sensitive in receiving the signals from the microenvironment and responding to diffuse molecules released from dying neurons or secreted from reactive glia following TBI. It will be interesting to explore these diffusible molecules and investigate how the intracellular programs of the QNPs are regulated to induce their cell cycle entry after the injury.
Recently, it has been shown that proliferation and fate determination of neural stem cells and intermediate progenitors during early neural development are regulated by different signaling transduction pathways (Mizutani et al., 2007). Notch signaling generated from progenitors is required for the maintenance of stem cells (Yoon et al., 2008). Knockdown of the Notch effector gene CBF1 (C-promoter binding factor) promotes the conversion of stem cells into intermediate progenitors; however, activation of CBF1 is insufficient to convert progenitors back into stem cells (Mizutani et al., 2007). These reports indicate that during early development, stem cells and their progeny are not only different in their morphology and activity, but their proliferation and fate determination are differentially regulated as well. It will be important to investigate how TBI impacts the signaling pathways in adult stem and progenitor cells and what causes their differential response to the TBI insult. Such information may enable efficient activation of specific subclasses of stem and progenitor cells in order to enhance neurogenesis following TBI.
Supplementary Material
Acknowledgments
This work was supported by a startup funding to J. Chen from Indiana University School of Medicine.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.expneurol.2009.07.007.
References
- Ariza M, Serra-Grabulosa JM, Junque C, Ramirez B, Mataro M, Poca A, Bargallo N, Sahuquillo J. Hippocampal head atrophy after traumatic brain injury. Neuropsychologia. 2006;44:1956–1961. doi: 10.1016/j.neuropsychologia.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee Y. Neuroscience. Shell shock revisited: solving the puzzle of blast trauma. Science. 2008;319:406–408. doi: 10.1126/science.319.5862.406. [DOI] [PubMed] [Google Scholar]
- Bonislawski DP, Schwarzbach EP, Cohen AS. Brain injury impairs dentate gyrus inhibitory efficacy. Neurobiol. Dis. 2007;25:163–169. doi: 10.1016/j.nbd.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull ND, Bartlett PF. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J. Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Chirumamilla S, Sun D, Bullock MR, Colello RJ. Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma. 2002;19:693–703. doi: 10.1089/08977150260139084. [DOI] [PubMed] [Google Scholar]
- Chua HC, Ng PY. Neuroprotection in acute stroke. Ann. Acad. Med. Singap. 2001;30:134–142. [PubMed] [Google Scholar]
- Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, Ellmo W, Kalmar K, Giacino JT, Harley JP, Laatsch L, Morse PA, Catanese J. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch. Phys. Med. Rehabil. 2005;86:1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- DeRidder MN, Simon MJ, Siman R, Auberson YP, Raghupathi R, Meaney DF. Traumatic mechanical injury to the hippocampus in vitro causes regional caspase-3 and calpain activation that is influenced by NMDA receptor subunit composition. Neurobiol. Dis. 2006;22:165–176. doi: 10.1016/j.nbd.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Ma X, Kline AE, Yan HQ, Ferimer H, Kochanek PM, Wisniewski SR, Jenkins LW, Marion DW. Acute etomidate treatment reduces cognitive deficits and histopathology in rats with traumatic brain injury. Crit. Care Med. 2003;31:2222–2227. doi: 10.1097/01.CCM.0000080493.04978.73. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Enikolopov G. Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol. 2008;85:243–272. doi: 10.1016/S0091-679X(08)85011-X. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Vazquez ME, Switzer RC, Chamberland DW, Nick H, Levine HG, Scarpa PJ, Enikolopov G, Steindler DA. Quiescent adult neural stem cells are exceptionally sensitive to cosmic radiation. Exp. Neurol. 2008;210:274–279. doi: 10.1016/j.expneurol.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol. Cell. Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Floyd CL, Lyeth BG. Astroglia: important mediators of traumatic brain injury. Prog. Brain Res. 2007;161:61–79. doi: 10.1016/S0079-6123(06)61005-4. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gao X, Chen J. Direct isolation of neural stem cells in the adult hippocampus after traumatic brain injury. J. Neurotrauma. 2008;25:985–995. doi: 10.1089/neu.2008.0460. [DOI] [PubMed] [Google Scholar]
- Gao X, Chen J. Conditional knockout of brain-derived neurotrophic factor in the hippocampus increases death of adult-born immature neurons following traumatic brain injury. J. Neurotrauma. doi: 10.1089/neu.2008.0744. in press doi:1089/neu.2008-0744. [DOI] [PubMed] [Google Scholar]
- Gao X, Deng-Bryant Y, Cho W, Carrico KM, Hall ED, Chen J. Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J. Neurosci. Res. 2008;86:2258–2270. doi: 10.1002/jnr.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Smith GM, Chen J. Impaired dendritic development and synaptic formation of postnatal-born dentate gyrus granular neurons in the absence of brain-derived neurotrophic factor signaling. Exp. Neurol. 2009;215:178–190. doi: 10.1016/j.expneurol.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Gupta M. Post traumatic epilepsy: a review of scientific evidence. Indian J. Physiol. Pharmacol. 2006;50:7–16. [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Isoniemi H, Kurki T, Tenovuo O, Kairisto V, Portin R. Hippocampal volume, brain atrophy, and APOE genotype after traumatic brain injury. Neurology. 2006;67:756–760. doi: 10.1212/01.wnl.0000234140.64954.12. [DOI] [PubMed] [Google Scholar]
