Abstract
Exposure to environmental tobacco smoke (ETS) is known to contribute to and exacerbate inflammatory diseases of the lung such as chronic obstructive pulmonary disease (COPD) and asthma. The effect of ETS on angiogenesis and leukocyte recruitment, both of which promote lung inflammation, was investigated using lung tissue from mice exposed to aged and diluted sidestream cigarette smoke or fresh air for 12 weeks and transplanted into dorsal skin-fold chambers in nude mice. Lung tissue from mice exposed to cigarette smoke for 12 weeks exhibited significantly increased vascular density (angiogenesis) associated with selectin-mediated increased intravascular leukocyte rolling and adhesion compared to fresh air–exposed lung tissue by intravital microscopy. Further, neutrophils from nicotine-exposed mice displayed significantly increased rolling and adhesion compared to control neutrophils in microvessels of nicotine-exposed lungs versus control lung microvessels, suggesting that nicotine in cigarette smoke can augment leukocyte-endothelial interactions. ETS-induced angiogenesis and leukocyte trafficking may play a key role in airway recruitment of inflammatory cells in ETS-associated disorders such as COPD bronchitis or asthma.
Keywords: environmental tobacco smoke, leukocyte trafficking, lung angiogenesis, nicotine
Environmental tobacco smoke (ETS) is a major cause of a vast number of diseases, including chronic inflammatory diseases of the lung such as chronic obstructive pulmonary disease (COPD) [1] and asthma [2]. Exposure to cigarette smoke induces inflammatory processes in the airways. Long-term, ETS exposure leads to swelling of the airway epithelium, mucus hypersecretion, and increased airway reactivity characteristic of chronic bronchitis and COPD [3, 4]. At a cellular level, lung inflammation is characterized by the recruitment of circulating leukocytes into extravascular spaces of the lungs, leading to tissue infiltration by inflammatory cells. The molecular events following exposure to ETS, or its constituents such as nicotine, that lead to the initial interaction of circulating leukocytes with vascular endothelial cells and their subsequent accumulation in the airways are only now emerging. In a murine model of cerebral microcirculation, suffusion with nicotine was found to enhance P-selectin–dependent leukocyte rolling [5], an early critical step in the cascade of events leading to the recruitment of inflammatory cells to sites of inflammation. More recent studies from our laboratory using a novel lung allograft model [6] demonstrate that exposure to nicotine via suffusion significantly enhances rolling and adhesion of leukocytes within normal murine lung microvessels in a selectin-dependent manner via mitogen-activated protein kinase activation [7]. Although these studies suggest that in vivo, airway inflammation associated with cigarette smoking could potentially be due to enhanced leukocyte interactions in the lung microcirculation caused by the nicotine in cigarette smoke (CS), it is important to bear in mind that in both these studies [5, 7], nicotine was administered by suffusion of the exposed microcirculation and not administered systemically or, more importantly, by direct exposure to the airways. Furthermore, although being a major constituent, nicotine is one of over four thousand compounds found in CS [8].
In addition to its effect on leukocytes, studies have shown that nicotine stimulates proliferation of human endothelial cells [9] and prolongs cell survival by exerting an antiapoptotic effect in vitro [10]. In vivo, exposure to nicotine for three weeks was found to induce angiogenesis by promoting fibrovascular ingrowth within nicotine-loaded discs embedded subcutaneously (s.c.) in the flanks of mice as well as in a mouse model of hind-limb ischemia, where it was shown to increase capillary and collateral growth and enhance tissue perfusion [11]. Recent studies have demonstrated that exposure to second-hand smoke stimulates tumor angiogenesis that is mediated in part by nicotinic acetylcholine receptors (nAChR) [12]. Although the significance of angiogenesis in development, wound repair, and cancer has received much attention [13–15], the role played by angiogenesis in the lung during normal alveolar development, injury, and repair [16] or during airway remodeling associated with chronic airway inflammation such as that seen in asthma is only recently being understood [17]. In chronic inflammatory diseases of the lung, in addition to the influx of inflammatory cells, the airways are exposed to excessive mechanical strain, especially during periods of acute exacerbations. Recent studies have shown that under excessive mechanical strain, human airway smooth muscle cells produce angiogenesis-promoting factors such as hypoxia-inducible factor-1α, a transcription factor required for vascular endothelial growth factor (VEGF) expression, as well as VEGF itself, which may contribute to the angiogenesis seen with repeated exacerbation of asthma and COPD [18].
We hypothesized that airway inflammation in response to ETS exposure is associated with increased angiogenesis in the lung, which in turn could enable enhanced leukocyte-endothelial interactions in lung microvessels. These factors can contribute to the overall exacerbation of the inflammatory process by facilitating increased pulmonary recruitment of inflammatory cells. Accordingly, in the present study, the effects of sustained exposure to aged and diluted sidestream CS (ADSCS) by inhalation on pulmonary angiogenesis and leukocyte trafficking within murine pulmonary microvasculature were investigated using a novel model of lung microcirculation.
Materials and Methods
Exposure of Mice to CS
Normal BALB/c mice, 8 to 12 weeks old, were exposed to ADSCS as a surrogate for ETS or filtered air (FA) for 6 hours each day, 5 days a week for 12 weeks in a smoking apparatus as described previously [19, 20]. Research cigarettes (1R4F) obtained from the Tobacco Research Institute at the University of Kentucky were used. Mice in the present study were exposed to ETS at a target concentration of 30 ± 1 mg/m3 total suspended particulate, resulting in a nicotine concentration of 4.8 ± 0.5 mg/m3 within the inhalation chamber. Sidestream smoke is one of the primary components of ETS and contains the same toxic components identified in mainstream smoke exhaled by smokers [8]. All studies involving animals were performed according to Institutional Animal Care and Use Committee (IACUC)-approved protocols.
