Abstract
We describe a novel method for creating luminescent lanthanide-containing nanoparticles in which the lanthanide cations are sensitized by the semiconductor nanoparticle’s electronic excitation. In contrast to previous strategies, this new approach creates such materials by addition of external salt to a solution of fully formed nanoparticles. We demonstrate this post-synthetic modification for the lanthanide luminescence sensitization of two visible emitting lanthanides (Ln), Tb3+ and Eu3+ ions, through ZnS nanoparticles in which the cations were added post-synthetically as external Ln(NO3)3·xH2O salt to solutions of ZnS nanoparticles. The post-synthetically treated ZnS nanoparticle systems display Tb3+ and Eu3+ luminescence intensities that are comparable to those of doped Zn(Ln)S nanoparticles, which we reported previously (J. Phys. Chem. A, 2011, 115, 4031–4041). A comparison with the synthetically doped systems is used to contrast the spatial distribution of the lanthanide ions, bulk versus surface localized. The post-synthetic strategy described in this work is fundamentally different from the synthetic incorporation (doping) approach and offers a rapid and less synthetically demanding protocol for Tb3+:ZnS and Eu3+:ZnS luminophores, thereby facilitating their use in a broad range of applications.
Keywords: Trivalent Lanthanide, Luminescence, II–VI Nanoparticles, Post-synthetic strategy
Introduction
Because of their unique luminescence properties,1–10 lanthanide cations (Ln3+) are of considerable interest for a broad range of applications; including telecommunications, energy conversion, bio-analysis, and biological imaging applications. Their 4f-4f transitions appear as sharp emission bands that are distributed over the visible and near-IR spectral regions, and their wavelength maxima do not shift upon change of external conditions, such as temperature, pH, or biological environment. Because of their minimal overlap and ‘fingerprint’ character,9 these luminescence bands are attractive candidates for use in multiplex assays. Because the lanthanide luminescence is long-lived (microsecond to millisecond time domain), time-gated luminescence measurements offer a method to discriminate the emission from nanosecond-lived background species, thus providing an improved signal-to-noise ratio and more sensitive detection. Unlike organic fluorophores, lanthanide luminescence is highly resistant to photobleaching, thereby offering promise for imaging applications.11
To exploit Ln3+ luminescence in applications, challenges posed by their small oscillator strength and non-radiative relaxation pathways7 must be overcome. Because of the electric-dipole forbidden nature of the f-f transitions, the molar extinction coefficient of the Ln3+ ions are typically extremely small, ≤ 10 M−1cm−1.12–14 To circumvent this limitation and more efficiently excite the lanthanide ions, workers use organic chromophores and/or semiconductor materials as sensitizers that absorb incident photons and transfer the excitation energy nonradiatively to excite the Ln3+ ions.15, 16 This indirect excitation of Ln3+ ions leads to the same well-defined and long-lived luminescence emission.2, 17–29 Doping of Ln3+ ions into an inorganic nanoparticle antenna provides an excellent strategy for both sensitizing the Ln3+ emission and increasing its quantum yield by protecting it from high frequency vibrational overtones of –OH, –NH, and –CH stretches, which can strongly quench the luminescence.30, 31 The superior promise of inorganic materials over organic chromophores as antennae derives from this feature. While the benefits of increasing the excitation efficiency and decreasing the nonradiative rates are well appreciated, the advantage of using a high number density of lanthanide ions is less well appreciated. Because detection sensitivity in an application depends directly on the number of photons emitted, using polymetallic lanthanide molecules and materials is a promising strategy for maximizing the number of photons per unit volume.32–34 In contrast to organic dyes the luminescence of Tb3+ and Eu3+ doped ZnS nanoparticles exhibit little self-quenching of the Ln3+ emission, thereby supporting the strategy of creating materials with a high density of Ln3+ ions. For these three reasons, inorganic nanoparticle hosts represent a very promising strategy for improving the efficiency of lanthanide ion luminescence.
Some previous studies have discussed the external addition of metal ions into nanoparticles from salt solution.35–41 For example, Alivisatos and coworkers35 showed that it is possible to produce Ag2Se nanoparticles by adding Ag+ ions to a solution of CdSe nanoparticles, and Banin and coworkers36 have demonstrated the doping of InAs nanoparticles with Cu2+, Ag+, and Au3+ through cation exchange. Murphy and coworkers37 compared the luminescence properties of Mn2+-doped ZnS nanoparticles with those for which the Mn2+ ions were added externally (post nanoparticle synthesis) and found that the luminescence was different. Cation exchange reactions in lanthanide fluoride nanoparticles were first reported by van Veggel and coworkers,38 however the host does not act as an antenna in this case. Chen and coworkers39 have added Tb3+ ions externally (post-synthesis) to ZnS nanoparticles in AOT [sodium bis(2-ethylhexyl)sulfosuccinate] reverse micelles and have observed enhanced Tb3+ emission; however, their study considers Tb3+ ions located on or near the surface of the nanoparticles.35, 38, 40, 41 In contrast to previous work, our current study compares the photophysical properties of the post-synthetically treated ZnS nanoparticles with those that have Ln3+ synthetically incorporated (doped). Sensitization of Tb3+ and Eu3+ luminescence is analyzed by studying the evolution of the ZnS nanoparticle spectra as Tb3+ or Eu3+ nitrate salts are added externally to their solutions, which are denoted as ZnS/Ln salt (where the slash indicates a post-synthesis Ln3+ salt addition), and by comparing those spectra to the synthetically incorporated Zn(Ln)S systems (the use of parentheses indicates that Ln3+ was added during the synthesis of the nanoparticle). The generality of this approach is illustrated by performing the same post-synthetic salt addition strategy to commercially purchased CdS nanoparticles. Finally, the asymmetry ratio and luminescence lifetime of Eu3+ cations are used to assess the relative contributions of core and surface localized Eu3+ ions.
The post-synthetically treated nanoparticles present a major advantage over the corresponding synthetically incorporated (doped) counterpart. For example, it is possible to synthesize Tb3+ and/or Eu3+ incorporated nanoparticles starting from one batch of undoped nanoparticles, whereas to prepare the corresponding doped systems requires two separate syntheses. Thus the novel post-synthetic protocol described in the present work reduces the synthetic effort, loss of yield, and other risks significantly. This advantage allows the synthesis of large amounts of luminescent-based materials when the synthesis of lanthanide based molecular complexes often requires multistep reactions which can require ‘man-months’ of effort.
