Abstract
Purpose
To compare neodymium-doped yttrium aluminum garnet laser posterior capsulotomy (LPC) rates between the Hoya FY60AD, Hoya PY60AD, and Alcon AcrySof SN60WF intraocular lenses (IOLs) after routine cataract surgery.
Methods
In this retrospective comparative study, patients undergoing uncomplicated cataract surgery over a 3-year period were included, and those subsequently undergoing LPC were identified from laser clinic records. LPC rates at 2 years postoperatively were compared between the round-edged Hoya FY60AD, the newer sharp-edged Hoya PY60AD three-piece IOLs, and the one-piece AcrySof SN60WF IOL.
Results
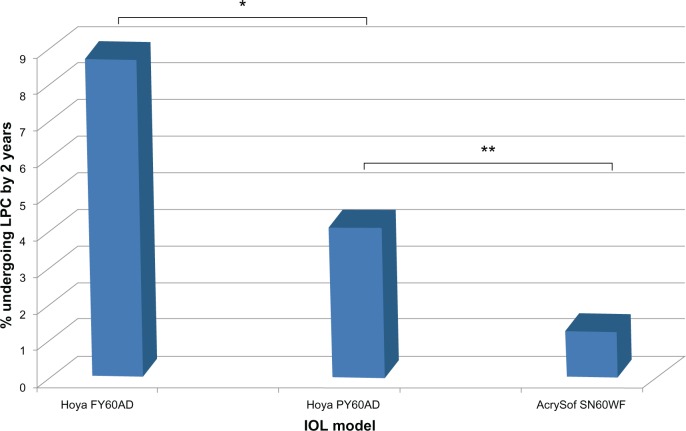
A total of 1,265 cataract operations were included, and 49 eyes (3.9%) underwent LPC within 2 years of surgery. Twenty-eight of 315 eyes (8.9%) implanted with the FY60AD underwent LPC by 2 years, compared to eleven of 254 (4.3%) with the newer sharp square-edged PY60AD and ten of 696 (1.4%) with the one-piece SN60WF (P < 0.05, Chi-squared analyses).
Conclusions
The newer, sharper-edged Hoya PY60AD IOL has a lower LPC rate than the Hoya FY60AD IOL at 2 years post-cataract surgery. The one-piece AcrySof SN60WF has a lower LPC rate than both the three-piece Hoya IOLs in the same time period postoperatively. Variations in IOL-edge design and material effect may have contributed to the different rates observed.
Keywords: intraocular lenses, Nd:YAG laser capsulotomy, posterior-capsule opacification
Introduction
Posterior-capsule opacification (PCO) is a well documented postoperative complication of modern cataract surgery, and although rates have reduced since the days of extracapsular cataract extraction, it is still the most common cause of decreased visual acuity and loss of contrast sensitivity following surgery.1,2 PCO develops as a result of metaplasia and migration of residual lens epithelial cells (LECs) from the equator of the capsular bag, causing development of an opaque membrane across the posterior capsule.1 Definitive treatment in the form of neodymium-doped yttrium aluminum garnet (Nd:YAG) laser posterior capsulotomy (LPC) is a quick and relatively easy outpatient procedure, although there are rare but significant complications associated with this treatment, including cystoid macular edema, retinal detachment, and raised intraocular pressure.3
The purpose of this study was to assess differences in postoperative LPC rates between patients receiving the older three-piece Hoya FY60AD intraocular lens (IOL) with the newer, sharper, square-edged three-piece Hoya PY60AD model following a change in manufacturing process aimed at reducing PCO development and the need for LPC. We also compared them with the commonly used one-piece AcrySof SN60WF IOL (Alcon Laboratories, Fort Worth, TX, USA).
