Abstract
Patient retention is critical to the management of chronic diseases such as human immunodeficiency virus (HIV); hence, accurate measures of loss to follow-up (LTF) are important. Many different LTF definitions have been proposed. In a cohort of 9,692 HIV-infected patients initiating antiretroviral therapy in Mozambique from 2006 to 2011, we investigated the impact of the definition of LTF on estimated rates of LTF, acquired immunodeficiency syndrome (AIDS)-defining events, and death by applying 17 different definitions of LTF gleaned from HIV literature. We further investigated the impact of 4 specific components of the LTF definitions. Cumulative incidences of LTF and AIDS-defining events were estimated by treating death as a competing risk; Kaplan-Meier techniques and variations to account for informative censoring were used to estimate rates of mortality. Estimates of LTF 2 years after treatment initiation were high and varied substantially, from 22% to 84% depending on the LTF definition used. Estimates of 2-year mortality varied from 11% to 16%, and estimates of 2-year AIDS-defining events varied from 6% to 8%. As seen here, the choice of LTF definition can greatly affect study conclusions and program evaluations. Selection of LTF definitions should be based on the study outcome, available data on clinical encounters, and the patients' visit schedules; we suggest some general guidelines.
Keywords: chronic disease, cohort studies, HIV, long-term care, lost to follow-up, program evaluation
The burden of chronic diseases, such as heart disease, cancer, and diabetes, is high in low- and middle-income countries and is forecasted to increase with population aging and urbanization (1). One example is human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), a chronic disorder manageable in the era of antiretroviral therapy (2). Properly managed AIDS patients take antiretroviral therapy for life, much as other patients might take statins, antihypertensives, or insulin (3).
Reducing loss to follow-up (LTF) is among the most important challenges in chronic disease care worldwide. Retention is essential to optimize patient outcomes, and, for HIV, to help limit the spread of disease (4, 5). However, high rates of LTF have been reported in HIV treatment programs in low- (6, 7), middle- (7–9), and high-income (10–12) settings. Rates of LTF also vary substantially across treatment programs, even in similar settings (6). In studies of chronic diseases with routine clinical data, LTF is not only a nuisance encountered in analysis but also an important study outcome itself. Accurate measures of LTF are valuable for understanding processes and programmatic details, and they are critical to evaluating clinical outcomes.
Disparate definitions of LTF have been applied by HIV investigators and program evaluators. A review of patient retention in antiretroviral therapy programs from 33 cohorts in sub-Saharan Africa incorporated 8 definitions of LTF (6). Multisite cohort collaborations often define LTF as no visit within 1 year of the study close (8, 13). Empirical data from 111 facilities in Africa, Asia, and Latin America were used to recommend adopting >180 days since the last clinic visit as a standard LTF definition (14). Additional definitions of patient retention have been proposed that capture constancy of care (15).
Several components comprise the definition of LTF. These include the following: 1) length of time without a visit (e.g., 60, 180, or 365 days) or number of missed consecutive visits (e.g., 2 or 3 visits); 2) what type of encounters count as visits (e.g., clinic visits, pharmacy pickups, or any encounter); 3) whether to define LTF retrospectively (e.g., patient seen within 180 days of study close) or prospectively (e.g., patient not seen at clinic for 180 days); and 4) from which date to start counting (e.g., time from last visit or from missed visit).
Using data from a single cohort of HIV-infected patients starting antiretroviral therapy in Mozambique, we studied the impact of different LTF definitions on estimates of LTF, AIDS-defining events, and death. First, we applied 17 definitions of LTF found in HIV/AIDS literature (13–44). Second, we varied components of the LTF definition to assess the impact on study estimates.
MATERIALS AND METHODS
The Friends in Global Health Mozambican cohort has been described elsewhere (45). Briefly, Friends in Global Health is a Vanderbilt-affiliated nongovernmental organization supporting the Mozambican Ministry of Health in the provision of HIV care and treatment at government health centers in 12 rural districts of Zambézia Province, a north-central region of 4.2 million persons with a 2010 estimated adult HIV prevalence of 12% (45). Districts began enrolling patients into antiretroviral therapy-based care in June 2006, Friends in Global Health support began in February 2007, and routine data entry began in March 2007. This study uses data from 10 districts receiving Friends in Global Health support; 2 districts did not have electronic databases installed at the time of analysis. Districts included Alto Molocue, Gile, Ile, Inhassunge, Lugela, Maganja, Mopeia, Murrumbala, Namacurra, and Pebane. Analyses included data from each district seat's hospital/health center and the following health centers: Nauela (Alto Molucue), Alto Ligonha (Gile), Mugulama (Ile), Gonhane (Inhassunge), Namagoa (Lugela), and 7 Abril (Pebane). Paper forms designed for clinical record documentation by the Ministry of Health were completed by clinicians, laboratory technicians, pharmacists, and counselors. Data were entered daily by Friends in Global Health staff. Data quality audits occurred every 6 months. Patients could be tracked across Friends in Global Health–supported clinics.
Adult (≥15 years) patients who started antiretroviral therapy before July 1, 2011, were included in this study. Regular clinic visits were scheduled at least once per 3 months; typically they were more frequent (approximately once per month), especially during the first year after antiretroviral therapy initiation. Medication pickup was scheduled at least once per month and may or may not have coincided with the clinic visit.
