Abstract
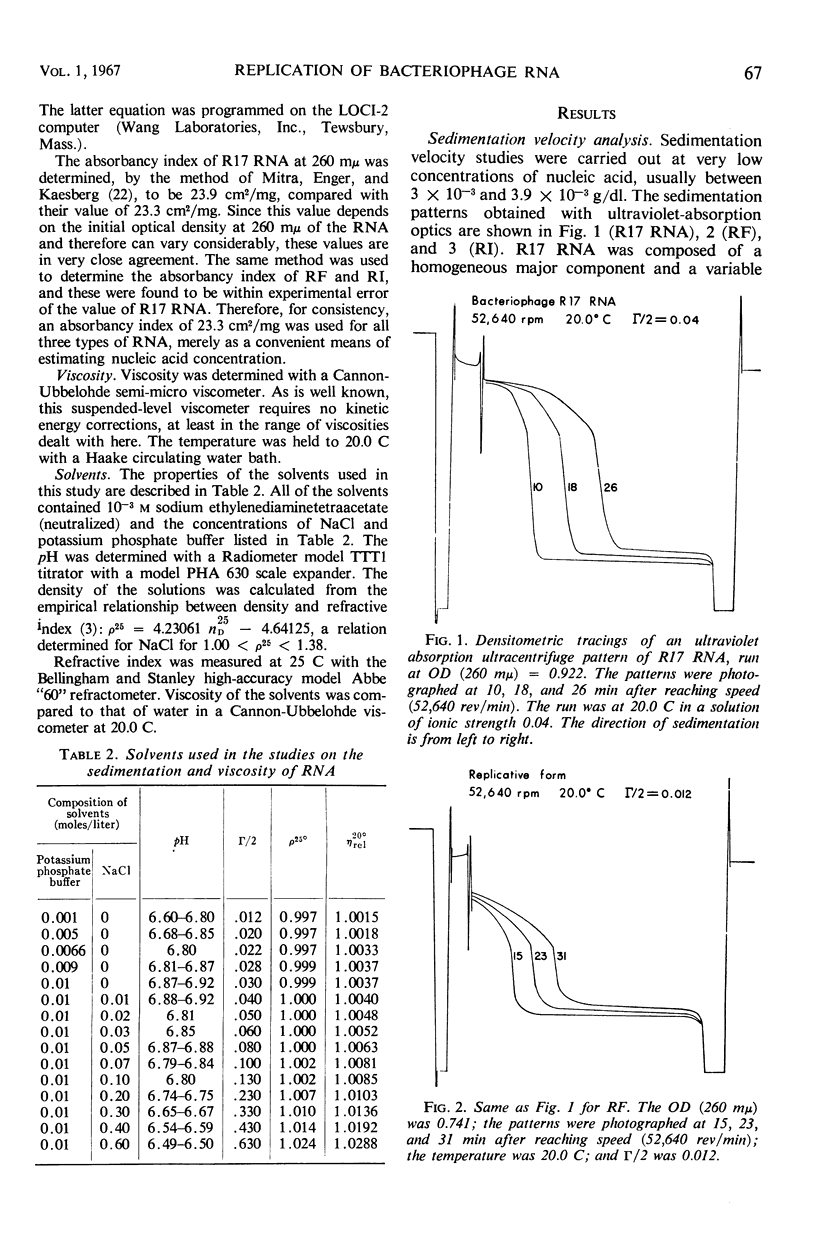
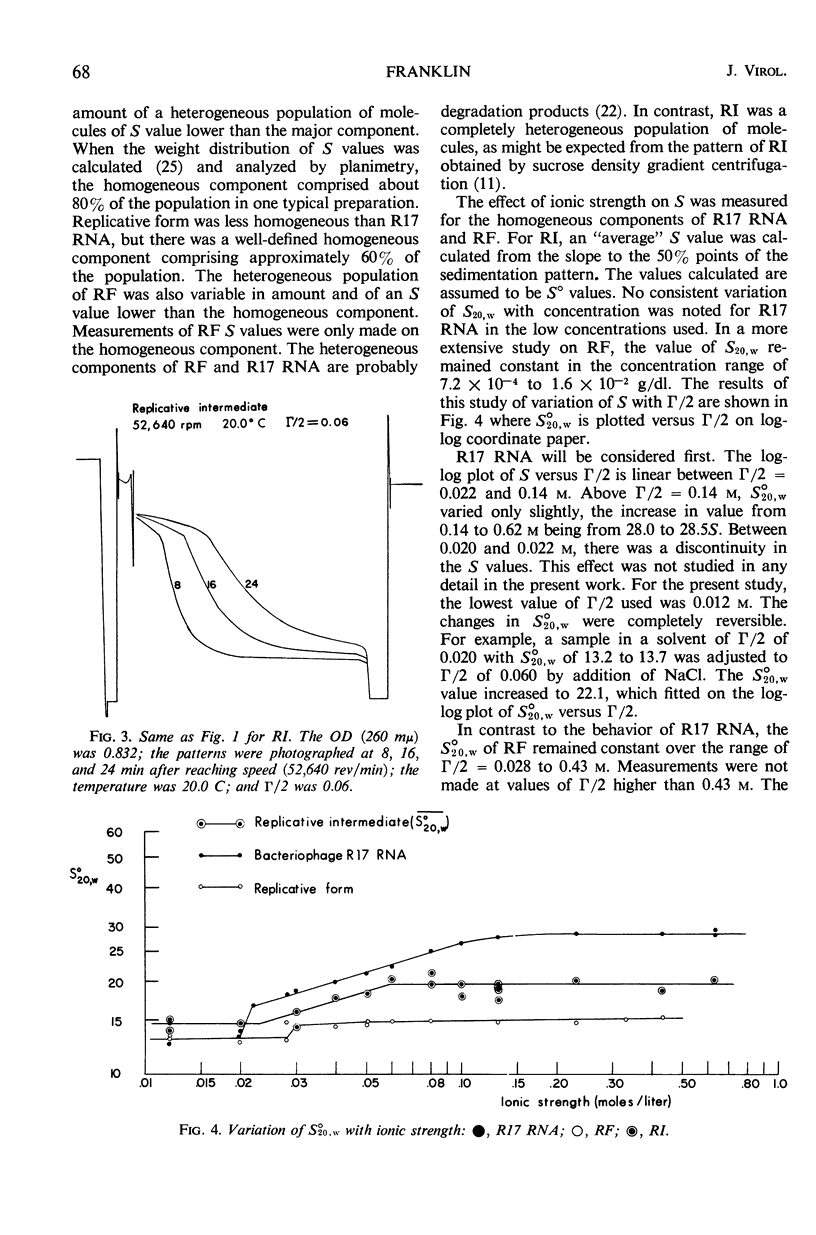
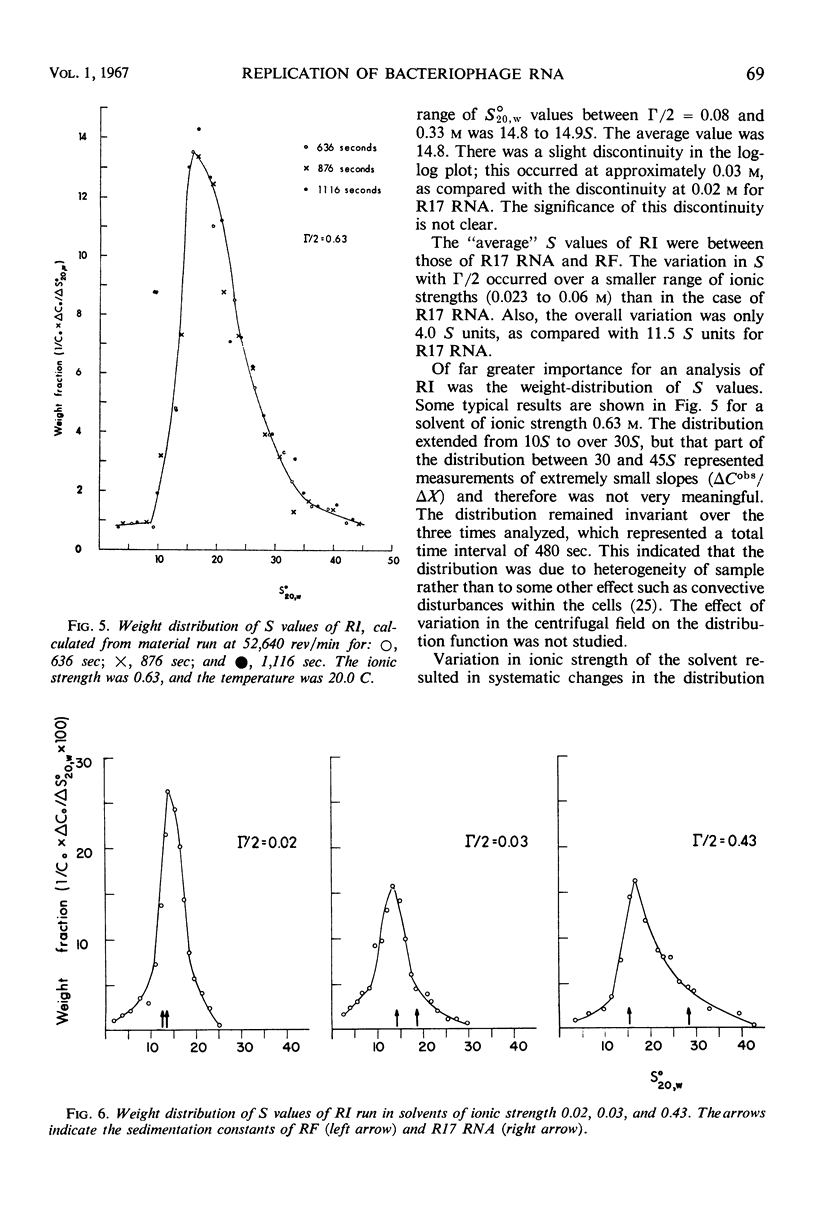
Replicative intermediate (RI) is considered to be the double-stranded ribo-nucleic acid (RNA) template for synthesis of viral RNA, with bound nascent single-stranded viral RNA. A theoretical description of RI is based on the analysis of a steady state of biopolymerization on a template which determines not only nucleotide sequence, but also chain length. The hydrodynamic properties of RI isolated from Escherichia coli infected with bacteriophage R17 are compared with those of RNA isolated from R17 (single-stranded RNA) and of replicative form (RF) isolated from E. coli infected with R17. RF is double-stranded RNA template without any single-stranded component. Whereas S for R17 RNA is a function of the ionic strength (Γ/2) of the solvent, S is almost invariant with Γ/2 for RF. By contrast S̄ for RI lies between the sedimentation constants for R17 RNA and RF and S̄ varies with Γ/2 as does R17 RNA. The weight distribution of S for RI demonstrates the heterogeneity of this material, and the variation in the weight distribution with ionic strength demonstrates the duality of structure in RI. Using S̄ and [η], the M̄w for RI is estimated to be 2.6 × 106 daltons, as compared with the theoretical value of 2.9 × 106 daltons.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMMANN J., DELIUS H., HOFSCHNEIDER P. H. ISOLATION AND PROPERTIES OF AN INTACT PHAGE-SPECIFIC REPLICATIVE FORM OF RNA PHAGE M12. J Mol Biol. 1964 Dec;10:557–561. doi: 10.1016/s0022-2836(64)80079-6. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Weissmann C., Warner R. C. Replication of viral ribonucleic acid. IX. Properties of double-stranded RNA from Escherichia coli infected with bacteriophage MS2. J Mol Biol. 1966 May;17(1):145–173. doi: 10.1016/s0022-2836(66)80101-8. [DOI] [PubMed] [Google Scholar]
- Bruner R., Vinograd J. The evaluation of standard sedimentation coefficients of sodium RNA and sodium DNA from sedimentation velocity data in concentrated NaCl and CsCl solutions. Biochim Biophys Acta. 1965 Sep 6;108(1):18–29. doi: 10.1016/0005-2787(65)90104-8. [DOI] [PubMed] [Google Scholar]
- Eigner J., Doty P. The native, denatured and renatured states of deoxyribonucleic acid. J Mol Biol. 1965 Jul;12(3):549–580. doi: 10.1016/s0022-2836(65)80312-6. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Franklin R. M. Symposium on replication of viral nucleic acids. I. Formation and properties of a replicative intermediate in the biosynthesis of viral ribonucleic acid. Bacteriol Rev. 1966 Jun;30(2):267–278. doi: 10.1128/br.30.2.267-278.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENWICK M. L., ERIKSON R. L., FRANKLIN R. M. REPLICATION OF THE RNA OF BACTERIOPHAGE R17. Science. 1964 Oct 23;146(3643):527–530. doi: 10.1126/science.146.3643.527. [DOI] [PubMed] [Google Scholar]
