Abstract
Objective
Few treatment options are available for advanced non-small cell lung cancer (NSCLC) patients who have failed of gefitinib or erlotinib treatment in second/third-line treatment. The aim of this study was to investigate the efficacy of re-administration of the same TKI after failure of gefitinib or erlotinib.
Patients and methods
The clinical data of 33 patients with advanced NSCLC were retrospectively analyzed. All of the patients were given the same TKI treatment after the failure of gefitinib or erlotinib. Survival analysis was evaluated by Kaplan-Meier method.
Results
Twenty patients (60.6%) were re-administration with gefitinib as the 2nd EGFR-TKI, and thirteen patients (39.4%) received erlotinib. One patient (3.0%) showed partial response (PR), 14 (42.4%) achieved stable disease (SD), and 18 (54.5%) had progressive disease (PD). The disease control rate was 45.5% and the median progression-free survival was 1.5 months (95% CI: 0.6-2.3 months). The PFS in patients who got disease control in the prior TKI was 2.2 and 1.2 months in the progression disease cases (P=0.29), the DCR was 54.5% and 27.3% in two group, respectively (P=0.26).
Conclusions
Re-administration of TKI seems to be a potential therapeutic option for treatment of selected advanced NSCLC patients after failure of gefitinib or erlotinib, especially for the patients with NSCLC who once responded from the prior TKI treatment.
KEY WORDS : Non-small cell lung cancer (NSCLC), erlotinib, gefitinib, retreatment, efficacy
Introduction
Gefitinib, an oral small molecule agent that inhibits epidermal growth factor receptor (EGFR) tyrosine phosphorylation (1), is the first targeted agent to be approved for the treatment of the patients with advanced non-small cell lung cancer (NSCLC), which has demonstrated clinical efficacy in the second or third-line treatment of NSCLC, especially among never-smokers, females, East Asians, and patients with adenocarcinoma (2,3). Erlotinib, another EGFR-TKI, also has shown a survival benefit in second-line or third-line treatment for advanced NSCLC (4,5).
Despite the high objective response rate (ORR) and disease control rate (DCR) in the EGFR mutation patients with the gefitinib or erlotinib treatment, most of cases would be with disease progression. For patients who previous treated with TKI and later showed tumor progression, currently, many patients with no further treatment options. Some studies have conducted trials to evaluate the efficacy of erlotinib after gefitinib failure in patients with NSCLC (6-11), but, few studies investigated the same EGFR-TKI re-administration and most of the data was from case report (12-17).
In the present study, we investigated the efficacy of re-administration of the same TKI after failure of gefitinib or erlotinib, and to explore which patients may benefit from re-administration.
Patients and methods
Patient eligibility
Six hundred and ninety-one consecutive, unselected NSCLC patients, who were admitted to Zhejiang Cancer Hospital from January 2007 to July 2011, were administrated with erlotinib or gefitinib treatment. NSCLC staging was performed for all the patients according to the 7th TNM classification. Inclusion criteria were as follows: (I) pathologically proven primary stage IIIB or IV NSCLC; (II) All the patients were supplied with the same TKI as subsequent salvage therapy after failure of gefitinib or erlotinib; (III) All patients received chemotherapy between the first TKI treatment and re-administration; (IV) The disease recurrence was confirmed using chest computed tomography (CT), brain MRI and bone scan as well as ultrasound examination and/or CT of the abdomen; (V) Without any local treatment like radiotherapy or interventional therapy during the period of gefitinib or erlotinib therapy; (VI) At least one measurable lesion and an Eastern Cooperative Oncology Group performance status of 0 to 3.
Response evaluation
All patients were followed up every 6 weeks with imaging examination during treatment with EGFR-TKIs or were evaluated early when significant tumor progression appeared. Objective tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Objective tumor responses included complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). DCR was defined as the addition of objective response and stabilization.
Toxicity evaluation
The toxicity profile of EGFR-TKI was assessed by reviewing medical records including skin rash, diarrhea, liver toxicity, and radiological evidence of interstitial pneumonitis. Severity of adverse reactions was determined based on the requirements of dosage reduction or discontinuation of EGFR-TKI. All such toxicities were evaluated according to the National Cancer Institute Common Toxicity Criteria version 3.0 (CTC 3.0).