Primary study outcomes were LTF (defined below), AIDS-defining events, and all-cause mortality. AIDS-defining events were defined as progression to World Health Organization (WHO) stage IV. Stage of disease was to be recorded at each clinic visit. Analyses of AIDS-defining events were limited to patients who started antiretroviral therapy with WHO stages I–III. Analyses of time to LTF and death used data from all included patients.
To study the definition of LTF, we considered the following 4 components:
Length of time to consider a patient lost: 60, 90, 180, or 365 days.
Type of qualifying visit: clinic (including laboratory), pharmacy, or both (labeled any encounter).
Retrospective or prospective assessment of patient loss (see below).
Time measured from last visit versus from missed visit.
The retrospective definition classifies patients as LTF if they do not attend a qualifying visit within x days prior to the database/study close (July 1, 2011, in this study). A patient who has a large gap in care but has a qualifying visit (or dies) within x days prior to the database close is therefore not LTF with the retrospective definition. The prospective definition defines a patient as LTF if they do not have a qualifying visit for x days. Thus, with the prospective definition, a patient who has a large gap (>x days) is considered LTF, regardless of return (or death) before the database/study close.
Most definitions of LTF comprise various combinations of the above 4 components. Mozambique national guidelines define LTF as no contact for >60 days from the last scheduled clinic visit or medication pickup (60 days, any encounter, prospective, time from last visit) (45). A recently suggested “universal definition” defined LTF as no clinic visit within 180 days prior to a specified date (180 days, clinic visit, retrospective, time from last visit) (14). The WHO definition of LTF (or ‘drop’) is >90 days from the missed clinical or drug-pickup appointment without any follow-up contacts (90 days, any encounter, prospective, time from missed visit) (46). Multisite cohort collaborations have defined LTF as no encounter within 365 days of study close (365 days, any encounter, retrospective, time from last visit) (8, 13).
The cumulative incidence of LTF and AIDS-defining events were estimated by treating death as a competing risk, acknowledging the fact that a patient who dies can no longer be LTF or have a future AIDS-defining event (43, 47). Mortality was computed by using standard Kaplan-Meier techniques. For mortality and AIDS-defining event analyses, patients who were LTF during the first 2 years of antiretroviral therapy were censored at the last pre-LTF visit date.
Standard estimation of AIDS-defining events and mortality requires the assumption of noninformative censoring—that patients LTF had an incidence of AIDS-defining events/death similar to that of patients not LTF. Such an assumption is improbable for our rural Mozambican data. To account for potential informative censoring, we also computed the 1-year incidence of death by using a nomogram approach developed by other HIV investigators (42) with the calculator provided at www.iedea-sa.org. This approach uses tracing data from other sub-Saharan Africa cohorts (48) to estimate the proportion of those LTF who died, and it incorporates this estimate into overall mortality estimates.
Analyses were performed at the Vanderbilt Institute for Global Health by using R statistical software, version 2.13.1 (available at http://www.R-project.org; R Foundation for Statistical Computing, Vienna, Austria). Analysis scripts are available at http:/biostat.mc.vanderbilt.edu/MozambiqueLTFU. The study was approved by the Mozambican Ministry of Health bioethics committee and the Vanderbilt University Institutional Review Board.
RESULTS
Our study includes 9,692 HIV-infected adults initiating antiretroviral therapy in 10 Mozambican districts. There were 98,223 patient visits (including laboratory) and 140,006 pharmacy visits. The median time from antiretroviral therapy initiation to last visit was 399 days (interquartile range, 122–803). The median age was 33 years (interquartile range, 27–40), and 60% were women. Among patients with available data at antiretroviral therapy initiation, 19% were classified as WHO stage IV, and the median CD4+ cell count was 184 cells/µL (interquartile range, 102–279). The WHO stage and CD4 count were not documented within 90 days before and 14 days after antiretroviral therapy initiation for 44% and 50% of subjects, respectively. Our AIDS-defining event analysis includes 4,390 HIV-infected adults classified as WHO stage I–III at antiretroviral therapy initiation.
