Abstract
Background
Conventional chemotherapy and radiation therapy against non-small cell lung cancer (NSCLC) are relatively insensitive and unsatisfactory. Para-toluenesulfonamide (PTS), a unique antitumor drug for local intratumoral injection, shows an efficacy of severely suppressing solid tumor growth with mild side effects in clinical trials. The aim of this study was to investigate the effect of PTS on lung cancer H460 cells in vivo in nude mice and its underlying mechanisms in vitro.
Methods
A lung cancer model for in vivo experiment was established in BALB/c nude mice using H460 cells to examine the effect of local injection of PTS on tumor suppression. We also assessed the injury to the normal tissue by subcutaneous injection of PTS. In vitro, PTS was diluted into different doses for study on its antitumor mechanisms. We evaluated the necrotic effect of PTS on H460 cells by PI and Hoechst 33342 staining. Cell viability and membrane permeability were also determined by using CCK-8 and LDH assays respectively. All these tests were conducted in comparison with traditional local injection of anhydrous ethanol.
Results
PTS was shown to significantly inhibit the growth of H460 tumor xenografts in nude mice by inducing necrosis of the tumor histologically. Its effect on tumor growth was significantly stronger than that of anhydrous ethanol. By contrast, the injured normal tissue by PTS injection was less than that by ethanol. In vitro, PTS still demonstrated excellent necrotizing effect on H460 cells when diluted to a lower concentration. Detailed analysis of PTS on H460 cells indicated that PTS had a better effect on attenuating the cell viability and increasing the cell membrane permeability than ethanol at the same level.
Conclusions
PTS exhibits excellent inhibition effect on the growth of lung cancer by necrotizing tumor in vivo and in vitro, reducing tumor cell viability and augmenting the membrane permeability in vitro, with only mild injury to normal tissue. The antitumor effect of PTS on lung cancer in vivo and in vitro is stronger than that of ethanol.
KEY WORDS : Para-toluenesulfonamide (PTS), lung cancer, necrosis, therapy, antitumor agent
Introduction
A national prospective cohort study showed that malignant neoplasms have become a major cause of death among Chinese adults (1). The mortality rate of lung cancer has increased by 464.84% over the past 3 decades, surpassing liver cancer as the first leading cause of death among people with malignant tumors in China since 2008 (2). Although surgical treatment may lead to a fine prognosis for lung cancer at early stage (3), asymptomatic primary lung cancer is difficult to diagnose early enough for its victims to have an optimal opportunity for surgical treatment. As a result, the prognosis for patients with lung cancer is very poor, with the overall 5-year survival rates varying from 5% to 10% worldwide (4). Advanced central bronchogenic cancer is often treated by a palliative therapy, such as laser, electrocautery, argon plasma coagulation and chemoradiation (5-7), to rapidly relieve the airway obstruction. These therapies, however, necessitate sophisticated facilities and, more importantly, advanced personal skills and expertise of the team which limit their clinical application. Intratumoral ethanol injection has been widely recognized and proves effective to treat solid tumors, especially for hepatocellular carcinoma (8-12). However, the outcomes are not always satisfactory. Recurrence frequently occurs at the original lesion or other sites (13,14) as the ethanol can not destroy all the tumor cells. In addition, complications (15-19) such as hemorrhage, alcohol intoxication, pain and fever, may result from the inevitable spread of injected ethanol in adjacent normal tissue because anhydrous ethanol is strongly permeable and a great amount should be injected to produce a sufficiently large area of necrosis. Consequently, intratumoral ethanol injection is not widely applied as a routine treatment because of its limitations.
Para-toluenesulfonamide (PTS), whose active ingredient is p-methylbenzenesulfonamide at 330 mg per milliliter, is a novel anticancer agent with good lipophilic ability. As an adjunct of conventional chemotherapy, PTS is often delivered by intratumoral or peritumoral injection. Primary basic research suggested that PTS effectively suppressed the proliferation of liver and lung cancers in vivo and in vitro (20-22). According to a preclinical study, PTS was relatively safe in co-administration with many drugs (23). Several on-going phase II clinical trials on PTS against solid tumors (24-27) proved its antitumor efficacy. Recently, a phase II clinical trial showed that chemotherapy with concurrent PTS local injection was well tolerated and efficient in the treatment of patients with peripherally advanced lung cancer (28). Therefore, we hypothesized that PTS could be widely used against lung cancers. However, the knowledge of how PTS works against lung tumor is limited. Therefore, the aim of the study was to investigate the efficacy of PTS against lung cancer in vivo and in vitro and the possible underlying mechanisms as well.
Methods
Cancer cell line and culture
Human NSCLC cell line H460 (Shanghai Cell Bank, Chinese Academy of Sciences, China) was cultured in DMEM with 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 µg/mL streptomycin (all from Gibco, USA). Cells were maintained in a humidified 37 degrees Celsius incubator (Thermo, USA) with 5% CO2. Cells in the exponential growth phase were used for the experiments.
In vivo study
Experimental design
A total of 40 female 4-week-old BALB/c (nu/nu) nude mice purchased from Guangdong Laboratory Animal Center (Guangzhou, China) weighing 18-22 g were raised in SPF conditions with a 12-hour day-night cycle at State Key Laboratory of Respiratory Disease. The protocol of the experiment was approved by the Ethics Committee for Animal Experiments at our hospital. After housing for a week, each mouse (n=40) was subcutaneously inoculated with 5×106 H460 cells in 0.2 mL PBS. 2 weeks after the implantation, the tumor diameter reached to 0.5 mL (1.0 cm ×1.0 cm in length and width). As PTS (Beijing Vision Drugs Development Ltd, China) is highly viscous, direct syringe injection is difficult. Therefore, according to the dissolving method for phase II and III clinical trials of NSCLC, PTS solution was mixed in a blend of 7 parts to 3 parts of ethanol (PTS:Ethanol =7:3) to facilitate the injection. The mice were randomly divided into 4 groups (n=10 each). Group A was treated with PTS solution (PTS) by intratumoral injection. Group B was treated with conventional local injection of anhydrous ethanol (E) in the same way. Corresponding controls groups C and D were treated with 30% ethanol (30% E) and normal saline (NS) respectively. The solutions for all the groups were injected intratumorally at 3 different puncture points using a microsyringe every 4 days. According to a previous study on dose-effect relationship of PTS (29) the total injection volume in each treatment for every mouse was 50 μL. Each group received 4 treatments before the end of the experiment.