Follow-up
All the patients were to be evaluated for tumor response and PFS. Follow-up rate was 100%. The last follow-up date was July 31, 2012. The median follow-up period was 30.2 months (6.7-56 months).
Statistical analysis
The Chi-square was applied to elucidate the differences between different treatment arms. PFS encompassed the time from the first day of TKI therapy to documented progression or death from any cause, or until the date of the last follow-up visit for patients who were still alive and who had not progressed. Survival analysis was conducted with a Kaplan-Meier analysis and log-rank test. A P-value of less than 0.05 was regarded as statistically significant. All statistical tests were analyzed using the computer software SPSS version 16.0 (SPSS Inc, Chicago, IL, USA).
Results
Patient characteristics
A total of 33 patients were included in the study and all of them were assessable for response and toxicity. There were 16 males and 17 females. PS 0-1 was present in 22 patients (66.7%) and PS 2-3 accounted for 33.3%. The median age of the patients was 57.9 years (range, 32-76 years). The majority of the tumors were adenocarcinoma (87.9%) and all of them were advanced stage on presentation. Thirty percent (10/33) had a smoking history. In 20 patients with adequate specimens for molecular analysis, 14 (70%) had EGFR mutations (8 with deletions within exon 19 and 6 with L858R messenger mutation in exon 21). All of the cases underwent cytotoxic chemotherapy between the first and second TKI therapy. Patients’ characteristics are shown in Table 1.
Table 1. Baseline characteristics of the study population (n=33).
| Variables | Number | Percent |
|---|---|---|
| Gender | ||
| Male | 16 | 48.5 |
| Female | 17 | 51.5 |
| PS | ||
| 0-1 | 22 | 66.7 |
| 2-3 | 11 | 33.3 |
| Age | ||
| Median | 59.0 | |
| Mean | 57.9±10.6 | |
| Smoking history | ||
| Yes | 10 | 30.3 |
| No | 23 | 69.7 |
| Histology | ||
| Adenocarcinoma | 28 | 84.8 |
| Non-adenocarcinoma | 5 | 15.2 |
| Staging | ||
| IIIB | 0 | 0 |
| IV | 33 | 100 |
| Chemotherapy before re-treatment | ||
| Yes | 33 | 100 |
| No | 0 | 0 |
| TKI | ||
| Gefitinib | 20 | 60.6 |
| Erlotinib | 13 | 39.4 |
| Brain metastasis | ||
| Yes | 6 | 18.2 |
| No | 27 | 81.8 |
Response data and survival analysis
Response data for gefitinib and erlotinib therapy are shown in Table 2. Thirteen patients had a PR and nine patients had SD in the initial gefitinib or erlotinib treatment, accounting for a DCR of 66.7%. There was only one patient with a PR to the retreatment (Figure 1), while 14 patients had SD and 18 patients had PD. No patients had PR and two with SD in the six patients with brain metastasis. Median PFS during initial gefitinib and erlotinib treatment was 8.9 months (95% CI: 5.0-12.8 months), but only 1.5 months during erlotinib or gefitinib retreatment (95% CI: 0.6-2.3 months). The median survival time for all patients was 27.5 months. The median OS from the beginning of the 2nd EGFR-TKI was 9.9 months (95% CI: 7.5-12.2 months).
Table 2. Response rate after treatment with gefitinib and retreatment.
| Initial treatment |
Retreatment |
||||
|---|---|---|---|---|---|
| Gefitinib | Erlotinib | Gefitinib | Erlotinib | ||
| PR, n [%] | 9 [45] | 4 [30.8] | 0 [0] | 1 [7.7] | |
| SD, n [%] | 4 [20] | 5 [38.4] | 9 [45] | 5 [38.5] | |
| PD, n [%] | 7 [35] | 4 [30.8] | 11 [55] | 7 [53.8] | |
| ORR, n [%] | 9 [45] | 4 [30.8] | 0 [0] | 1 [7.7] | |
| DCR, n [%] | 13 [65] | 9 [69.2] | 9 [45] | 5 [46.2] | |
Figure 1.
