Abstract
Introduction
Although several large studies showed roflumilast (Rof) has demonstrated efficacy in patients with chronic obstructive pulmonary disease (COPD), the efficacy of Rof in dyspnea remains unclear. We therefore undertook a meta-analysis to assess the efficacy of Rof in dyspnea for COPD patients.
Methods
A computerized search through electronic databases was performed to obtain randomized controlled trials (RCTs). Dyspnea was assessed by the transition dyspnea index (TDI) and the UCSD Shortness of Breath Questionnaire (SOBQ). The quality of the included studies was assessed by the Jadad score. Weighted mean differences (WMDs) and 95% confidence intervals (CIs) were calculated and heterogeneity was assessed with the I2 test. The effect sizes were compared with the minimum clinically important difference (MCID).
Results
Four RCTs involving 4,767 patients with forced expiratory volume in one second (FEV1) <80% predicted met the inclusion criteria. The Jadad score of each study was 5 scores. Rof statistically improved the TDI focal score (WMD =0.30 units; 95% CI: 0.14-0.46), but failed to decrease the SOBQ (WMD =–1.10 units; 95% CI: –4.24 to 2.04). The overall effect sizes were lower than the MCID of the TDI and the SOBQ, respectively.
Conclusions
Sufficient evidence to support Rof relieving dyspnea in COPD patients is currently lacking. Further studies are needed to investigate the effects of Rof in dyspnea, especially for COPD patients with a different phenotype.
KEY WORDS : Chronic obstructive pulmonary disease (COPD), roflumilast (Rof), dyspnea, meta-analysis
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by a specific pattern of chronic lung inflammation and progressive, irreversible airflow limitation (1-3). It is an important cause of morbidity and mortality worldwide and results in an economic and social burden (1,4-6). Exacerbations of symptoms such as dyspnea may contribute to the severity of COPD in individual patients (1,7). Currently, treatment for COPD primarily focuses on pharmacological therapy such as bronchodilators and anti-inflammatory agents (2). The latter includes phosphodiesterase 4 (PDE4) inhibitor. Inhibiting PDE4 reduces cellular inflammatory activity, which may account for the beneficial efficacy (8). The latest Global Initiative for Chronic Obstructive Lung Disease (GOLD) consensus report proposed that roflumilast (Rof), a new selective PDE4 inhibitor, may also be used to reduce exacerbation rate of patients with forced expiratory volume in one second (FEV1) <50% predicted, chronic bronchitis, and frequent exacerbations (2).
To our knowledge, there are published randomized controlled trials (RCTs) regarding the effect of Rof in COPD patients (9-16). Rof can significantly increase pre- and post-bronchodilator FEV1 and decrease exacerbation rate of COPD. In addition, a Cochrane review comparing PDE4 inhibitors with placebo in the treatment of COPD patients was published in 2011, which summarised the evidence in favour of Rof treatment, especially in spirometry parameters and exacerbation rate (17). The latest studies suggested that treatment with Rof shifts patients from the frequent to the more stable infrequent exacerbator state and Rof treatment is associated with progressive improvement of airway function but not lung hyperinflation (13,18). However, these authors did not assess the efficacy of Rof in the topic of dyspnea. Whether Rof can improve dyspnea remains unclear because these studies convey inconclusive results. Therefore, we performed a meta-analysis of the relevant literature to further critically assess the effects of Rof in dyspnea during the treatment of COPD patients.
Methods
Data sources and searches
A computerized search was performed through PubMed, Cumulative Index to Nursing and Allied Health (CINAHL), EMBASE, Physiotherapy Evidence Database (PEDro), and the Cochrane Central Register of Controlled Trials up to Mar 2013 for original research articles following the major keywords: “Rof” and “COPD”. Results limited to studies with human subjects and RCTs were included. No language restriction was imposed. Bibliographies of all potentially relevant retrieved studies, identified relevant articles (including meta-analysis studies, a follow-up from reference lists of relevant articles and personal contact with experts in this field) and international guidelines were searched by hand.
Article selection
The following inclusive selection criteria in patients, intervention, comparator, outcome/s, study design (PICOS) order included: (I) population: patients with diagnosed COPD according to the American Thoracic Society standard guidelines; (II) intervention: Rof (500 μg) with or without other pharmacological treatments; (III) comparison intervention: placebo with or without other pharmacological treatments; and (IV) outcome measures: dyspnea; and (V) study design: RCT.