- Kelly KM. Modeling traumatic brain injury and posttraumatic epilepsy. Epilepsy Curr. 2004;4:160–161. doi: 10.1111/j.1535-7597.2004.44015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Neurogenesis in the adult hippocampus. Novartis Found. Symp. 2000;231:220–235. discussion 235–241, 302–226. [PubMed] [Google Scholar]
- Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J. Neurosci. Res. 2001;66:317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippo-campus respond differently to physiologic neurogenic stimuli. J. Comp. Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy ML, MacKenzie EJ, Durbin DR, Aitken ME, Jaffe KM, Paidas CN, Slomine BS, Dorsch AM, Berk RA, Christensen JR, Ding R. The Pediatric Quality of Life Inventory: an evaluation of its reliability and validity for children with traumatic brain injury. Arch. Phys. Med. Rehabil. 2005;86:1901–1909. doi: 10.1016/j.apmr.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J. Comp. Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, McIntosh TK. Animal models of post-traumatic epilepsy. J. Neurotrauma. 2006;23:241–261. doi: 10.1089/neu.2006.23.241. [DOI] [PubMed] [Google Scholar]
- Prigatano GP. Impaired self-awareness after moderately severe to severe traumatic brain injury. Acta Neurochir. Suppl. 2005;93:39–42. doi: 10.1007/3-211-27577-0_5. [DOI] [PubMed] [Google Scholar]
- Pullela R, Raber J, Pfankuch T, Ferriero DM, Claus CP, Koh SE, Yamauchi T, Rola R, Fike JR, Noble-Haeusslein LJ. Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Dev. Neurosci. 2006;28:396–409. doi: 10.1159/000094166. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Goings GE, Soderstrom KE, Szele FG, Kozlowski DA. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Rice AC, Khaldi A, Harvey HB, Salman NJ, White F, Fillmore H, Bullock MR. Proliferation and neuronal differentiation of mitotically active cells following traumatic brain injury. Exp. Neurol. 2003;183:406–417. doi: 10.1016/s0014-4886(03)00241-3. [DOI] [PubMed] [Google Scholar]
- Rola R, Mizumatsu S, Otsuka S, Morhardt DR, Noble-Haeusslein LJ, Fishman K, Potts MB, Fike JR. Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp. Neurol. 2006;202:189–199. doi: 10.1016/j.expneurol.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J. Neurotrauma. 2006;23:1241–1253. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Sahakian BJ. Cognitive outcome in traumatic brain injury survivors. Curr. Opin. Crit. Care. 2005;11:111–116. doi: 10.1097/01.ccx.0000155358.31983.37. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp. Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J. Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM. Astrocytes Give Rise to New Neurons in the Adult Mammalian Hippocampus. J. Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, Ribak CE. Integration of newly born dentate granule cells into adult brains: hypotheses based on normal and epileptic rodents. Brain Res. Brain Res. Rev. 2005;48:43–56. doi: 10.1016/j.brainresrev.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Statler KD. Pediatric posttraumatic seizures: epidemiology, putative mechanisms of epileptogenesis and promising investigational progress. Dev. Neurosci. 2006;28:354–363. doi: 10.1159/000094162. [DOI] [PubMed] [Google Scholar]
- Stiles J, Reilly J, Paul B, Moses P. Cognitive development following early brain injury: evidence for neural adaptation. Trends Cogn. Sci. 2005;9:136–143. doi: 10.1016/j.tics.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J. Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Colello RJ, Daugherty WP, Kwon TH, McGinn MJ, Harvey HB, Bullock MR. Cell proliferation and neuronal differentiation in the dentate gyrus in juvenile and adult rats following traumatic brain injury. J. Neurotrauma. 2005;22:95–105. doi: 10.1089/neu.2005.22.95. [DOI] [PubMed] [Google Scholar]
- Tasker RC. Changes in white matter late after severe traumatic brain injury in childhood. Dev. Neurosci. 2006;28:302–308. doi: 10.1159/000094156. [DOI] [PubMed] [Google Scholar]
- Tran LD, Lifshitz J, Witgen BM, Schwarzbach E, Cohen AS, Grady MS. Response of the contralateral hippocampus to lateral fluid percussion brain injury. J. Neurotrauma. 2006;23:1330–1342. doi: 10.1089/neu.2006.23.1330. [DOI] [PubMed] [Google Scholar]
- Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. J. Clin. Exp. Neuropsychol. 2005;27:977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- Yavuz BB, Ariogul S, Cankurtaran M, Oguz KK, Halil M, Dagli N, Cankurtaran ES. Hippocampal atrophy correlates with the severity of cognitive decline. Int. Psychogeriatr. 2006:1–11. doi: 10.1017/S1041610206004303. [DOI] [PubMed] [Google Scholar]
- Yoon KJ, Koo BK, Im SK, Jeong HW, Ghim J, Kwon MC, Moon JS, Miyata T, Kong YY. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron. 2008;58:519–531. doi: 10.1016/j.neuron.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J. Neurosci. 2008;28:12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Cho HJ, Lee S, Lee JH, Choi SH, Ryu V, Yoo SB, Lee JY, Kim DG, Jahng JW. Impairments in water maze learning of aged rats that received dextromethorphan repeatedly during adolescent period. Psychopharmacology (Berl) 2007;191:171–179. doi: 10.1007/s00213-006-0548-3. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr., Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.