Materials and methods
This retrospective study was registered with the Clinical Governance Department at Wye Valley NHS Trust, Hereford, UK. Patients undergoing cataract surgery and IOL implantation under a single consultant (JMAS) were included if they had undergone surgery over a 3-year period, representing a time of transition between the older Hoya FY60AD IOL and the newer PY60AD IOL, with the AcrySof SN60WF IOL also in routine use. Patients were excluded if the surgery was complicated by posterior-capsule rupture, vitreous loss, or IOL not fixated within the capsular bag, or if they required a combined procedure, such as indirect laser photocoagulation or intraocular injection of corticosteroid for diabetic retinopathy. Surgery was performed or directly supervised by AS, according to a standard approach under sub-Tenon’s anesthesia. Clear corneal incision and side-port paracentesis were made, and viscoelastic (Healon, Abbott Medical Optics, Santa Ana, CA, USA) was used to inflate the anterior chamber. Continuous curvilinear capsulorhexis (CCC) was performed with a cystotome, followed by hydrodissection and phacoemulsification with a “divide-and-conquer” technique. Cortical lens matter was removed with automated coaxial irrigation/aspiration, followed by viscoelastic to inflate the capsular bag prior to insertion of the IOL. The viscoelastic was removed during completion of the surgery.
Characteristics of the three IOL models implanted are summarized in Table 1. The FY60AD and PY60AD have a 360° square-edge profile, although the edge of the latter is sharper than the former.4 The AcrySof SN60WF has a square edge that is interrupted at the optic–haptic junction, and sharpness of the square edge lies between that of the two Hoya models (Table 1).4
Table 1.
| Hoya FY60AD | Hoya PY60AD | AcrySof SN60WF |
|---|---|---|
| IOL design | ||
| Three-piece | Three-piece | One-piece |
| monofocal | monofocal | monofocal |
| Aspheric, | Aspheric, | Aspheric, |
| 6 mm optic | 6 mm optic | 6 mm optic |
| Posterior square-edge structure | ||
| Continuous 360° | Continuous 360° | Interrupted at optichaptic junction |
| Edge sharpness (area-measurement deviation from perfect square) | ||
| 329.7 μm2 | 39.1 μm2 | 100.1 μm2 |
| IOL material | ||
| Hydrophobic acrylic | Hydrophobic acrylic | Hydrophobic acrylic |
| Phenylethyl methacrylate | Phenylethyl methacrylate | Phenylethyl methacrylate |
| N-butylacrylate | N-butylacrylate | Phenylethyl acrylate |
| Fluoroalkyl methacrylate | Fluoroalkyl methacrylate | Butanediol diacrylate |
| Blue blocking | Blue blocking | Blue blocking |
Patients from this surgical cohort who subsequently underwent LPC within 2 years postoperatively were identified from the departmental laser clinic logbook. LPC was performed based on subjective patient complaints or measurable decrease in visual acuity, with concomitant clinical evidence of PCO on dilated examination with slit-lamp biomicroscopy. Details of the laser treatment were recorded for each patient.
The primary comparison was of LPC rates between the older Hoya FY60AD and the newer PY60AD IOLs. A secondary comparison was between the PY60AD and the Alcon SN60WF IOL. LPC rates between groups receiving the different IOLs were compared by Chi-squared analysis. Comparison between groups of other variables, including patient age, corrected distance visual acuity (CDVA), duration between primary surgery and subsequent LPC, and laser energy delivered was undertaken by analysis of variance using SPSS version 19 (IBM, Armonk, NY, USA). Statistical significance was reported at the P < 0.05 level.
Results
A total of 1,265 cataract operations were included. Average age was 72.3 (range 52–88) years at the time of surgery, with mean (± standard deviation) preoperative CDVA of 0.52 ± 0.32 logarithm of minimum angle of resolution (logMAR; approximately 6/19 Snellen) and postoperative CDVA 0.24 ± 0.19 logMAR (approximately 6/10 Snellen). Overall, 49 (3.9%) patients subsequently received LPC within 2 years of cataract surgery, on average 16.4 (range 10–24) months postoperatively. LPC rates for each IOL type are presented in Table 2 and Figure 1.
Table 2.
Neodymium-doped yttrium aluminum garnet (Nd: YAG) laser capsulotomy rates by intraocular lens at 2 years postoperatively
| Hoya FY60AD | Hoya PY60AD | AcrySof SN60WF | |||
|---|---|---|---|---|---|
| Implanted | 315 | 254 | 696 | ||
| Nd: YAG LPC (%) | 28 (8.9) | 11 (4.3) | 10 (1.4) | ||
| P = 0.03* | P = 0.007* |
Note:
Chi-squared test.
Abbreviation: LPC, laser posterior capsulotomy.