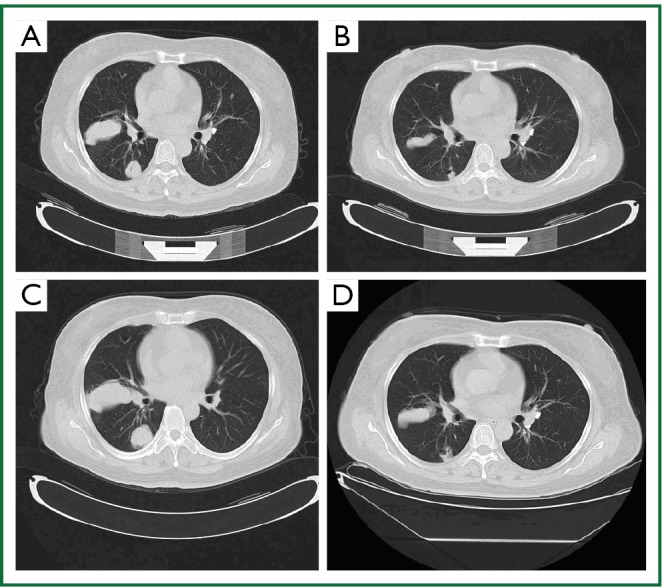
Chest CT scan of a patient who had a PR with retreatment of erlotinib. A. Chest CT scan before treatment with erlotinib; B. Chest CT scan 2 months after treatment with erlotinib; C. Chest CT scan before retreatment with erlotinib; D. Chest CT scan after retreatment with 2 months of erlotinib.
The relationship between initial treatment and retreatment efficacy
The overall DCR in the retreatment group was 45.5% (15/33). The retreatment DCR was 54.5% (12/22) in patients who got disease control in the prior TKI and 27.3% (3/11) in the initial PD group (P=0.26), and the PFS was 2.2 and 1.2 months in two group, respectively, (P=0.29) (Figure 2). No difference was found of the PFS between the erlotinib and gefitinib group (1.9 vs.1.4 months, P=0.98). The median PFS was 2.4 months in 12 patients with EGFR mutation and 1.2 months in EGFR wild-type patients (P=0.09).
Figure 2.
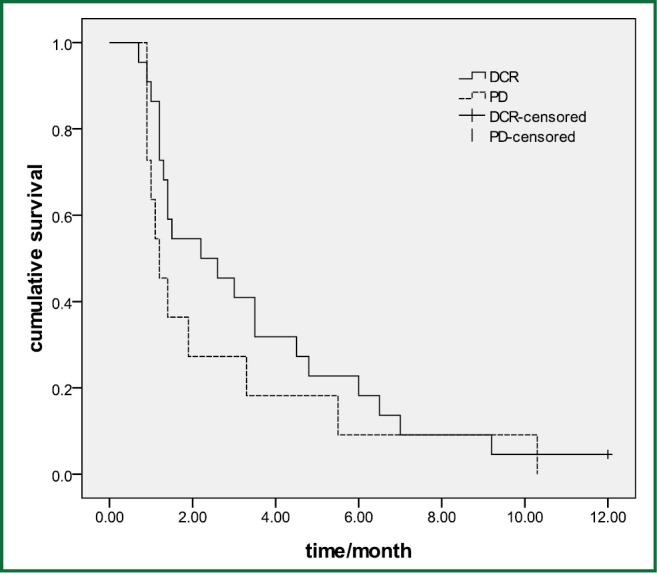
The retreatment PFS in patients who got disease control in the prior TKI and in the initial PD group (P=0.29).
Toxicities of treatment
Toxicity was evaluated in all the patients. The most common adverse event was skin toxicity in 15 patients (45.5%), including 3 patients with grade 3. Other common toxicity included diarrhea (eleven cases), fatigue (twelve cases). Two patients demonstrated hepatic function injuries after being retreated with erlotinib therapy. No dosage reduction was occurred. Overall, toxicity appeared similar to the previously published trials of gefitinib and erlotinib monotherapy (Table 3).
