Abstract
Aims
In HIV-infected individuals, heavy drinking compromises survival. In HIV primary care, the efficacy of brief motivational interviewing (MI) to reduce drinking is unknown, alcohol-dependent patients may need greater intervention and resources are limited. Using interactive voice response (IVR) technology, HealthCall was designed to enhance MI via daily patient self-monitoring calls to an automated telephone system with personalized feedback. We tested the efficacy of MI-only and MI+HealthCall for drinking reduction among HIV primary care patients.
Design
Parallel random assignment to control (n = 88), MI-only (n = 82) or MI+HealthCall (n = 88). Counselors provided advice/education (control) or MI (MI-only or MI+HealthCall) at baseline. At 30 and 60 days (end-of-treatment), counselors briefly discussed drinking with patients, using HealthCall graphs with MI+HealthCall patients.
Setting
Large urban HIV primary care clinic.
Participants
Patients consuming ≥4 drinks at least once in prior 30 days.
Measurements
Using time-line follow-back, primary outcome was number of drinks per drinking day, last 30 days.
Findings
End-of-treatment number of drinks per drinking day (NumDD) means were 4.75, 3.94 and 3.58 in control, MI-only and MI+HealthCall, respectively (overall model χ2, d.f. = 9.11,2, P = 0.01). For contrasts of NumDD, P = 0.01 for MI+HealthCall versus control; P = 0.07 for MI-only versus control; and P = 0.24 for MI+HealthCall versus MI-only. Secondary analysis indicated no intervention effects on NumDD among non-alcohol-dependent patients. However, for contrasts of NumDD among alcohol-dependent patients, P < 0.01 for MI+HealthCall versus control; P = 0.09 for MI-only versus control; and P = 0.03 for MI+HealthCall versus MI-only. By 12-month follow-up, although NumDD remained lower among alcohol-dependent patients in MI+HealthCall than others, effects were no longer significant.
Conclusions
For alcohol-dependent HIV patients, enhancing MI with HealthCall may offer additional benefit, without extensive additional staff involvement.
Keywords: Alcohol dependence, brief intervention, drinking, HIV, interactive voice response, IVR, motivational interviewing, primary care, randomized trial, technology
INTRODUCTION
Among HIV-infected individuals, liver disease is now a common cause of mortality [1,2]. Heavy drinking also predicts mortality in HIV patients, with or without hepatitis [3]. Despite this, in HIV primary care clinics, where staff time and resources are often limited, drinking-reduction interventions are rare [4-6] and referrals to alcoholism treatment seldom followed [7,8]. Only one trial has focused primarily on drinking reduction in HIV primary care: an intervention in Kenya consisting of six 90-minute group sessions [9]. For dissemination possibilities, interventions should be briefer. Showing that brief motivational interviewing (MI) [10] could reduce heavy drinking among HIV patients would have important implications, but no such study has been conducted in HIV primary care. The study reported below was designed to address this issue.
We were initially concerned that a single brief intervention might be insufficient to reduce heavy drinking in this multi-problem clinical population [11]. After we began the study, reviews [8,12] identified alcohol dependence as an additional issue. In general primary care, brief MI reduces drinking in patients without alcohol dependence [8,10,12-15]. However, trials combining patients with and without alcohol dependence did not show efficacy [8,14], suggesting that brief intervention among alcohol-dependent primary care patients is insufficient. Unfortunately, in HIV primary care, where drinking reduction is critical to survival, extended staff-delivered interventions are not feasible. The problem is how to extend intervention in a feasible manner.
Technology offers innovative ways to extend health interventions [16,17]. Automated telephone interactive voice response (IVR) can provide user-friendly questions for self-monitoring, with patients’ answers stored in a database. IVR is used increasingly to improve medical management and health behaviors [18-22]. We designed HealthCall to utilize IVR as an extension of brief MI for drinking reduction. After an MI session, HealthCall consists of 60 days of patient IVR calls (1–3 minutes) to self-monitor alcohol- and other health-related behaviors. Call data are summarized in personalized feedback graphs presented to patients by their MI counselors at 30 and 60 days to facilitate brief (~10 minutes) discussions of patients’ drinking. Preliminary work [11] showed that MI+HealthCall was acceptable to heavy-drinking urban HIV primary care patients, who preferred 60-day interventions over longer or shorter periods, and who demonstrated drinking reduction at 60 days.
We now report a randomized trial comparing MI-only and MI+HealthCall to an advice/education control condition among heavily drinking HIV primary care patients. Given the lack of information about brief MI in HIV primary care, as well as our concern that these patients might need longer intervention, our original aim was to investigate whether MI-only and MI+HealthCall were superior to advice/education in reducing drinking. Given concerns about brief intervention for alcohol-dependent patients arising after our trial began [8,12], an important secondary aim was to test whether results differed between patients with and without alcohol dependence.