Figure 1.
Neodymium-doped yttrium aluminum garnet laser posterior capsulotomy rates by intraocular lens (IOL) at 2 years postoperatively.
Notes: *P < 0.05; **P < 0.01; Chi-squared test.
The average duration between cataract surgery and LPC was 15.8 ± 4.6 (range 8–24) months for patients receiving the FY60AD, 17.2 ± 4.3 (range 10–23) months for the PY60AD, and 17.1 ± 3.1 (range 13–22) months for the SN60WF (Table 3). The trend toward earlier LPC in the FY60AD group did not reach significance (P = 0.56). There were no significant differences between IOL groups for patient age, CDVA at the time of LPC, or amount of laser energy required (Table 3).
Table 3.
Comparison between IOL groups for patient age, duration between primary surgery and LPC, CDVA at presentation for LPC and laser energy delivered. Data presented are mean ± SD for all variables and range where indicated. *analysis of variance (ANOVA) without post-hoc comparison.
| Hoya FY60AD | Hoya PY60AD | AcrySof SN60WF | P* | |
|---|---|---|---|---|
| Patient age (years) | 72.3 ± 9.1 | 70.7 ± 8.5 | 74.2 ± 8.1 | 0.67 |
| Range | 52–88 | 64–85 | 59–88 | |
| Duration to laser after surgery (months) | 15.8 ± 4.6 | 17.2 ± 4.3 | 17.1 ± 3.1 | 0.56 |
| Range | 8–24 | 10–23 | 13–22 | |
| CDVA at LPC (logMAR) | 0.43 ± 0.47 | 0.45 ± 0.24 | 0.31 ± 0.27 | 0.66 |
| Laser energy delivered (mJ) | 68.4 ± 43.8 | 55.2 ± 22.34 | 62.9 ± 43.6 | 0.65 |
Abbreviations: CDVA, corrected distance visual acuity; LPC, laser posterior capsulotomy; logMAR, logarithm of minimum angle of resolution; IOL, intraocular lens; SD, standard deviation.
Discussion
We report that the newer, sharper-edged Hoya PY60AD IOL had a significantly lower LPC rate compared to the older, rounder-edged Hoya FY60AD IOL, a reduction of 52% at 2 years postoperatively. Furthermore, the AcrySof SN60WF had a significantly lower rate than the Hoya PY60AD model. There was a trend toward earlier LPC requirement in the FY60AD group, although this did not reach statistical significance.
Whilst patient variables may influence the development of PCO and subsequent requirement for LPC, such as younger age, female sex, and ocular comorbidity, preventive strategies include variations in surgical technique, such as meticulous cortical cleanup, CCC in contact with the anterior surface of the IOL optic, posterior-capsule polishing, and placement of the IOL in the capsular bag.5,6 Hydrophilic IOLs are associated with higher rates of PCO and greater PCO severity than hydrophobic IOLs, acrylic IOLs are associated with less PCO formation than other biomaterials,7–11 and LPC rates are reportedly lower in eyes implanted with aspheric compared to spherical IOLs.12
The importance of IOL-edge structure is widely accepted, and the evidence in favor of a square posterior optic edge in reducing PCO and LPC requirement is overwhelming. A square edge on the posterior IOL surface provides a barrier to LEC migration by inducing a capsular bend where it is in contact with the IOL edge.13–17 A recent systematic review found significantly lower PCO scores in sharp-edged IOLs compared to round-edged models, although no clear differences between IOL materials.18 However, not all square edges are the same, as edge sharpness is subject to variation between IOL models. Using scanning electron microscopy and computer-aided imaging, it is possible to determine the area of the lateral-posterior IOL edge deviating from a perfect square, demonstrating a large variation both between IOL designs and between different powers of the same design (Figure 2). Area-measurement values for hydrophilic acrylic IOLs as a group are higher than for hydrophobic acrylic or silicone IOLs, indicating a more rounded edge in the former and suggesting that differences reported between IOL materials may actually be related in part to IOL-edge sharpness.4,19 In addition, a prospective, single surgeon, fellow-eye comparison study found higher PCO rates and poorer visual acuity with the Hoya AF-1 YA-60BB IOL compared to the Alcon AcrySof SN60AT, and electron microscopy showed a much sharper posterior-edge profile in the Alcon IOL compared to the Hoya IOL.20 This type of evaluation can help manufacturers optimize their IOL optic edges. For example, Hoya Surgical Optics have recently altered their IOL-polishing process in order to sharpen the posterior edge of their IOLs, reducing the area of deviation from a perfect square from 329.7 μm2 in the older FY60AD model to a sharper 39.1 μm2 in the newer PY60AD and 251 IOLs, the latter now amongst the sharpest square-edge profiles of any commercially available IOL.19 Our study supports the validity of this approach, with fewer patients requiring LPC after implantation with the sharper-edged PY60AD compared to the older FY60AD by 2 years after surgery.