Table 3. Toxicities of gefitinib and erlotinib as initial and retreatment therapy for patients with advanced NSCLC.
| Toxicity | Initial treatment, n=33 (%) |
Retreatment, n=33 (%) |
|||
|---|---|---|---|---|---|
| Grades 1-2 | Grades 3-4 | Grades 1-2 | Grades 3-4 | ||
| Rash | 14 (42.4) | 3 (9.1) | 12 (36.4) | 3 (9.1) | |
| Fatigue | 8 (24.2) | 2 (6.1) | 8 (24.2) | 4 (12.1) | |
| Diarrhoea | 11 (33.3) | 1 (3.0) | 9 (27.3) | 2 (6.1) | |
| Nausea | 10 (30.3) | 1 (3.0) | 10 (30.3) | 2 (6.1) | |
| Anorexia | 6 (18.2) | 1 (3.0) | 6 (18.2) | 3 (9.1) | |
| Dyspnoea | 3 (9.1) | 2 (6.1) | 3 (9.1) | 2 (6.1) | |
| Vomiting | 5 (15.2) | 1 (3.0) | 7 (21.2) | 1 (3.0) | |
| Neurotoxicity | 2 (6.1) | 0 (0) | 1 (3.0) | 0 (0) | |
| Cough | 3 (9.1) | 3 (9.1) | 6 (18.2) | 4 (12.1) | |
| Stomatitis | 3 (9.1) | 1 (3.0) | 3 (9.1) | 3 (9.1) | |
| Dry skin | 5 (15.2) | 3 (9.1) | 1 (1.6) | 0 (0.0) | |
| Febrile neutropenia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hepatic injure | 2 (6.1) | 1 (3.0) | 3 (9.1) | 2 (9.1) | |
Discussion
To the best of our knowledge, our represents the largest data to assess whether gefitinib and erlotinib re-administration confers any clinical benefit in patients with advanced NSCLC. In our series, we obtained a DCR of 45.5% with a median duration of this control of 1.5 months with TKI re-administration. In particular, our study suggested that the overall DCR was 54.5% in patients who got disease control in the prior TKI, in contrast, the DCR was only 27.3% in the initial TKI PD group.
Riely et al. reported that in patients who develop acquired resistance, stopping gefitinib or erlotinib results in symptomatic progression, and increased tumor size, while restarting EGFR-TKI results in a decreasing in tumor diameter, and improvement of symptoms (15). Ten patients who previously responded to erlotinib or gefitinib suggested that these patients continued to benefit from treatment with erlotinib or gefitinib despite progression of disease in Riely et al. study. At our knowledge, a total of 55 cases, treated with gefitinib after failure of gefitinib and only six cases with erlotinib after failure of erlotinib were described (12-14,16,17). Table 4 lists all the studies recently published with the same TKI retreatment, which showed promising results to re-administration of gefitinib and erlotinib.
Table 4. All the articles published about retreatment as a salvage treatment after failure of gefitinib or erlotinib.
| Author | TKI sequence | No. of patients | Response to TKI |
Response to 2nd TKI |
DCR to 2nd TKI | |||
|---|---|---|---|---|---|---|---|---|
| CR/PR/SD | PD | CR/PR/SD | PD | |||||
| Yokouchi H (12) | G-G | 9 | 9 | 0 | 8 | 1 | 88.90% | |
| Yoshimoto A (13) | G-G | 1 | 1 | 0 | 1 | 0 | 100% | |
| Yano S (18) | G-G | 3 | 3 | 0 | 2 | 1 | 66.70% | |
| Hashimoto N (19) | G-G | 1 | 1 | 0 | 0 | 1 | 0% | |
| Kurata T (17) | G-G | 1 | 1 | 0 | 1 | 0 | 100% | |
| Watanabe S (20) | G-G | 3 | 3 | 0 | 2 | 1 | 66.70% | |
| Guo RH (21) | G-G | 1 | 1 | 0 | 1 | 0 | 100% | |
| Asahina H (14) | G-G | 16 | 16* | 0 | 7 | 8 | 46.7% | |
| Tomizawa Y (22) | G-G | 20 | 20 | 0 | 13 | 7 | 65% | |
| Guo RH (21) | E-E | 1 | 1 | 0 | 1 | 0 | 100% | |
| Becker A (23) | E-E | 8 | UK | UK | UK | UK | UK | |
| Current study | G-G | 20 | 13 | 7 | 9 | 13 | 45% | |
| Current study | E-E | 13 | 9 | 4 | 5 | 8 | 46.2% | |
*, including one patient can not evaluate efficacy; G, gefitinib; E, erlotinib; UK, unknown.