METHODS
Procedures
In a parallel three-arm individually randomized design (1 : 1 : 1 allocation ratio), 258 participants were assigned to advice/education control, MI-only or MI+HealthCall between August 2007 and May 2010, with groups balanced on depression, drug abuse, unstable housing and hepatitis using urn randomization [23]. Counselors did not know the computer-generated random number sequence; counselors and patients were not blinded to treatments after assignment. Clinic staff referred patients to bilingual (English/Spanish) counselors for eligibility assessment, informed consent, assessment, treatment group assignment (viewed by counselors on-screen, post-assessment) and intervention. Procedures were in English or Spanish (patients’ preference). Patient compensations for assessments were gift certificates ($20; $40 at last two post-treatment follow-ups).
Setting and participants
The study was conducted at a large urban HIV primary care clinic. Institutional review boards at Columbia University, St Vincent’s Hospital, and Mt Sinai Medical Center approved all procedures. For generalizability, we minimized inclusion/exclusion criteria. Inclusion criteria were: ≥4 US drinks of alcohol at least once, in the prior 30 days; HIV-positive; English- or Spanish-speaking; aged ≥18 years; and treated at the clinic. Exclusion criteria were: active psychosis; suicidality; or gross cognitive impairment (Halstead–Reitan Trails A) [24].
Interventions
The 60-day intervention period began after randomization (baseline). Two counselors (supervised in weekly meetings by E.A.) administered interventions; one had a MA in health education and the other a BA in psychology. Both had experience as HIV health educators but not alcoholism counselors.
Advice/education (control) arm
At baseline, counselors informed patients that they drank more than medically advisable and showed them a 30-minute HIV self-care DVD without alcohol content. Counselors then provided a National Institute on Alcohol Abuse and Alcoholism (NIAAA) pamphlet about drinking reduction techniques [25] and a digital alarm watch, suggesting it as a medication reminder. At 30 and 60 days, patients returned for assessment and brief (~5 minutes) counselor meetings, where drinking reduction was encouraged.
MI-only arm
At baseline, counselors administered a 20–25-minute individual MI using standard techniques to motivate reduced drinking [10], encouraging patients to set a drinking-reduction goal. Counselors then provided the pamphlet and watch. At 30 and 60 days, counselor and patient met for 10–15 minutes, discussed the patient’s drinking during the past month, evaluated the drinking goal and set a new goal if patients wished.
MI+HealthCall arm
Counselors conducted baseline activities described for MI, then showed patients how to use HealthCall, asking them to call daily for the next 30 days. Patient and counselor practised using HealthCall to ensure correct use, identified an accessible telephone and best time for calls, and set the watch alarm to this time as a reminder. Patients subsequently accessed HealthCall via a toll-free number for daily 1–3-minute calls, answering pre-recorded questions about ‘yesterday’ (morning, afternoon, evening) to ensure consistent reporting periods regardless of the hour called. Brief self-monitoring questions covered alcohol consumption (e.g. ‘How many beers did you drink yesterday?’) and reasons for drinking or not drinking. Additional questions covered mood, medication adherence and wellbeing.
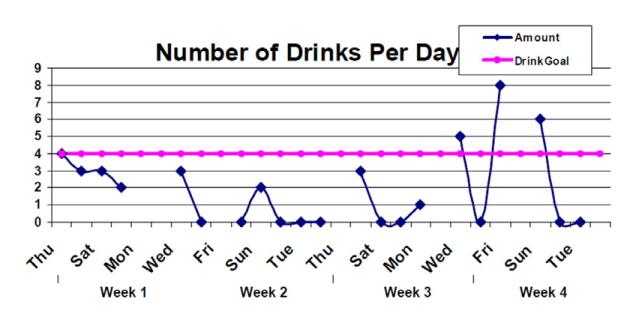
If patients skipped two consecutive calls, counselors briefly reminded patients to continue calling. IVR data were compiled and used to produce personalized feedback, including a graph showing individual drinking goals and number of drinks reported daily to the IVR (Fig. 1) and summary statistics (average drinks per drinking day; reasons for drinking). After 30 days, counselors met individually with patients, provided their graph, and used it as the basis for a 10–15-minute discussion of patients’ drinking, evaluating the drinking goal and re-setting it if patients wished. Counselors then asked patients to call for 30 more days. At 60 days, counselors met again with patients to review a similar graph for the 31–60-day period.
Figure 1.
Sample personalized patient feedback graph given to patients in motivational interviewing (MI)+HealthCall arm at 30 and 60 days, based on the drinking they reported in calls to HealthCall
MI training and fidelity
An MI Network Trainer trained the counselors and made standardized fidelity ratings [26] of 64.7% of the 170 MI sessions. These showed acceptable to excellent fidelity on seven of eight domains, e.g. mean % complex reflections (50.4%), reflection/question ratio (1.1), MI spirit/empathy (4.7; 4.6) and number of MI non-adherent statements (0.6).
Assessments
Self-administered computerized assessments were conducted prior to randomization, at 30 and 60 days (during treatment), and during post-treatment follow-up (3, 6 and 12 months). As explained to patients, counselors did not receive self-assessment results. Baseline assessment of DSM-IV alcohol dependence used the NIAAA Alcohol Use Disorders and Associated Disabilities Interview Schedule (AUDADIS-IV) [27-29]. Alcohol consumption was assessed at all time-points via 30-day time-line follow-back (TLFB) [30]. If patients missed an assessment but returned for subsequent assessments, a retrospective TLFB was administered covering the missed time-periods.