Sensitizing Ln3+ Luminescence with II–VI Semiconductor Nanoparticles
General Characterization
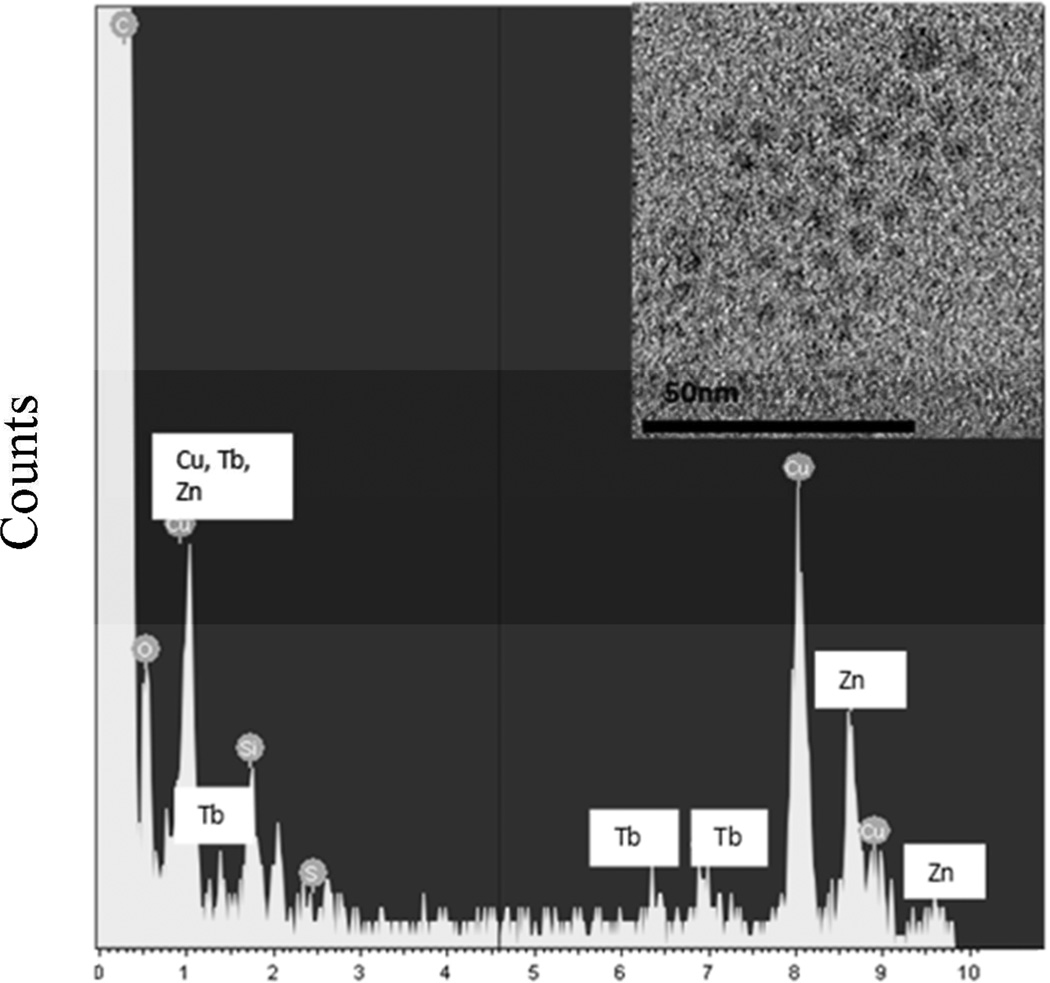
High resolution Transmission Electron Microscopy (HRTEM) images were obtained using a JEOL-2100 CF instrument operating between 120 kV and 200 kV. A TEM image and the corresponding EDS data for Zn(Tb)S nanoparticles are shown in Figure 1. The EDS data were collected with a micron scale spot size and demonstrate the presence of terbium. Various other researchers have also presented experimental observations on doping nano-sized ZnS materials with Tb3+ 39, 42 and Eu3+ 43–51 cations. The size distribution of the Zn(Tb)S nanoparticles were calculated using the Image J software and found to be 3.7 ± 0.7 nm in average diameter.
Figure 1.
The upper right panel shows an HRTEM image of Zn(Tb)S nanoparticles. An energy dispersive X-ray spectrum (EDS) of doped ZnS which labels the metal peaks is shown. Note that Cu most likely comes from the mounting assembly.
Absorption spectra of ZnS nanoparticles show a band centered around 295 nm (see Figure S1 in the supporting information). A simple estimation for the size of the nanoparticle based on the method proposed by Brus52 gives a value of 3.3 nm diameter and is consistent with the TEM measurement. The dependence of the exciton band position on ZnS nanoparticle growth time was shown previously.31 Peng and coworkers have also demonstrated growth time dependent absorption spectra for ZnS nanoparticles.53
Luminescence Spectra for ZnS Nanoparticle Tb(or Eu) Salt Solutions
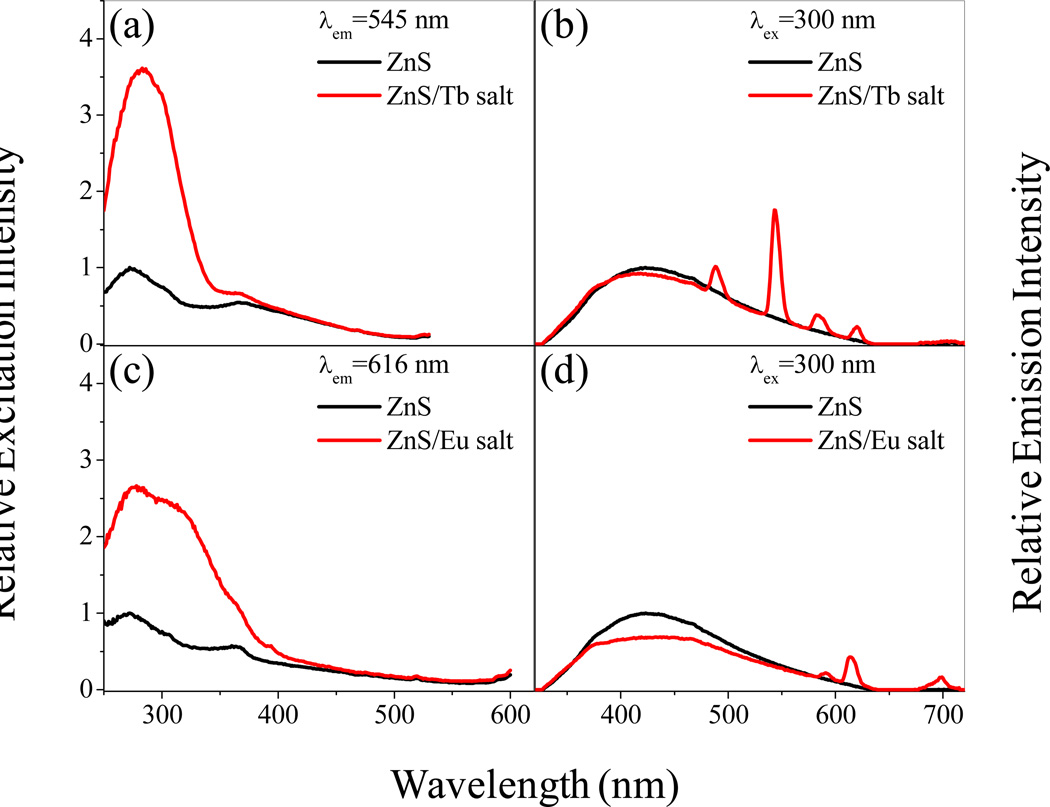
Figure 2 presents the steady-state excitation and emission spectra of ZnS/Tb salt, ZnS/Eu salt, and ZnS solutions. Under λex = 300 nm excitation, solutions of ZnS nanoparticles show a broad emission band (panels b and d, black) with an apparent maximum at ~420 nm, which is qualitatively consistent with previous work and is assigned to emission arising from various defect states of the nanoparticles.31 The panels a and c (black curve) show the ZnS excitation spectra at two different emission wavelengths.
Figure 2.
Representative steady-state excitation and emission spectra of ZnS/Tb salt and ZnS/Eu salt solutions are shown and compared to those for ZnS solutions. The nanoparticles were dissolved in chloroform: for the corresponding lanthanide salts, 0.1% water (v/v) was added to the nanoparticle containing chloroform solution. In each panel, the ZnS nanoparticle’s emission (black curve) is normalized to unity at the maximum signal intensity and the ZnS/Tb (or Eu) salt spectra (red curve) are shown with respect to it by scaling to the same optical density at the excitation wavelength. Figure S2 in the supporting information presents the subtracted excitation spectra.