Lung Allograft Model for Angiogenesis and Measurement of Vascular Density
At the end of 12 weeks of exposure to ADSCS, mice were sacrificed and lung sections from both groups of mice (ADSCS and FA) were transplanted into skin-fold chambers in nude mice and allowed to undergo revascularization as described in our previous studies [6]. Briefly, longitudinal lung slices (1 to 3 slices, approximately 1 to 5 mm2) obtained from the periphery were fluorescently labeled with 5-(and 6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR; 1 mg/mL; Molecular Probes, Eugene, OR) and transplanted aseptically into skin-fold chambers placed in recipient nude mice under anesthesia. The chamber containing the lung allograft was then suffused with 25 to 50 μL of sterile saline and covered with a sterile siliconized coverslip. The microvasculature of the transplanted lung was observed periodically over two-weeks with the help of a Leitz Biomed intravital microscope (IVM) and all images were recorded on an S-VHS videocassette recorder (HC-6600; JVC, Tokyo, Japan) for play back off-line analysis. Our previous studies have demonstrated that over 10 to 14 days, the blood vessels within transplanted normal lung tissue sections completely establish connections (anestemosis) with host vessels and demonstrate efficient blood flow (revascularize) [6]. Still images of the vascular network on day 14 were recorded at various magnifications and tracings of the images recorded with a 10× objective were scanned. The dense dark regions on the scanned images, which represent the blood vessels, were selected and the area measured as pixels using Adobe Photoshop CS [21]. The dense area is expressed as a percent of the total number of pixels of the whole image. Comparisons were made between transplanted lung tissues from FA- and ADSCS-exposed mice with respect to density of the vascular network, which serves as a measure of angiogenesis (n = 2 different regions/allograft, 2 allografts each for ADSCS and ETS).
Evaluation of Leukocyte-Endothelial Interactions in ADSCS-Exposed Lung Microvessels and Antibody Blockade Studies
On day 14 after transplantation, mice bearing ADSCS- and FA-exposed lung allografts were administered intravenously (i.v.) with acridine orange (0.5 mg/mouse; Sigma Chemical, St. Louis, MO) to fluorescently label circulating leukocytes in vivo [7]. The interaction of the labeled leukocytes with the vascular endothelium of revascularized lung microvessels was evaluated by IVM (n = 3 experiments with 4 mice per experiment for ADSCS and 2 experiments with 3 mice per experiment for FA) followed by offline analysis of recorded video images as described in our previous studies [22]. Leukocytes visibly interacting with the lung microvascular endothelium (postcapillary venules and arterioles) and passing at a slower rate than the main blood stream were considered as rolling cells and were quantitated by manually counting the number of rolling cells passing through a reference point in a vessel segment. The number of rolling cells was expressed as rolling fraction, which was a percentage of the total number of cells (interacting and free-flowing or noninteracting) passing through the same reference point. Adherent cells were defined as those cells remaining stationary for >30 s and expressed as the number of adherent cells/100-μm length of lung microvessel. Rolling and adhesion in 4 to 6 lung microvessels (including postcapillary venules and arterioles) were analyzed per mouse on an average. In certain experiments, the involvement of endothelial P- and E-selectin in mediating ETS-induced leukocyte rolling and adhesion in these microvessels was investigated by i.v. administration of monoclonal antibody (mAb) against P- and E-selectin (mAb 5H1 and 9A9, respectively, at 2 mg/kg body weight) to mice bearing ADSCS-exposed lung allografts prior to microscopic observation (n = 2 experiments, 3 mice per group per experiment) [7]. Normal rat immunoglobulin G (IgG) used as a control exhibited the same level of rolling and adhesion observed with saline, which was considered as baseline rolling and adhesion.
Exposure to Nicotine and Evaluation of Neutrophil Rolling and Adhesion in Nicotine-Exposed Lung Microvessels
Female BALB/c mice (7 to 8 weeks) were implanted (s.c.) with 21-day slow-release nicotine pellets (5 mg/pellet; Innovative Research of America, Sarasota, FL) as described in our previous studies [23] (group I). One week after initiation of nicotine pellet exposure in these mice, lung tissue collected from age- and sex-matched untreated control BALB/c mice housed under similar conditions was transplanted into nude mice and allowed to undergo revascularization for 14 days. Nicotine pellet–exposed mice were euthanized on day 21 and blood as well as lung tissue was collected for neutrophil isolation and transplantation into nude mice, respectively. Neutrophils isolated (described below) from nicotine pellet–treated and control mice were labeled with carboxyfluorescein diacetate (CFDA; Invitrogen, Carlsbad, CA) and injected into the tail vein of nude mice bearing revascularized lung allografts from the control mice to evaluate rolling and adhesion of nicotine-exposed versus control neutrophils in normal lung microvessels. Two weeks after initiation of nicotine pellet exposure in the first group of mice described above, nicotine pellets were implanted in a second set of BALB/c mice (group II) to obtain nicotine-exposed neutrophils for infusion into mice bearing revascularized lung allografts from nicotine-exposed mice (from group I). These latter studies were designed to evaluate rolling and adhesion of nicotine-exposed versus control neutrophils in nicotine-exposed lung microvessels. Rolling and adhesion in all groups of mice was evaluated by IVM as described above for ADSCS-exposed mice (n = 8 mice for nicotine pellet-treated and 5 mice for control).