Application of 17 LTF definitions
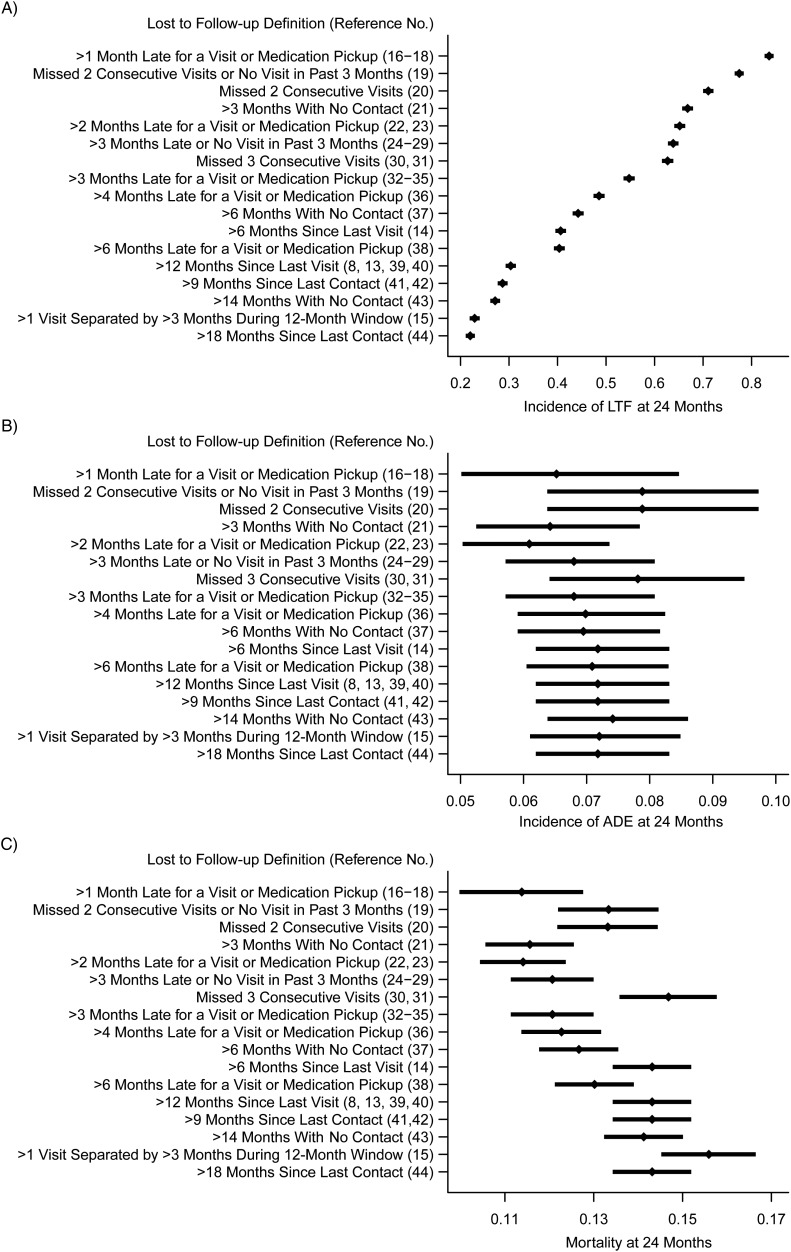
We estimated the incidence of LTF, AIDS-defining events, and mortality 2 years after antiretroviral therapy initiation, applying 17 definitions of LTF found in the HIV/AIDS literature (Figure 1) (13–44). Estimates of the cumulative incidence of LTF after 2 years ranged from 22% (retrospective definition of >18 months since last contact (44)) to 84% (prospective definition of >1 month late for scheduled encounter (16–18)).
Figure 1.
Seventeen definitions of lost to follow-up used in the literature applied to data from a single, rural Mozambican cohort, 2006–2011. Subfigures demonstrate the following: cumulative incidence of LTF (A); cumulative incidence of AIDS-defining events (ADE) (B); and probability of death at 24 months for each definition (C). AIDS, acquired immunodeficiency syndrome; LTF, lost to follow-up.
The choice of definition for LTF also affected estimates of the cumulative incidence of AIDS-defining events and mortality. Estimates of 2-year incidence of AIDS-defining events ranged from 6% (using >2 months late for scheduled encounter definition (22, 23)) to 8% (combination of missed 2 consecutive visits or no visit in past 3 months (19)). Estimates of 2-year mortality ranged from 11% (using >1 month late for scheduled encounter definition (16–18)) to 16% (visit constancy definition: ≥2 visits separated by >3 months during a 1-year period (15)).
Impact of components of LTF definition
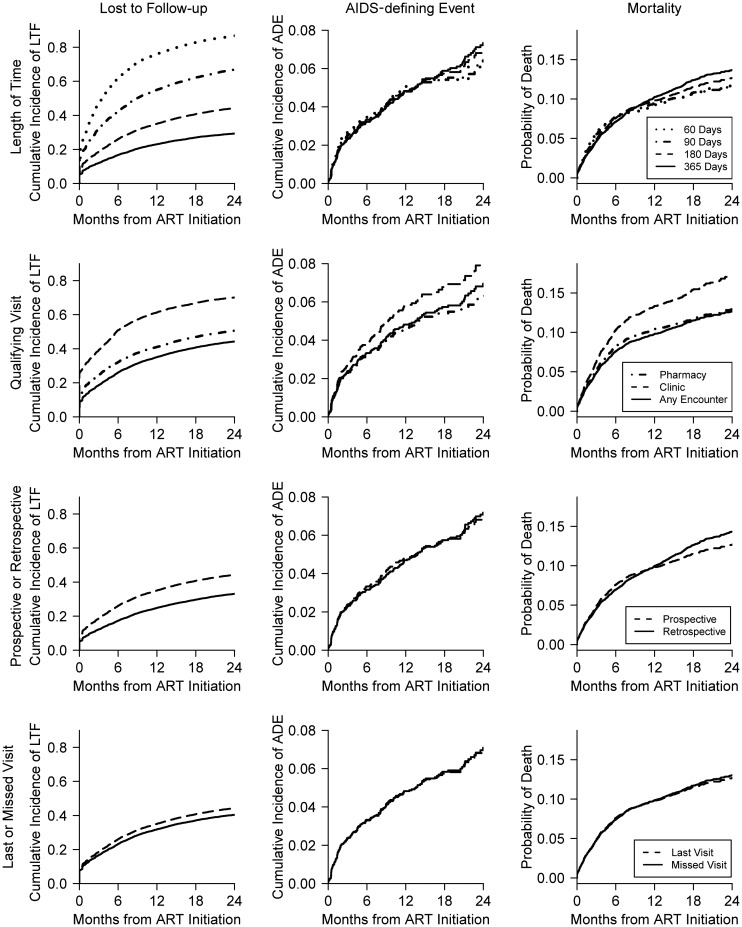
In Figure 2, we demonstrate the impact of various components of the LTF definition. Estimates are centered at a definition that classifies patients as LTF if they go 180 days from their last visit without any encounter (i.e., 180 days, any encounters, prospective, time from last visit). As expected, rates of LTF monotonically increased as the qualifying number of days to be LTF decreased (Figure 2, row 1, column 1), ranging from 29% (365 days) to 87% (60 days).Varying the number of days also impacted estimates of the cumulative incidence of AIDS-defining events and mortality, although these relationships were less pronounced and curves crossed (Figure 2, row 1, columns 2 and 3).