ZnS/Tb Salt (Strategy I, 0.1% water, see Materials and Methods section)
Upon addition of Tb3+ nitrate salt to ZnS nanoparticle solutions, prominent sharp Tb3+ luminescence bands appear with well-defined maxima located at ~490 nm (5D4→7F6), ~545 nm (5D4→7F5), ~585 nm (5D4→7F4), and ~620 nm (5D4→7F3). (Figure 2, panel b). The Tb3+ emission of the ZnS/Tb salt is similar in intensity to the synthetically incorporated Zn(Tb)S system31 (see Table 1). By monitoring the sharp emission band centered at ~545 nm (Tb3+ emission) and scanning the excitation wavelength, a broad excitation profile which resembles that of the ZnS excitation spectrum is evident (Figure 2, panel a); however the band at ~285 nm is strongly enhanced for the ZnS/Tb salt solution which implies that it is more efficient in sensitizing the Tb3+ emission. In order to evaluate the stability of the lanthanide containing nanoparticles, corresponding spectra were acquired over many days and weeks; even in samples that are ~2.5 months old, no degradation of the lanthanide centered luminescence is evident. These data indicate that the sensitization of the Tb3+ luminescence operates through the electronic excitation of the ZnS nanoparticles in the 285 nm region; the ZnS nanoparticle acts as an antenna.
Table 1.
Comparison of Quantum Yields in Different Systems Studied.a
| Chloroform Solutions | Relative ΦF (Ln3+) |
|---|---|
| Zn(Tb)S | 1 |
| Zn(Tb)Sb | 0.93 ± 0.03 |
| ZnS/Tb saltb | 1.2 ± 0.2 |
| Zn(Tb)S/Tb saltb | 2.5 ± 0.3 |
| Zn(Eu)S | 1 |
| Zn(Eu)Sb | 0.77 ± 0.05 |
| ZnS/Eu saltb | 0.9 ± 0.2 |
| Zn(Eu)S/Eu saltb | 1.3 ± 0.2 |
All values were calculated relative to the value found for the synthetically incorporated Zn(Ln)S system at a sample absorbance of 0.2 at 300 nm. Its value was normalized to unity.
Solutions include 0.1% water.
ZnS/Eu Salt (Strategy I, 0.1% water, see Materials and Methods section)
External addition of Eu3+ ions to a ZnS nanoparticle solution generates Eu3+ luminescence bands, which appear at ~590 nm (5D0→7F1), ~616 nm (5D0→7F2), and ~700 nm (5D0→7F4) (Figure 2, panel d). The ZnS/Eu salt system displays a luminescence efficiency that is similar to the synthetically incorporated Zn(Eu)S system (see Table 1). Monitoring the sharp emission signal at 616 nm (Eu3+ emission) and scanning the excitation wavelength generates a broad excitation profile that closely resembles that of the ZnS excitation spectrum; however the band near 300 nm is enhanced over that for the pure ZnS. These data show that the ZnS nanoparticle absorbance sensitizes the Eu3+ luminescence (Figure 2, panel c), similar to the situation observed for the ZnS/Tb salt system described above.
ZnS/Eu Salt (Strategy II; no water, see Materials and Methods section)
Figure 3 compares the steady-state emission spectra of Eu3+ luminescence bands in a chloroform solution of Eu(NO3)3 with that for a ZnS/Eu salt solution, after subtraction of the intrinsic ZnS nanoparticle emission. The two solutions have the same concentration of Eu ions, and the spectra are scaled to unity for the Eu(NO3)3 solutions in order to simplify the comparisons. These data demonstrate that the solutions containing ZnS nanoparticles generate ~10 times more intensity for the Eu3+ luminescence.54 To better quantify the increase in Eu3+ luminescence which results from the ZnS nanoparticle’s antenna effect, control experiments were performed in which the Eu(NO3)3 was dissolved in chloroform containing TOP/TOPO/octadecene/tetracosane/0.1% water (the amount of these compounds were adjusted to closely mimic the synthetic conditions) and ZnS nanoparticles were added. The amount of ZnS nanoparticles in the solution was systematically varied and quantified by measuring the absorbance at 300 nm both before and after their addition. Figure 3b plots the change in the Eu3+ emission, scaled to the intensity without ZnS nanoparticles, as a function of the solution’s absorbance change (ΔA) at 300 nm. These data reveal a correlation between the increase in the Eu3+ 5D0→7F2 luminescence band centered at ~616 nm and the increase in absorbance at 300 nm (Figure 3, panel b). These data establish that the ZnS nanoparticles can increase the luminescence of Eu3+ ions by at least 60 times. Further control experiments are described in the supporting information (see Table S1). These findings show that the observed Eu3+ luminescence signal originates from a transfer of excitation energy from the ZnS nanoparticles to Eu3+; i.e., ZnS nanoparticles act as an antenna for Eu3+ in these systems.
Figure 3.
Representative steady-state emission spectra of Eu3+ containing systems with and without ZnS nanoparticles are shown in panel a. The experiments were performed by dissolving the salt in chloroform - see experimental section. Panel b shows a plot of how the 5D0→7F2 Eu3+ luminescence band intensity increases upon addition of ZnS nanoparticles to a ~50 µM overall concentration of Eu(NO3)3 solution in chloroform (see text for additional details). The relative intensity values are scaled with respect to the corresponding intensity value without the ZnS nanoparticles, which is normalized to unity at the maximum intensity.
Luminescence Spectra for Zn(Ln)S Doped Nanoparticles in Ln Salt Solutions
Zn(Tb)S/Tb Salt (Strategy I; 0.1% water, see Materials and Methods section)
Figure 4 (top panels) shows steady-state excitation and emission spectra of the Zn(Tb)S (black and red curves) and Zn(Tb)S/Tb salt (blue curve) solutions. As a control, the spectra of Zn(Tb)S nanoparticles were collected with ~0.1% water added to the chloroform solution (red curve), and they reveal little effect of the water on the excitation and emission spectra. Table 1 reports the relative Tb3+ band centered quantum yields. The excitation spectra of the Zn(Tb)S (Figure 4a, λem=545 nm, the Tb3+ centered emission) reveal a prominent feature at ~275 nm, which is different from that of ZnS (see Figure 2 and Figure S3 left panel in the supporting information) and indicates a change in the ZnS electronic transition because of the Tb3+ ion incorporation. The emission spectra of Zn(Tb)S and Zn(Tb)S/Tb3+ (Figure 4b) show prominent Tb3+ bands like those shown in Figure 2b for ZnS/Tb3+; see discussion of Figure 2b for band assignment.
Figure 4.
Representative steady-state excitation and emission spectra are shown for the Zn(Tb)S and Zn(Eu)S nanoparticle solutions in chloroform (black curve), for the Zn(Tb)S and Zn(Eu)S nanoparticle solutions in chloroform with 0.1% water (red curve), and for the Zn(Tb)S/Tb salt and Zn(Eu)S/Eu salt solutions in chloroform with 0.1% water (blue curve). For clarity, an enlarged part of the Eu3+ emission bands is shown in the inset of panel d. The excitation spectra for the Zn(Tb)S and Zn(Eu)S nanoparticles (black curve) were scaled to unity at the maximum intensity (~275 nm), and the other spectra are plotted with respect to it. The emission spectra were normalized to unity for the Zn(Tb)S and Zn(Eu)S nanoparticles at the peak of the broad, blue emission band (~420 nm); the red and blue curves were scaled with respect to the black curve in each panel. The spectra are corrected for optical density at the excitation wavelength.
Zn(Eu)S/Eu Salt (Strategy I, 0.1% water, see Materials and Methods section)
Figure 4 (bottom panels) depicts steady-state excitation and emission spectra of the Zn(Eu)S (black and red curves) and the Zn(Eu)S/Eu salt (blue curve) solutions. Addition of 0.1% water to the Zn(Eu)S solution (red curve) results in some small changes in the spectra. The Zn(Eu)S/Eu3+ solutions show some increase in the Eu3+ emission over that observed for Zn(Eu)S solutions, however the effect is less dramatic than in the case of Tb3+. The excitation spectrum, obtained by monitoring the Eu3+ centered emission, reveals a prominent feature at ~275 nm, which is different than that found for the ZnS and ZnS/Eu3+ salt solutions (see Figure 2c; also see Figure S3 (right panel)). This result is similar to that observed in the Zn(Tb)S/Tb salt system. The similarity of the excitation spectral changes for Zn(Eu)S and Zn(Tb)S suggests that it arises from changes in the ZnS electronic transition because of the creation of localized defects (Eu3+ and Tb3+ dopant sites).