Tumor measurement and histological analysis
The tumor volume was determined using a caliper and calculated by the following formula (30): 0.5× length × width2. All the groups were measured every 4 days to obtain tumor growth curves.
Animals were sacrificed 24 hours after the fourth treatment. Tumors were extirpated and compared for weight and volume. Samples randomly chosen from both control and treatment groups were prepared for histopathologic analysis. Samples were fixed in 10% buffered formalin. Then, tumors were embedded in paraffin and cut into 4-5 μm thick and stained with hematoxylin and eosin (H & E). Images were obtained from a standard light microscope (Leica, Germany) for measurement of the necrosis area by software Image Pro Plus (Media Cybernetics, USA).
In vitro study
Dilution of injection agent
As PTS is difficult to dissolve in the medium, we used DMSO, a solvent commonly used in study of water-insoluble elicitors, to make PTS dissolvable in DMEM. When PTS was diluted to 1/50, 2% DMSO was added to make it soluble in DMEM. Anhydrous ethanol can be easily deliquated by DMEM. The test groups were incubated with different concentrations of PTS and ethanol respectively. Control ones were treated with medium containing 2% DMSO or DEME only. The results of PTS and ethanol on cell necrosis, viability and membrane permeability at the same diluted level were observed and calculated.
Cell necrosis assay
Cells were stained with Hoechst 33342 and propidium iodide (PI) (Beyotime Institute of Biotechnology, China) to investigate the necrotic effect of PTS. H460 cells (5×105/well) were seeded in 6-well plates and grown to 80% confluence. The growth of H460 cells were arrested with the medium containing 1% FBS for 12 h. Next, the medium was removed and cells were incubated with PTS and ethanol (at concentrations: 1/50, 1/100, 1/150 and 1/200), added with DMEM, and incubated for 2 h respectively. Then 1 mL phosphate buffer was added in each well after washed by PBS (4 degrees Celsius). 5 μL (10 ng/mL) of Hoechst 33342 and 5 μL (10 ng/mL) of PI were stained and mixed thoroughly into each well. The incubation time was 30 min at 4 degrees Celsius. After the incubation, each well was washed by PBS at 4 degrees Celsius twice. Images were randomly taken from 5 different views by a fluorescence microscope (Leica, Germany). Hoechst 33342 stained and PI stained cells were counted by software Image Pro Plus. The necrosis rate was calculated by the percentage of necrotic cells to the total number of cells. The total number of cells should be counted to a minimum of 500 from at least 100 cells at each view.
Cell viability assay
Effects of PTS on cell viability were examined using the CCK-8 assay (31) (Dojindo Molecular Technologies, Japan). The kit contains water-soluble tetrazolium salt, WST-8, which is generated by the activities of mitochondrial succinate dehydrogenases in cells and is directly proportional to the number of living cells. The detection sensitivity of CCK-8 is higher than that of the other tetrazolium salts such as MTT, XTT and MTS. First, cells (8×103/well) were seeded into 96-well plates and cultured overnight with arrested growth in DMEM containing 1% FBS for 12 h. Next cells were respectively treated with PTS and ethanol (concentrations: 1/10, 1/50, 1/100 and 1/200) for 2 h. After the medium was removed, 110 μL fresh DMEM containing 10 μL CCK-8 was added in each well. Controls were grown under the same conditions (2% DMSO or DMEM only) but without any drug. The absorbance at 490 nm was measured using a spectrometer reader (Thermo, USA). The survival rate was calculated using the following formula: viability rate = [(ODtest group – ODBlank)/(ODcontrol group – ODBlank] ×100. Data of each experimental series of PTS were tested against those of ethanol for statistical significance.
Cell membrane permeability assay
Effects of PTS on cell membrane permeability were tested by the lactate dehydrogenase (LDH) release assay (32) (Beyotime Institute of Biotechnology, China). The assay was performed to investigate the cytosolic enzyme release which was induced by PTS. According to the results from the CCK-8 assay, the concentrations of PTS are 1/200, 1/400, 1/800 and 1/1,600. Since PTS is noncytotoxic (survival rate is over 90%) within this range, the enzyme release observed can be ascribed to an increase in cell membrane permeability as opposed to general lysis due to cell death (33). Cells (8×103/well) were prepared in 96 well plates and cultured overnight with arrested growth (DMEM containing 1% FBS). Next, cells were respectively incubated with PTS and ethanol (concentrations: 1/200, 1/400, 1/800 and 1/1,600). The supernatants were then collected at 2 time points (2 and 6 h). Cell debris was removed by centrifugation of the supernatant (at 400 g for 5 min). The supernatant (120 μL) was mixed with 60 μL of LDH reagent solution, sheltered from light and incubated at room temperature for 30 min. The measured LDH activities were calculated by percentage of LDH released in the supernatant to that of cell lysates from intact cells (% LDH released). The LDH released rate was calculated by the following formula: LDH released rate = [(ODtest group – ODcontrol group)/(ODlysates of intact cells – ODcontrol group] ×100. The absorbance at 490 nm was tested by the spectrometer reader.
Injury to normal tissue
10 female 7-week-old nude mice (weight: 25-27 g) were purchased and anaesthetized. 50 μL PTS was subcutaneously injected into the hypodermia at one side of the back in each mouse and 50 μL ethanol was injected on the other side in the same way. The injury area was measured by a caliper to access the damage range caused by PTS and ethanol to normal tissue. Injury area (S) was calculated using the ellipse formula, according to the following equation: S = Length × Width × π/4. The epidermis and subcutaneous tissues at 0, 0.2 and 0.5 cm away from the injection point along the length axis were embedded in paraffin, cut into 4-5 μm thick and stained with H&E as well. Images were obtained from a standard light microscope to assess the severity of damage from PTS and ethanol to normal tissue.
Statistical analysis
Data were expressed as means with 95% confidence interval. A one-way ANOVA and Student-Newman-Keuls tests were used for statistical analysis and a value of P<0.05 was considered statistically significant. If the homogeneity of variances was statistically significant, the data were analyzed by nonparametric tests. All statistical analyses were performed using SPSS 13.0 (IBM, USA).
