Abstract
As an ancillary report to a large National Institutes of Health (NIH)–funded trial, we examined the effects of 6 months of exercise training at 50%, 100%, and 150% of the NIH Consensus Recommendations for physical activity (i.e., 4, 8, and 12 kcal/kg of energy expenditure/wk [KKW]) versus a nonexercise control group on the metabolic syndrome (MS) in sedentary, overweight, moderately hypertensive, postmenopausal women. We examined the clinically defined National Cholesterol Education Program MS, individual components scores, and summed z-scores, expressed as a continuous variable (zMS), using chi-square and general linear models to assess the clinical and progressive nature of MS, respectively. Our results showed significant improvements in zMS for all exercise groups and MS for the 8- and 12 KKW groups only (all, p for trend = 0.02). Post hoc analyses showed that 12 KKW for zMS and 8 and 12 KKW for MS was significant versus the control group (all, p <0.05). When examining the composite scores, we observed significant trends for improvement in waist circumference (p for trend = 0.001), fasting glucose (p for trend = 0.01), and systolic blood pressure (p for trend = 0.02), which appeared to be dose dependent, given the additive nature for incorporating the within-group improvements in waist circumference (4, 8, and 12 KKW), fasting glucose (8 and 12 KKW), and systolic blood pressure (12 KKW). Our results suggest that low-to-moderate intensity cardiorespiratory exercise appears to improve components of the MS in postmenopausal women at levels at or greater than NIH recommendations and that zMS improves at half the NIH recommendations. Greater levels of energy expenditure appear to enhance this effect by incorporating a greater number of requisite MS composite scores.
The metabolic syndrome (MS) is a combination of risk factors composed of abdominal obesity, insulin resistance, hypertension, and lipid abnormalities and represents the erosion of the individual component parts associated with its diagnosis.1 We hypothesized that given the categorical nature of the MS, a full appreciation for improvement might not be adequately portrayed by simply measuring the MS cutpoints. We have recently published results from this hypothesis in a cross-sectional analysis from the Aerobics Center Longitudinal Study.2 We based this hypothesis on the observation that MS is constructed by the presence or absence of a component score composed of defined cutpoints. It is also unclear which components drive the MS or, when applicable, reduce its prevalence after exercise training. Thus, the categorical nature of the MS assessment might not fully explain the benefits of an exercise intervention, given the nature of the assessment as a failure to meet a particular component's cutpoint, despite marked improvement, would still qualify a patient for the MS. In a recent randomized controlled trial, we demonstrated that cardiorespiratory exercise administered at 50%, 100%, and 150% of the National Institutes of Health (NIH) Consensus Panel physical activity recommendation increases maximum cardiorespiratory fitness in a dose-wise fashion.3 We present here an analysis of the effects of moderate intensity exercise training on the MS in sedentary, overweight or obese, postmenopausal women with elevated blood pressure considered to have an elevated risk of cardiovascular disease.
Methods
The complete design, methods, and primary outcomes of the Dose-Response to Exercise in Women Aged 45 to 75 Years (DREW) study have been previously published.3,4 In brief, the DREW study was a randomized, dose–response exercise training trial complying with the Declaration of Helsinki and comparing a nonexercise control group and 3 groups exercising at incremental doses (50%, 100%, and 150%) of the minimal NIH Consensus Development Panel's recommendation for energy expenditure.5 The Cooper Institute and Pennington Biomedical Research Center's institutional review boards initially and subsequently reviewed our protocol annually. The primary outcomes for the DREW study included maximum cardiorespiratory capacity, which was calculated as the average of 2 baseline and 2 follow-up exercise tests and the blood pressure at rest. The clinicaltrials.gov identifier is NCT00011193.
After an initial evaluation and run-in period, we randomized 464 postmenopausal women (age 45 to 75 years) to 1 of 3 exercise training groups or a nonexercise control group for a 6-month intervention period. The exercise intensity for the present study was fixed at 50% of the measured maximum cardiorespiratory capacity. During the exercise portion of the study, there were distinct and separate intervention and assessment teams, and all assessment staff were kept unaware of the participant randomization assignment. The study participants were sedentary (exercising <20 minutes; <3 days/wk; <8,000 steps/day assessed during a 1-week period), overweight or obese (body mass index 25.0 to 43.0 kg/m2), and had a systolic blood pressure of 120 to 160 mm Hg. We excluded women who had a history of stroke, myocardial infarction, or any serious medical condition that prevented participants from adhering to the protocol or exercising safely. Our present analysis was limited to 408 women with complete data for MS risk factors at baseline and follow-up.