Luminescence Spectra for CdS Nanoparticles in Ln Salt Solutions
The generality of the post-synthetic salt addition for sensitizing Ln3+ emission was tested by replacing the ZnS nanoparticles with CdS nanoparticles. CdS nanoparticles were chosen because of our previous work with Cd(Ln)S doped nanoparticles and their luminescence sensitization efficiency.31 Experiments were undertaken in which Tb(III) nitrate or Eu(III) nitrate were added separately to solutions of the commercially available oleic acid capped CdS nanoparticles (see Figure 5). Note that the capping ligand for the CdS nanoparticles is different from that used in the synthesis of ZnS nanoparticles. A comparison of the observed Tb3+ (Eu3+) luminescence intensities reveals an enhanced Tb3+ (Eu3+) luminescence signal in the presence of CdS nanoparticles (black curves, Figures 5b and 5d), than for the Tb3+ (Eu3+) in chloroform salt solutions (red curves, Figures 5b and 5d). The overlap of the Eu3+ 5D0 → 7F1 transition at ~590 nm and 5D0 → 7F2 transition at ~616 nm is caused by the wider slit widths, which were needed to acquire the weaker signals in these time-gated spectra (see Materials and Methods section). The excitation spectra (black curve), reveal two bands centered at around 300 nm and 360 nm. The 360 nm band in the excitation spectra coincides with the first exciton band of the CdS absorption spectrum (red curve), demonstrating that the CdS nanoparticles act as an antenna for sensitizing the Tb3+ and Eu3+ luminescence. In addition, the excitation spectrum for the CdS/Eu salt solutions display significant band intensities in the region of 300–360 nm, where no direct excitation of Eu3+ bands occurs, see Figure S4 in the supporting information. Both the Tb3+ and Eu3+ excitation spectra have a dominant peak in the 300 nm range, which demonstrates that higher energy photons are more efficient at sensitizing the lanthanide emission. Collectively, the measurements described above demonstrate that it is possible to sensitize the luminescence of Tb3+ and Eu3+ cations by the post-synthetic addition of the corresponding nitrate salts to solutions of the II–VI sulfide nanoparticles.
Figure 5.
Time-gated normalized excitation and relative emission spectra of CdS/Tb salt and CdS/Eu salt systems are shown; the excitation spectra in panels (a) and (c) are compared to the absorption spectrum of the pure CdS nanoparticles. The samples were dissolved in toluene with an overall total lanthanide cation concentration of approximately 50 µM. The emission intensities in the presence of CdS nanoparticles were scaled with respect to the corresponding value in the absence of the nanoparticles, for which the emission intensity was normalized to unity at the band maximum.
Assessing the Role of Core versus Surface Sites in Sensitizing Eu3+ Luminescence
The data and the analyses provided above do not allow for the determination of the lanthanide ions’ location in the nanoparticle; i.e., in the bulk of the ZnS (or CdS) nanoparticle or near its surface. The determination of the precise location of dopant ions in a host nanoparticle lattice is a significant challenge and few researchers have addressed this issue. Sarma and coworkers55 have used a spectral shape analysis of luminescence bands to determine the location of dopant Mn2+ ions in CdS nanoparticles of different sizes. Other strategies for the identification of surface related species rely on the modification of dopant properties with changes in the surface ligand coating of the nanoparticles and/or the synthesis of core-shell materials. Van Veggel and coworkers56 have explored these methods by analyzing how Eu3+ band intensities are altered in Eu3+ doped LaF3 nanoparticles. Our work distinguishes surface localized lanthanide ions from core localized lanthanide ions by analyzing the excited state decay rate of the Eu3+ centered emission and the relative luminescence intensities of the Eu3+ bands, which are sensitive to the symmetry of the local coordination environment.
Asymmetry Ratio
Although the intra-configurational 4f-4f lanthanide luminescence bands are shielded, several transitions are sensitive to the environment and some of them are considered to be hypersensitive.9 For Eu3+ the 5D0→7F2 transition (centered at ~616 nm), which is of electric dipole character and is forbidden in a perfectly octahedral environment, becomes more allowed and has higher oscillator strength in a less symmetric environment.9 In contrast, the 5D0→7F1 transition of the Eu3+ ion (centered at ~590 nm) is an f-f magnetic dipole transition that is largely insensitive to the environment around the Eu3+ ion. Thus the intensity ratio of the 5D0→7F2 transition to the 5D0→7F1 transition is a parameter that can be used to gauge the asymmetry around the Eu3+ ions. The higher the value of this intensity ratio, the more asymmetric is the electronic environment around the Eu3+ ions.a By postulating that surface sites are more asymmetric than core sites, the intensity ratio can be used to assess the relative contribution of core and surface bound Eu3+ ions to the overall emission.
For this analysis, the asymmetry parameter AS is defined as
| (1) |
where the band intensities are integrated over frequency ν. The AS values are reported in Table 2, and the values in parentheses indicate the number of independent measurements. The error in the AS determination arises predominantly from the error in sample preparation and reflects the difficulty in adding a precise and reproducible amount of salt to the chloroform solutions.57 For the systems investigated here, the post-synthesis addition of Eu3+ ions, either the ZnS/Eu3+ or Zn(Eu)S/Eu3+ nanoparticle solutions, causes an increase in the average asymmetry of the Eu3+ coordination environment, in comparison to the Zn(Eu)S system (a ratio of ~5.5 compared to one of ~4.6).
Table 2.
Asymmetry Parameter Values for Eu3+ in Different Systems Studied.a
| Chloroform Solutions | ∫ Iem[5D0 − 7F2] dν / ∫ Iem[5D0 − 7F1] dν |
|---|---|
| Pure Eu salt | 3.7 ± 0.2 (2)a |
| Zn(Eu)S | 4.8 ± 0.1 (4)a |
| Zn(Eu)Sc | 4.6 ± 0.2 (4)a |
| ZnS/Eu saltb,c | 5.5 ± 0.6 (5)a |
| Zn(Eu)S/Eu saltb,c | 5.6 ± 0.1 (3)a |
The numbers in the parenthesis indicate the number of independent measurements used to calculate the average and standard deviation values.
The europium (III) nitrate.xH2O salt was used for the experiments.
Samples dissolved in chloroform with 0.1% water.
The data presented in the Table 2 display some clear trends. First, the addition of a small amount of water (~0.1% v/v) causes a small change in the asymmetry parameter for the Zn(Eu)S system; however it still lies within the margin of error and the dominant effect is a decrease in the overall quantum yield ΦF (see Table 1). Second, the ZnS/Eu salt and Zn(Eu)S/Eu salt solutions have higher asymmetry ratios than the Zn(Eu)S solutions. The increase in the asymmetry parameter can be explained by postulating that more of the Eu3+ ions reside near (or on) the surface of the nanoparticles and these coordination environments are more asymmetric. Third, the free Eu3+ in chloroform has the lowest asymmetry ratio among the different systems studied in the present work.