Figure 2.
Impact of varying specific components of the lost to follow-up definition, Mozambican cohort, 2006–2011. We investigated the impact of various components of the definition of LTF on estimates. All estimates are centered at a definition that classifies patients as LTF if they go 180 days from their last visit without any encounter (180 days, any encounters, prospective, time from last visit). The rows demonstrate the sensitivity of results to variation in these choices: The top row varies the length of time, the second row (from the top) varies what qualifies as a clinic visit, the third row compares prospective and retrospective definitions, and the bottom row compares time from last visit with time from missed visit. The columns correspond to the different study outcomes: The first column contains the cumulative incidence of LTF, the second column contains the cumulative incidence of AIDS-defining events (ADE), and the third column contains the probability of death. AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; LTF, lost to follow-up.
If only clinic (including laboratory) visits were incorporated, the cumulative incidence of LTF after 2 years tended to be much higher (70%) than the incidences based on definitions incorporating only pharmacy visits (51%) and incorporating both clinic and pharmacy visits (44%) (Figure 2, row 2, column 1). Counting only clinic encounters also led to higher mortality estimates because many of the people considered lost were still alive (and visiting the pharmacy) but were censored and treated as those who were actively remaining (and dying) in the study (Figure 2, row 2, column 3). Results were similar for the AIDS-defining events endpoint.
Estimates changed further if one altered the definition from prospective to retrospective (Figure 2, row 3). The incidence of LTF was higher when the prospective definition was used, because a patient was considered lost if he/she did not have a visit for 180 days at any point during the 2-year follow-up period. In contrast, with the retrospective definition, the 180-day gap must have occurred at the end of study. Therefore, patients considered LTF with the prospective definition had an opportunity to return to follow-up with the retrospective definition. The estimated incidences of AIDS-defining events and death were similar under the retrospective and prospective definitions (Figure 2, row 3, columns 2 and 3).
If LTF required 180 days from the last visit versus 180 days from the missed visit, the rate of LTF was only slightly higher (Figure 2, row 4, column 1). In our setting, visits occurred approximately monthly (including pharmacy visits), so the 180-day period from the missed visit is similar to the 210-day period from the last visit.
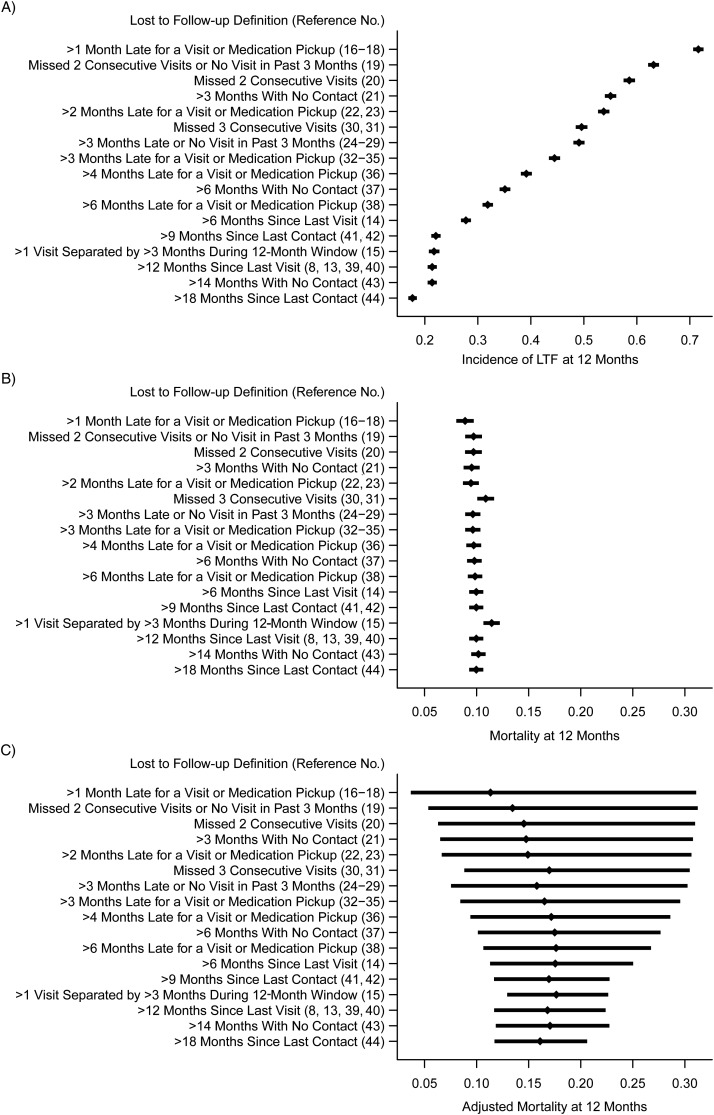
Informative censoring
Standard estimates of mortality are shown at 1 year for different definitions of LTF making the noninformative-censoring assumption (Figure 3B) and instead using a nomogram approach that assumes a proportion of those lost died on the basis of tracing data from elsewhere in sub-Saharan Africa (42) (Figure 3C). (Note, the nomogram approach was developed for 1 (not 2)-year mortality; Figure 3A shows the corresponding 1-year estimates of LTF to facilitate comparisons.) The estimates based on the nomogram were higher and more variable than standard estimates (11%–18% vs. 9%–11%, respectively). The ordering of point estimates across definitions of LTF was approximately the same when using the nomogram and standard approaches.