In an attempt to elucidate the progression or improvements surrounding the MS, we approached our analysis by examining categorically defined MS and MS according to the z-scores (zMS) from the National Cholesterol and Education Program components described in the Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity for subjects from the United States.6 We calculated the presence of MS by taking each participant's individual score at baseline and follow-up and subtracting it from the cohort's baseline mean value. The resultant value of each was then divided by the cohort's SD for that score, summed, and then divided by the number of the composite scores. This approach has been used previously in several other studies.7–9
We used chi-square and generalized linear models to analyze the influences of the differing doses of exercise training on the MS and zMS. When significant trends were observed, we explored our findings further for pairwise comparisons between the exercise training groups and the control group using a Dunnett-Hsu post hoc assessment. The Dunnett-Hsu test allows specific multiple pairwise comparisons while still protecting against type I statistical errors. We covaried all our analyses with the participant's age, baseline MS scores for each composite viable, as appropriate, and hypertension medication (28% of cohort), hyperlipidemia medication (16% of cohort), hormonal therapy (15% of cohort), thyroid medication (15% of cohort), antidepressants (18% of cohort), and glucose control medication (1% of cohort). Within-group differences between baseline and the follow-up duration are reported as the mean and 95% confidence intervals (CIs). All reported p values are 2 sided (p <0.05), and our analyses were performed using the Statistical Package for Social Sciences, version 19.0 (IBM, Somers, New York).
Results
The present report details the effects of 6 months of exercise training on the MS in 408 participants. The cohort's demographic, anthropometric, blood pressure, and cardiorespiratory capacity data are listed in Table 1. The hematologic and MS score characteristics are listed in Table 2. Medication use is listed in Table 3. Overall, the participants in the present analysis were 45 to 75 years old, weighed 58.2 to 112.8 kg, and had a body mass index of 23.2 to 40.9 kg/m2. Similar to our primary outcome study,3 we observed a significant, dose-dependent increase in maximum cardiorespiratory capacity (p for trend = 0.0001) for the 4 KKW (0.64 ml/kg/min, 95% CI 0.38 to 0.95), 8 KKW (1.28 ml/kg/min, 95% CI 0.91 to 1.65), 12 KKW (1.67 ml/kg/min, 95% CI 1.32 to 2.03) groups, with each group having statistically significant differences (p <0.001) versus the control group (−0.18 ml/kg/min, 95% CI −0.54 to 0.18). Although we did not observe a significant difference between baseline and follow-up regarding medication use, a significant percentage of our cohort presented to the study with MS (78%).
Table 1. Demographic, anthropometric, blood pressure and cardiorespiratory capacity of Dose-Response to Exercise in Women Aged 45 to 75 Years (DREW) study participants (n = 408).
| Variable | All | Exercise Treatment Group | |||