Data extraction and outcome measure
For each study, we recorded the first author, year of publication, study ID numbers, the sample size of the study population (male/female), COPD grade, mean body mass index (BMI, kg/m2) and mean age (intervention/control), current smoking status, intervention protocol, study design, treatment duration and oral drug frequency, and dyspnea measurement. To assess eligibility, data and trial quality information were extracted independently by two investigators (LP and YZG). Any disagreements were resolved by discussion and consensus. A third investigator (BZ) was consulted in case of disagreement to improve accuracy. The analytical data missing from the primary reports were requested from their authors. When the same population was reported in several publications, we retained only the most informative article or complete study to avoid duplication of information. The outcome measure was dyspnea. In addition, dyspnea was assessed by the transition dyspnea index (TDI) focal score and the UCSD Shortness of Breath Questionnaire (SOBQ). Higher scores for the TDI indicate more favourable, but higher scores for the SOBQ indicate less favourable.
Quality assessment and risk-of-bias assessment
The methodological quality of each study was evaluated using the Jadad scale (19). The quality scale ranged from 0 to 5 points. A score ≤2 indicates low quality and a score ≥3 indicates high quality (20). The risk of bias was assessed using the Cochrane Handbook for Systematic Reviews of Interventions (Revman version 5.1.0, The Cochrane Collaboration 2011).
Statistical analysis
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (21). All data were combined using Revman 5.1.0., Mean difference weighted mean difference (WMD) was calculated for continuous outcomes. All measures were estimated from each study with the associated 95% confidence intervals (CIs) and pooled across studies using a random effects model (22). Heterogeneity across studies was tested by using the I2 statistic. Studies with an I2 statistic of 25% to 50% were considered to have low heterogeneity, those with an I2 statistic of 50% to 75% were considered to have moderate heterogeneity, and those with an I2 statistic of >75% were considered to have a high degree of heterogeneity (23). If I2 >50%, potential sources of heterogeneity were identified by sensitivity analyses conducted by omitting one study in each turn and investigating the influence of a single study on the overall pooled estimate. We undertook subgroup analyses to explore observed heterogeneity and examine the influence of various exclusion criteria according to duration of therapy, basic grade of COPD, and use of monotherapy vs. combination therapy. Potential publication bias for each analysis was assessed visually using a funnel plot. However, publication bias was not assessed because of the limited number (below 10) of studies included in each analysis. A P value <0.05 was considered statistically significant. The overall treatment effect was compared with its minimum clinically important difference (MCID).
Results
Bibliographic search results
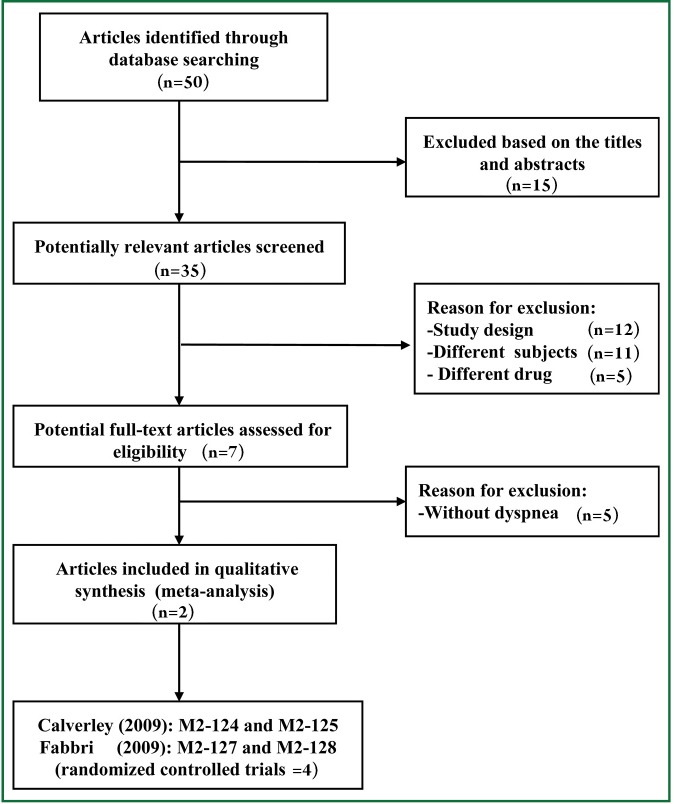
Fifty potential studies were retrieved from the computer searches. Following screening of study titles and abstracts, seventeen articles were deemed unrelated and excluded. Thirty-three potentially relevant studies identified for analysis. Following inclusion criteria further applied, twelve of them were excluded due to their study design (e.g., narrative review, comment or editorial), eleven were excluded because of applying the different subjects, and five were excluded because of applying the different drug (i.e., cilomilast). Furthermore, five RCTs of them were excluded due to without dyspnea measurement (12-16). Reasons for exclusion are presented in Figure 1. Finally, two articles including 4 RCTs were selected for this meta-analysis because every article included two respective RCTs resulted from the different population (9,10). All RCTs were published in English.