Figure 3.
Correcting 1-year mortality for lost to follow-up, Mozambican cohort, 2006–2011. Subfigures demonstrate the following: cumulative incidence of LTF at 12 months (A); probability of death at 12 months (B); and nomogram-adjusted probability of death at 12 months (C). LTF, lost to follow-up.
DISCUSSION
The definition of LTF has a major impact on estimates and interpretations of LTF, and it can affect estimates of the incidence of death and other events. Applying the different definitions of LTF found in the literature to a single cohort in Mozambique, we obtained estimates of LTF in the first 2 years of antiretroviral therapy ranging from 22% to 84%. Estimated probabilities of 2-year mortality varied from 11% to 16%. We posit that much of the heterogeneity in the incidence of LTF seen in the literature and in some of the differences between estimates of mortality is due to differences in LTF definitions, which may not represent true programmatic outcome differences.
The large impact of the LTF definition on results provides strong motivation to establish a uniform definition across cohorts and studies to allow more equitable comparisons (14). However, we believe that there are reasons why one might require several standardized definitions depending on cohort characteristics and intended study outcomes. In addition to summarizing the impact of specific components of LTF definitions on estimates, Appendix Table 1 contains general recommendations for selecting the definition of LTF based on study outcomes.
If the study outcome is mortality, we recommend using the retrospective definition based on any encounter (clinic or pharmacy). If a patient has a long period without care but then returns (or dies) near the end of the study, then vital status is known, and the patient need not be counted as lost. In contrast, if incidence of a soft endpoint or clinical event (e.g., AIDS-defining events) is the primary outcome, it is preferable to define LTF by using a prospective definition based on clinic encounters only. For example, it is possible for someone to have an AIDS-defining event during a gap in care and then to return to care; a retrospective definition for LTF would implicitly assume that any AIDS-defining event during a gap in care would be accurately reported to providers upon a patient's return, which is not necessarily true. Similarly, because HIV-related diagnoses are made during clinic visits, pharmacy visits should not be incorporated into the LTF definition when the primary outcome is a clinical event.
If incidence of LTF itself is the primary outcome, then the definition should largely depend on what the investigator wants to capture. There are many ways to think about being LTF, for example, lost to the clinic, not retained in care, vital status unknown. Different definitions serve different goals, each of which might be valuable in certain contexts (15). With that said, a reasonable choice could be a prospective definition counting any encounter (clinic or pharmacy) as a qualifying visit, with patients defined as lost at the time of their missed visit. From a clinician's perspective, a patient is first considered lost at the time of his/her missed visit (prior to that time, the patient was in care as actively as anyone who then attended his/her regular scheduled visit), and such a definition could be useful to prompt tracing of patients recently deemed LTF.
We have not recommended a specific number of days for the LTF definition, because it should depend on the frequency of visits and other components of the definition. For example, if patients are scheduled at clinic once every 3 months, defining LTF as 90 days without a clinic visit will overclassify patients as lost. However, for a clinic with monthly visits, or if any encounter qualifies and there are monthly pharmacy pickups, then defining LTF as >90 days from the last visit may be reasonable.
The challenge then becomes how to compare results across cohorts. Chi et al. (14) empirically selected a retrospective LTF definition of 180 days from last visit, counting only clinic encounters. Their definition is applicable to many circumstances, especially since some sites do not record pharmacy pickup or missed visits. However, their definition was empirically selected to minimize the misclassification of being truly LTF, defined as not being seen during a 1-year period. One could therefore argue that Chi et al.'s (14) “gold standard” LTF definition was actually 365 days without any encounter. Furthermore, their definition is impractical in developed country settings where scheduled visits may be as infrequent as once per 6 months. For example, in a subsequent paper combining data from the Swiss cohort and Zambia, the authors, including many from the same group of researchers advocating the universal definition, used yet another definition for LTF (>14 months without a visit) (43). This suggests that the optimal length of time should be a function of the frequency of visits. LTF definitions that incorporate both numbers of missed visits and length of time (e.g., >180 days without a visit and at least 1 missed visit) may be most valid in combined cohort studies with heterogeneous visit schedules. Multisite cohorts, such as the Antiretroviral Therapy in Lower Income Countries (ART-LINC), the Antiretroviral Therapy Cohort Collaborative (ART-CC), and the Caribbean and Central and South American Network for HIV Research (CCASAnet), have used a retrospective LTF definition of >365 days without any encounter (8, 13). Such definitions may be useful for evaluating mortality; HIV-related clinic visits are more frequent than once per year, which avoids problems of length of time being shorter than the frequency of visits. However, these definitions are insensitive when the study outcomes are retention and AIDS-defining events, and they may still result in poor cross-cohort mortality comparisons if rates of patient tracking and death ascertainment differ across cohorts.