Results
In vivo study
General condition of the mice during the study in vivo
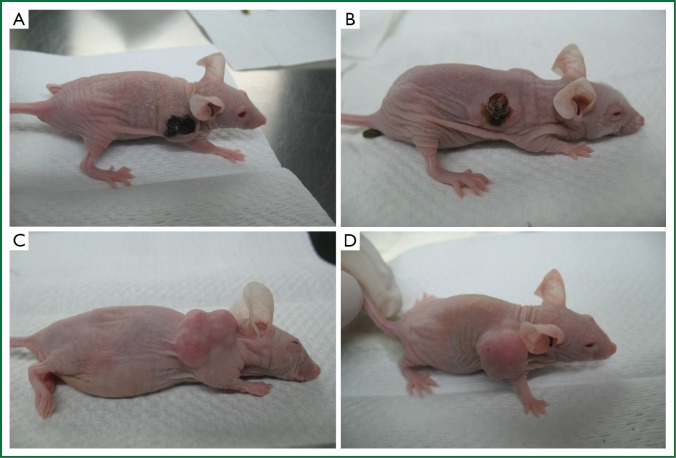
All the mice tolerated the injection and no mouse died during the treatment. Most of the mice in the two test groups showed poor appetite and sluggishness after the injection until the next day when all the mice recovered to normal. These signs were not observed in the two control groups. The weight of each mouse gradually declined in the control groups during the study as the tumor volume augmented. 24 hours after the first injection of PTS and anhydrous ethanol respectively in groups A and B, scabs formed in the tumors while escharosis was not observed in the controls (Figure 1).
Figure 1.
General condition of the mice at 24 h after first injection. A. PTS; B. Ethanol; C. 30% ethanol; D. Normal saline (NS).
PTS suppressed growth of H460 xenograft tumor in nude mice
The initial tumor volumes before injection were not significantly different among the 4 groups (P=0.873). In the control groups, the tumors displayed rapid and continued growth during the course of the experiment. PTS and ethanol effectively inhibited the tumor growth as the tumor volume was much smaller than in the control groups at the end of the injection treatment (Figure 2A, Table 1). Both PTS and ethanol groups had significantly lower tumor weight (P<0.05) than the controls and the PTS group also had significantly lower tumor weight than the ethanol group (P<0.05) (Figure 2B, Table 2). There was no significant difference regarding the tumor weight between the two control groups (P=0.893).
Figure 2.
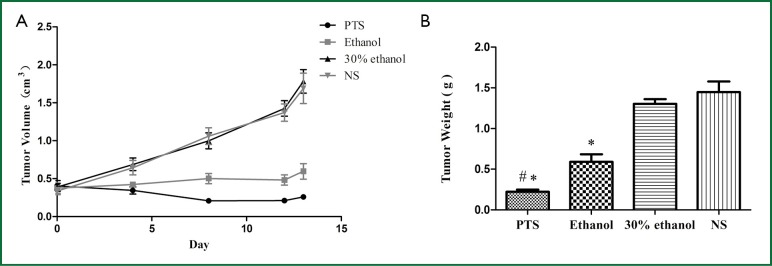
The volume and weight of tumor xenografts. A. Growth curves of tumor xenografts in nude mice; B. Weights of extirpated tumor xenografts (#P<0.05 vs. Ethanol; *P<0.05 vs. 30% ethanol and NS).
Table 1. Tumor volumes before treatment and at 4, 8, 12 and 13 days during treatment (cm3) (n=10).
| Group | Before treatment | During treatment |
|||
|---|---|---|---|---|---|
| 4 d | 8 d | 12 d | 13 d | ||
| PTS | 0.403 | 0.343 | 0.208ab | 0.209ab | 0.259ab |
| [0.240-0.565] | [0.237-0.449] | [0.133-0.282] | [0.133-0.285] | [0.180-0.338] | |
| Ethanol | 0.374 | 0.422 | 0.501c | 0.481c | 0.595c |
| [0.219-0.528] | [0.345-0.500] | [0.348-0.655] | [0.330-0.631] | [0.367-0.823] | |
| 30% ethanol | 0.387 | 0.687 | 1.000 | 1.425 | 1.781 |
| [0.284-0.490] | [0.497-0.878] | [0.760-1.235] | [1.196-1.165] | [1.429-2.133] | |
| NS | 0.336 | 0.644 | 1.059 | 1.37 | 1.69 |
| [0.228-0.443] | [0.429-0.859] | [0.809-1.309] | [1.111-1.629] | [1.240-2.138] | |
aP<0.05 vs. Ethanol; bP<0.05 vs. 30% ethanol and NS; cP<0.05 vs. 30% ethanol and NS.
Table 2. Weights of extirpated tumors.
| Group | Tumor weight (g) |
|---|---|
| PTS | 0.222 [0.163-0.281]ab |
| Ethanol | 0.588 [0.375-0.801]c |
| 30% ethanol | 1.304 [1.179-1.429] |
| NS | 1.446 [1.146-1.746] |
aP<0.05 vs. Ethanol; bP<0.05 vs. 30% ethanol and NS; cP<0.05 vs. 30% ethanol and NS.