Isolation and Labeling of Murine Neutrophils
Murine peripheral blood neutrophils were purified by discontinuous density gradient centrifugation as described previously with minor modifications [24]. Blood from nicotine pellet–treated and control mice was first incubated with hetastarch (6% in normal saline) for 50 minutes to sediment erythrocytes. The upper plasma layer was collected and centrifuged on a discontinuous Percoll gradient consisting of 55% (v/v), 65% (v/v), and 75% (v/v) Percoll (Amersham Biosciences, Piscataway, NJ) in phosphate-buffered saline (PBS) at 1500 rpm for 20 minutes at room temperature (RT). The upper plasma and monocyte layers were carefully removed. Mature neutrophils were recovered at the interface of the 65% and 75% fractions. The viability and purity of the neutrophils determined by Wright-Giemsa staining and Trypan blue exclusion was >95% and 90%, respectively. Murine neutrophils thus isolated were labeled with CFDA as described in our earlier studies [25] and resuspended at a concentration of 2 × 106/200 μL prior to infusion.
Statistical Analysis
Values are presented as the mean ± standard error, unless otherwise stated. Statistical significance between two groups was estimated using the 2-tailed Student's t test. A P value of less than .05 was considered to be significant.
Results
Exposure to ADSCS Results in Increased Angiogenesis
Our previous studies have shown that normal lung tissue sections, when transplanted into skin-fold chambers, undergo revascularization by establishing connections with the host blood vessels and demonstrating blood flow [6]. In the current study, we used this model to evaluate the effect of CS on revascularization of lung microvessels. Whereas FA-exposed lung allografts exhibited the normal pattern of revascularization, continuous exposure to ADSCS for 12 weeks resulted in a highly branched and dense vascular network within revascularized lung allografts (Figure 1A). A quantitation of the vascular density demonstrated that exposure to ADSCS induced >2.5-fold increase in vascular density compared to mice that were exposed to FA (29.2% ± 9.88% versus 11.09% ± 13.64%) (Figure 1B).
Figure 1.
Exposure to ADSCS induces pulmonary angiogenesis. (A) Lung allografts obtained from FA-and ADSCS-exposed mice were transplanted into skin-fold chambers in nude mice and allowed to undergo revascularization. Representative photomicrographs of the microvasculature of the revascularized lung allografts from FA- and ADSCS-exposed mice observed by IVM on day 14 at magnifications of 40× (upper panels) and 100× (lower panels) are shown. (B) The density of the vascular network in revascularized lung allografts from ADSCS- and FA-exposed mice was determined from scanned images of the vascular network as described in Materials and Methods. Individual data of 4 different regions from 2 allografts each for FA and ADSCS is shown. The inset shows mean ± range of the data.
Exposure to ADSCS Induces Leukocyte Rolling and Adhesion Within Revascularized Lung Microvessels That Is E- and P-Selectin Dependent
Lung inflammation is associated with increased recruitment of inflammatory leukocytes to the airways, a process that is orchestrated in part by increased cellular trafficking mediated by cell adhesion molecules on both leukocytes and endothelial cells. IVM studies of leukocytes within lung microvessels of allografts from ADSCS- and FA-exposed mice revealed that acridine orange–labeled leukocytes demonstrate significantly increased rolling in microvessels of ADSCS-exposed lung allografts (42.66% ± 1.83%) compared to vessels in lung allografts from FA-exposed mice (5.95% ± 2.27%; P < .01) (Figure 2A). Fluorescent cells within microvessels of ADSCS- and FA-exposed lung allografts are shown in Figure 2B. Although the number of adherent leukocytes in microvessels of ADSCS-exposed lung allografts was low (1.15 ± 0.39), the number of adherent cells in microvessels of FA-exposed allografts was observed to be even lower (0.18 ± 0.1; P < .01).
Figure 2.
Exposure to ADSCS induces leukocyte rolling and adhesion within revascularized lung microvessels. (A) The interaction of acridine orange-labeled circulating leukocytes with the endothelium of the revascularized microvessels of lung allografts from ADSCS- and FA-exposed mice was evaluated by IVM and the number of rolling and adherent cells was determined by off-line analysis of recorded video images. Data represent mean ± SE. *P < .01 (B) Representative photomicrographs of acridine orange-labeled leukocytes within microvessels of lung allografts from ADSCS- (left panel) and FA- (right panel) exposed mice at magnification of 100× are shown.
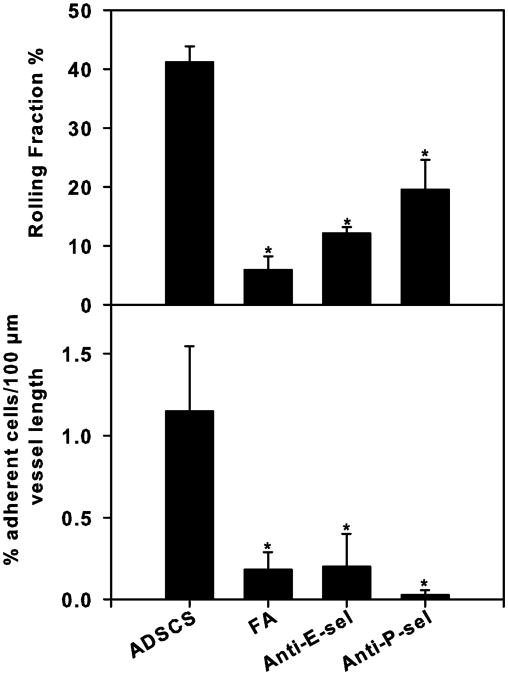
The increased rolling observed in revascularized microvessels of ADSCS-exposed lung allografts was significantly inhibited by mAbs against E-selectin (67.29% ± 1.45%; P < .01) and P-selectin (47.53% ± 13.62%; P < .01), whereas leukocyte adhesion was reduced to levels observed in FA-exposed lung microvessels by both of these antibodies (Figure 3). These studies demonstrate that endothelial-expressed P- and E-selectins play a prominent role in mediating ADSCS-induced rolling and adhesion.