Figure 2.
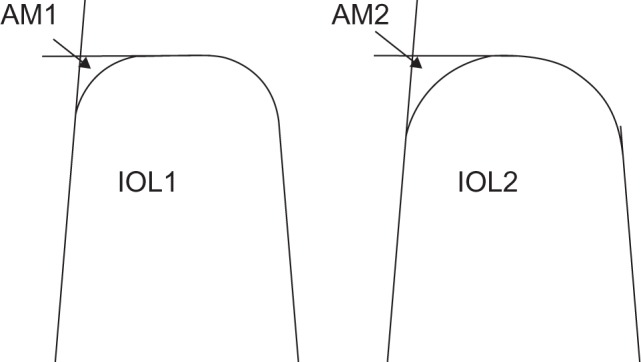
Graphical representation of the assessment of intraocular lens (IOL)-edge sharpness, based on measurement of the area deviating from a perfect square on electron photomicrographs (see text). in these stylized IOLs drawn in section, IOL1 has a smaller area measurement (AM1) than IOL2 (AM2), and therefore has a squarer edge.
The one-piece AcrySof IOL had superior results over the Hoya models, despite the edge of the former being less sharp than the newer Hoya PY60AD IOL. In addition, the posterior square edge of the AcrySof IOL optic is interrupted at the optic–haptic junction. This feature of most single-piece IOLs has been shown to be a factor in the development of PCO, as this point provides an opportunity for uninhibited LEC migration across the posterior capsule.21,22 However, studies have consistently failed to reach a consensus on the relative merits of one-piece versus three-piece IOLs per se in protecting against PCO development. Animal models and cadaveric studies report more PCO in eyes implanted with one-piece IOLs compared to three-piece models, with the optic–haptic junction as the primary site of initiation of PCO development.7,22,23 Some clinical trials support this notion, eg, a retrospective review of 434 eyes implanted with either one-piece or three-piece AcrySof IOLs found a greater incidence of LPC at 24 months in the one-piece group (7.5%) compared to the three-piece group (3.6%).5 The thin haptics of the three-piece IOL reportedly allow for better adhesion between the anterior and posterior capsules and bend formation, compared to the bulky haptics of one-piece models, which enable enhanced posterior LEC migration. However, two systematic reviews showed no significant differences in PCO intensity or laser-treatment rates between one- and three-piece IOLs.24,25 Extending the posterior square edge across the optic–haptic junction to create a 360° sharp edge in one-piece IOLs reduces PCO development and LPC rates compared to single piece IOLs in which the sharp edge is interrupted by the haptic base.26,27 It therefore appears that a sharp posterior edge is a more critical factor in protecting against PCO than whether an IOL is of one-piece or three-piece design.
In addition to beneficial preventive effects on PCO development and LPC requirement, sharp IOL-edge profiles have been associated with increased troublesome glare and photic phenomena. Light rays entering the eye and refracted by the posterior surface of round-edged IOLs are dispersed over a relatively large retinal area, whereas sharper-edged IOLs cause refracted light to be concentrated in a crescent-like distribution on the peripheral retina.28 Glare phenomena induced by the sharp edges of implanted IOLs settle with time in the majority, but a small proportion of patients may experience intractable symptoms and require IOL explantation.29 Texturing the IOL edge is effective in reducing subjective photic symptoms postoperatively,30 although IOL-edge profiles act in combination with other IOL characteristics in glare formation, and modern IOL designs are associated with reduced glare and spherical aberrations overall.31
The favorable results of the single piece AcrySof IOL in our study cannot be fully explained by the edge design. It is possible that whilst a sharp square edge is important for inducing a capsular bend to prevent LEC migration, there may be a threshold degree of edge sharpness beyond which no further mechanical benefit is conferred, and that once an appropriate degree of contact inhibition is induced by a sharp square edge, other factors may come into play to explain variable PCO rates with different IOLs.