|---|---|---|---|---|---|
|
|
|||||
| Control | 4 KKW | 8 KKW | 12 KKW | ||
| Age (yrs) | 57.23 ± 6.4 | 57.12 ± 5.8 | 57.94 ± 6.6 | 56.91 ± 6.6 | 56.57 ± 6.5 |
| Height (cm) | |||||
| Baseline | 162.89 ± 5.7 | 162.87 ± 6.2 | 162.77 ± 5.8 | 162.40 ± 5.3 | 163.53 ± 5.5 |
| Follow-up | 162.98 ± 5.7 | 162.99 ± 6.2 | 162.81 ± 5.8 | 162.56 ± 5.3 | 163.59 ± 5.5 |
| Weight (kg) | |||||
| Baseline | 84.28 ± 11.9 | 85.52 ± 12.5 | 83.73 ± 11.4 | 85.03 ± 12.4 | 83.23 ± 11.4 |
| Follow-up | 82.83 ± 11.9 | 84.12 ± 12.5 | 82.42 ±11.4 | 83.14 ± 12.4 | 81.91 ± 11.6 |
| BMI (kg/m2) | |||||
| Baseline | 31.72 ± 3.8 | 32.18 ± 4.0 | 31.57 ± 3.7 | 32.19 ± 4.0 | 31.07 ± 3.6 |
| Follow-up | 31.15 ± 3.8 | 31.65 ± 4.0 | 31.06 ± 3.7 | 31.42 ±4.1 | 30.54 ± 3.6 |
| Waist circumference (cm)* | |||||
| Baseline | 100.85 ± 11.6 | 102.28 ± 11.9 | 100.27 ±11.2 | 101.87 ± 11.4 | 99.42 ± 12.0 |
| Follow-up | 98.76 ± 11.4 | 102.42 ± 12.3 | 97.48 ± 10.0 | 99.10 ± 12.0 | 96.84 ± 11.2 |
| Hip circumference (cm) | |||||
| Baseline | 114.88 ± 9.0 | 116.02 ± 9.5 | 114.43 ± 8.5 | 115.25 ± 9.2 | 114.12 ± 9.2 |
| Follow-up | 112.82 ± 9.6 | 114.29 ± 9.5 | 112.49 ±9.1 | 113.18 ± 10.8 | 111.57 ± 9.4 |
| Systolic blood pressure (mm Hg)* | |||||
| Baseline | 139.55 ± 13.1 | 141.68 ± 12.4 | 138.65 ± 13.3 | 139.90 ± 13.8 | 138.53 ± 12.8 |
| Follow-up | 138.06 ± 13.5 | 138.78 ± 11.7 | 139.78 ± 13.5 | 137.38 ± 13.9 | 135.38 ± 14.3 |
| Diastolic blood pressure (mm Hg)* | |||||
| Baseline | 80.69 ± 8.4 | 80.64 ± 7.9 | 80.43 ± 8.7 | 80.81 ± 8.3 | 81.03 ± 8.7 |
| Follow-up | 80.82 ± 8.2 | 80.18 ± 7.6 | 81.40 ± 8.0 | 80.58 ± 9.4 | 80.78 ± 8.2 |
| Relative VO2max (ml/kg/min) | |||||
| Baseline | 15.50 ± 2.9 | 15.64 ± 2.9 | 15.43 ± 3.0 | 14.98 ± 2.4 | 15.93 ± 3.0 |
| Follow-up | 16.33 ± 3.0 | 15.43 ± 2.9 | 16.08 ±3.1 | 16.38 ± 2.6 | 17.54 ± 3.0 |
| Absolute VO2max (L/min) | |||||
| Baseline | 1.30 ± 0.3 | 1.33 ± 0.3 | 1.28 ± 0.3 | 1.27 ± 0.2 | 1.31 ± 0.2 |
| Follow-up | 1.34 ± 0.3 | 1.29 ± 0.3 | 1.31 ± 0.2 | 1.35 ± 0.3 | 1.43 ± 0.3 |
| Maximum heart rate (beats/min) | |||||
| Baseline | 151.56 ± 16.4 | 151.69 ± 15.7 | 150.50 ± 17.3 | 151.59 ± 15.7 | 153.01 ± 16.6 |
| Follow-up | 150.53 ± 16.4 | 147.88 ± 16.5 | 149.74 ± 16.0 | 152.23 ± 16.0 | 152.72 ± 17.0 |
Data are presented as mean ± SD.
VO2max = maximum cardiorespiratory capacity.
Component of NCEP Adult Treatment Panel III metabolic syndrome.
Table 2. Hematologic and metabolic syndrome (MS) characteristics of Dose-Response to Exercise in Women Aged 45 to 75 Years (DREW) study participants (n = 408).
| All | Exercise Treatment Group | ||||
|---|---|---|---|---|---|
|
|
|||||
| Control | 4 KKW | 8 KKW | 12 KKW | ||
| Blood parameters† | |||||
| Cholesterol (mg/dl) | |||||
| Baseline | 201.54 ± 29.9 | 201.33 ± 29.7 | 200.60 ±31.6 | 202.11 ± 29.0 | 202.66 ± 28.6 |
| Follow-up | 203.03 ± 31.7 | 205.19 ± 35.4 | 202.18 ± 31.8 | 201.94 ± 27.9 | 203.23 ±31.4 |
| HDL (mg/dl)* | |||||
| Baseline | 57.47 ± 14.4 | 57.02 ± 14.2 | 57.67 ± 14.3 | 57.28 ± 15.7 | 57.78 ± 13.5 |
| Follow-up | 57.01 ± 13.3 | 56.78 ± 12.2 | 57.42 ± 13.3 | 57.35 ± 15.1 | 56.32 ± 12.8 |
| LDL (mg/dl) | |||||
| Baseline | 118.19 ± 26.7 | 117.92 ± 26.2 | 116.99 ± 27.6 | 118.46 ± 25.4 | 120.03 ± 27.3 |