Figure 3.
ADSCS-induced rolling and adhesion of leukocytes in lung microvessels is selectin-mediated. The effect of anti-P- and anti-E-selectin mAb treatment on leukocyte rolling and adhesion in revascularized lung microvessels of ADSCS- and FA-exposed mice was investigated by IVM. Anti-selectin antibodies were used at a concentration of 2 mg/kg body weight. Rolling (upper panel) and adhesion (lower panel) of circulating acridine orange–labeled leukocytes was analyzed by off-line analysis of recorded video images. Data represent mean ± SE. *P < .05 versus rolling and adhesion in ADSCS-exposed microvessels.
Nicotine Induces Neutrophil Rolling and Adhesion
To evaluate whether ADSCS-induced leukocyte rolling and adhesion was due to nicotine, in these next set of experiments, neutrophils isolated from nicotine-exposed or control (non–nicotine-exposed) mice were fluorescently tagged with CFDA and infused into mice implanted with lung allografts from nicotine-exposed or control mice. Neutrophils from control mice demonstrated significantly increased rolling and adhesion in revascularized microvessels of nicotine-exposed lungs, in comparison to the negligible levels of rolling and adhesion observed in microvessels of control lungs, and the increased rolling was associated with a significant reduction in rolling velocities of these neutrophils (Figure 4A, solid bars in upper, middle, and lower panels; *P < .01; Figure 4B, upper right and left panels). Neutrophils isolated from nicotine-exposed mice also exhibited substantially higher rolling and adhesion associated with reduced rolling velocities in microvessels of nicotine-exposed lungs compared to microvessels of control lung allografts (Figure 4A, lined bars in upper, middle, and lower panels; **P < .01; Figure 4B, lower right and left panels). These results suggest that the increased leukocyte rolling and adhesion observed in lung microvessels from ADSCS-exposed mice (Figure 2) is mediated by nicotine, which alters the adhesive properties of the microvessels, enhancing neutrophil-endothelial interactions. Interestingly, neutrophils from nicotine-exposed mice exhibited significantly increased rolling and adhesion compared to neutrophils from control non–nicotine-exposed mice not only in microvessels from nicotine pellet–exposed lungs but also in microvessels from control lungs (Figure 4A; §P < .05), suggesting that exposure to nicotine may either directly or indirectly (through the activation of other cell types such as platelets) alter the adhesive properties of leukocytes, accounting for the enhanced rolling and adhesion of nicotine-exposed neutrophils.
Figure 4.
Nicotine induces neutrophil rolling and adhesion in lung microvessels. (A) Rolling and adhesion of CFDA-labeled neutrophils from control and nicotine pellet-exposed mice in revascularized lung microvessels of allografts from control and nicotine-exposed mice was evaluated by IVM and off-line analysis of recorded video images. Data represent mean ± SE. *, **P < .01 versus rolling and adhesion in control lungs; §P < .05 versus rolling and adhesion of control neutrophils. (B) Representative photomicrographs of CFDA-labeled neutrophils from control and nicotine-exposed mice within microvessels of lung allografts from control and nicotine-exposed mice at a magnification of 100× are shown. Upper left panel: control neutrophils in control lung microvessels; lower left panel: neutrophils from nicotine-exposed mice in control lung microvessels; upper right panel: control neutrophils in nicotine-exposed lung microvessels; lower right panel: neutrophils from nicotine-exposed mice in nicotine-exposed lung microvessels.
Discussion
Over the last several decades, it has become increasingly clear that exposure to ETS has serious adverse effects on lung health, contributing to overall poor pulmonary function, diseases such as COPD bronchitis, and even exacerbation of asthma [2, 26]. In addition to the increased cellular infiltration, the chronic bronchitis phenotype of COPD and chronic asthma are associated with structural remodeling of the airways, including vascular remodeling or angiogenesis [27, 28]. In fact, enhanced expression of VEGF, a major proangiogenic factor [29], has been demonstrated in pulmonary arteries of smokers and patients with moderate COPD [30] as well as in the sputum of patients with COPD bronchitis [31], but not in patients with severe COPD and emphysema where VEGF expression has been shown to be decreased [32]. Our aim in the present study was to understand the impact of ETS on angiogenesis in the chronic bronchitis phenotype associated with mild to moderate COPD rather than severe emphysema. Previous time course studies in mice have shown that exposure to CS up to 24 weeks until the development of pulmonary emphysema results in a progressive biphasic increase in the total number of inflammatory cells in the bronchoalveolar lavage, with the 12-week mid–time point being the initiation of the second phase of increase in lung inflammatory cells [33]. Further, exposure to ETS for 12 weeks enables determination of the effects of ETS following a relatively extended period of exposure. Therefore, a 12-week end point was selected in the current study.