IOL material is important in preventing PCO, and acrylic IOLs consistently perform better than other materials.32–34 The advantageous effect of acrylic has been attributed to its bioadhesive properties; tight adherence of the IOL surface to the capsular bag may help minimize the space available for proliferation and migration of LECs.35,36 All three IOLs assessed in this study were hydrophobic acrylic lenses, although the exact formulation of IOL biomaterial does vary between manufacturers (Table 1).37 Subtle variations in biomaterial composition, the blue-light-filtering chromophores, or the IOL-manufacturing process may contribute to the likelihood of LEC migration. There is no clear evidence linking the blue-light-filtering chromophore to PCO development, although a small study in children implanted with either the tinted or nontinted version of an AcrySof IOL reported a slight increase in transient intraocular inflammation in the tinted IOL group, but no differences in long-term inflammatory sequelae or PCO rates.38 Further studies looking specifically at the effect of blue-blocking chromophores on PCO development may help to resolve this issue further.
Two main subtypes of PCO have been described, with “pearl” and “fibrous” forms thought to result from the proliferation of different LEC subpopulations.39 Pearl-type PCO is reportedly associated with worse CDVA and contrast sensitivity than the fibrous type, and LPC is effective at improving both these parameters,40 although different PCO subtypes may require variable degrees of laser energy for effective LPC.41 We cannot report whether different subtypes of PCO were associated with the different IOLs in this study, due to the retrospective nature of the data collection and the focus on LPC rates rather than anatomical details of PCO. Laser-energy requirement for LPC was not significantly different between our IOL groups (Table 3).
This study helps validate the alteration in the polishing process of the Hoya IOLs in order to sharpen the edge of the posterior optic surface as an effective strategy in reducing the requirement for LPC. However, we acknowledge several limitations with the study. As a retrospective analysis, study end points were not defined prior to any intervention. The observation that patient ages were similar in all three groups and that there were no significant differences in CDVA between groups at the time of LPC suggests no considerable bias toward any one of the IOLs. However, the relatively small number of patients in each group undergoing LPC means that statistical tests may be underpowered to detect a significant difference between groups in some parameters, so intergroup differences cannot be ruled out.
Analysis of LPC rates is an indirect measure of PCO, as selection for laser treatment is largely subjective based on patient symptoms, coupled with the identification of PCO signs on slit-lamp examination. Such an indirect marker for PCO is less accurate than formal analysis of the degree of capsular opacity, but it does provide a functional measure of the development of PCO, and the same limitation is applied to all groups under investigation. Other studies comparing IOL performance include those reporting either LPC rates only,5,26,42 qualitative/quantitative measures of PCO,7,20,24,27 or combinations of both.9,16
The relationship between IOL and anterior capsule was not specifically investigated. Surgery aimed for a large rhexis, with CCC just within the optic diameter, although inevitably there would be some variation in this. The majority of patients were operated on by a single consultant (AS), although some patients included in the analysis had their surgery performed by different trainee surgeons, albeit under direct supervision and according to the same approach described. Therefore, surgical technique may also contribute in part to our observed capsulotomy rates. Patients with coexistent ocular pathology may be less likely to be symptomatic from the development of PCO, and therefore may not progress to LPC, despite a degree of PCO that other patients may find troublesome enough to warrant intervention. Finally, our data are limited to 2 years postoperatively, which may be inadequate to validate LPC requirement in the long term, but this duration is commonly used to report IOL performance after cataract surgery.26,27,43
Conclusions
Our results suggest that IOL-edge microstructure may contribute to reducing LPC rates following cataract surgery, and indicate the first demonstration of reduced LPC requirement for Hoya IOLs after a change in manufacturing process. However, variations in IOL-material composition may also play a role in determining susceptibility to PCO development independent of edge sharpness.
Acknowledgments
This work was originally presented at the Congress of the European Society of Cataract and Refractive Surgeons in Milan, Italy (September 8–12, 2012).