| Follow-up | 120.74 ± 29.0 | 122.55 ± 33.3 | 120.34 ± 28.2 | 119.21 ± 26.7 | 121.00 ± 28.4 |
| VLDL (mg/dl) | |||||
| Baseline | 26.09 ± 12.6 | 27.36 ± 14.0 | 25.94 ±11.9 | 26.36 ± 12.1 | 24.85 ± 12.8 |
| Follow-up | 25.00 ± 12.2 | 25.70 ± 11.0 | 24.42 ±12.1 | 25.38 ± 13.1 | 24.85 ± 12.8 |
| Cholesterol/HDL ratio | |||||
| Baseline | 3.69 ±1.0 | 3.71 ± 0.9 | 3.66 ±1.0 | 3.74 ± 1.0 | 3.68 ± 0.9 |
| Follow-up | 3.73 ± 1.0 | 3.78 ± 1.0 | 3.68 ± 1.0 | 3.73 ± 1.0 | 3.76 ± 0.9 |
| Triglycerides (mg/dl)* | |||||
| Baseline | 131.20 ± 64.9 | 136.58 ± 70.0 | 129.44 ± 59.3 | 131.89 ± 60.2 | 128.01 ± 72.3 |
| Follow-up | 129.39 ± 85.9 | 141.24 ± 132.2 | 122.19 ± 60.6 | 126.95 ± 65.7 | 131.00 ± 77.5 |
| Glucose (mg/dl)* | |||||
| Baseline | 94.61 ± 9.9 | 95.20 ± 13.3 | 94.08 ± 8.7 | 94.40 ±9.1 | 95.02 ± 8.5 |
| Follow-up | 93.89 ± 9.1 | 96.44 ± 10.2 | 93.22 ± 9.0 | 92.99 ± 8.8 | 93.25 ± 8.2 |
| Insulin (pmol/L) | |||||
| Baseline | 140.18 ± 410.9 | 121.95 ± 384.9 | 163.67 ± 443.7 | 128.42 ± 395.2 | 133.21 ± 403.0 |
| Follow-up | 143.39 ± 416.0 | 137.83 ± 409.0 | 163.48 ± 443.7 | 127.55 ± 395.4 | 132.99 ± 403.1 |
| Metabolic Syndrome | |||||
| Score | |||||
| Baseline | 3.20 ± 0.9 | 3.30 ± 1.0 | 3.19 ± 0.9 | 3.15 ± 0.9 | 3.16 ± 0.9 |
| Follow-up | 3.05 ± 0.9 | 3.24 ± 0.9 | 3.10 ± 0.9 | 2.94 ± 0.9 | 2.87 ± 1.0 |
| Prevalence | |||||
| Baseline | 319, 78% | 69, 77% | 113, 81% | 65, 77% | 72, 77.4% |
| Follow-up | 299, 73% | 72, 80% | 110, 79% | 59, 69% | 58, 62.4% |
Data are presented as mean ± SD.
HDL = high-density lipoprotein; LDL = low-density lipoprotein; VLDL = very-low-density lipoprotein.
Component of National Cholesterol Education Program Adult Treatment Panel III MS.
SI conversions: to convert total, low-density lipoprotein, and high-density lipoprotein cholesterol to mmol/L, multiply by 0.0259; triglycerides to mmol/L, multiply by 0.0113; glucose to mmol/L, multiply by 0.0555; and insulin to pmol/L multiply by 6.945.
Table 3. Medication use for Dose-Response to Exercise in Women Aged 45 to 75 Years (DREW) study participants.
| Medication | All (n) | Exercise Treatment Group (n) | |||
|---|---|---|---|---|---|
|
|
|||||
| Control | 4 KKW | 8 KKW | 12 KKW | ||
| Blood pressure | |||||
| Baseline | 113 (28) | 22 (24) | 37 (26) | 26 (31) | 28 (30) |
| Follow-up | 108 (28) | 20 (24) | 37 (29) | 29 (36) | 22 (25) |
| Antidepressant | |||||
| Baseline | 75 (18) | 14 (16) | 26 (19) | 16 (19) | 19 (20) |
| Follow-up | 71 (19) | 16 (19) | 24 (19) | 14 (17) | 17 (20) |
| Cholesterol | |||||
| Baseline | 64 (16) | 14 (16) | 27 (19) | 13 (15) | 10 (11) |
| Follow-up | 57 (15) | 14 (17) | 22 (17) | 13 (16) | 8 (9) |
| Glucose regulation | |||||
| Baseline | 2 (1) | 1 (1) | 0 (0) | 0 (0) | 1 (1) |
| Follow-up | 4 (1) | 1 (1) | 1 (1) | 1 (1) | 1 (1) |
| Thyroid | |||||
| Baseline | 62 (15) | 14 (16) | 17 (12) | 14 (17) | 17 (18) |
| Follow-up | 53 (14) | 12 (14) | 14 (11) | 13 (16) | 14 (16) |
| Current hormonal therapy | |||||
| Baseline | 183 (45) | 47 (52) | 58 (41) | 35 (41) | 43 (46) |
| Follow-up | 152 (37) | 38 (42) | 47 (34) | 31 (37) | 36 (39) |
Data in parentheses are percentages.