The impact of the LTF definition on estimates of death in our Mozambican cohort was less substantial than the impact on estimates of LTF, but it still resulted in estimates of 2-year mortality ranging from 11% to 16%. Whether this represents a large range depends on one's perspective. In our study, estimates of mortality tended to increase with decreasing estimates of LTF (Figure 1). This suggests that Mozambican patients who were counted as LTF using definitions resulting in higher estimates of LTF were more likely to have died than those remaining in the cohort and not classified as LTF. Most analyses of AIDS-defining events or death, including those presented in Figure 1, B and C, and in Figure 2, assume that the rate of AIDS-defining events/death among patients who are lost is similar to the rate of AIDS-defining events/death among patients remaining in the study. Note that the choice of LTF definition can affect the plausibility/impact of this noninformative censoring assumption.
The noninformative censoring assumption is often unreasonable. In sub-Saharan Africa HIV care settings, patients who are LTF tend to be less healthy than those remaining in care (42, 48), whereas in other settings (e.g., upper income nations), patients who are LTF are often more healthy than those remaining in care (8). Statistical approaches that account for informative censoring using patient covariates exist and can yield unbiased estimates under certain (often unrealistic) assumptions (49, 50). Other methods that adjust overall mortality for LTF advocate tracing a randomly sampled subset of lost patients or using vital registration data (not available in Mozambique) to estimate mortality among those LTF (51–53). In the absence of tracing data, a simple approach is to assume that a proportion of those LTF had the event and to investigate the sensitivity of results to this assumed event rate (10). An analogous approach advocated by Egger et al. (42) and applied here assumes that the rate of mortality among those LTF is similar to that seen elsewhere in sub-Saharan Africa, where sites with lower LTF rates had higher mortality rates among actively traced LTF patients (48). Given that we tended to observe lower mortality rates when using definitions that yielded higher rates of LTF, when applied to our data, this nomogram approach not only yielded higher 1-year mortality estimates but also magnified differences in estimates across LTF definitions. The approach advocated by Egger et al. (42) appears reasonable in our rural impoverished setting and may be the best we can do without an ability to trace a subset of lost patients. However, it is unlikely that rates of mortality among those who are lost can be accurately imputed across cohorts, particularly considering the impact of the definition of LTF on initial estimates of LTF and death used to develop the nomogram.
Our study applies different LTF definitions to a single cohort; some findings may not hold when definitions are applied to other cohorts or study settings. Study weaknesses include our inability to ascertain deaths or trace lost patients. Our study assessed antiretroviral therapy retention, but we acknowledge that LTF also occurs pre–antiretroviral therapy initiation where LTF definitions may not be identical (e.g., there are no regular pharmacy visits pre–antiretroviral therapy). Interpretation of the AIDS-defining event analyses is limited, as more than half of the patients were excluded from this analysis, many because of missing information on clinical stage of disease at initiation of antiretroviral therapy. Finally, we censored all lost patients at their last visit, which could bias mortality estimates because time after the last visit was therefore included only in the subset of observed deaths. Some authors have addressed this potential bias by adding follow-up time to lost patients (30), although the best approach to address this potential bias warrants further study. Study strengths include the relatively large number of patients and our application of these methods with data from a high-volume, resource-limited, patient-care setting, reflecting the type of data actually seen in practice.
In conclusion, we found that the choice of LTF definition greatly affected study results and inferences. The choice of definition can also significantly impact programmatic details such as projections for medication and staff requirements, as well as strategies adopted to improve retention. Although our focus has been on HIV, LTF is important for all chronic diseases; careful consideration of the various components of LTF definitions is warranted when evaluating clinical outcomes or studying chronic disease management. Our recommendations for LTF definition are to base the definition on the study/programmatic outcome of interest, available encounter data, and the cohort visit schedule. Except in the context of collaborative networks, we do not advocate a universal standard as the definition of LTF should depend on the intended application and the cohort(s) of study.
ACKNOWLEDGMENTS
Author affiliations: Vanderbilt Institute for Global Health, Vanderbilt University School of Medicine, Nashville, Tennessee (Bryan E. Shepherd, Meridith Blevins, Lara M. E. Vaz, Troy D. Moon, Aaron M. Kipp, Sten H. Vermund); Department of Biostatistics, Vanderbilt University School of Medicine, Nashville, Tennessee (Bryan E. Shepherd, Meridith Blevins); Department of Preventive Medicine, Vanderbilt University School of Medicine, Nashville, Tennessee (Lara M. E. Vaz, Sten H. Vermund); Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, Tennessee (Troy D. Moon); Department of Epidemiology, Vanderbilt University School of Medicine, Nashville, Tennessee (Aaron M. Kipp); and Friends in Global Health, Quelimane and Maputo, Mozambique (Lara M. E. Vaz, Troy D. Moon, Sten H. Vermund, Eurico José, Ferreira G. Ferreira).
Bryan E. Shepherd and Meridith Blevins contributed equally to this work as co–first authors.
This research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention, US Department of Health and Human Services, under the terms of Cooperative Agreement U2GPS000631 and National Institutes of Health grant 1R01 AI093234-01.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest: none declared.
Appendix Table 1.