Although the process of development of new vessels (angiogenesis) plays an important role in normal health and is fundamental for ensuring adequate metabolic supply to tissues, in some disease states such as cancer, there is a perpetuation of angiogenesis that favors the pathological state [15]. CS extract has been shown to induce the release of proangiogenic factors such as VEGF and matrixmetalloproteinases 2 and 9 by human colon adenocarcinoma cells in addition to indirectly inducing endothelial cell proliferation in vitro [34]. In addition, exposure to second-hand smoke has been shown to induce angiogenesis (capillary density) in vivo within tumors in tumor angiogenesis models [12]. Previous studies using bronchial biopsy specimens of preneoplastic lesions from smokers who were at high risk for developing lung carcinoma and from normal subjects suggest that smoking appears to induce a proliferative response as well as neovascularization in the bronchial mucosa [35]. This effect of CS may in part be due to nicotine, a major constituent of CS, as studies in different tumor models have shown that angiogenesis is induced by nicotine [11, 36–38] and that nicotine-induced angiogenesis can be suppressed by inhibitors of nAChR [39, 40]. Recent studies have shown that an endothelial nAChR, which can be activated by exogenous nicotine, mediates endothelial proliferation, survival, migration, and tube formation in vitro, and angiogenesis in vivo [41].
Although the effects of ETS on tumor angiogenesis are evident from the above studies, little is known regarding the effects of ETS or its components on nonpathological angiogenesis. In contrast to the findings described above on the angiogenic effect of CS, previous in vitro studies have demonstrated that acute exposure of 5-day chick chorioallantoic membranes (CAMs) to mainstream and sidestream CS solutions inhibits growth and angiogenesis [42]. In other in vitro studies, CS extract was found to inhibit angiogenesis (endothelial monolayer wound repair, tube formation, migration, and proliferation) of pulmonary artery endothelial cells [43] as well as primary cultured human umbilical vein endothelial cells (HUVECs) [44]. Although these studies provide useful information regarding the short-term (24- to 48-hour) effects of CS extract on angiogenesis in vitro, they do not address the in vivo effects of sustained exposure to ETS. We have previously shown that blood vessels within normal lung tissue sections are able to reestablish connections with host vessels when transplanted into skin-fold chambers, demonstrating a network of arterioles, capillaries, and postcapillary venules with continuous blood flow [6]. In the present study, we used this model to investigate the effect of sustained exposure (12 weeks) to AD-SCS on the vascular structure within lung sections once they have undergone revascularization (14 days after transplantation). In contrast to the in vitro findings with acute CS exposure described above with CAM or cultured endothelial cells, our studies demonstrate that revascularized lung allografts from sustained ADSCS-exposed mice exhibited significantly increased microvascular density and branching (angiogenesis) compared to allografts from FA-exposed mice (Figure 1A and B).
Inflammatory lung diseases such as COPD bronchitis and asthma that are exacerbated by ETS exposure are associated with the recruitment of increased numbers of inflammatory cells to the airways [45, 46]. Recruitment of cells to sites of inflammation involves initial leukocyte-endothelial adhesive interactions (rolling and adhesion) followed by chemoattractant induced transmigration into extravascular sites of inflammation [47]. In the current study, an evaluation of the effect of ETS on leukocyte-endothelial interactions within revascularized lung microvessels demonstrated that circulating leukocytes exhibit significantly increased rolling and adhesion within microvessels of lung allografts from ADSCS-exposed mice compared to those from FA-exposed mice (Figure 2A and B). Previous IVM studies in hamsters have shown that a 5-minute exposure to the main-stream CS of one cigarette can augment leukocyte rolling and adhesion in postcapillary venules [48]. In addition, enhanced leukocyte rolling and adhesion within the microcirculation of the cremaster muscle was recently demonstrated in rats exposed to CS for 4 weeks [49]. Our studies further support these findings and, more importantly, demonstrate that sustained exposure to ETS (up to 12 weeks) can exert long-term effects on angiogenesis and leukocyte-endothelial interactions within the angiogenic microvessels because these effects are apparent even 14 days after exposure to ETS has been terminated and lung allografts have undergone revascularization. Increased rolling and adhesion in microvessels of lung allografts from ADSCS-exposed mice in the present study was found to be E- and P-selectin mediated (Figure 3). This increased rolling and adhesion is most likely due to increased expression of adhesion molecules. Previous studies in vitro have shown that CS and nicotine can up-regulate the expression of adhesion molecules such as intercellular cell adhesion molecule (ICAM)-1, E-selectin, and vascular cell adhesion molecule (VCAM)-1 on HUVECs [50–52]. In addition, studies have also shown that administration of anti-P-selectin antibodies to hamsters inhibits leukocyte rolling and adherence induced by CS exposure in the cremaster muscle microcirculation [49]. Increased rolling and adhesion observed in the present study may be mediated in part by nicotine because neutrophils from control (non–nicotine-exposed) mice demonstrated a similar augmentation of rolling and adhesion in revascularized microvessels of lung allografts from mice that were systemically exposed to nicotine (via embedded pellets) compared to microvessels of control lung allografts (Figure 4A, upper and middle panels, solid bars, and Figure 4B, upper right and left panels, respectively). Further, previous studies by us and others have demonstrated that suffusion of blood vessels in the dorsal skin-fold chamber of mice with nicotine induces a dose-dependent increase in leukocyte rolling and adhesion in lung microvessels [7] as well as cerebral microcirculation [5] in an E- and P-selectin–dependent manner. However, nicotine is only one of many compounds present in ETS and it is possible that other components of CS can also contribute to increased leukocyte rolling and adhesion in addition to nicotine. Overall, the above studies clearly demonstrate that both vascular E- and P-selectin participate in ETS-induced leukocyte-endothelial interactions.