Luminescence Lifetime Measurements
A comparison between the luminescence decay curves obtained for the Tb3+ and Eu3+ salts in chloroform and those measured for the ZnS/Tb salt and ZnS/Eu salt solutions (strategy I, 0.1% water) reveals that the average emission lifetime of the Tb3+ and Eu3+ luminescence is greatly lengthened in the presence of ZnS (see Figure 6). The average lifetime obtained for the Tb3+ and Eu3+ salts in chloroform were found to be in the range of ~250 and ~125 microseconds respectively, whereas the addition of ZnS nanoparticles caused a millisecond lifetime component to become apparent. The lifetime decay profiles for the nanoparticle solutions are well described by a bi-exponential decay law with a shorter lifetime component of ~500 µs (for both ZnS/Tb salt and ZnS/Eu salt) and a longer millisecond timescale component of 2.4 ms for ZnS/Tb salt and 1.5 ms for ZnS/Eu salt. Previous literature examples of luminescence lifetime values for well protected Tb3+ and Eu3+ molecular complexes are 1.3 ms and 0.78 ms respectively.58 A comparison of the longer lifetime components obtained in the presence of nanoparticles with those from molecular complexes and ions in solution reveals that ZnS nanoparticles are better at protecting the Tb3+ and Eu3+ cations from non-radiative decay processes arising from the solvent.
Figure 6.
Examples of Tb3+ and Eu3+ band-centered luminescence decay traces and their fits by a double-exponential decay law are shown for solutions with and without ZnS nanoparticles. The adjusted R2 values for the ZnS/Tb salt, Tb salt, ZnS/Eu salt, and Eu salt fits are 1.00, 0.90, 1.00, and 0.91 respectively. For pure lanthanide salts, the decays were acquired by dissolving them in chloroform. The ZnS/Tb and ZnS/Eu salt solutions were prepared in chloroform with 0.1% (v/v) water. Samples were excited at 354 nm and the emission was collected at 545 nm (Tb3+) and 616 nm (Eu3+).
The two different lifetime components could originate from two different environments in the ZnS nanoparticles; namely, the lanthanide ions in the core correspond to the millisecond timescale component and the lanthanide ions located near the ZnS surface correspond to the 500 µs component. In order to more clearly see the trend in the amplitudes of the lifetime components, the decay traces were best fit by fixing the lifetime components. Table 3 summarizes the lifetime decay parameters of the Eu3+ band centered luminescence decays for the different cases, with lifetimes fixed at 500 µs and 1.5 ms. The average lifetime values for the fitting with fixed lifetime parameters were calculated according to equation 2,
| (2) |
The corresponding value calculated from freely varying all parameters in the fitting procedure were calculated according to equation 3,
| (3) |
The average difference between <τ>vary and <τ>fix lifetime values was found to be less than 2%. This comparison shows that the fitting by fixed lifetime parameters provides an acceptable parameterization of the decay profile. The Eu3+ lifetime parameters in Table 3 reveal that the amplitude of the short lifetime (0.5 ms) component in the ZnS/Eu salt and Zn(Eu)S/Eu salt is only higher by ~5–10% as compared to the Zn(Eu)S system.
Table 3.
| System | a1 | τ1 (ms)c | a2 | τ2 (ms)c | <τ>fix (ms) |
|---|---|---|---|---|---|
| Zn(Eu)S | 0.49 ± 0.03 | 0.5 | 0.51 ± 0.03 | 1.5 | 1.01 |
| ZnS/Eu salt | 0.54 ± 0.04 | 0.5 | 0.46 ± 0.04 | 1.5 | 0.96 |
| Zn(Eu)S/Eu salt | 0.59 ± 0.01 | 0.5 | 0.41 ± 0.01 | 1.5 | 0.91 |
Samples were dissolved in chloroform with 0.1% water.
λem = 616 nm, Eu3+ 5D0→7F2 transition.
Luminescence lifetime components were kept fixed in the fitting process.
In order to further characterize the Eu3+ band centered luminescence decays, the decay curves were fit by a distribution of lifetimes for the Zn(Eu)S and Zn(Eu)S/Eu salt solutions (see Figure 7). Comparison of the lifetime distribution shows that for the Zn(Eu)S/Eu salt solutions, the shorter lifetime components are more prevalent than for the Zn(Eu)S solutions. The increase in the amplitude of the shorter lifetime components in the Zn(Eu)S/Eu salt solutions is consistent with an enhancement of surface related Eu3+ population and corroborates the trend of an increased amplitude for the shorter lifetime component in the double-exponential decay fitting analysis (see Table 3).
Figure 7.
Luminescence lifetime distribution of the Zn(Eu)S and Zn(Eu)S/Eu salt systems studied. In each case, the area under the curve has been normalized to unity.
Conclusion
This work presents and demonstrates a novel strategy to create Tb3+ and Eu3+ luminophores by post-synthetic, external addition of Tb3+ and Eu3+ salts to solutions of II–VI semiconductor nanoparticles (ZnS and CdS). The lanthanide luminescence in the externally added salt systems is demonstrated to be similar and of comparable intensity to the corresponding synthetically incorporated systems with the ZnS nanoparticles acting as an antenna.31 Photophysical characterization, based on the analysis of the asymmetry ratio and the luminescence decay, reveals a small but discernible enhancement of the surface related contribution to the luminescence of the post-synthetically treated systems, as compared to the corresponding synthetically incorporated (doped) system. These data indicate that the distribution of lanthanide ions in the ZnS nanoparticles show a small preference for surface localized sites for the post-synthetic addition, as compared to the synthetically doped ZnS. Similar post-synthetic salt addition experiments with CdS nanoparticles reveal an increase in Tb3+ and Eu3+ luminescence in the presence of nanoparticles, as compared to the free salt case, suggesting that this approach is broadly applicable. The lanthanide luminescence sensitization that is found by post-synthetic salt addition offers a rapid and less synthetically demanding approach to sensitize the Tb3+ and Eu3+ luminescence, facilitating the scope of their applications.
Materials and Methods
Chemicals
Trioctylphosphine [TOP] (90%), trioctylphosphine oxide [TOPO] (90%), zinc stearate (tech.), and octadecene (90% tech.) were purchased from Sigma-Aldrich-Fluka, St. Louis, MO. Tetracosane (99%) was purchased from Acros. Chloroform was purchased from J. T. Baker, Phillipsburg, NJ. Toluene was purchased from Fisher Scientific, Pittsburgh, PA. Sulfur was purchased from Fisher Scientific, Pittsburgh, PA. Terbium (III) nitrate (99.9%), lanthanum (III) nitrate (99.99%), and lutetium (III) nitrate (99.9%) were purchased from Strem, europium (III) nitrate (99.99%) was purchased from Aldrich, and gadolinium (III) nitrate (99.99%) was purchased from Alfa Aesar, Ward Hill, MA. In all cases, hydrated lanthanide salts were used. n-Hexane and 1-octanol were purchased from Acros, and ethyl acetate was purchased from EMD, Gibbstown, NJ. Argon gas was purchased from Valley National, Pittsburgh, PA. All chemicals were used as purchased without additional purification.
ZnS Based Nanoparticle Synthesis
All ZnS nanoparticle systems were synthesized using a non-coordinating solvent system consisting of octadecene and tetracosane. Zinc stearate and lanthanide nitrate salts were used as cation precursors, and elemental sulfur served as the anion precursor. Tetracosane (4.0 g), octadecene (3.0 mL), TOPO (1.7 g), and 0.68 mmol of zinc stearate were loaded into a three neck, round-bottom flask and refluxed at 300 °C while stirring under nitrogen. The lanthanide stock solution (0.12 mmol lanthanide nitrate dissolved in trioctylphosphine) was injected after approximately ~2 h of heating and allowed to stir within the reaction mixture for 1 hour. The sulfur stock solution (sulfur powder dissolved in 2.5 mL octadecene) was injected ~1 h after the lanthanide stock solution injection. After 20 minutes of growth, the reaction was quenched by removal of the heat source and all of the reaction mixture was collected. The resulting nanoparticles were then re-dispersed in chloroform for spectroscopic analysis.