Footnotes
Disclosure
The authors declare no conflicts of interest with this submission.
References
- 1.Trivedi RH, Werner L, Apple DJ, Pandey SK, Izak AM. Post cataract-intraocular lens surgery opacification. Eye (Lond) 2002;16:217–241. doi: 10.1038/sj.eye.6700066. [DOI] [PubMed] [Google Scholar]
- 2.Buehl W, Sacu S, Findl O. Association between intensity of posterior capsule opacification and contrast sensitivity. Am J Ophthalmol. 2005;140:927–930. doi: 10.1016/j.ajo.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Steinert RF, Puliafito CA, Kumar SR, Dudak SD, Patel S. Cystoid macular oedema, retinal detachment and glaucoma after Nd:YAG laser posterior capsulotomy. Am J Ophthalmol. 1991;112:373–378. doi: 10.1016/s0002-9394(14)76242-7. [DOI] [PubMed] [Google Scholar]
- 4.Werner L, Müller M, Tetz M. Evaluating and defining the sharpness of intraocular lenses. Microedge structure of commercially available square-edged hydrophobic lenses. J Cataract Refract Surg. 2008;34:310–317. doi: 10.1016/j.jcrs.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Mian SI, Fahim K, Marcovitch A, Gada H, Musch DC, Sugar A. Nd:YAG capsulotomy rates after use of AcrySof acrylic three piece and one piece intraocular lenses. Br J Ophthalmol. 2005;89:1453–1457. doi: 10.1136/bjo.2005.067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng Q, Apple DJ, Visessok N, et al. Surgical prevention of posterior capsule opacification. Part 2: Enhancement of cortical cleanup by focusing on hydrodissection. J Cataract Refract Surg. 2000;26:188–197. doi: 10.1016/s0886-3350(99)00354-5. [DOI] [PubMed] [Google Scholar]
- 7.Heatley CJ, Spalton DJ, Kumar A, Jose R, Boyce J, Bender LE. Comparison of posterior capsule opacification rates between hydrophilic and hydrophobic single-piece acrylic intraocular lenses. J Cataract Refract Surg. 2005;31:718–724. doi: 10.1016/j.jcrs.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 8.Vasavada AR, Raj SM, Shah A, Shah G, Vasavada V, Vasavada V. Comparison of posterior capsule opacification with hydrophobic acrylic and hydrophobic acrylic intraocular lenses. J Cataract Refract Surg. 2011;37:1050–1059. doi: 10.1016/j.jcrs.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 9.Iwase T, Nishi Y, Oveson BC, Jo YJ. Hydrophobic versus double-square-edged hydrophilic foldable acrylic intraocular lens: effect on posterior capsule opacification. J Cataract Refract Surg. 2011;37:1060–1068. doi: 10.1016/j.jcrs.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 10.Ursell PG, Spalton DJ, Pande MV, et al. Relationship between intraocular lens biomaterials and posterior capsule opacification. J Cataract Refract Surg. 1998;24:352–360. doi: 10.1016/s0886-3350(98)80323-4. [DOI] [PubMed] [Google Scholar]
- 11.Hollick EJ, Spalton DJ, Ursell PG, et al. The effect of polymethyl-methacrylate, silicone and polyacrylic intraocular lenses on posterior capsule opacification 3 years after cataract surgery. Ophthalmology. 1999;106:49–54. doi: 10.1016/S0161-6420(99)90047-7. [DOI] [PubMed] [Google Scholar]
- 12.Biber JM, Sandoval HP, Trivedi RH, de Castro LE, French JW, Solomon KD. Comparison of the incidence and visual significance of posterior capsule opacification between multifocal spherical, monofocal spherical, and monofocal aspheric intraocular lenses. J Cataract Refract Surg. 2009;35:1234–1238. doi: 10.1016/j.jcrs.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Nishi O, Nishi K, Wickström K. Preventing lens epithelial cell migration using intraocular lenses with sharp rectangular edges. J Cataract Refract Surg. 2000;26:1543–1549. doi: 10.1016/s0886-3350(00)00426-0. [DOI] [PubMed] [Google Scholar]