In addition to their effect on the endothelium described above, nicotine in CS can have an effect on leukocytes. Increased rolling and adhesion of infused neutrophils from control mice within revascularized lung microvessels of nicotine-exposed mice clearly demonstrates the effect of nicotine on the endothelium during neutrophil-endothelial interactions (Figure 4A, upper panel, solid bars). Infusion of neutrophils from nicotine-exposed mice demonstrated increased rolling and adhesion not only in lung microvessels of nicotine-exposed mice, but also within lung microvessels of control mice compared to control neutrophils (Figure 4A, upper and middle panels, lined bars, and Figure 4B, lower right and left panels, respectively). These studies suggest that nicotine can augment leukocyte-endothelial interactions by exerting its effect not only on the endothelium but also on neutrophils, either directly or indirectly (by activating other factors/cells such as platelets, which in turn might effect neutrophil-endothelial interactions). Nonetheless, studies in humans [53] and mice have shown that exposure to CS increases the number of neutrophils in the lungs [54] and the increased nicotine-mediated neutrophil-endothelial interactions described herein may constitute an important early event in neutrophil recruitment to the airways.
Overall, our studies demonstrate that exposure to ETS induces angiogenesis of lung microvessels as well as increased leukocyte rolling and adhesion within these microvessels that is mediated by E- and P-selectin. ETS-induced endothelial-leukocyte interactions appear to be mediated by nicotine. The perpetuation of angiogenesis in the lungs associated with increased leukocyte trafficking within the angiogenic microvessels in response to ETS exposure may aid the ingress of inflammatory cells to the airways during chronic inflammatory diseases such as COPD bronchitis or asthma as described in the case of other inflammatory diseases such as rheumatoid arthritis [55]. In fact, studies in a murine model of asthma have demonstrated that treatment of mice with a potent antiangiogenic factor, endostatin, inhibits recruitment of inflammatory cells to the airways as well as airway hyperresponsiveness [56].
Acknowledgments
This study was supported by grants from the California Tobacco-related Disease Research Program (10 RT-0171) and National Institutes of Health (AI 35796) to P.S.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
M. Reza Hosseinkhani, Department of Veterinary and Biomedical Sciences and Department of Medicine, University of Minnesota, St. Paul, Minnesota, USA.
Kent E. Pinkerton, Center for Health and the Environment, University of California, Davis, California, USA
P. Sriramarao, Department of Veterinary and Biomedical Sciences and Department of Medicine, University of Minnesota, St. Paul, Minnesota, USA
References
- 1.Hylkema MN, Sterk PJ, de Boer WI, Postma DS. Tobacco use in relation to COPD and asthma. Eur Respir J. 2007;29:438–445. doi: 10.1183/09031936.00124506. [DOI] [PubMed] [Google Scholar]
- 2.Eisner MD. Environmental tobacco smoke and adult asthma. Clin Chest Med. 2002;23:749–761. doi: 10.1016/s0272-5231(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 3.Dwyer TM. Cigarette smoke-induced airway inflammation as sampled by the expired breath condensate. Am J Med Sci. 2003;326:174–178. doi: 10.1097/00000441-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87:1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 5.Yong T, Zheng MQ, Linthicum DS. Nicotine induces leukocyte rolling and adhesion in the cerebral microcirculation of the mouse. J Neuroimmunol. 1997;80:158–164. doi: 10.1016/s0165-5728(97)00151-3. [DOI] [PubMed] [Google Scholar]
- 6.Sikora L, Johansson ACM, Rao SP, Hughes GK, Broide DH, Sriramarao P. A murine model to study leukocyte rolling and intravascular trafficking in lung microvessels. Am J Pathol. 2003;162:2019–2028. doi: 10.1016/S0002-9440(10)64334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikora L, Rao SP, Sriramarao P. Selectin-dependent rolling and adhesion of leukocytes in nicotine-exposed microvessels of lung allografts. Am J Physiol Lung Cell Mol Biol. 2003;285:L654–L663. doi: 10.1152/ajplung.00448.2002. [DOI] [PubMed] [Google Scholar]
- 8.Chan-Yeung M, Dimich-Ward H. Respiratory health effects of exposure to environmental tobacco smoke. Respirology. 2003;8:131–139. doi: 10.1046/j.1440-1843.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 9.Villablanca AC. Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J Appl Physiol. 1998;84:2089–2098. doi: 10.1152/jappl.1998.84.6.2089. [DOI] [PubMed] [Google Scholar]
- 10.Zeidler R, Albermann K, Lang S. Nicotine and apoptosis. Apoptosis. 2007;12:1927–1943. doi: 10.1007/s10495-007-0102-8. [DOI] [PubMed] [Google Scholar]
- 11.Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, Tsao PS, Johnson FL, Cooke JP. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 12.Zhu BQ, Heeschen C, Sievers RE, Karliner JS, Parmley WW, Glantz SA, Cooke JP. Second hand smoke stimulates tumor angiogenesis and growth. Cancer Cell. 2003;4:191–196. doi: 10.1016/s1535-6108(03)00219-8. [DOI] [PubMed] [Google Scholar]