Histological analysis
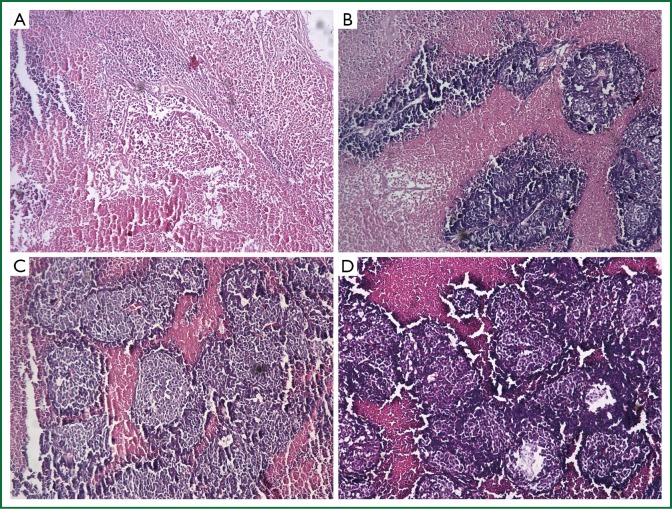
Histologically, tumors in the therapeutic groups were composed of massive necrotizing tissue on eosin staining (red) (Figure 3A,B). The necrotic cells were difficult to distinguish except for the boundary between the viable and necrotizing tumor tissues. There was only a small portion of viable neoplastic tissue which was hemalum stained (blue) containing pleomorphic cells with large, irregular, hyperchromatic nuclei. In contrast, there was massive viable tumor tissue and a small portion of necrotizing tissue in the control groups (Figure 3C,D). The area of necrotizing tissue was evaluated by Image Pro Plus (Figure 4, Table 3). The two therapeutic groups had significantly larger area of necrotic tissue than the two control ones (P<0.05) and the necrotizing area was significantly larger in the PTS group than in the ethanol one (P<0.05). There was no significant difference between the two control groups (P=0.122).
Figure 3.
Histopathological features of the tumors in nude mice (Magnification, ×100). A. PTS; B. Ethanol; C. 30% ethanol; D. NS.
Figure 4.
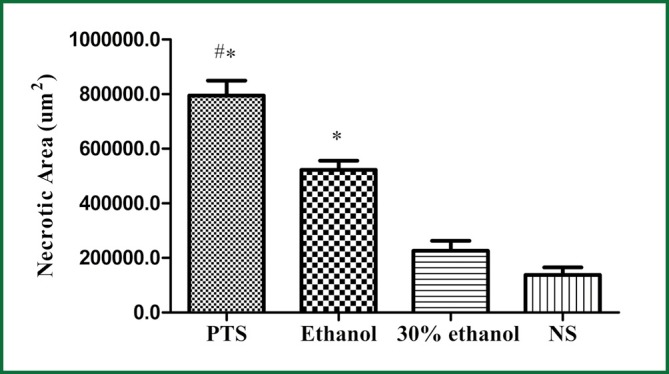
Necrotic areas of the tumors from histopathological images (#P<0.05 vs. Ethanol; *P<0.05 vs. 30% ethanol and NS).
Table 3. Necrotic areas calculated from histopathological images.
| Group | Area (μm2) |
|---|---|
| PTS | 794263.9 [667226.2-921301.6]ab |
| Ethanol | 522792.4 [446014.8-599570.0]c |
| 30% ethanol | 226291.0 [142766.5-309815.6] |
| NS | 137537.4 [72796.0-202278.8] |
aP<0.05 vs. Ethanol; bP<0.05 vs. 30% ethanol and NS; cP<0.05 vs. 30% ethanol and NS.
In vitro study
PTS induced necrosis in H460 cells
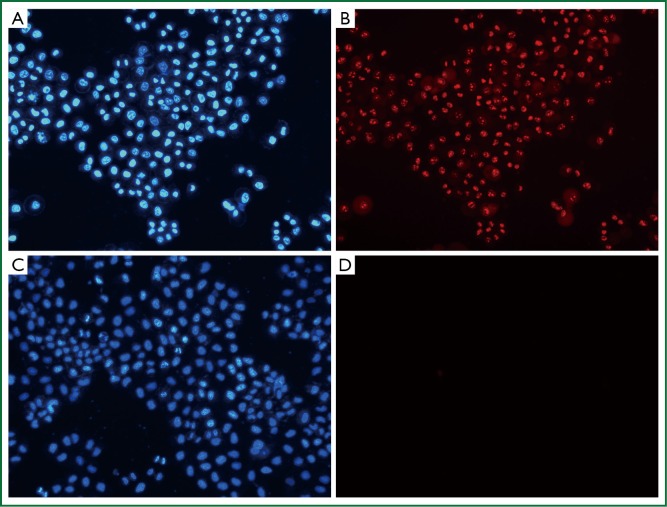
Hoechst 33342, a kind of blue-fluorescence dye, stains the chromatin in normal, apoptotic and necrotic cells. It stains the condensed chromatin in apoptotic cells more brightly than it does the chromatin in normal cells. PI, a red-fluorescence dye, is only permeant to dead cells. The simultaneous use of these dyes makes it possible to distinguish normal, apoptotic, and necrotic cell populations by fluorescence microscopy. Normal and apoptotic cells are only stained by Hoechst 33342 but the apoptotic ones exhibit brighter blue-fluorescence; necrotic cells are stained by both Hoechst 33342 and PI. When PTS was diluted to 1/100, it still had a strong necrotizing effect on H460 cells (Figure 5, Table 4). However, ethanol did not show this effect at the same diluted dose (Table 5). Therefore, PTS had a greater necrotizing effect than ethanol.
Figure 5.
Necrotic effects of PTS and ethanol on H460 cells at the concentration of 1/100 under fluorescence microscopy (Magnification, ×200). A. PTS with Hoechst 33342 stained; B. PTS with PI stained in the same view; C. Hoechst 33342 stained; D. PI stained in the same view. Blue spots represented the cell nuclei stained by Hoechst 33342 and red spots were PI conjugates. Cells incubated with PTS were double stained, indicating necrosis.
Table 4. Necrotic rates of H460 cells incubated with PTS at different concentrations.
| Concentration | Necrotic rate (%) |
|---|---|
| 1/50 | 98.5 [98.0-99.1] |
| 1/100 | 98.1 [97.5-98.7] |
| 1/150 | 3.9 [1.0-6.9] |
| 1/200 | 0.2 [0.2-0.5] |
| 2% DMSO | 0.6 [0.4-0.8] |
| Control (DMEM) | 0.5 [0.3-1.3] |
Table 5. Necrotic rates of H460 cells incubated with ethanol at different concentrations.
| Group | Necrotic rate (%) |
|---|---|
| 1/50 | 99.2 [98.0-100.2] |
| 1/10 | 98.8 [97.4-100.2] |
| 1/15 | 1.5 [0.4-3.5] |
| 1/20 | 1.4 [0.6-3.4] |
| 1/100 | 0.6 [0.1-1.3] |
| Control (DMEM) | 0.2 [0.2-0.7] |
PTS inhibited cell viability
The effects of PTS on viability of H460 cells are shown in Figure 6. PTS significantly inhibited the viability of H460 cells under the experimental conditions (continuous exposure for 2, 6 and 24 h) at the concentration of 1/10, 1/50 and 1/100. At the concentration of 1/10, both PTS and ethanol greatly suppressed the viability of H460 cells (viability rate <10%). There was no significant difference in the percentage of viable cells between the two groups at this level (P=0.454). When the two injection agents were diluted to 1/50 and 1/100, PTS maintained its suppressing activity on cell viability while the inhibitory influence of ethanol on H460 cells sharply declined. PTS had a stronger inhibitory effect than ethanol on the viability of H460 cells.