Pure (not incorporating lanthanides) ZnS nanoparticles were prepared using the methods described above; however, the zinc stearate precursor was increased to 0.80 mmol, and the lanthanide stock solution preparation was omitted. For purification of nanoparticles, solid raw material from the synthesis was dissolved in chloroform. To this solution, methanol was added drop wise until a precipitate formed. The precipitate was isolated by centrifugation and was used for TEM measurements.
CdS Nanoparticles
CdS nanoparticles with oleic acid as capping ligand, size ~2.3 nm, were purchased from NN Labs as a solution in toluene and were used as received.
Sample Preparation
To prepare the solutions for the external addition experiments with the ZnS nanoparticles, two different strategies were used. In strategy I, a known (maintaining the weight ratio of Ln3+ ions to the total weight of the synthesized nanoparticles) and quantitative amount of Ln3+ salt was added to the nanoparticle solution. The externally added salts were dissolved by adding a small amount of water (~0.1% v/v). Incorporation of a small amount of polar solvent to dissolve the externally added salt is a well-known procedure described in the literature.35 In strategy II, Eu3+ salt was sonicated in chloroform for approximately one week at ~40–45 °C. The resulting suspension was filtered and an additional amount of chloroform was added to the filtrate to obtain a total volume of ~3 mL. Synthesized ZnS nanoparticles were added to this solution. In this way, the solution contains a quantifiable (that is, the total amount of Eu3+ in the solution does not change before and after addition of ZnS nanoparticles) but uncertain amount of Eu3+ ions in the solution (the extremely low value of molar extinction coefficient of Eu3+ ions in chloroform prevented the determination of its exact amount by UV-Vis absorption technique). Supplementary water was not added to these solution preparations of the solutions described under strategy II. It is to be noted that the exact amount of Eu3+ ions in these samples does not impact the conclusions in a significant way; as long as the total amount of Eu3+ ions remains fixed. This experiment reveals the role of ZnS nanoparticles for the sensitization of Eu3+ luminescence in these systems.
For performing experiments with CdS nanoparticles, a comparison was made between the respective lanthanide bands’ luminescence in the presence and in the absence of nanoparticles. The samples were dissolved in toluene. Lanthanide salts were introduced into the solutions from a methanol stock solution with 0.1% (v/v) methanol content.
Steady-state Optical Measurements
Steady-state absorption spectra were obtained with a Perkin-Elmer Lambda 9 or an Agilent 8453 UV-visible spectrophotometer. Steady-state excitation and emission spectra were recorded using either a Jobin Yvon Horiba Fluorolog-322 or a Jobin Yvon Horiba Fluoromax-3. No significant differences were observed by comparing results obtained from these two instruments. In all cases, spectra were corrected for excitation and emission response (lamp, detector, and monochromators). Time-gated spectra were collected in a Cary Eclipse fluorometer. A quartz 1-cm pathlength cuvette was used for all spectroscopic experiments. All measurements were performed at room temperature. Steady-state spectra were acquired with excitation and emission slits of 4 nm and 4 nm respectively. The steady-state spectra were acquired with long pass filters that eliminated the excitation light. For time-gated measurements, the spectra were acquired with excitation and emission slits of 20 nm and 20 nm respectively. For the acquisition of time-gated spectra, the filter arrangement was adjusted from the software by selecting the “Auto” option to eliminate the excitation light.
Quantum Yields
Absorption spectra were recorded on a Perkin-Elmer Lambda 9 spectrometer that was coupled to a personal computer using software supplied by Perkin-Elmer. Relative quantum yields were calculated by scaling the emission intensities to the value for the synthetically incorporated Zn(Ln)S system, whose quantum yield was set to unity. All spectra were corrected for the excitation and emission instrument responses. The quantum yield values were calculated using equation 4:
| (4) |
where the subscript r denotes the reference and x denotes the sample; A is the absorbance at the excitation wavelength (λ), I is the intensity of the excitation light, η is the refractive index (η = 1.446 in chloroform), and D is the measured integrated luminescence intensity. For the ZnS/Ln3+ systems studied, the lanthanide quantum yields were determined by integrating over the narrow Ln3+ emission bands, with the nanoparticle’s broad band steady-state emission subtracted. The integration was performed over all the Tb3+ and Eu3+ luminescence bands that were visible in the steady-state mode.
Asymmetry Ratios
Asymmetry ratios for the Eu3+ ions were calculated by subtracting the nanoparticle’s emission contribution from the total steady-state emission spectrum. Typically the region of interest in the spectrum was first interpolated with three data points to establish a baseline. This baseline was then plotted with a 1-nm resolution and subsequently subtracted from the acquired experimental spectrum. This process assumes that two adjacent Eu3+ bands are always separated by a zero baseline. Because of the presence of experimental noise, the subtraction process sometimes resulted in the generation of some small negative values. In such occurrences, the intensity was set to zero. The respective bands were integrated on the wavenumber scale and the ratios were then calculated.
Lifetime Measurements
Luminescence lifetime measurements on the Tb3+ and Eu3+ signals were performed by excitation of solutions in 1-cm quartz cuvettes using an Nd-YAG Continuum Powerlite 8100 laser (354 nm, third harmonic) as the excitation source. Emission was collected at a right angle to the excitation beam, and wavelengths were selected by a monochromator (Spectral Products CM 110). The signal was monitored using a Hamamatsu R928 photomultiplier tube, collecting the signal output with a 500 MHz bandpass digital oscilloscope (Tektronix TDS 620B). Signals from ~ 2000 flashes were collected and averaged. Typically, all decay traces were collected with 2 ms/division of the oscilloscope. Luminescence lifetimes were averaged from multiple (at least three) measurements. Experimental luminescence decay curves were imported into Origin 8.0 and analyzed using the Advanced Fitting Tool. In all cases, the first 200 µs data points were systematically removed and the time axis was shifted to zero before fitting. This procedure removes any fast contribution in the decay trace that could originate from scattering of the excitation light or the nanosecond lived nanoparticle emission. However, this preliminary data treatment results in the loss of early time(< 200 µs) information. Luminescence lifetime distributions were calculated using the Edinburgh’s FAST decay analysis software. The distribution curves have been generated with 200 logarithmically spaced time points.
Supplementary Material
Acknowledgement
We acknowledge financial support from the National Institutes of Health via NIH Grant R21-EB008257-01A1. Stéhane Petoud acknowledges support in France from la Ligue contre le Cancer and from Institut National de la Santé et de la Recherche Méicale (INSERM). Prasun Mukherjee and David Waldeck acknowledge support in part from the National Science Foundation (CHE 1057981). The work in France was carried out within the COST Action D38 and CM1006. We thank Dr. Susheng Tan of the Petersen Institute of Nanoscience and Engineering at the University of Pittsburgh for help with the HRTEM measurements.
Footnotes
The intensity ratio of the Eu3+ bands results from the spatial arrangement of coordinating units (ligands) and their electronic character (chemical nature).
Supporting Information
Additional information as noted in text. This information is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
David H. Waldeck, Email: dave@pitt.edu.
Stéphane Petoud, Email: stephane.petoud@cnrs-orleans.fr.