- 14.Nishi O, Nishi K, Akura J, Nagata T. Effect of round-edged acrylic intraocular lenses on preventing posterior capsule opacification. J Cataract Refract Surg. 2001;27:608–613. doi: 10.1016/s0886-3350(00)00644-1. [DOI] [PubMed] [Google Scholar]
- 15.Peng Q, Visessook N, Apple DJ, et al. Surgical prevention of posterior capsule opacification. Part 3: Intraocular lens optic barrier effect as a second line of defense. J Cataract Refract Surg. 2000;26:198–213. doi: 10.1016/s0886-3350(99)00352-1. [DOI] [PubMed] [Google Scholar]
- 16.Kohnen T, Fabian E, Gerl R, et al. Optic edge design as long-term factor for posterior capsular opacification rates. Ophthalmology. 2008;115:1308–1314. doi: 10.1016/j.ophtha.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Buehl W, Findl O. Effect of intraocular lens design on posterior capsule opacification. J Cataract Refract Surg. 2008;24:1976–1985. doi: 10.1016/j.jcrs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database Syst Rev. 2010;17:CD003738. doi: 10.1002/14651858.CD003738.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Werner L, Tetz M. Edge profiles of currently available intraocular lenses and recent improvements. Eur Ophthalmic Rev. 2009;3:74–76. [Google Scholar]
- 20.Hancox J, Spalton DJ, Cleary G, et al. Fellow-eye comparison of posterior capsule opacification with AcrySof SN60 AT and AF-1 YA-60BB blue-blocking intraocular lenses. J Cataract Refract Surg. 2008;34:1489–1494. doi: 10.1016/j.jcrs.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Nixon DR, Apple DJ. Evaluation of lens epithelial cell migration in vivo at the haptic-optic junction of a one-piece hydrophobic acrylic intraocular lens. Am J Ophthalmol. 2006;142:557–562. doi: 10.1016/j.ajo.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 22.Ness PJ, Werner L, Maddula S, et al. Pathology of 219 human cadaver eyes with 1-piece or 3-piece hydrophobic acrylic intraocular lenses: capsular bag opacification and sites of square-edge barrier breach. J Cataract Refract Surg. 2011;37:923–930. doi: 10.1016/j.jcrs.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 23.Nishi O, Nishi K, Osakabe Y. Evaluation of posterior capsule opacification using a new posterior view method in rabbits: single-piece acrylic versus 3-piece acrylic intraocular lens. J Cataract Refract Surg. 2005;31:2369–2374. doi: 10.1016/j.jcrs.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Leydolt C, Davidovic S, Sacu S, et al. Long-term effect of 1-piece and 3-piece hydrophobic acrylic intraocular lens on posterior capsule opacification: a randomised trial. Ophthalmology. 2007;114:1663–1669. doi: 10.1016/j.ophtha.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Sacu S, Findl O, Menapace R, Buehl W, Wirtitsch M. Comparison of posterior capsule opacification between the 1-piece and 3-piece Acrysof intraocular lenses: two-year results of a randomised trial. Ophthalmology. 2004;111:1840–1846. doi: 10.1016/j.ophtha.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Mathew RG, Coombes AG. Reduction of Nd:YAG capsulotomy rates after implantation of a single-piece acrylic hydrophobic intraocular lens with 360° squared edge: 24 month results. Ophthalmic Surg Lasers Imaging. 2010;41:651–655. doi: 10.3928/15428877-20100929-04. [DOI] [PubMed] [Google Scholar]
- 27.Nixon DR, Woodcock MG. Pattern of posterior capsule opacification models 2 years postoperatively with 2 single-piece acrylic intraocular lenses. J Cataract Refract Surg. 2010;26:929–934. doi: 10.1016/j.jcrs.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 28.Holladay JT, Lang A, Portney V. Analysis of edge glare phenomena in intraocular lens edge designs. J Cataract Refract Surg. 1999;25:748–752. doi: 10.1016/s0886-3350(99)00038-3. [DOI] [PubMed] [Google Scholar]