- Francke B., Hofschneider P. H. Infectious nucleic acids of e. Coli bacteriophages, ix. Sedimentation constants and strand integrity of infectious m12 phage replicative-form RNA. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1883–1890. doi: 10.1073/pnas.56.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GESTELAND R. F., BOEDTKER H. SOME PHYSICAL PROPERTIES OF BACTERIOPHAGE R17 AND ITS RIBONUCLEIC ACID. J Mol Biol. 1964 Apr;8:496–507. doi: 10.1016/s0022-2836(64)80007-3. [DOI] [PubMed] [Google Scholar]
- GIERER A. Grösse und Struktur der Ribosenucleinsäure des Tabakmosaikvirus. Z Naturforsch B. 1958 Aug;13B(8):477–484. [PubMed] [Google Scholar]
- GOMATOS P. J., STOECKENIUS W. ELECTRON MICROSCOPE STUDIES ON REOVIRUS RNA. Proc Natl Acad Sci U S A. 1964 Dec;52:1449–1455. doi: 10.1073/pnas.52.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGRIDGE R., GOMATOS P. J. The structure of RNA. Reovirus RNA and transfer RNA have similar three-dimensional structures, which differ from DNA. Science. 1963 Aug 23;141(3582):694–698. doi: 10.1126/science.141.3582.694. [DOI] [PubMed] [Google Scholar]
- LITTAUER U. Z., EISENBERG H. Ribonucleic acid from Escherichia coli; preparation, characterization and physical properties. Biochim Biophys Acta. 1959 Apr;32:320–337. doi: 10.1016/0006-3002(59)90604-3. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Zinder N. D. Replication of the RNA of Bacteriophage f2. Science. 1966 Apr 15;152(3720):372–377. doi: 10.1126/science.152.3720.372. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Zinder N. D. Semi-conservative replication of bacteriophage f2 RNA. J Mol Biol. 1966 Oct 28;21(1):207–209. doi: 10.1016/0022-2836(66)90090-8. [DOI] [PubMed] [Google Scholar]