Figure 1.
Search strategy and flow chart of screened, excluded, and eventually analyzed articles.
Characteristics of the included trials
The principal characteristics of the selected studies are presented in Table 1. Four RCTs involving a total of 4,767 patients (male vs. female: 3,483 vs. 1,284) were published in 2009. Patients were administered either a single dose of roflumilast or placebo once daily for 52 weeks for two RCTs (M2-124 and M2-125) (9). However, patients were administered either a dose of roflumilast plus salmeterol or placebo plus salmeterol once daily for 24 weeks for one RCT (M2-127), and patients were administered either a dose of roflumilast plus tiotropium or placebo plus tiotropium once daily for 24 weeks for another RCT (M2-128) (10).
Table 1. Characteristics of randomized controlled trials included in the meta-analysis.
| First author, year | Study design/Jadad score | Patients No. (M/F); grade | BMI (kg/m2); age (yrs), (I/C), mean | Current smoker, n (%), (I/C) | Chronic cough and sputum, n (%), (I/C) | Intervention protocol | Endpoint |
|---|---|---|---|---|---|---|---|
| Calverley et al., 2009 (9) (HERMES M2-124) | Phase III, double-blind, multicenter, RCT/5 | 1,523 (1,078/445); severe to very severe | 26.4/26.0; 64/63 | 365 (48%)/361 (48%) | NA | Rof 500 µg (n=765), placebo (n=758), once daily for 52 wk | Dyspnea (TDI) |
| Calverley et al., 2009 (9) (AURA M2-125) | Phase III, double-blind, multicenter, RCT/5 | 1,568 (1,258/310); severe to very severe | 25.2/25.4; 64/64 | 270 (35%)/282 (35%) | NA | Rof 500 µg (n=772), placebo (n=796), once daily for 52 wk | Dyspnea (TDI) |
| Fabbri et al., 2009 (10) (EOS M2-127) | Phase III, double-blind, multicenter, RCT/5 | 933 (618/315); moderate to severe | NA; 65/65 | 184 (39%)/184 (39%) | 367 (79%)/362 (78%) | Rof 500 µg + salmeterol (n=466), placebo + salmeterol (n=467) once daily for 24 wk | Dyspnea (TDI, SOBQ) |
| Fabbri et al., 2009 (10) (HELIOS M2-128) | Phase III, double-blind, multicenter, RCT/5 | 743 (529/214); moderate to severe | NA; 64/64 | 147 (40%)/146 (39%) | 371 (100%)/372 (100%) | Rof 500 µg + tiotropium (n=371), placebo + tiotropium (n=372), once daily for 24 wk | Dyspnea (TDI, SOBQ) |
M/F, male/female; BMI, body-mass index; I/C, intervention/control; RCT, randomized controlled trial; NA, not available; TDI, the transition dyspnea index; Rof, roflumilast; SOBQ, the UCSD Shortness of Breath Questionnaire.
Patients could use shortacting β2 agonists as needed and could continue treatment with longacting β2 agonists or shortacting anticholinergic drugs at stable doses. However, inhaled corticosteroids and longacting anticholinergic drugs were not allowed during the study. Eligible patients were stratified according to their use of longacting β2 agonists and smoking status.
Quality and risk-of-bias assessment
Two investigators (LP and YZG) agreed on every item of the Jadad scores. The Jadad score of each study was 5 scores. Risk of bias analysis showed that all RCTs applied double-blind, reported the randomization protocol, and described a method used to conceal the allocation (Figure 2). Analysis was by intention to treat for all RCTs.