- 13.Velazquez OC. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vas Surg. 2007;45:A39–A47. doi: 10.1016/j.jvs.2007.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otrock ZK, Mahfouz RAR, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis. 2007;39:212–220. doi: 10.1016/j.bcmd.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 16.Thébaud B. Angiogenesis in lung development, injury and repair: implications for chronic lung disease of prematurity. Neonatology. 2007;91:291–297. doi: 10.1159/000101344. [DOI] [PubMed] [Google Scholar]
- 17.Mauad T, Bel EH, Sterk PJ. Asthma therapy and airway remodeling. J Allergy Clin Immunol. 2007;120:997–1009. doi: 10.1016/j.jaci.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Hasaneen NA, Zucker S, Lin RZ, Vaday GG, Panettieri RA, Foda HD. Angiogenesis is induced by airway smooth muscle strain. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1059–L1068. doi: 10.1152/ajplung.00480.2006. [DOI] [PubMed] [Google Scholar]
- 19.Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S, Jenkins RA, Moneyhun JH. A sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhal Toxicol. 1994;6:79–93. [Google Scholar]
- 20.Zhong CY, Zhou YM, Joad JP, Pinkerton KE. Environmental tobacco smoke suppresses nuclear factor-{kappa}b signaling to increase apoptosis in infant monkey lungs. Am J Respir Crit Care Med. 2006;174:428–436. doi: 10.1164/rccm.200503-509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuster MM, Wang L, Castagnola J, Sikora L, Reddi K, Lee PHA, Radek KA, Schuksz M, Bishop JR, Gallo RL, Sriramarao P, Esko JD. Genetic alteration of endothelial heparan sulfate selectively inhibits tumor angiogenesis. J Cell Biol. 2007;177:539–549. doi: 10.1083/jcb.200610086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borgström P, Hughes G, Hansell P, Wolitzky B, Sriramarao P. Leukocyte adhesion in angiogenic blood vessels. Role of E-selectin P-selectin and β2 integrin in lymphotoxin-mediated leukocyte recruitment in tumor microvessels. J Clin Invest. 1997;99:2246–2253. doi: 10.1172/JCI119399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandit TS, Sikora L, Muralidhar G, Rao SP, Sriramarao P. Sustained exposure to nicotine leads to extramedullary hematopoiesis in the spleen. Stem Cells. 2006;24:2373–2381. doi: 10.1634/stemcells.2005-0447. [DOI] [PubMed] [Google Scholar]
- 24.Allport JR, Lim YC, Shipley JM, Senior RM, Shapiro SD, Matsuyoshi N, Vestweber D, Luscinskas FW. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J Leukoc Biol. 2002;71:821–828. [PubMed] [Google Scholar]
- 25.Sriramarao P, von Andrian UH, Butcher EC, Bourdon MA, Broide DH. L-selectin and very late antigen-4 integrin promote eosinophil rolling at physiological shear rates in vivo. J Immunol. 1994;53:4238–4246. [PubMed] [Google Scholar]
- 26.Reardon JZ. Environmental tobacco smoke: respiratory and other health effects. Clin Chest Med. 2007;28:559–573. doi: 10.1016/j.ccm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Kanazawa H, Asai K, Nomura S. Vascular endothelial growth factor as a non-invasive marker of pulmonary vascular remodeling in patients with bronchitis-type of COPD. Respir Res. 2007;8:22–28. doi: 10.1186/1465-9921-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqui S, Sutcliffe A, Shikotra A, Woodman L, Doe C, McKenna S, Wardlaw A, Bradding P, Pavord I, Brightling C. Vascular remodeling is a feature of asthma and nonasthmatic eosinophilic bronchitis. J Allergy Clin Immunol. 2007;120:813–819. doi: 10.1016/j.jaci.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Ferrara N. VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol. 2000;11:617–624. doi: 10.1016/s0958-1669(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 30.Santos S, Peinado VI, Ramirez J, Morales-Blanhir J, Bastos R, Roca J, Rodriguez-Roisin R, Barbera J. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1250–1256. doi: 10.1164/rccm.200210-1233OC. [DOI] [PubMed] [Google Scholar]
- 31.Rovina N, Papapetropoulos A, Kollintza A, Michailidou M, Simoes DC, Roussos C, Gratziou C. Vascular endothelial growth factor: an angiogenic factor reflecting airway inflammation in healthy smokers and in patients with bronchitis type of chronic obstructive pulmonary disease? Respir Res. 2007;8:53–60. doi: 10.1186/1465-9921-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanazawa H, Asai K, Hirata K, Yoshikawa J. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med. 2003;114:354–358. doi: 10.1016/s0002-9343(02)01562-0. [DOI] [PubMed] [Google Scholar]
- 33.D'Hulst AI, Vermaelen KY, Brusselle GG, Joos GF, Pauwels RA. Time course of cigarette smoke-induced pulmonary inflammation in mice. Eur Respir J. 2005;26:204–213. doi: 10.1183/09031936.05.00095204. [DOI] [PubMed] [Google Scholar]
- 34.Ye YN, Liu ESL, Shin VY, Wu WKK, Cho CH. Contributory role of 5-lipoxygenase and its association with angiogenesis in the promotion of inflammation-associated colonic tumorigenesis by cigarette smoking. Toxicology. 2004;203:179–188. doi: 10.1016/j.tox.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Hiroshima K, Iyoda A, Shibuya K, Hoshino H, Haga Y, Toyozaki T, Shiba M, Baba M, Fujisawa T, Ohwada H. Evidence of neoangiogenesis and an increase in the number of proliferating cells within the bronchial epithelium of smokers. Cancer. 2002;95:1539–1545. doi: 10.1002/cncr.10850. [DOI] [PubMed] [Google Scholar]