Figure 6.
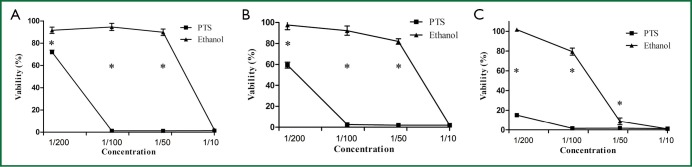
Viability of H460 cells incubated with PTS and ethanol at different concentrations (1/200, 1/100, 1/50, 1/10) for 2 h (A), 6 h (B) and 24 h (C).
PTS increased cell membrane permeability in H460 cells
Plasma membrane permeability was examined by the release of LDH from the cells which were incubated with PTS and ethanol (concentrations: 1/200, 1/400, 1/800 and 1/1,600) for 2 and 6 h respectively. Both PTS and ethanol increased the LDH release at the same diluted concentration and significantly more LDH was released from the cells induced by PTS than from those by ethanol (P<0.05) (Figures 7A,B). PTS increased the cell membrane permeability more effectively than ethanol at an equivalent concentration.
Figure 7.
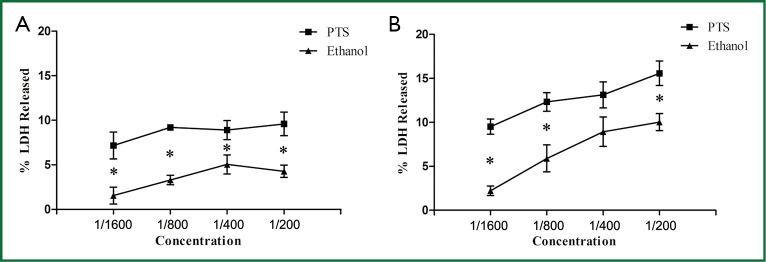
LDH release from H460 cells with continuous exposure to PTS and ethanol at different concentrations (1/1600, 1/800, 1/400, 1/200) for 2 h (A) and 6 h (B) (*P<0.05 PTS vs. ethanol).
Injury to normal tissue
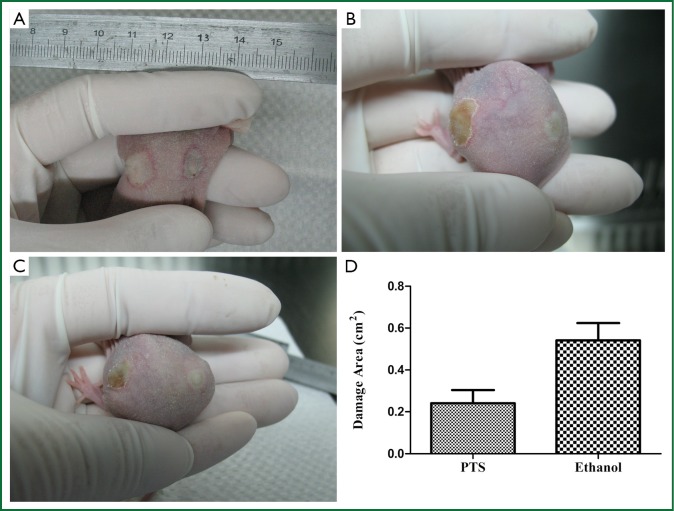
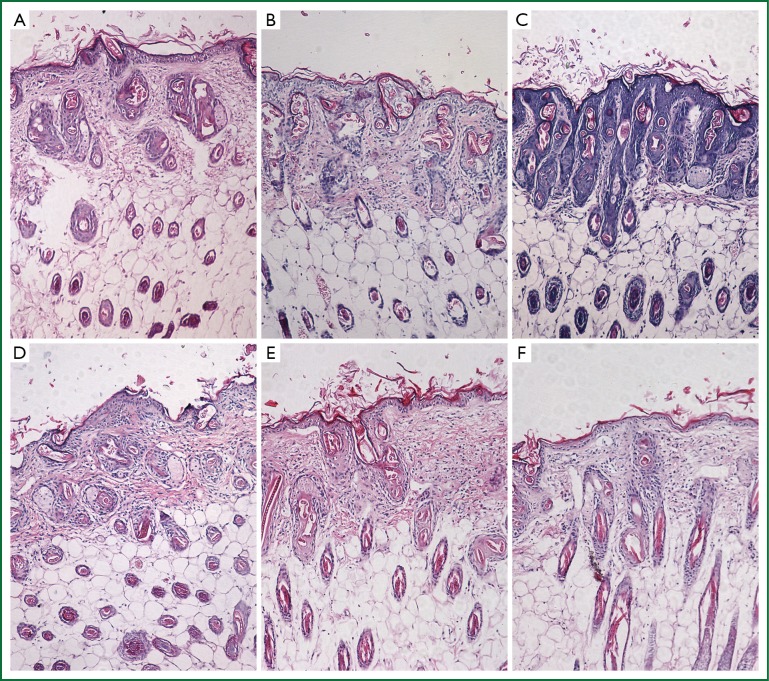
After PTS and ethanol were injected into the hypodermia, both were disseminated easily at the back of the mice. The area of damage to normal tissue by PTS was visibly less than that by ethanol (Figure 8A,B,C), which was consistent with the statistical results (Figure 8D, Table 6) (P<0.05). At the sites where PTS and ethanol diffused, masses of necrotic epidermic and hair follicular cells (eosin stained) were observed. By contrast, at the sites where the disseminated injection agents did not reach, lots of viable cells (hemalum stained) were observed. In order to histologically testify the differences in damage range induced by PTS and ethanol to the normal tissue, the epidermis and subcutaneous tissues along the length axis at 0, 0.2 and 0.5 cm away from the injection spot were analyzed (Figure 9). Although necrotizing views were revealed in the tissues at 0 and 0.2 cm away from the injection site along the length axis, no difference was observed between the two injection agents (Figure 9A,B,D,E). However, at the 0.5 cm points, lots of necrotic cells were observed in the ethanol group (Figure 9F) while there were numerous viable cells in the PTS one (Figure 9C), indicating that the necrotic area caused by PTS was smaller than by ethanol.