- Mitra S., Enger M. D., Kaesberg P. PHYSICAL AND CHEMICAL PROPERTIES OF RNA FROM THE BACTERIAL VIRUS R17. Proc Natl Acad Sci U S A. 1963 Jul;50(1):68–75. doi: 10.1073/pnas.50.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUMAKER V. N., SCHACHMAN H. K. Ultracentrifugal analysis of dilute solutions. Biochim Biophys Acta. 1957 Mar;23(3):628–639. doi: 10.1016/0006-3002(57)90386-4. [DOI] [PubMed] [Google Scholar]
- SINHA N. K., FUJIMURA R. K., KAESBERG P. RIBONUCLEASE DIGESTION OF R17 VIRAL RNA. J Mol Biol. 1965 Jan;11:84–89. doi: 10.1016/s0022-2836(65)80173-5. [DOI] [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Vasquez C., Granboulan N., Franklin R. M. Structure of the ribonucleic acid bacteriophage R17. J Bacteriol. 1966 Dec;92(6):1779–1786. doi: 10.1128/jb.92.6.1779-1786.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P., BURDON R. H., BILLETER M. A., OCHOA S. REPLICATION OF VIRAL RNA, III. DOUBLE-STRANDED REPLICATIVE FORM OF MSW PHAGE RNA. Proc Natl Acad Sci U S A. 1964 Apr;51:682–690. doi: 10.1073/pnas.51.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P., BURDON R. H., BILLETER M. A., OCHOA S. REPLICATION OF VIRAL RNA. IV. PROPERTIES OF RNA SYNTHETASE AND ENZYMATIC SYNTHESIS OF MS2 PHAGE RNA. Proc Natl Acad Sci U S A. 1964 May;51:890–897. doi: 10.1073/pnas.51.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]