At the baseline visit, we observed that the control (77%), 4 KKW (81%), 8 KKW (76%), and 12 KKW (77%) groups all had an extremely high, and similar, prevalence of MS. After 6 months of exercise training, we observed that the prevalence of MS had increased by 4% in the control group compared with significant improvements in the MS in the 4 KKW (−3%), 8 KKW (−9%), and 12 KKW (−19%) groups (p for trend <0.02). However, only the 8 and 12 KKW group improvements were statistically significant, with both groups being significantly different from those for the control group (all comparisons, p <0.05).
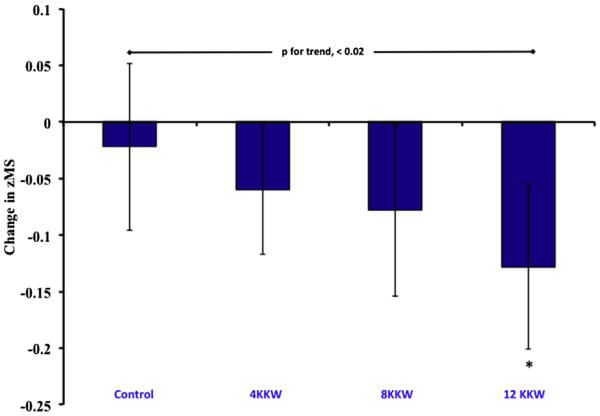
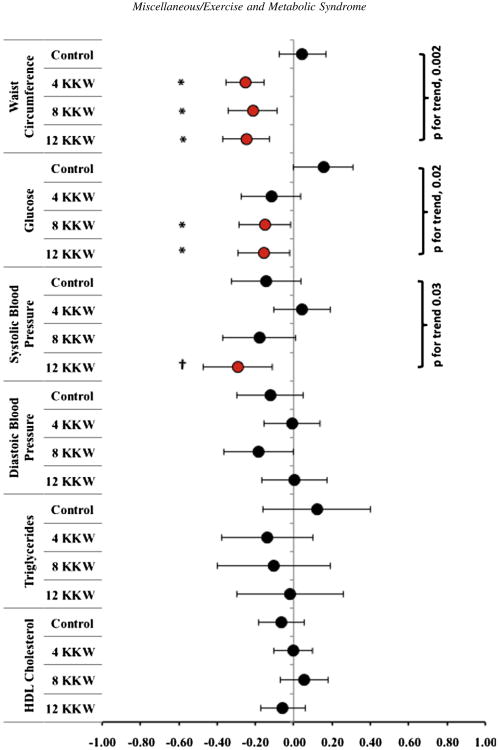
We observed a significant dose response for the improvement of zMS for each treatment group (p for trend = 0.02; Figure 1). The post hoc analyses also showed that the 12 KKW group had significant differences compared with the control group (p <0.05). Overall, the increased magnitude of the zMS response within the 3 treatment groups appeared to be related to exercise energy expenditure. Specifically, each succeeding level of exercise energy expenditure added another significant composite score to the zMS effect, whereby all 3 exercise groups showed significant improvements in waist circumference (p for trend = 0.002; Figure 2), but only the 8 and 12 KKW groups demonstrated significant improvements in the fasting glucose level (p for trend = 0.02). Despite a significant trend (p for trend = 0.03), only the 12 KKW group demonstrated a significant within-group improvement for systolic blood pressure.
Figure 1.
Data represent mean and 95% confidence interval reduction in MS score after 6 months of exercise training. *Different from control (p <0.05).
Figure 2.
Data represent mean and 95% confidence interval changes for each zMS composite score constituting the MS for postmenopausal women undertaking 6 months of monitored aerobic exercise training at 4, 8, and 12 KKW. *Findings significantly different from the control group (p <0.05). †Findings significantly different from the control group (p <0.003).
In our post hoc analysis, we found that the reductions observed for waist circumference for each exercise treatment group were significant compared with the control group (all, p <0.001). For fasting glucose, each exercise group was also significantly different from the control group, although only the 8 KKW and 12 KKW groups showed significant within-group changes. These particular post hoc effects were likely a result of a significant increase in the control group's fasting glucose level. Finally, for systolic blood pressure, only the 12 KKW group reached a statistically significant difference compared with the control group (p = 0.05).