Several studies have suggested a possible explanation for the clinical benefit of EGFR-TKI retreatment. Some cytotoxic agents have been reported to restore the sensitivity of NSCLC cells to gefitinib in vitro by increasing EGFR phosphorylation (24,25). It is also possible that chemotherapy during the EGFR-TKI-free interval could decrease EGFR-TKI resistant tumor cells. All of the previous patients including our cases were received chemotherapy between the first and TKI retreatment.
Another explanation may be contributed to T790M mutation in the EGFR gene and amplification of the MET gene, which are some of the mechanisms of the resistance to gefitinib and erlotinib (26-28). However, the mechanisms of the resistance or re-sensitization to gefitinib or erlotinib have not been clearly defined. It may be explained the proportion of sensitive or resistant cells might have been modified or some genetic changes in EGFR associated resistance to gefitinib or erlotinib (17).
A limitation of this study was the retrospective design with its inherent shortcomings. In addition, EGFR mutation status is not fully available for the patients enrolled in our present study. However, with few cases in previous clinical studies, our retrospective study may also be considered to be meaningful.
In conclusion, our results indicated that re-administration of TKI could be consider as one of treatment option for the patients who responded to treatment of initial TKI. The erlotinib and gefitinib retreatment had a similar efficacy. It is necessary to explore the mechanisms induced resistance and re-sensitivity for EGFR-TKI.
Acknowledgements
This work was supported by grants from the Medical Scientific Research Foundation of Zhejiang Province (No. 2013KYB049) and fund of development center for medical science and technology ministry of health (W2012FZ134).
Disclosure: The authors declare no conflicts of interest.
References
- 1.Ciardiello F, Tortora G.A novel approach in the treatment of cancer: targeting the epidermal growth factor receptor. Clin Cancer Res 2001;7:2958-70 [PubMed] [Google Scholar]
- 2.Thatcher N, Chang A, Parikh P, et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527-37 [DOI] [PubMed] [Google Scholar]
- 3.Maruyama R, Nishiwaki Y, Tamura T, et al. Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 2008;26:4244-52 [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32 [DOI] [PubMed] [Google Scholar]
- 5.Ciuleanu T, Stelmakh L, Cicenas S, et al. Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 2012;13:300-8 [DOI] [PubMed] [Google Scholar]
- 6.Chang JW, Chou CL, Huang SF, et al. Erlotinib response of EGFR-mutant gefitinib-resistant non-small-cell lung cancer. Lung Cancer 2007;58:414-7 [DOI] [PubMed] [Google Scholar]
- 7.Cho BC, Im CK, Park MS, et al. Phase II study of erlotinib in advanced non-small-cell lung cancer after failure of gefitinib. J Clin Oncol 2007;25:2528-33 [DOI] [PubMed] [Google Scholar]
- 8.Lee DH, Kim SW, Suh C, et al. Phase II study of erlotinib as a salvage treatment for non-small-cell lung cancer patients after failure of gefitinib treatment. Ann Oncol 2008;19:2039-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sim SH, Han SW, Oh DY, et al. Erlotinib after Gefitinib failure in female never-smoker Asian patients with pulmonary adenocarcinoma. Lung Cancer 2009;65:204-7 [DOI] [PubMed] [Google Scholar]
- 10.Wong MK, Lo AI, Lam B, et al. Erlotinib as salvage treatment after failure to first-line gefitinib in non-small cell lung cancer. Cancer Chemother Pharmacol 2010;65:1023-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song ZB, Yu YF, Chen ZW, et al. Erlotinib as a salvage treatment for patients with advanced non-small cell lung cancer after failure of gefitinib treatment. Chin Med J (Engl) 2011;124:2279-83 [PubMed] [Google Scholar]