References
- 1.Richardson FS. Terbium(III) and Europium(III) Ions as Luminescent Probes and Stains for Biomolecular Systems. Chem. Rev. 1982;82:541–552. [Google Scholar]
- 2.Moore EG, Samuel APS, Raymond KN. From Antenna to Assay: Lessons Learned in Lanthanide Luminescence. Acc. Chem. Res. 2009;42:542–552. doi: 10.1021/ar800211j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hildebrandt N, Löhmannsröben H-G. Quantum Dot Nanocrystals and Supramolecular Lanthanide Complexes – Energy Transfer Systems for Sensitive In Vitro Diagnostics and High Throughput Screening in Chemical Biology. Curr. Chem. Biol. 2007;1:167–186. [Google Scholar]
- 4.Binnemans K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009;109:4283–4374. doi: 10.1021/cr8003983. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery CP, Murray BS, New EJ, Pal R, Parker D. Cell-Penetrating Metal Complex Optical Probes: Targeted and Responsive Systems Based on Lanthanide Luminescence. Acc. Chem. Res. 2009;42:925–937. doi: 10.1021/ar800174z. [DOI] [PubMed] [Google Scholar]
- 6.Bünzli J-CG, Piguet C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005;34:1048–1077. doi: 10.1039/b406082m. [DOI] [PubMed] [Google Scholar]
- 7.Eliseeva SV, Bünzli J-CG. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010;39:189–227. doi: 10.1039/b905604c. [DOI] [PubMed] [Google Scholar]
- 8.Bünzli J-CG, Eliseeva SV. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths. 2010;28:824–842. [Google Scholar]
- 9.Bünzli J-CG. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010;110:2729–2755. doi: 10.1021/cr900362e. [DOI] [PubMed] [Google Scholar]
- 10.Thibon A, Pierre VC. Principles of Responsive Lanthanide-Based Luminescent Probes. Anal. Bioanal. Chem. 2009;394:107–120. doi: 10.1007/s00216-009-2683-2. [DOI] [PubMed] [Google Scholar]
- 11.Nockemann P, Beurer E, Driesen K, Deun RV, Hecke KV, Meervelt LV, Binnemans K. Photostability of a highly luminescent europium b-diketonate complex in imidazolium ionic liquids. Chem. Comm. 2005:4354–4356. doi: 10.1039/b506915g. [DOI] [PubMed] [Google Scholar]
- 12.Carnall WT, Fields PR. Lanthanide/Actinide Chemistry. Vol. 71. Washington D. C.: American Chemical Society; 1967. [Google Scholar]
- 13.Tsien RY, Ernst L, Waggoner A. Fluorophores for Confocal Microscopy: Photophysics and Photochemistry. In: Pawley JB, editor. Handbook of Biological Confocal Microscopy. third edition. Vol. New York: SpringerScience+Business Media; 2006. [Google Scholar]
- 14.Waggoner A. Fluorescent labels for proteomics and genomics. Current Opinion in Chemical Biology. 2006;10:62–66. doi: 10.1016/j.cbpa.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Petoud S. Novel Antennae for Luminescent Lanthanide Cations Emitting in the Visible and in the Near-Infrared: From Small Molecules to Polymetallic Lanthanide Containing Nanocrystals. CHIMIA International Journal for Chemistry. 2009;63:745–752. [Google Scholar]
- 16.Uh H, Petoud S. Novel antennae for the sensitization of near infrared luminescent lanthanide cations. Comptes Rendus Chimie. 2010;13:668–680. [Google Scholar]
- 17.Chengelis DA, Yingling AM, Badger PD, Shade CM, Petoud S. Incorporating Lanthanide Cations with Cadmium Selenide Nanocrystals: A Strategy to Sensitize and Protect Tb(III) J. Am. Chem. Soc. 2005;127:16752–16753. doi: 10.1021/ja0511725. [DOI] [PubMed] [Google Scholar]
- 18.Bhargava RN. Doped nanocrystalline materials - Physics and applications. J. Lumin. 1996;70:85–94. [Google Scholar]
- 19.Brennan JD, Capretta A, Yong K, Gerritsma D, Flora KK, Jones A. Sensitization of Lanthanides by Nonnatural Amino Acids. Photochem. Photobiol. 2002;75:117–121. doi: 10.1562/0031-8655(2002)075<0117:solbna>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Kim YH, Baek NS, Kim HK. Sensitized Emission of Luminescent Lanthanide Complexes Based on 4-Naphthalen-1-yl-Benzoic Acid Derivatives by a Charge-Transfer Process. Chem. Phys. Chem. 2006;7:213–221. doi: 10.1002/cphc.200500291. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, Wada M. Relations between Intramolecular Energy Transfer Efficiencies and Triplet State Energies in Rare Earth β-diketone Chelates. Bull. Chem. Soc. Jap. 1970;43:1955–1962. [Google Scholar]
- 22.Yang C, Fu L-M, Wang Y, Zhang J-P, Wong W-T, Ai X-C, Qiao Y-F, Zou B-S, Gui L-L. A Highly Luminescent Europium Complex Showing Visible-Light-Sensitized Red Emission:Direct Observation of the Singlet Pathway. Angew. Chem. Int. Ed. 2004;43:5010–5013. doi: 10.1002/anie.200454141. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Lu Z. Dye sensitized luminescent europium nanoparticles and its time-resolved fluorometric assay for DNA. Anal. Chim. Acta. 2007;587:180–186. doi: 10.1016/j.aca.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 24.Sivakumar S, van Veggel FCJM, Raudsepp M. Sensitized Emission from Lanthanide-Doped Nanoparticles Embedded in a Semiconductor Sol–Gel Thin Film. Chem. Phys. Chem. 2007;8:1677–1683. doi: 10.1002/cphc.200700283. [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Luo W, Liu Y, Liu G. Recent Progress on Spectroscopy of Lanthanide Ions Incorporated in Semiconductor Nanocrystals. J. Rare Earths. 2007;25:515–525. [Google Scholar]
- 26.Anderson WW. Tb3+ as a Recombination Center in ZnS. Phys. Rev. 1964;136:A556–A560. [Google Scholar]
- 27.Palm J, Gan F, Zheng B, Michel J, Kimerling LC. Electroluminescence of erbium-doped silicon. Phys. Rev. B. 1996;54:17603–17615. doi: 10.1103/physrevb.54.17603. [DOI] [PubMed] [Google Scholar]
- 28.Klik MAJ, Gregorkiewicz T, Bradley IV, Wells J-PR. Optically Induced Deexcitation of Rare-Earth Ions in a Semiconductor Matrix. Phys. Rev. Lett. 2002;89:227401-1–227401-4. doi: 10.1103/PhysRevLett.89.227401. [DOI] [PubMed] [Google Scholar]
- 29.Planelles-Arago J, Cordoncillo E, Ferreira RAS, Carlos LD, Escribano P. Synthesis, characterization and optical studies on lanthanide-doped CdS quantum dots: new insights on CdS / lanthanide energy transfer mechanisms. J. Mater. Chem. 2011;21:1162–1170. [Google Scholar]
- 30.Beeby A, Clarkson IM, Dickins RS, Faulkner S, Parker D, Royle L, deSousa AS, Williams JAG, Woods M. Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: an improved luminescence method for establishing solution hydration states. J. Chem. Soc. Perkin Trans. 1999;2:493–503. [Google Scholar]
- 31.Mukherjee P, Shade CM, Yingling AM, Lamont DN, Waldeck DH, Petoud S. Lanthanide Sensitization in II–VI Semiconductor Materials: A Case Study with Terbium (III) and Europium (III) in Zinc Sulfide Nanoparticles. J. Phys. Chem. A. 2011;115:4031–4041. doi: 10.1021/jp109786w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White KA, Chengelis DA, Zeller M, Geib SJ, Szakos J, Petoud S, Rosi NL. Near-infrared emitting ytterbium metal–organic frameworks with tunable excitation properties. Chem. Commun. 2009:4506–4508. doi: 10.1039/b909658b. [DOI] [PubMed] [Google Scholar]