Figure 8.
Injury to normal tissue by PTS and ethanol. A, B, C. Morphological damage to normal tissue induced by PTS and ethanol (left side injected with ethanol and right side with PTS); D. Areas of damage to normal tissue (*P<0.05 vs. ethanol).
Table 6. Areas of damage to normal tissue (n=10).
| Group | Area (cm2) |
|---|---|
| PTS | 0.2632 [0.1517-0.3078]a |
| Ethanol | 0.5253 [0.4165-0.6874] |
aP<0.05 vs. Ethanol
Figure 9.
Histopathological features of the normal tissue incubated with PTS and ethanol in the nude mice. A, B and C were samples taken from the tissue damaged by PTS injection respectively at 0, 0.2 and 0.5 cm away from the injection spot. Accordingly, D, E and F were samples from the tissue damaged by ethanol injection respectively at 0, 0.2 and 0.5 cm away from the injection spot.
Discussion
In the present study, we tested anticancer effects of a novel injection agent PTS in vivo and in vitro. A xenograft model of nude mice with H460 cells was used to determine the in vivo inhibitory effect of PTS. PTS showed a strong tumor inhibition in mice and was superior to ethanol, which is in accordance with a former study of PTS on hepatoma in vivo (20). Moreover, in vitro evidence revealed that PTS exhibited an excellent anticancer activity by rapidly necrotizing tumor cells, reducing the cell viability and increasing the cellular membrane permeability. Additionally, the influences of PTS in vitro were stronger than those of ethanol at the same diluted dose. The present study demonstrated that PTS had a strong therapeutic effect on tumors.
Although PTS had a stronger necrotic ability against tumors than ethanol, the extent of damage caused by PTS to the normal tissue was less than that by ethanol. We suppose that this may be attributed to the viscous characteristic of PTS. It is harder for PTS to get disseminated into the neighboring tissue, resulting in a less range of injured normal tissue than ethanol.
Unlike conventional drugs for chemotherapy, whose antitumor functions are mainly inhibiting proliferation, inducing apoptosis and suppressing migration of cancer cells, PTS acts rapidly on and necrotizes the tumor cells. It is well known that the progress of apoptosis is time-consuming and ATP-requiring. It is evident that the ATP availability may become a switch in determining the pattern of cell death by apoptosis or necrosis (34,35). Moreover, it is shown that a relatively intact mitochondrial function is critical for proceeding the apoptotic progress in cells (36,37) and the mitochondria are the main source of ATP in cells with high metabolic demand. With limited mitochondrial activity and lack of ATP for apoptosis, necrosis program emerges. Although ATP levels were not determined in this study, the severe decrease in WTS-8 metabolism observed by CCK-8 assay after PTS treatment at a necrotizing dose (concentrations: 1/10, 1/50 and 1/100) (Figure 6) indirectly supported that the inadequate mitochondrial function insulted by PTS contributed to the process of necrosis. We thus suggest that the impaired mitochondrial function caused by PTS may induce necrosis of the tumor cells.
Data from Figure 5 showed that PTS had a necrotizing influence 10 times stronger than ethanol. PTS induced severe cell death with the dilution to 1/100 while the similar necrotic effect of ethanol was only shown at a concentration of above 1/10. This is consistent with the results in vivo. We concluded that because PTS may cause severe and rapid necrosis in tumor cells it is superior to the conventional injection agent ethanol. The less injury to normal tissue by PTS is first due to its viscous lipophilic capability and next to the complicated structure of different tissues at the injection spot.
Local injection therapy is effective as an alleviative treatment to reduce the tumor burden. Anhydrous ethanol is widely used as an intratumoral injection agent in the treatment of patients with advanced cancer, especially hepatocellular carcinoma (38). Anhyrous ethanol destroys tumor tissue mainly due to its dehydrating and protein degenerating properties (39-41). Although percutaneous ethanol injection is a relatively safe procedure with low mortality, fatal events after the injection have been reported (9). Local ethanol injection is also applied to treat patients with malignant lung tumor, particularly for tracheal tumors by bronchoscopy (10,42,43). However, the necrotic margin of the tumor tissue caused by ethanol injection is unmanageable because the chemical characteristics of ethanol require injections for much more times to ensure thorough necrosis of the lesion. Consequently, complications (esp. tracheo-broncheal perforation) in the lung may follow. In addition, the necrosis induced by the injection is sometimes multifocal, probably due to transbronchial spread of the injected ethanol (44). All these drawbacks therefore prevent the wide clinical application of percutaneous ethanol injection in the treatment of lung cancers. In the present study, we proved that PTS had a better necrotic effect on lung tumor. Because of its viscous property, it induced less range of damage to normal tissue than ethanol. Furthermore, several reports had proven that local injection of PTS was well-tolerated, safe and effective in the treatment of patients with solid tumor (45,46). Therefore, PTS may replace ethanol as an intratumoral injection agent in the treatment of solid tumors, especially lung cancer.
The present study has two major limitations. First, only one cell line of NSLC cells was used in vivo and in vitro. The antitumor effect of PTS on other cell lines should be investigated in further studies. Second, we only addressed the rapid necrotic changes in cancer cells caused by PTS and the influences of PTS on invasiveness and apoptosis under continuous exposure at lower concentrations were not observed. Therefore, more studies in vitro and in vivo are needed to support our findings in the future.