- 12.Yokouchi H, Yamazaki K, Kinoshita I, et al. Clinical benefit of readministration of gefitinib for initial gefitinib-responders with non-small cell lung cancer. BMC Cancer 2007;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimoto A, Inuzuka K, Kita T, et al. Remarkable effect of gefitinib retreatment in a patient with nonsmall cell lung cancer who had a complete response to initial gefitinib. Am J Med Sci 2007;333:221-5 [DOI] [PubMed] [Google Scholar]
- 14.Asahina H, Oizumi S, Inoue A, et al. Phase II study of gefitinib readministration in patients with advanced non-small cell lung cancer and previous response to gefitinib. Oncology 2010;79:423-9 [DOI] [PubMed] [Google Scholar]
- 15.Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res 2007;13:5150-5 [DOI] [PubMed] [Google Scholar]
- 16.Tomizawa Y, Fujita Y, Tamura A, et al. Effect of gefitinib re-challenge to initial gefitinib responder with non-small cell lung cancer followed by chemotherapy. Lung Cancer 2010;68:269-72 [DOI] [PubMed] [Google Scholar]
- 17.Kurata T, Tamura K, Kaneda H, et al. Effect of re-treatment with gefitinib (‘Iressa’, ZD1839) after acquisition of resistance. Ann Oncol 2004;15:173-4 [DOI] [PubMed] [Google Scholar]
- 18.Yano S, Nakataki E, Ohtsuka S, et al. Retreatment of lung adenocarcinoma patients with gefitinib who had experienced favorable results from their initial treatment with this selective epidermal growth factor receptor inhibitor: a report of three cases. Oncol Res 2005;15:107-11 [PubMed] [Google Scholar]
- 19.Hashimoto N, Imaizumi K, Honda T, et al. Successful re-treatment with gefitinib for carcinomatous meningitis as disease recurrence of non-small-cell lung cancer. Lung Cancer 2006;53:387-90 [DOI] [PubMed] [Google Scholar]
- 20.Watanabe S, Tanaka J, Ota T, et al. Clinical responses to EGFR-tyrosine kinase inhibitor retreatment in non-small cell lung cancer patients who benefited from prior effective gefitinib therapy: a retrospective analysis. BMC Cancer 2011;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo R, Chen X, Wang T, et al. Subsequent chemotherapy reverses acquired tyrosine kinase inhibitor resistance and restores response to tyrosine kinase inhibitor in advanced non-small-cell lung cancer. BMC Cancer 2011;11:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomizawa Y, Fujita Y, Tamura A, et al. Effect of gefitinib re-challenge to initial gefitinib responder with non-small cell lung cancer followed by chemotherapy. Lung Cancer 2010;68:269-72 [DOI] [PubMed] [Google Scholar]
- 23.Becker A, Crombag L, Heideman DA, et al. Retreatment with erlotinib: Regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR-TKI treatment. Eur J Cancer 2011;47:2603-6 [DOI] [PubMed] [Google Scholar]
- 24.Van Schaeybroeck S, Karaiskou-McCaul A, Kelly D, et al. Epidermal growth factor receptor activity determines response of colorectal cancer cells to gefitinib alone and in combination with chemotherapy. Clin Cancer Res 2005;11:7480-9 [DOI] [PubMed] [Google Scholar]
- 25.Van Schaeybroeck S, Kyula J, Kelly DM, et al. Chemotherapy-induced epidermal growth factor receptor activation determines response to combined gefitinib/chemotherapy treatment in non-small cell lung cancer cells. Mol Cancer Ther 2006;5:1154-65 [DOI] [PubMed] [Google Scholar]
- 26.Kosaka T, Yatabe Y, Endoh H, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res 2006;12:5764-9 [DOI] [PubMed] [Google Scholar]
- 27.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A 2007;104:20932-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43 [DOI] [PubMed] [Google Scholar]