- 29.Ellis MF. Sharp-edged intraocular lens design as a cause of permanent glare. J Cataract Refract Surg. 2001;27:1061–1064. doi: 10.1016/s0886-3350(00)00856-7. [DOI] [PubMed] [Google Scholar]
- 30.Meacock WR, Spalton DJ, Khan S. The effect of texturing the intraocular lens edge on postoperative glare symptoms. Arch Ophthalmol. 2002;120:1294–1298. doi: 10.1001/archopht.120.10.1294. [DOI] [PubMed] [Google Scholar]
- 31.Casprini F, Balestrazzi A, Tosi GM, et al. Glare disability and spherical aberration with five foldable intraocular lenses: a prospective randomized study. Acta Ophthalmol Scand. 2005;83:20–25. doi: 10.1111/j.1600-0420.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- 32.Nishi O, Nishi K, Okasabe Y. Effect of intraocular lenses on preventing posterior capsule opacification: design versus material. J Cataract Refract Surg. 2004;30:2170–2176. doi: 10.1016/j.jcrs.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Abhilakh Missier KA, Nuijts RM, Tjia KF. Posterior capsule opacification: silicone plate-haptic versus AcrySof intraocular lenses. J Cataract Refract Surg. 2003;29:1569–1574. doi: 10.1016/s0886-3350(02)02046-1. [DOI] [PubMed] [Google Scholar]
- 34.Apple DJ, Peng Q, Visessock N, et al. Eradication of posterior capsule opacification. Documentation of a marked decrease in Nd:YAG laser posterior capsulotomy rates noted in an analysis of 5416 pseudophakic eyes obtained post-mortem. Ophthalmology. 2001;108:505–518. doi: 10.1016/s0161-6420(00)00589-3. [DOI] [PubMed] [Google Scholar]
- 35.Linnola RJ, Sund M, Ylonen R, Pihlajaniemi T. Adhesion of soluble fibronectin, laminin, and collagen type IV to intraocular lens materials. J Cataract Refract Surg. 1999;25:1486–1491. doi: 10.1016/s0886-3350(99)00238-2. [DOI] [PubMed] [Google Scholar]
- 36.Lane SS, Burgi P, Milios GS, Orchowski MW, Vaughan M, Schwarte E. Comparison of the biomechanical behaviour of foldable intraocular lenses. J Cataract Refract Surg. 2004;30:2397–2402. doi: 10.1016/j.jcrs.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 37.McIntyre S, Werner L, Mamalis N. Hydrophobic acrylic IOLs: a primer. Cataract Refract Surg Today Eur. 2011:39–44. [Google Scholar]
- 38.Beauchamp CL, Stager DR, Jr, Weakley DR, Jr, Wang X, Felius J. Surgical findings with the tinted AcrySof intraocular lens in children. J AAPOS. 2007;11:166–169. doi: 10.1016/j.jaapos.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Pandey SK, Apple DJ, Werner L, et al. Posterior capsule opacification: a review of the aetiopathogenesis, experimental and clinical studies and factors for prevention. Indian J Ophthalmol. 2004;52:99–112. [PubMed] [Google Scholar]
- 40.Cheng CY, Yen MY, Chen SJ, et al. Visual acuity and contrast sensitivity in different types of posterior capsule opacification. J Cataract Refract Surg. 2001;27:1055–1060. doi: 10.1016/s0886-3350(00)00867-1. [DOI] [PubMed] [Google Scholar]
- 41.Bhargava R, Kumar P, Prakash A, Chaudhary KP. Estimation of mean Nd:YAG laser capsulotomy energy levels for membranous and fibrous posterior capsule opacification. Nepal J Ophthalmol. 2012;4:108–113. doi: 10.3126/nepjoph.v4i1.5861. [DOI] [PubMed] [Google Scholar]
- 42.Shah VC, Russo C, Cannon R, et al. Incidence of Nd:YAG capsulotomy after implantation of AcrySof multifocal and monofocal intraocular lenses: a case controlled study. J Refract Surg. 2010;26:565–568. doi: 10.3928/1081597X-20100303-01. [DOI] [PubMed] [Google Scholar]
- 43.Kugelberg M, Wejde G, Jayaram H, Zetterström C. Two-year follow-up of posterior capsule opacification after implantation of a hydrophilic or hydrophobic acrylic intraocular lens. Acta Ophthalmol. 2008;86:533–536. doi: 10.1111/j.1600-0420.2007.01094.x. [DOI] [PubMed] [Google Scholar]