- 36.Shin VY, Wu WKK, Chu KM, Wong HPS, Lam EKY, Tai EKK, Koo MWL, Cho CH. Nicotine induces cyclooxygenase-2 and vascular endothelial growth factor receptor-2 in association with tumor-associated invasion and angiogenesis in gastric cancer. Mol Cancer Res. 2005;3:607–615. doi: 10.1158/1541-7786.MCR-05-0106. [DOI] [PubMed] [Google Scholar]
- 37.Jarzynka MJ, Guo P, Bar-Joseph I, Hu B, Cheng SY. Estradiol and nicotine exposure enhances A549 bronchioloalveolar carcinoma xenograft growth in mice through the stimulation of angiogenesis. Int J Oncol. 2006;28:337–344. [PMC free article] [PubMed] [Google Scholar]
- 38.Wong HPS, Yu L, Lam EKY, Tai EKK, Wu WKK, Cho CH. Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation. Toxicol Sci. 2007;97:279–287. doi: 10.1093/toxsci/kfm060. [DOI] [PubMed] [Google Scholar]
- 39.Dasgupta P, Chellappan S. Nicotine-mediated cell proliferation and angiogenesis: new twists to an old story. Cell Cycle. 2006;5:2324–2328. doi: 10.4161/cc.5.20.3366. [DOI] [PubMed] [Google Scholar]
- 40.Kiuchi K, Matsuoka M, Wu JC, Lima e Silva R, Kengatharan M, Verghese M, Ueno S, Yokoi K, Khu NH, Cooke JP, Campochiaro PA. Mecamylamine suppresses basal and nicotine-stimulated choroidal neovascularization. Invest Ophthalmol Vis Sci. 2008;49:1705–1711. doi: 10.1167/iovs.07-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooke JP. Angiogenesis and the role of the endothelial nicotinic acetylcholine receptor. Life Sci. 2007;80:2347–2351. doi: 10.1016/j.lfs.2007.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melkonian G, Cheung L, Marr R, Tong C, Talbot P. Mainstream and Sidestream Cigarette smoke inhibit growth and angiogenesis in the day 5 chick chorioallantoic membrane. Toxicol Sci. 2002;68:237–248. doi: 10.1093/toxsci/68.1.237. [DOI] [PubMed] [Google Scholar]
- 43.Su Y, Cao W, Han Z, Block ER. Cigarette smoke extract inhibits angiogenesis of pulmonary artery endothelial cells: the role of calpain. Am J Physiol Lung Cell Mol Physiol. 2004;287:L794–L800. doi: 10.1152/ajplung.00079.2004. [DOI] [PubMed] [Google Scholar]
- 44.Yang YM, Liu GT. Damaging effect of cigarette smoke extract on primary cultured human umbilical vein endothelial cells and its mechanism. Biomed Environ Sci. 2004;17:121–134. [PubMed] [Google Scholar]
- 45.Bathoorn E, Kerstjens H, Postma D, Timens W, MacNee W. Airways inflammation and treatment during acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:217–229. doi: 10.2147/copd.s1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moerloose KB, Pauwels RA, Joos GF. Short-term cigarette smoke exposure enhances allergic airway inflammation in mice. Am J Respir Crit Care Med. 2005;172:168–172. doi: 10.1164/rccm.200409-1174OC. [DOI] [PubMed] [Google Scholar]
- 47.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 48.Lehr HA, Kress E, Menger MD, Friedl HP, Hubner C, Arfors KE, Messmer K. Cigarette smoking elicits leukocyte adhesion to endothelium in hamsters: inhibition by CuZn-SOD. Free Radic Biol Med. 1993;14:573–581. doi: 10.1016/0891-5849(93)90138-k. [DOI] [PubMed] [Google Scholar]
- 49.Chen SHT, Chang TC, Chen LMJ, Chen PHH, Huang CY, Wei FC. The effects of anti-P-selectin antibody on leucocyte activity related to cigarette smoke in rats. Scand J Plast Reconstr Surg Hand Surg. 2006;40:1–7. doi: 10.1080/02844310500410153. [DOI] [PubMed] [Google Scholar]
- 50.Chen HW, Chien ML, Chaung YH, Lii CK, Wang TS. Extracts from cigarette smoke induce DNA damage and cell adhesion molecule expression through different pathways. Chem Biol Interact. 2004;150:233–241. doi: 10.1016/j.cbi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Ueno H, Pradhan S, Schlessel D, Hirasawa H, Sumpio BE. Nicotine Enhances Human Vascular endothelial cell expression of ICAM-1 and VCAM-1 via protein kinase C, p38 mitogen-activated protein kinase, NF-κB, and AP-1. Cardiovasc Toxicol. 2006;6:39–50. doi: 10.1385/ct:6:1:39. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Wang Z, Zhou Y, Liu L, Zhao Y, Yao C, Wang L, Qiao Z. Nicotine stimulates adhesion molecular expression via calcium influx and mitogen-activated protein kinases in human endothelial cells. Int J Biochem Cell Biol. 2006;38:170–182. doi: 10.1016/j.biocel.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Chalmers GW, MacLeod KJ, Thomson L, Little SA, McSharry C, Thomson NC. Smoking and airway inflammation in patients with mild asthma. Chest. 2001;120:1917–1922. doi: 10.1378/chest.120.6.1917. [DOI] [PubMed] [Google Scholar]
- 54.Seagrave J, Barr EB, March TH, Nikula KJ. Effects of cigarette smoke exposure and cessation on inflammatory cells and matrix metalloproteinase activity in mice. Exp Lung Res. 2004;30:1–15. doi: 10.1080/01902140490252858. [DOI] [PubMed] [Google Scholar]
- 55.Szekanecz Z, Koch AE. Mechanisms of disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3:635–643. doi: 10.1038/ncprheum0647. [DOI] [PubMed] [Google Scholar]
- 56.Suzaki Y, Hamada K, Sho M, Ito T, Miyamoto K, Akashi S, Kashizuka H, Ikeda N, Nakajima Y, Iwase M, Homma I, Kobzik L, Kimura H. A potent antiangiogenic factor, endostatin prevents the development of asthma in a murine model. J Allergy Clin Immunol. 2005;116:1220–1227. doi: 10.1016/j.jaci.2005.08.052. [DOI] [PubMed] [Google Scholar]