Summary of Impact and General Recommendations for Different Components of the Definition of Loss to Follow-up, Mozambican Cohort, 2006–2011
| Component of LTF Definition | Outcome |
||
|---|---|---|---|
| Lost to Follow-up | Soft Endpoint (e.g., AIDS-defining Event) | Mortality | |
| Length of time (e.g., 60, 180, or 365 days) | Impact: Substantial and monotonic | Impact: Lesser and variable | Impact: Lesser and variable |
| Recommendation: Depends on frequency of visits and desired strictness of LTF definition | Recommendation: Depends on frequency of visits (perhaps 2–3 times the typical length of time between visits where the endpoint is measured) | Recommendation: Depends on frequency of visits (perhaps 2–3 times the typical length of time between visits) | |
| Qualifying visit (e.g., clinic, pharmacy, or any) | Impact: Substantial and monotonic | Impact: Moderate and variable | Impact: Moderate and variable |
| Recommendation: Depends on goals | Recommendation: Visits where endpoint is measured (e.g., clinic visits for AIDS-defining event) | Recommendation: Any encounter | |
| Prospective or retrospective | Impact: Substantial and monotonic | Impact: Lesser and variable | Impact: Lesser and variable |
| Recommendation: Depends on goals, although typically prospective | Recommendation: Prospective | Recommendation: Retrospective | |
| Last or missed visit | Impact: Minor and monotonic | Impact: Minor and variable | Impact: Minor and variable |
| Recommendation: Depends on goals, although typically missed visit | Recommendation: Time from last visit | Recommendation: Time from last visit | |
Abbreviations: AIDS, acquired immunodeficiency syndrome; LTF, lost to follow-up.
REFERENCES
- 1.Department of Chronic Diseases and Health Promotion. Preventing Chronic Diseases: A Vital Investment. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 2.Beaglehole R, Epping-Jordan J, Patel V, et al. Improving the prevention and management of chronic disease in low-income and middle-income countries: a priority for primary health care. Lancet. 2008;372(9642):940–949. doi: 10.1016/S0140-6736(08)61404-X. [DOI] [PubMed] [Google Scholar]
- 3.Harries AD, Zachariah R, Kapur A, et al. The vital signs of chronic disease management. Trans R Soc Trop Med Hyg. 2009;103(6):537–540. doi: 10.1016/j.trstmh.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clin Infect Dis. 2010;51(6):725–731. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montaner JS, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368(9534):531–536. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 6.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86(7):559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuboi SH, Schechter M, McGowan CC, et al. Mortality during the first year of potent antiretroviral therapy in HIV-1-infected patients in 7 sites throughout Latin America and the Caribbean. J Acquir Immune Defic Syndr. 2009;51(5):615–623. doi: 10.1097/QAI.0b013e3181a44f0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tassie JM, Baijal P, Vitoria MA, et al. Trends in retention on antiretroviral therapy in national programs in low-income and middle-income countries. J Acquir Immune Defic Syndr. 2010;54(4):437–441. doi: 10.1097/QAI.0b013e3181d73e1b. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd BE, Sterling TR, Moore RD, et al. Cross-cohort heterogeneity encountered while validating a model for HIV disease progression among antiretroviral initiators. J Clin Epidemiol. 2009;62(7):729–737. doi: 10.1016/j.jclinepi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giordano TP, Gifford AL, White AC, Jr, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 12.Gerver SM, Chadborn TR, Ibrahim F, et al. High rate of loss to clinical follow up among African HIV-infected patients attending a London clinic: a retrospective analysis of a clinical cohort. J Int AIDS Soc. 2010;13(1) doi: 10.1186/1758-2652-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 14.Chi BH, Yiannoutsos CT, Westfall AO, et al. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med. 2011;9(10):e1001111. doi: 10.1371/journal.pmed.1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mugavero MJ, Davila JA, Nevin CR, et al. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24(10):607–613. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bisson GP, Gaolathe T, Gross R, et al. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS One. 2008;3(3):e1725. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296(7):782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 18.McGuire M, Munyenyembe T, Szumilin E, et al. Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health. 2010;15(suppl 1):55–62. doi: 10.1111/j.1365-3156.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- 19.Hosseinipour MC, Neuhann FH, Kanyama CC, et al. Lessons learned from a paying antiretroviral therapy service in the public health sector at Kamuzu Central Hospital, Malawi: 1-year experience. J Int Assoc Physicians AIDS Care (Chic) 2006;5(3):103–108. doi: 10.1177/1545109706288722. [DOI] [PubMed] [Google Scholar]
- 20.Wester CW, Kim S, Bussmann H, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immune Defic Syndr. 2005;40(3):336–343. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 21.Yu JK, Chen SC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85(7):550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chi BH, Cantrell RA, Mwango A, et al. An empirical approach to defining loss to follow-up among patients enrolled in antiretroviral treatment programs. Am J Epidemiol. 2010;171(8):924–931. doi: 10.1093/aje/kwq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367(9519):1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 24.Wools-Kaloustian K, Kimaiyo S, Diero L, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa: experience from western Kenya. AIDS. 2006;20(1):41–48. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- 25.Djomand G, Roels T, Ellerbrock T, et al. Virologic and immunologic outcomes and programmatic challenges of an antiretroviral treatment pilot project in Abidjan, Cote d'Ivoire. AIDS. 2003;17(suppl 3):S5–S15. doi: 10.1097/00002030-200317003-00002. [DOI] [PubMed] [Google Scholar]