Discussion
In the present study, we found a dose response relation between weekly exercise energy expenditure, cardiometabolic risk factors, and the prevalence of MS in a large randomized study of postmenopausal women. We also observed significant improvements in zMS for all exercise training levels, with dose-dependent improvements in waist circumference (4, 8, and 12 KKW), fasting glucose (8 and 12 KKW), and systolic blood pressure (12 KKW). The prevalence of MS was reduced significantly in the 8 and 12 KKW levels. These results indicate that low-to-moderate intensity cardiorespiratory exercise training improves components of the MS in postmenopausal women at levels at or greater than the NIH recommendations, and the zMS improves at exercise levels half the NIH recommendations.
It is well recognized that women with MS are at a high risk of type 2 diabetes mellitus and cardiovascular disease.6,10 When taken as a whole, the manifestation of MS represents the worsening of several physiologic systems to the extent that the sum of a sufficient number of qualifying categories leads to a clinical diagnosis. This is of considerable importance to women's health, because it is also recognized that the MS increases throughout the transition period from pre- to postmenopause, which in turn, could explain the increase in cardiovascular disease in women after menopause, given the initial protection offered to women before menopause.11 As menopause transitions to the postmenopausal status, common changes include an increase in central adiposity/obesity, increased glucose and insulin levels, elevations in systolic and diastolic blood pressure, and unfavorable changes in lipid concentrations, especially increases in triglycerides and reductions in the cardioprotective high-density lipoprotein cholesterol.12 Although exercise training generally improves these risk factors, the categorical nature of the MS features might not necessarily reflect the true nature of the improvements.
Our present analysis has demonstrated this premise, as our categorical analysis showed a reduction in the MS in the 8 and 12 KKW exercise groups, and our zMS analysis demonstrated a significant improvement for all exercise groups. Therefore, healthcare practitioners should consider that the categorical nature of the MS might not fully reflect the effect of treatment if a participant fails to transcend the qualifying cutoff points for each category proposed in the published data.6 Nonetheless, we have shown that exercise at a level at or greater than the NIH recommendations improves MS scores in postmenopausal women. We further suggest that even levels at 50% of the NIH recommendations are beneficial for the treatment of cardiometabolic risk in post-menopausal women with a high prevalence of MS and cardiovascular disease risk.
Although physical activity is recommended to postmenopausal women as a method of decreasing cardiovascular disease risk, MS, and the associated co-morbidity prevalence, the level of exercise required to affect changes in the MS is not clear.5,13 In our present analysis, the beneficial effects of exercise training to reduce cardiometabolic risk were primarily driven by improvements in the individual composite scores of waist circumference, fasting glucose, and systolic blood pressure. These improvements appeared to be dose dependent, because each increase in the level of exercise energy expenditure (50%, 100%, and 150% of the consensus public health guidelines) was accompanied by a significant decrease in another component of the MS score. For example, we observed a significant within-group improvement in waist circumference for all exercise treatment groups (i.e., 4, 8, and 12 KKW), for the fasting glucose composite score in those exercising at a higher level (i.e., 8 and 12 KKW), and for the systolic blood pressure score in participants exercising at the highest level (12 KKW). Therefore, the exercise level (i.e., fixed intensity, different volumes) in the present study might be an important clinical consideration when providing advice on exercise training.
Several reviews have examined the role of exercise and its influence on MS.12,14 Within the published data are a paucity of reports examining postmenopausal women. Katzmarzyk et al15 reported that 30% of participants from the HERITAGE Family Study, presenting with MS and undertaking 20 weeks of aerobic training, no longer qualified as having National Cholesterol Education Program-determined MS at the end of the study. Others have reported similar findings.16,17 However, in a very small cohort (n = 48) of HERITAGE participants, no effects were observed regarding exercise training and MS in postmenopausal women.18 In our study, we observed that the prevalence of MS increased by 4% in the control group, demonstrating continued erosion over time, with by dose-dependent reductions in MS prevalence in the 4 KKW (−3%), 8 KKW (−9%), and 12 KKW (−19%) groups.