Figure 2.
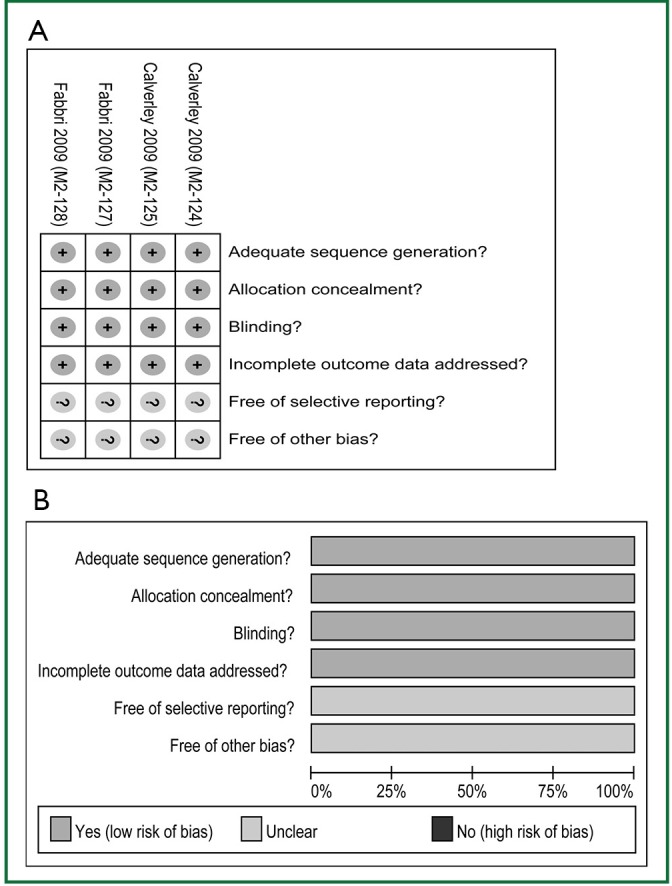
Risk-of-bias analysis. A. Risk-of-bias summary, the authors’ judgments about each risk-of-bias item for the each included studies; B. Risk-of-bias graph, the authors’ judgments about each risk-of-bias item presented as percentages across all included studies.
Meta-analysis of outcome measure
All RCTs reported dyspnea assessed by the TDI (9,10). The aggregate results of these studies suggested that Rof was associated with a statistical improving on the TDI focal score (WMD =0.30 units; 95% CI: 0.14-0.46; P for heterogeneity =0.24; I2 =28%) (Figure 3). The overall treatment effect was lower than the MCID of the TDI (≥1 unit) (24). Two RCTs (M2-127 and M2-128) reported dyspnea assessed by the SOBQ (10). The aggregate results suggested that Rof failed to increase the SOBQ (WMD =–1.10 units; 95% CI: –4.24 to 2.04 units; P for heterogeneity =0.02; I2 =81%) (Figure 4). The test heterogeneity was significant for the SOBQ. Owning to only two RCTs, we couldn’t perform sensitivity analyses to explore potential source of heterogeneity. In addition, the overall treatment effect was lower than the MCID of the SOBQ (25) (≥5 units).
Figure 3.
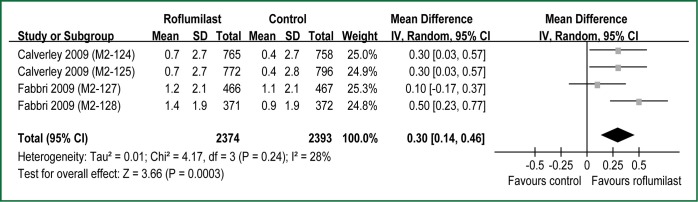
Meta-analysis of randomized controlled trials evaluating effects of dyspnea assessed by the transition dyspnea index. Each block represents a study and the area of each block is proportional to the precision of the mean treatment effect in that study. The horizontal line represents each study’s 95% confidence interval (CI) for the treatment effect. The center of the diamond is the average treatment effect across studies, and the width of the diamond denotes its 95% CI.
Figure 4.