In summary, the present study demonstrated that PTS exhibits a greater anti-lung cancer effect than anhydrous ethanol both in vivo and in vitro. Incubation of PTS may result in speedy necrosis, decrease viability and increase cellular membrane permeability of H460 cells in vitro. We suppose that the ongoing phase II and III clinical trials on PTS against lung cancer (47,48) will yield positive results consistent with ours. We conclude that PTS, as a novel local injection agent, may play a promising role in the concurrent chemotherapy for patients with advanced lung cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.He J, Gu D, Wu X, et al. Major causes of death among men and women in China. N Engl J Med 2005;353:1124-34 [DOI] [PubMed] [Google Scholar]
- 2.She J, Yang P, Hong Q, et al. Lung cancer in China: challenges and interventions. Chest 2013;143:1117-26 [DOI] [PubMed] [Google Scholar]
- 3.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7 [DOI] [PubMed] [Google Scholar]
- 4.Janssen-Heijnen ML, Gatta G, Forman D, et al. Variation in survival of patients with lung cancer in Europe, 1985-1989. EUROCARE Working Group. Eur J Cancer 1998;34:2191-6 [DOI] [PubMed] [Google Scholar]
- 5.Bolliger CT, Sutedja TG, Strausz J, et al. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J 2006;27:1258-71 [DOI] [PubMed] [Google Scholar]
- 6.Allen AM, Rabin MS, Reilly JJ, et al. Unresectable adenoid cystic carcinoma of the trachea treated with chemoradiation. J Clin Oncol 2007;25:5521-3 [DOI] [PubMed] [Google Scholar]
- 7.Haresh KP, Prabhakar R, Rath GK, et al. Adenoid cystic carcinoma of the trachea treated with PET-CT based intensity modulated radiotherapy. J Thorac Oncol 2008;3:793-5 [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Xiong W, Gao Y, et al. EUS-guided ethanol injection for treatment of pancreatic cancer. Endoscopy 2011;43:A154 [Google Scholar]
- 9.Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology 1995;197:101-8 [DOI] [PubMed] [Google Scholar]
- 10.Fujisawa T, Hongo H, Yamaguchi Y, et al. Intratumoral ethanol injection for malignant tracheobronchial lesions: a new bronchofiberscopic procedure. Endoscopy 1986;18:188-91 [DOI] [PubMed] [Google Scholar]
- 11.Komorowski J, Kuzdak K, Pomorski L, et al. Percutaneous ethanol injection in treatment of benign nonfunctional and hyperfunctional thyroid nodules. Cytobios 1998;95:143-50 [PubMed] [Google Scholar]
- 12.Lippi F, Ferrari C, Manetti L, et al. Treatment of solitary autonomous thyroid nodules by percutaneous ethanol injection: results of an Italian multicenter study. The Multicenter Study Group. J Clin Endocrinol Metab 1996;81:3261-4 [DOI] [PubMed] [Google Scholar]
- 13.Ebara M, Ohto M, Sugiura N, et al. Percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Study of 95 patients. J Gastroenterol Hepatol 1990;5:616-26 [DOI] [PubMed] [Google Scholar]
- 14.Ishii H, Okada S, Nose H, et al. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer 1996;77:1792-6 [DOI] [PubMed] [Google Scholar]
- 15.Shiina S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy of hepatocellular carcinoma: analysis of 77 patients. AJR Am J Roentgenol 1990;155:1221-6 [DOI] [PubMed] [Google Scholar]
- 16.Di Stasi M, Buscarini L, Livraghi T, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma. A multicenter survey of evaluation practices and complication rates. Scand J Gastroenterol 1997;32:1168-73 [DOI] [PubMed] [Google Scholar]
- 17.Da Ines D, Buc E, Petitcolin V, et al. Massive hepatic necrosis with gastric, splenic, and pancreatic infarctions after ethanol ablation for hepatocellular carcinoma. J Vasc Interv Radiol 2010;21:1301-5 [DOI] [PubMed] [Google Scholar]
- 18.Ohmoto K, Kunieda T, Shibata N, et al. Intraperitoneal hemorrhage as a major complication of percutaneous ethanol injection therapy for hepatocellular carcinoma. Hepatogastroenterology 2000;47:1199-202 [PubMed] [Google Scholar]
- 19.Zardi EM, Di Matteo F, Santini D, et al. Pancreatitis after percutaneous ethanol injection into HCC: a minireview of the literature. J Exp Clin Cancer Res 2008;27:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li MY, Meng H, Zhou SZ, et al. Effect of percutaneous para-toluenesulfonamide injection in treatment of hepatocarcinoma in rats. World chinese journal of digestology 2008;16:1232 [Google Scholar]
- 21.Meng H, Li MY, Zhu WL, et al. Therapeutic effect of para-toluenesulfonamide on transplanted hepatocarcinoma in nude mice. Nan Fang Yi Ke Da Xue Xue Bao 2009;29:1024-5 [PubMed] [Google Scholar]
- 22.Wang T, Li Y, Liu M, et al. Anti-cancer Effect of PTS in Vitro. Practic J Cancer 2004;19:1-4 [Google Scholar]
- 23.Zhou JQ, Tang ZQ, Zhang JN, et al. Metabolism and effect of para-toluene-sulfonamide on rat liver microsomal cytochrome P450 from in vivo and in vitro studies. Acta Pharmacol Sin 2006;27:635-40 [DOI] [PubMed] [Google Scholar]
- 24.Chinese Clinical Trial Register [Internet]. Chengdu (Sichuan): Ministry of Health (China). 2007 Jun 27. Identifier ChiCTR-ONC-12002948, A Phase IIa Clinical Trial Treating Patients With Early Stage Head and Neck Tumor (SCCHN & SCSC) for Not Exceeding 7 Days Prior Surgery With PTS (para-toluenesulfonamide injection), A Novel Local Invasive Anticancer Drug, Administered By Local and Intratumoral Injection Therapy; 2012 Dec 31 [cited 2013 May 7]; [1 page]. Available online: http://www.chictr.org/en/proj/show.aspx?proj=4136
- 25.Chinese Clinical Trial Register [Internet]. Chengdu (Sichuan): Ministry of Health (China). 2007 Jun 27. Identifier ChiCTR-ONC-12002946, A Phase IIa Clincal Trial Treating Patients With Early Stage Breast Cancer for Not Exceding 7 Days Prior Surgery With PTS(para-toluenesulfonamide injection), A Novel Local Invasive Anticancer Drug, Administered By Local and Intratumoral Injection Therapy; 2012 Dec 31 [cited 2013 May 7]; [1 page]. Available online: http://www.chictr.org/en/proj/show.aspx?proj=4134