- 33.Cross JP, Lauz M, Badger PD, Petoud S. Polymetallic Lanthanide Complexes with PAMAM-Naphthalimide Dendritic Ligands: Luminescent Lanthanide Complexes Formed in Solution. J. Am. Chem. Soc. 2004;126:16278–16279. doi: 10.1021/ja045706y. [DOI] [PubMed] [Google Scholar]
- 34.White KA, Chengelis DA, Gogick KA, Stehman J, Rosi NL, Petoud S. Near-Infrared Luminescent Lanthanide MOF Barcodes. J. Am. Chem. Soc. 2009;131:18069–18071. doi: 10.1021/ja907885m. [DOI] [PubMed] [Google Scholar]
- 35.Son DH, Hughes SM, Yin Y, Alivisatos AP. Cation Exchange Reactions in Ionic Nanocrystals. Science. 2004;306:1009–1012. doi: 10.1126/science.1103755. [DOI] [PubMed] [Google Scholar]
- 36.Mocatta D, Cohen G, Schattner J, Millo O, Rabani E, Banin U. Heavily Doped Semiconductor Nanocrystal Quantum Dots. Science. 2011;332:77–81. doi: 10.1126/science.1196321. [DOI] [PubMed] [Google Scholar]
- 37.Sooklal K, Cullum BS, Angel SM, Murphy CJ. Photophysical Properties of ZnS Nanoclusters with Spatially Localized Mn2+ J. Phys. Chem. 1996;100:4551–4555. [Google Scholar]
- 38.Dong C, van Veggel FCJM. Cation Exchange in Lanthanide Fluoride Nanoparticles. ACS Nano. 2009;3:123–130. doi: 10.1021/nn8004747. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Zhang J, Lu S, Ren X, Wang X. On the energy transfer from nanocrystalline ZnS to Tb3+ ions confined in reverse micelles. Chemical Physics Letters. 2005;409:144–148. [Google Scholar]
- 40.Robinson RD, Sadtler B, Demchenko DO, Erdonmez CK, Wang L-W, Alivisatos AP. Spontaneous Superlattice Formation in Nanorods Through Partial Cation Exchange. Science. 2007;317:355–358. doi: 10.1126/science.1142593. [DOI] [PubMed] [Google Scholar]
- 41.Sadtler B, Demchenko DO, Zheng H, Hughes SM, Merkle MG, Dahmen U, Wang L-W, Alivisatos AP. Selective Facet Reactivity during Cation Exchange in Cadmium Sulfide Nanorods. J. Am. Chem. Soc. 2009;131:5285–5293. doi: 10.1021/ja809854q. [DOI] [PubMed] [Google Scholar]
- 42.Jing-hua N, Rui-nian H, Wen-lian L, Ming-tao L, Tian-zhi Y. Electroluminescent properties of a device based on terbium-doped ZnS nanocrystals. J. Phys. D: Appl. Phys. 2006;39:2357–2360. [Google Scholar]
- 43.Planelles-Aragó J, Julián-López B, Cordoncillo E, Escribano P, Pellé F, Viana B, Sanchez C. Lanthanide doped ZnS quantum dots dispersed in silica glasses: an easy one pot sol–gel synthesis for obtaining novel photonic materials. J. Mater. Chem. 2008;18:5193–5199. [Google Scholar]
- 44.Ehrhart G, Capoen B, Robbe O, Beclin F, Boy P, Turrell S, Bouazaoui M. Energy transfer between semiconductor nanoparticles (ZnS or CdS) and Eu3+ ions in sol–gel derived ZrO2 thin films. Optical Materials. 2008;30:1595–1602. [Google Scholar]
- 45.Dong L, Liu Y, Zhuo Y, Chu Y. General Route to the Fabrication of ZnS and M-Doped (M = Cd2+, Mn2+, Co2+, Ni2+, and Eu3+) ZnS Nanoclews and a Study of Their Properties. Eur. J. Inorg. Chem. 2010:2504–2513. [Google Scholar]
- 46.Hou S, Yuen Y, Mao H, Wang J, Zhu Z. Photoluminescence properties of the Eu3+-doped ZnS nanocrystals and the crystal-field analysis. J. Phys. D: Appl. Phys. 2009;42:215105. (5pp). [Google Scholar]
- 47.Qu SC, Zhou WH, Liu FQ, Chen NF, Wang ZG, Pan HY, Yu DP. Photoluminescence properties of Eu3+-doped ZnS nanocrystals prepared in a water/methanol solution. Appl. Phys. Lett. 2002;80:3605–3607. [Google Scholar]
- 48.Sun XL, Zhang GL, Tang GQ, Chen WJ. The Site Symmetry of Eu3+ in ZnS:Eu Nanoparticle. Chin. Chem. Lett. 1999;10:807–810. [Google Scholar]
- 49.Sun L, Yan C, Liu C, Liao C, Li D, Yu J. Study of the optical properties of Eu3+-doped ZnS nanocrystals. Journal of Alloys and Compounds. 1998;275–277:234–237. [Google Scholar]
- 50.Wang L, Xu X, XinYuan Preparation and photoluminescent properties of doped nanoparticles of ZnS by solid-state reaction. J. Lumin. 2010;130:137–140. [Google Scholar]
- 51.Yang H, Yu L, Shen L, Wang L. Preparation and luminescent properties of Eu3+-doped zinc sulfide nanocrystals. Materials Letters. 2004;58:1172–1175. [Google Scholar]
- 52.Brus LE. Electron–electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984;80:4403–4409. [Google Scholar]
- 53.Li LS, Pradhan N, Wang Y, Peng X. High Quality ZnSe and ZnS Nanocrystals Formed by Activating Zinc Carboxylate Precursors. Nano Lett. 2004;4:2261–2264. [Google Scholar]
- 54., One might argue that the observed increase in the Ln3+ luminescence intensity in Figure 3 results only from a concentration effect. Based on the following arguments, we, argue that this is not the case. (i) Although after sonication, some solid salt remains visible in the vial, experiments with salt in chloroform were performed after filtration. Therefore the filtrate was visibly clear, eliminating the possibility of the availability of an excess amount of salt available for ZnS to dissolve, and (ii) the nanoparticles used are hydrophobic, so there should not be any strong preference to dissolve the salts in the nanoparticles.
- 55.Nag A, Cherian R, Mahadevan P, Gopal AV, Hazarika A, Mohan A, Vengurlekar AS, Sarma DD. Size-Dependent Tuning of Mn2+ d Emission in Mn2+-Doped CdS Nanocrystals: Bulk vs Surface. J. Phys. Chem. C. 2010;114:18323–18329. [Google Scholar]
- 56.Sudarsan V, van Veggel FCJM, Herring RA, Raudsepp M. Surface Eu3+ ions are different than “bulk” Eu3+ ions in crystalline doped LaF3 nanoparticles. J. Mater. Chem. 2005;15:1332–1342. [Google Scholar]
- 57., At this point, it is worth briefly summarizing the trend in asymmetry ratios that has been reported in the literature. By studying the Eu3+ luminescence with a wide range of ligands, van Veggel and coworkers56 have reported the Eu3+ asymmetry ratio that ranges between 0.98 and 1.66. Selvan and coworkers (J. Phys. Chem. B 1999, 103, 7064–7067) have observed a 1.1 times increase in Eu3+ asymmetry ratio in gel systems with increasing Ag concentration. Deki and coworkers (J. Phys. Chem. B 2003, 107, 9161–9164) have found a 1.3 times increase in the asymmetry around the Eu3+ ions in their systems. Levy and coworkers (Chem. Phys. Lett. 1984, 109, 593–597) have found a 12 times increase in Eu3+ asymmetry on going from Eu/SiO2 gel at 298 K to Eu/SiO2 glass at 873 K. Except for a few reports, in general the Eu3+ asymmetry ratio values do not change drastically, which is probably at least qualitatively consistent with the core-like nature of the f electrons.
- 58.Petoud S, Muller G, Moore EG, Xu J, Sokolnicki J, Riehl JP, Le UN, Cohen SM, Raymond KN. Brilliant Sm, Eu, Tb, and Dy Chiral Lanthanide Complexes with Strong Circularly Polarized Luminescence. J. Am. Chem. Soc. 2007;129:77–83. doi: 10.1021/ja064902x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.