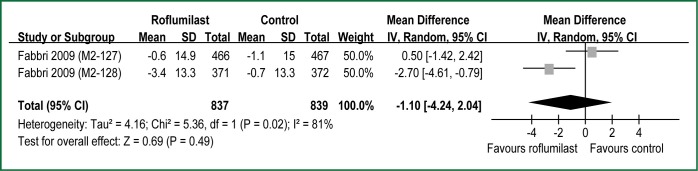
Meta-analysis of randomized controlled trials evaluating effects of dyspnea assessed by the UCSD Shortness of Breath Questionnaire. See Figure 3 legend for explanation of symbols used.
Furthermore, we performed subgroup analyses on the TDI focal score according to duration of therapy, basic grade of COPD, and use of monotherapy vs. combination therapy (Table 2). In term of the SOBQ, post hoc subgroup analyses were not performed due to the small number of trials.
Table 2. Subgroup analyses based on various exclusion criteria for the TDI focal score.
| Outcome | n [N] | WMD (95% CI) | P value | I2 (%) | Pheterogeneity |
|---|---|---|---|---|---|
| All included trials (9,10) | 4,767 [4] | 0.30 (0.14 to 0.46) | 0.0003 | 28 | 0.24 |
| Duration: 52 wk (9) | 3,091 [2] | 0.30 (0.11 to 0.49) | 0.002 | 0 | 1.00 |
| Duration: 24 wk (10) | 1,676 [2] | 0.30 (–0.09 to 0.69) | 0.13 | 76 | 0.04 |
| Monotherapy (9) | 3,091 [2] | 0.30 (0.11 to 0.49) | 0.002 | 0 | 1.00 |
| Combination therapy (10) | 1,676 [2] | 0.30 (–0.09 to 0.69) | 0.13 | 76 | 0.04 |
| Roflumilast + salmeterol (10) (M2-127) | 933 [1] | 0.10 (–0.17 to 0.37) | 0.47 | — | — |
| Roflumilast + tiotropium (10) (M2-128) | 743 [1] | 0.50 (0.23 to 0.77) | 0.0003 | — | — |
| Moderate to severe (10) | 1,676 [2] | 0.30 (–0.09 to 0.69) | 0.13 | 76 | 0.04 |
| Severe to very severe (9) | 3,091 [2] | 0.30 (0.11 to 0.49) | 0.002 | 0 | 1.00 |
TDI, the transition dyspnea index; n, number of patients; N, number of trials; WMD, weighted mean difference.
Discussion
The purpose of the present meta-analysis of existing data is to evaluate the role of Rof in improving dyspnea for COPD patients. Our results suggested that Rof may improve dyspnea in COPD patient especially for those with chronic cough and sputum, and Rof combining an anticholinergic drug may be more effective in alleviating dyspnea. However, there is currently a lack of clinical evidence to support these. Further studies are a priority needed to substantiate our current findings and investigate the effects of combination therapy in patients with COPD.
Dyspnea is one of the most important and debilitating symptoms of COPD patients (7). At present, one of the primary goals in the management of COPD is to alleviate dyspnea (2). Although several medication classes are used for COPD treatment, none of these medications have been shown to significantly improve long-term dyspnea. Recently, potential anti-inflammatory therapies including PDE4 inhibitors are being evaluated (8,26,27). The latest statement of COPD management suggested that Rof may decrease exacerbation rate of severe patients with COPD (2). Up to date, whether Rof can improve dyspnea remains unknown due to inconclusive results from published RCTs.
Our results showed that Rof was associated with a statistical improvement in dyspnea assessed by the TDI not the SOBQ. However, when interpreting clinical measures, it is important to remember not all statistically significant differences are clinically relevant (28). The MCID, defined as the smallest difference considered significant by the average patients, is the latest standard for determining effectiveness of interventions in clinical trials (29). A 1-unit change of the TDI in a multinational clinical trial is confirmed as the MCID (24), and the proposed MCID of 5 units for the SOBQ is recommended (25). The overall effect sizes were lower than the MCID of the TDI and the SOBQ, which showed that the TDI improvements in COPD patients were insufficient evidence to support Rof benefiting clinical effects on dyspnea. The results of published RCTs showed that improvement changes of pre- and post-bronchodilator FEV1 were lower than the MCID of FEV1 (≥100 mL) (30), which may account for lack of clinical positive efficacy of Rof related to dyspnea.