In one of a few studies examining exercise intensity and MS, Johnson et al assessed both the volume and the intensity implications surrounding exercise levels in men and women assigned to a 6-month sedentary control group or 3 exercises levels: (1) low amount/moderate intensity (equivalent to walking ∼ 19 km/wk), (2) low amount/vigorous intensity (equivalent to jogging ∼ 19 km/wk), or (3) high amount/vigorous intensity (equivalent to jogging ∼ 32 km/wk). The results of their study showed that although the low-amount/moderate-intensity and high-amount/vigorous-intensity exercise groups showed improvement in the MS relative to the inactive controls, the low-amount/vigorous-intensity group did not. Given that the mean ± SD age of the women in that study was 55 ± 5 years, many were likely postmenopausal. However, no statistical adjustments were made for menopausal status in their study. Two subsequent exercise trials have now shown that interval training, which is shortduration and high-intensity exercise by nature, has a greater effect in reducing the MS.19, 20 Although one study included a small number of women (n = 15) divided among 3 groups, the other included only men at risk of diabetes, which of course includes insulin resistance. 19,20
A major strength of our study was that we examined previously sedentary, overweight women with elevated blood pressure who were exercising at low-to-moderate intensities. These levels are synonymous with the NIH Consensus Panel's physical activity recommendations.5 Although a potential limitation of the present study might have been our use of low-to-moderate intensity exercise, it should be noted that the central hypothesis for the DREW study was stated a priori at its initiation as examining the “minimum” or “threshold” level of exercise needed to improve fitness and health surrounding the NIH recommendations for cardiorespiratory fitness. When considering zMS, we observed that women exercising at 50% of the NIH recommendations also significantly improved their MS status. The primary limitation of our study is that we cannot extend our findings to pre- or perimenopausal women. However, our findings are generalizable to aging women (45 to 75 years) who have completed menopause and remain sedentary, overweight, and, very likely, owing to the nature of MS progression, continue to accrue MS risk. We were also unable to address which factors associated with the MS carry greater weight regarding risk. Important to our findings is that physical activity remains a steadfast recommendation for the management of MS across all ages and genders.21 Moreover, the exercise levels we used in the present trial are easily attained by most women with a minimum weekly time commitment, resulting in significantly improved cardiometabolic status, with waist circumference the singular component most affected by exercise training, followed by the fasting glucose level and systolic blood pressure.
The results of the present study introduces a potentially interesting paradox to the discussion surrounding MS in that Reaven's original hypothesis22,23 was predicated on insulin resistance being the central mediating factor for syndrome × or the MS. In contrast, several consensus statements have suggested the prominent role of central adiposity.6,13 Given our present results, the MS and waist circumference were tightly coupled, and a recent report from our group has also shown waist circumference to correlate highly with fitness and the MS, using the same method for scoring used in our present report.7–9
Our results demonstrate the impact of cardiorespiratory exercise training dose on cardiometabolic risk factors and prevalence of MS in a large randomized study of postmenopausal women with a very high prevalence of MS and risk for subsequent CVD. Low-to-moderate intensity cardiorespiratory exercise appears to improve MS in postmenopausal women at levels at or above NIH recommendations, while zMS improves at half the NIH recommendations. Higher levels of energy expenditure appear to enhance this effect by incorporating a greater number of requisite MS composite scores.
Acknowledgments
This work was performed at the Cooper Institute (Dallas, Texas). We would like to thank the staff of DREW, the Cooper Institute Scientific Advisory Board, and the DREW participants for their work, advice, and participation in the DREW Study.
This work was supported by grant HL66262 from the National Institutes of Health (Bethesda, Maryland) and the donation of equipment from Life Fitness (Schiller Park, Illinois).
Footnotes
Disclosures: S.N.B. receives book royalties (<$5,000/year) from Human Kinetics (Champaign, Illinois); honoraria for service on the Scientific/Medical Advisory Boards for Alere (San Diego, California), Technogym (Fairfield, New Jersey), Santech (San Diego, California), Clarity (Seattle, Washington), and Jenny Craig (Carlsbad, California); honoraria for lectures and consultations from scientific, corporate, educational, and lay groups; research grants from the National Institutes of Health (Bethesda, Maryland), Coca-Cola Company (Atlanta, Georgia), Department of Defense (Washington, DC), and Body Media (Pittsburgh, Pennsylvania). T.S.C. has received honoraria for lectures from scientific, educational, and lay groups; has a book titled “Move Yourself: The Cooper Clinic Medical Director's Guide to All the Healing Benefits of Exercise”; has received research funding from the American Heart Association (Wiley Press, Indianapolis, Indiana) and the National Institutes of Health (Bethesda, Maryland), and unrestricted research funding from the Coca-Cola Company (Atlanta, Georgia); has overseen study sites for large pharmaceutical trials funded by Sanofi Aventis (Cambridge, Massachusetts), Orexigen (La Jolla, California), Arena (San Diego, California), and Amylin (San Diego, California); is a member of the Jenny Craig Medical Advisory Board; has served as a consultant to Technogym (Fairfield, New Jersey), Trestle Tree (Fayetteville, Arkansas), Vivus (Mountain View, California), Lockton-Dunning (Dallas, Texas), and Neuliven Health (San Diego, California); and serves as the Senior Medical Advisor for Catapult Health (Dallas, Texas). No other disclosures are reported.