- 26.Laurent C, Meilo H, Guiard-Schmid JB, et al. Antiretroviral therapy in public and private routine health care clinics in Cameroon: lessons from the Douala antiretroviral (DARVIR) initiative. Clin Infect Dis. 2005;41(1):108–111. doi: 10.1086/430712. [DOI] [PubMed] [Google Scholar]
- 27.Zachariah R, Teck R, Buhendwa L, et al. Community support is associated with better antiretroviral treatment outcomes in a resource-limited rural district in Malawi. Trans R Soc Trop Med Hyg. 2007;101(1):79–84. doi: 10.1016/j.trstmh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18(6):887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 29.Sow PS, Otieno LF, Bissagnene E, et al. Implementation of an antiretroviral access program for HIV-1-infected individuals in resource-limited settings: clinical results from 4 African countries. J Acquir Immune Defic Syndr. 2007;44(3):262–267. doi: 10.1097/QAI.0b013e31802bf109. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan S, Wu K, Smurzynski M, et al. Incidence rate of and factors associated with loss to follow-up in a longitudinal cohort of antiretroviral-treated HIV-infected persons: an AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) analysis. HIV Clin Trials. 2011;12(4):190–200. doi: 10.1310/HCT1204-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuong DD, Thorson A, Sonnerborg A, et al. Survival and causes of death among HIV-infected patients starting antiretroviral therapy in north-eastern Vietnam. Scand J Infect Dis. 2012;44(3):201–208. doi: 10.3109/00365548.2011.631937. [DOI] [PubMed] [Google Scholar]
- 32.Rosen S, Long L, Sanne I. The outcomes and outpatient costs of different models of antiretroviral treatment delivery in South Africa. Trop Med Int Health. 2008;13(8):1005–1015. doi: 10.1111/j.1365-3156.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 33.Lurton G, Akonde A, Madec Y, et al. Looking for lost to follow-up patients: experience of Segou, Mali [abstract] Presented at the International AIDS Conference, Mexico City, Mexico, August 3–8, 2008. [Google Scholar]
- 34.Joshi K, Jhanwar S, Mathur A, et al. Barriers in adherence of ART (anti retroviral treatment): a experience of ART Centre of Western Rajasthan, India [abstract] Presented at the International AIDS Conference, Mexico City, Mexico, August 3–8, 2008. [Google Scholar]
- 35.Muwanga A, Easterbrook P, Schaefer P, et al. Losses to follow-up in a large ART program in Uganda [abstract] Presented at the Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, February 3–6, 2008. [Google Scholar]
- 36.Karcher H, Omondi A, Odera J, et al. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12(5):687–694. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 37.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53(3):405–411. doi: 10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etard JF, Ndiaye I, Thierry-Mieg M, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20(8):1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 39.Ndiaye B, Ould-Kaci K, Salleron J, et al. Characteristics of and outcomes in HIV-infected patients who return to care after loss to follow-up. AIDS. 2009;23(13):1786–1789. doi: 10.1097/QAD.0b013e32832e3469. [DOI] [PubMed] [Google Scholar]
- 40.Lemly DC, Shepherd BE, Hulgan T, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis. 2009;199(7):991–998. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hulgan T, Shepherd BE, Raffanti SP, et al. Absolute count and percentage of CD4+ lymphocytes are independent predictors of disease progression in HIV-infected persons initiating highly active antiretroviral therapy. J Infect Dis. 2007;195(3):425–431. doi: 10.1086/510536. [DOI] [PubMed] [Google Scholar]
- 42.Egger M, Spycher BD, Sidle J, et al. Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med. 2011;8(1):e1000390. doi: 10.1371/journal.pmed.1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoni-Affolter F, Keiser O, Mwango A, et al. Estimating loss to follow-up in HIV-infected patients on antiretroviral therapy: the effect of the competing risk of death in Zambia and Switzerland. PLoS One. 2011;6(12):e27919. doi: 10.1371/journal.pone.0027919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4(+) counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr. 2007;44(2):179–187. doi: 10.1097/01.qai.0000247229.68246.c5. [DOI] [PubMed] [Google Scholar]
- 45.Moon TD, Burlison JR, Blevins M, et al. Enrolment and programmatic trends and predictors of antiretroviral therapy initiation from President's Emergency Plan for AIDS Relief (PEPFAR)-supported public HIV care and treatment sites in rural Mozambique. Int J STD AIDS. 2011;22(11):621–627. doi: 10.1258/ijsa.2011.010442. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. Operations Manual for Delivery of HIV Prevention, Care and Treatment at Primary Health Centres in High-Prevalence, Resource-constrained Settings. Geneva, Switzerland: World Health Organization; 2008. Monitoring services, patients and programmes; p. 120. Edition 1 for field testing and country adaptation. [PubMed] [Google Scholar]
- 47.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: Wiley; 1980. [Google Scholar]
- 48.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 50.Hogan JW, Laird NM. Model-based approaches to analysing incomplete longitudinal and failure time data. Stat Med. 1997;16(1-3):259–272. doi: 10.1002/(sici)1097-0258(19970215)16:3<259::aid-sim484>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 51.Fox MP, Brennan A, Maskew M, et al. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15(4):405–413. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geng EH, Emenyonu N, Bwana MB, et al. Sampling-based approach to determining outcomes of patients lost to follow-up in antiretroviral therapy scale-up programs in Africa. JAMA. 2008;300(5):506–507. doi: 10.1001/jama.300.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yiannoutsos CT, An MW, Frangakis CE, et al. Sampling-based approaches to improve estimation of mortality among patient dropouts: experience from a large PEPFAR-funded program in western Kenya. PLoS One. 2008;3(12):e3843. doi: 10.1371/journal.pone.0003843. [DOI] [PMC free article] [PubMed] [Google Scholar]