Subsequently, we performed subgroup analyses on the TDI focal score according to various criteria (Table 2). Fortunately, we found several valuable information. First, the long-term Rof therapy (52 wk) did not significantly reduce dyspnea compared with the short-term of 24 wk (M2-128). Second, combination therapy may more significantly reduce dyspnea than monotherapy. Although there was no statistical difference between Rof group and placebo group in the study of M2-127, Rof plus salmeterol significantly increased the TDI focal score of intervention group compared with monotherapy in M2-124 and M2-125. Third, Rof combining an anticholinergic drug may more improve dyspnea in COPD patient, especially in those with chronic cough and sputum.
From Table 1, we found that percentage of patients with chronic cough and sputum was approximately 79% in the study of M2-127, which was lower than 100% in the study of M2-128. Moreover, intervention protocol (i.e., combination therapy) was different: Rof plus salmeterol and placebo plus salmeterol were applied in the study of M2-127, while Rof plus tiotropium and placebo plus tiotropium were applied in the study of M2-128. In a word, differences of COPD phenotype and combination drug between the two studies may contribute to the heterogeneity and the inconsistent result of the TDI focal score. On the basis of these findings, we believe that Rof combining an anticholinergic drug may more significantly improve dyspnea in COPD patients, especially in those with chronic cough and sputum. However, this reason also may be related to a more advantageous tiotropium than salmeterol (31). Further studies are needed to investigate the effects of combination therapy in COPD patients with a different phenotype.
The current meta-analysis was comprehensive in its scope and search. We conducted it in line with contemporary recommendations and complied with the PRISMA statement. Our search of literature aimed to minimise the risk of selection and publication bias. Next, we performed a rigorous quality assessment and risk-of-bias assessment. Reliable data were identified on a clinically important outcome related to dyspnea. We explored for sources of heterogeneity when required. The validity of a meta-analysis depends on the quality of the component studies, heterogeneity observed, and the risk of publication bias. The Jadad score of each study included was 5 scores. Risk of bias analysis showed that all RCTs applied double-blind, reported the randomization protocol, and described a method used to conceal the allocation. Finally, the test heterogeneity was insignificant for the TDI and four large-scale RCTs involving 4,767 patients were included and pooled into our analysis, which increase the validity and reliability of our meta-analysis.
However, several limitations of the present meta-analysis should be taken into account. Firstly, our analysis is actually based on only four RCTs which account for failure to undertake a funnel plot to assess potential publication bias for analysis. Secondly, there was a potential risk of bias and heterogeneity among the included trials. The targeted population varied (e.g., sex, ethnicity and grade). Adopted intervention protocol (e.g., treatment duration and combination therapy) differed. These factors may explain the heterogeneity and may potentially affect our results. Thirdly, in all the trials the primary outcome assessment was not dyspnea, which is prone to selective bias. Moreover, there was an inconsistent result regarding the SOBQ. Owning to only two RCTs, we couldn’t perform sensitivity analyses to explore potential source of heterogeneity. Hence, the evidence was not robust and the results should be interpreted with caution. Finally, several clinical trials remain unpublished that are identified as “completed” when searching ClinicalTrials.gov. So some missing and unpublished data may lead to bias.
Despite of some demerits abovementioned, the present study provides additional interesting clues that may be useful for future research on the topic. First of all, future study needs to further focus on the topic of dyspnea. Different measurement of dyspnea can be applied in evaluating the efficacy of Rof during treatment COPD patients. We can evaluate the change of dyspnea by the Borg scale during exercising and activities of daily living with the identical work level in order to better understand the benefits, mechanisms, and role of Rof in the management of dyspnea. Next, further research should pay more attention on combination therapy to play a maximum therapeutic effect with a synergistic effect between the drugs and elucidate the best combination regimen for COPD patients. In addition, it remains unclear what specific mechanisms of Rof are responsible for the potential beneficial effects on dyspnea, and how Rof differs from other drugs. Concentrating on these aspects therefore may be of interest for future research on the subject. Finally, novel therapies should continue to be developed.