References
- 1.Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109:551–556. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 2.Earnest CP, Artero EG, Sui X, Lee DC, Church TS, Blair SN. Cross-sectional association between cardiorespiratory fitness, cardiometabolic risk factors and metabolic syndrome in the Aerobics Center Longitudinal Study. Mayo Clin Proc. 2013;88:259–270. doi: 10.1016/j.mayocp.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 4.Morss GM, Jordan AN, Skinner JS, Dunn AL, Church TS, Earnest CP, Kampert JB, Jurca R, Blair SN. Dose Response to Exercise in Women aged 45-75 yr (DREW): design and rationale. Med Sci Sports Exerc. 2004;36:336–344. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- 5.Physical activity and cardiovascular health: NIH consensus development panel on physical activity and cardiovascular health. JAMA. 1996;276:241–246. [PubMed] [Google Scholar]
- 6.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 7.Brage S, Wedderkopp N, Ekelund U, Franks PW, Wareham NJ, Andersen LB, Froberg K. Features of the metabolic syndrome are associated with objectively measured physical activity and fitness in Danish children: the European Youth Heart Study (EYHS) Diabetes Care. 2004;27:2141–2148. doi: 10.2337/diacare.27.9.2141. [DOI] [PubMed] [Google Scholar]
- 8.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ. Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care. 2005;28:1195–1200. doi: 10.2337/diacare.28.5.1195. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JL, Slentz CA, Houmard JA, Samsa GP, Duscha BD, Aiken LB, McCartney JS, Tanner CJ, Kraus WE. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise) Am J Cardiol. 2007;100:1759–1766. doi: 10.1016/j.amjcard.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIH Publication No 02–3284. Bethesda: National Heart, Lung, and Blood Institute; [Accessed on May 17, 2007]. International Position Paper on Women's Health and Menopause: A Comprehensive Approach. Available at: http://www.nhlbi.nih.gov/health/prof/heart/other/wm_menop.htm. [Google Scholar]
- 11.Mesch VR, Boero LE, Siseles NO, Royer M, Prada M, Sayegh F, Schreier L, Benencia HJ, Berg GA. Metabolic syndrome throughout the menopausal transition: influence of age and menopausal status. Climacteric. 2006;9:40–48. doi: 10.1080/13697130500487331. [DOI] [PubMed] [Google Scholar]
- 12.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 13.Earnest CP, Artero EG, Sui X, Lee DC, Church TS, Blair SN. Maximal estimated cardiorespiratory fitness, cardiometabolic risk factors, and metabolic syndrome in the Aerobics Center Longitudinal Study. Mayo Clin Proc. doi: 10.1016/j.mayocp.2012.11.006. Epub 2013 Feb 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford ES, Li C. Physical activity or fitness and the metabolic syndrome. Exp Rev Cardiovasc Ther. 2006;4:897–915. doi: 10.1586/14779072.4.6.897. [DOI] [PubMed] [Google Scholar]
- 15.Katzmarzyk PT, Leon AS, Wilmore JH, Skinner JS, Rao DC, Rankinen T, Bouchard C. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003;35:1703–1709. doi: 10.1249/01.MSS.0000089337.73244.9B. [DOI] [PubMed] [Google Scholar]
- 16.Stewart KJ, Bacher AC, Turner K, Lim JG, Hees PS, Shapiro EP, Tayback M, Ouyang P. Exercise and risk factors associated with metabolic syndrome in older adults. Am J Prev Med. 2005;28:9–18. doi: 10.1016/j.amepre.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Watkins LL, Sherwood A, Feinglos M, Hinderliter A, Babyak M, Gullette E, Waugh R, Blumenthal JA. Effects of exercise and weight loss on cardiac risk factors associated with syndrome X. Arch Int Med. 2003;163:1889–1895. doi: 10.1001/archinte.163.16.1889. [DOI] [PubMed] [Google Scholar]
- 18.Green JS, Stanforth PR, Rankinen T, Leon AS, Rao Dc D, Skinner JS, Bouchard C, Wilmore JH. The effects of exercise training on abdominal visceral fat, body composition, and indicators of the metabolic syndrome in postmenopausal women with and without estrogen replacement therapy: the HERITAGE family study. Metabolism. 2004;53:1192–1196. doi: 10.1016/j.metabol.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Earnest CP, Lupo M, Thibodeaux J, Hollier C, Butitta B, Lejeune E, Johannsen NM, Gibala M, Church TS. Interval training in men at risk for insulin resistance. Int J Sports Med. doi: 10.1055/s-0032-1311594. Epub 2012 Nov 23. [DOI] [PubMed] [Google Scholar]
- 20.Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slordahl SA, Kemi OJ, Najjar SM, Wisloff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118:346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 22.Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 23.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu Rev Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]