- 26.Chinese Clinical Trial Register [Internet]. Chengdu (Sichuan): Ministry of Health (China). 2007 Jun 27-. Identifier ChiCTR-ONC-12002944, A Phase IIb Clincal Trial Treating Patients with Advanced Palpable Solid Tumor After Failure From Conventional Treatments and Patients Who are Suitable for Palliative Treatments with PTS(para-toluenesulfonamide injection), A Novel Local Invasive Anticancer Drug, by Intratumoral Injection Therapy; 2012 Dec 31 [cited 2013 May 7]; [1 page]. Available online: http://www.chictr.org/en/proj/show.aspx?proj=4132
- 27.Chinese Clinical Trial Register [Internet]. Chengdu (Sichuan): Ministry of Health (China). 2007 Jun 27. Identifier ChiCTR-ONC-12002943, A Phase IIb Clincal Trial Treating Patients with Advanced Liver Cancer with PTS (Para-Toluenesulfonamide Injection), A Novel Local Invasive Anticancer Drug, by Percutaneous Intratumoral Injection Therapy; 2012 Dec 31 [cited 2013 May 7]; [1 page]. Available from: http://www.chictr.org/en/proj/show.aspx?proj=4133
- 28.He J, Ying W, Yang H, et al. Gemcitabine plus cisplatin chemotherapy with concurrent para-toluenesulfonamide local injection therapy for peripherally advanced nonsmall cell lung cancer larger than 3 cm in the greatest dimension. Anticancer Drugs 2009;20:838-44 [DOI] [PubMed] [Google Scholar]
- 29.Li MY, Meng H, Zhu WL, et al. Dose-effect relationship of para-toluenesulfonamide for treatment of hepatocellular carcinoma in rats. Nan Fang Yi Ke Da Xue Xue Bao 2008;28:249-51 [PubMed] [Google Scholar]
- 30.Naito S, von Eschenbach AC, Giavazzi R, et al. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res 1986;46:4109-15 [PubMed] [Google Scholar]
- 31.Liu X, Zhang B, Guo Y, et al. Down-regulation of AP-4 inhibits proliferation, induces cell cycle arrest and promotes apoptosis in human gastric cancer cells. PLoS One 2012;7:e37096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton K, Meyer JC, Bellamy AR, et al. Rotavirus nonstructural glycoprotein NSP4 alters plasma membrane permeability in mammalian cells. J Virol 1997;71:9458-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong S, Leroueil PR, Janus EK, et al. Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjug Chem 2006;17:728-34 [DOI] [PubMed] [Google Scholar]
- 34.Tsujimoto Y.Apoptosis and necrosis: intracellular ATP level as a determinant for cell death modes. Cell Death Differ 1997;4:429-34 [DOI] [PubMed] [Google Scholar]
- 35.Leist M, Single B, Castoldi AF, et al. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med 1997;185:1481-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ankarcrona M, Dypbukt JM, Bonfoco E, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 1995;15:961-73 [DOI] [PubMed] [Google Scholar]
- 37.Zamzami N, Hirsch T, Dallaporta B, et al. Mitochondrial implication in accidental and programmed cell death: apoptosis and necrosis. J Bioenerg Biomembr 1997;29:185-93 [DOI] [PubMed] [Google Scholar]
- 38.Shiina S, Tagawa K, Niwa Y, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma: results in 146 patients. AJR Am J Roentgenol 1993;160:1023-8 [DOI] [PubMed] [Google Scholar]
- 39.Shiina S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer 1991;68:1524-30 [DOI] [PubMed] [Google Scholar]
- 40.Fujimoto T.The experimental and clinical studies of percutaneous ethanol injection therapy (PEIT) under ultrasonography for small hepatocellular carcinoma. Acta Hepatol Jpn 1988; 29:52-9 [Google Scholar]
- 41.Shiina S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy for the treatment of hepatocellular carcinoma. AJR Am J Roentgenol 1990;154:947-51 [DOI] [PubMed] [Google Scholar]
- 42.Sawa T, Ikoma T, Yoshida T, et al. Intratumoral ethanol injection therapy using endoscopic video information system. Gan To Kagaku Ryoho 1999;26:1865-8 [PubMed] [Google Scholar]
- 43.Morio H, Osegawa M, Matsuoka Y, et al. Effect of ethanol injection in tracheal large cell carcinoma--a case report. Nihon Kyobu Shikkan Gakkai Zasshi 1990;28:623-7 [PubMed] [Google Scholar]
- 44.Suzuki K, Moriyama N, Yokose T, et al. Preliminary study of percutaneous alcohol injection into the lung. Jpn J Cancer Res 1998;89:89-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He Q, Kuang AR, Guan YS, et al. Puncture injection of para-toluenesulfonamide combined with chemoembolization for advanced hepatocellular carcinoma. World J Gastroenterol 2012;18:6861-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S, Yang J, Chen R, et al. Treatment of large airway obstruction due to lung cancer with local injection of Immusyn via bronchoscopy. Guangdong Medical Journal 2001;3:2 [Google Scholar]
- 47.Chinese Clinical Trial Register [Internet]. Chengdu (Sichuan): Ministry of Health (China). 2007 Jun 27. Identifier ChiCTR-ONC-12002942, A Phase IIb Clinical Study of PTS (Para-Toluenesulfonamide Injection), A Novel Local Invasive Anticancer Drug, Administered Locally and Intratumorally to Patients With Advanced Lung Cancer; 2012 Dec 31 [cited 2013 May 7]; [1 page]. Available online: http://www.chictr.org/en/proj/show.aspx?proj=4137
- 48.Chinese Clinical Trial Register [Internet]. Chengdu (Sichuan): Ministry of Health (China). 2007 Jun 27. Identifier ChiCTR-TNC-12002648, A Phase III Single Arm Trial of PTS (Para toluenesulfonamide Injection) via Bronchoscopy Intervention Intratumoral Injection in Patients with Central Air Way NSCLC Tumor Severe Obstruction; 2012 Nov 05 [cited 2013 May 7]; [1 page]. Available online: http://www.chictr.org/en/proj/show.aspx?proj=3665