Conclusions
In summary, clinically relevant evidence that Rof therapy improves dyspnea in COPD patients with FEV1 <80% predicted, is still limited. Therefore, further studies are urgently needed to substantiate our current findings as well as finding the best beneficiary population and the optimal mixed formulations in COPD patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155:179-91 [DOI] [PubMed] [Google Scholar]
- 2.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347-65 [DOI] [PubMed] [Google Scholar]
- 3.Decramer M, Janssens W, Miravitlles M.Chronic obstructive pulmonary disease. Lancet 2012;379:1341-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397-412 [DOI] [PubMed] [Google Scholar]
- 5.Dalal AA, Shah M, Lunacsek O, et al. Clinical and economic burden of patients diagnosed with COPD with comorbid cardiovascular disease. Respir Med 2011;105:1516-22 [DOI] [PubMed] [Google Scholar]
- 6.García-Polo C, Alcázar-Navarrete B, Ruiz-Iturriaga LA, et al. Factors associated with high healthcare resource utilisation among COPD patients. Respir Med 2012;106:1734-42 [DOI] [PubMed] [Google Scholar]
- 7.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012;185:435-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross NJ. Novel antiinflammatory therapies for COPD. Chest 2012;142:1300-7 [DOI] [PubMed] [Google Scholar]
- 9.Calverley PM, Rabe KF, Goehring UM, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009;374:685-94 [DOI] [PubMed] [Google Scholar]
- 10.Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009;374:695-703 [DOI] [PubMed] [Google Scholar]
- 11.Rennard SI, Calverley PM, Goehring UM, et al. Reduction of exacerbations by the PDE4 inhibitor roflumilast--the importance of defining different subsets of patients with COPD. Respir Res 2011;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SD, Hui DS, Mahayiddin AA, et al. Roflumilast in Asian patients with COPD: a randomized placebo-controlled trial. Respirology 2011;16:1249-57 [DOI] [PubMed] [Google Scholar]
- 13.O’Donnell DE, Bredenbröker D, Brose M, et al. Physiological effects of roflumilast at rest and during exercise in COPD. Eur Respir J 2012;39:1104-12 [DOI] [PubMed] [Google Scholar]
- 14.Rabe KF, Bateman ED, O’Donnell D, et al. Roflumilast--an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005;366:563-71 [DOI] [PubMed] [Google Scholar]
- 15.Calverley PM, Sanchez-Toril F, McIvor A, et al. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:154-61 [DOI] [PubMed] [Google Scholar]
- 16.Grootendorst DC, Gauw SA, Verhoosel RM, et al. Reduction in sputum neutrophil and eosinophil numbers by the PDE4 inhibitor roflumilast in patients with COPD. Thorax 2007;62:1081-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong J, Poole P, Leung B, et al. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011;(5):CD002309. [DOI] [PubMed] [Google Scholar]
- 18.Wedzicha JA, Rabe KF, Martinez FJ, et al. Efficacy of roflumilast in the COPD frequent exacerbator phenotype. Chest 2013;143:1302-11 [DOI] [PubMed] [Google Scholar]
- 19.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12 [DOI] [PubMed] [Google Scholar]
- 20.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 2001;135:982-9 [DOI] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N.Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Witek TJ, Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J 2003;21:267-72 [DOI] [PubMed] [Google Scholar]
- 25.Kupferberg DH, Kaplan RM, Slymen DJ, et al. Minimal clinically important difference for the UCSD Shortness of Breath Questionnaire. J Cardiopulm Rehabil 2005;25:370-7 [DOI] [PubMed] [Google Scholar]
- 26.Tamimi A, Serdarevic D, Hanania NA. The effects of cigarette smoke on airway inflammation in asthma and COPD: therapeutic implications. Respir Med 2012;106:319-28 [DOI] [PubMed] [Google Scholar]
- 27.Vignola AM. PDE4 inhibitors in COPD--a more selective approach to treatment. Respir Med 2004;98:495-503 [DOI] [PubMed] [Google Scholar]
- 28.Beaton DE, Bombardier C, Katz JN, et al. Looking for important change/differences in studies of responsiveness. OMERACT MCID Working Group. Outcome measures in rheumatology. Minimal clinically important difference. J Rheumatol 2001;28:400-5 [PubMed] [Google Scholar]
- 29.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15 [DOI] [PubMed] [Google Scholar]
- 30.Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005;2:111-24 [DOI] [PubMed] [Google Scholar]
- 31.Vogelmeier C, Fabbri LM, Rabe KF, et al. Effect of tiotropium vs. salmeterol on exacerbations: GOLD II and maintenance therapy naïve patients. Respir Med 2013;107:75-83 [DOI] [PubMed] [Google Scholar]