Outcomes
The primary study outcome, mean number of drinks per drinking day during the prior 30 days (NumDD), was created with TLFB data for all time-points, including the primary focus of analysis, end-of-treatment (60 days). NumDD was selected as the primary outcome due to the potential for liver toxicity and damage from large alcohol quantities. The on-time and retrospective TLFB data for 60 days did not differ in the total sample, in any treatment arm, or in alcohol-dependent and non-dependent subgroups (all P > 0.24; Table S1), and patients with on-time and retrospective data did not differ on NumDD at baseline [mean NumDD 6.99 (SD 3.74) and 6.96 (SD 4.22) respectively; t-test P = 0.96]. Therefore, on-time and retrospective data were pooled for analysis. A secondary TLFB outcome was percentage of days abstinent (PDA) over the prior 30 days. Breathalyzer assessments showed no inconsistencies with TLFBs.
Statistical analysis
Pre-study power computations [31] indicated that n = 90 per group would provide 80% power at α = 0.05 to detect a moderate treatment effect on NumDD (d = 0.4 [31]), guided by pilot results [11]. We stopped enrollment before reaching our target due to hospital ownership transition. All tests were two-tailed; P < 0.05 indicated significance, with trends towards significance at P = 0.05–0.09. Patients were all analyzed in their originally assigned groups.
Our primary analysis focused on NumDD at end-of-treatment (60 days). A generalized linear model with a negative binomial distribution (PROC GENMOD, SAS) was used to test the main effect of treatment on NumDD at this primary end-point among patients with 60-day data. Parameter estimates and associated P-values from this model were used to indicate paired contrasts between conditions.
We conducted two secondary analyses of outcome at 60 days using generalized linear models with a negative binomial distribution. We tested an overall interaction effect of treatment × alcohol dependence on NumDD. Given the significant interaction effect, contrasts for this model are presented separately for alcohol-dependent and non-dependent patients. We also conducted a main effects analysis for our secondary outcome, PDA.
We conducted three sensitivity analyses to understand the robustness of our NumDD findings. Two involved multiple imputation (MICE procedure, R software [32,33]) to evaluate robustness of results to missing observations. Multiple imputation avoids the bias that has been demonstrated for single-imputation methods such as last-value-carried-forward [34-40]. Imputation variables included baseline NumDD and all other variables in Table 1 except PDA. The third sensitivity analysis involved an alternative analytical method, a generalized linear mixed effects model of NumDD up to end-of-treatment (known to be robust to effects of missing data), incorporating time, treatment, and alcohol dependence, with a log-link function and random intercept representing pre-treatment between-individual heterogeneity, thereby accounting for within-individual correlation (PROC GLIMMIX, SAS).
Table 1.
Baseline characteristics of study participantsa.
| DVD Control (N=88) |
MI Only (N=82) |
MI+HealthCall (N=84) |
Full Sample (N=254) |
||
|---|---|---|---|---|---|
| No. (%) | P-value | ||||
| Female | 17 (19.3) | 20 (24.4) | 19 (22.6) | 56 (22.1) | 0.72 |
| Ethnicity | 0.85 | ||||
| African American | 40 (45.5) | 43 (52.4) | 42 (50.0) | 125 (49.2) | b |
| Hispanic | 44 (50.0) | 34 (41.5) | 37 (44,1) | 115 (45.3) | b |
| Other | 04 (04.6) | 05 (06.1) | 05 (06.0) | 15 (05.9) | b |
| Spanish-speaking | 23 (26.1) | 15 (18.3) | 15 (17.9) | 53 (20.9) | 0.32 |
| High school education | 55 (62.5) | 49 (59.8) | 44 (52.4) | 148 (58.3) | 0.38 |
| Married / stable relationship | 10 (11.4) | 12 (14.6) | 11 (13.1) | 33 (13.0) | 0.82 |
| Employed | 13 (14.8) | 7 (8.5) | 12 (14.3) | 32 (12.6) | 0.40 |
| Current DSM-IV alcohol dependence | 41 (46.6) | 39 (47.6) | 43 (51.2) | 123 (48.4) | 0.82 |
| Mean (SD) | |||||
| Age, years | 44.5 (08.3) | 46.5 (07.9) | 46.3 (08.0) | 45.7 (08.1) | 0.19 |
| Years since HIV diagnosis | 13.0 (07.5) | 12.2 (07.5) | 13.1 (07.7) | 12.8 (07.5) | 0.68 |
| Drinks per drinking day (NumDD)c | 06.8 (03.6) | 06.6 (03.9) | 07.5 (04.0) | 07.0 (03.8) | 0.25 |
| Percent days abstinent (PDA)c | 68.1 (25.1) | 69.8 (23.8) | 66.5 (24.1) | 68.1 (24.3) | 0.69 |
MI = motivational interviewing; SD = standard deviation.
Study of HealthCall enhancement of Motivational Interviewing for drinking reduction: New York City HIV primary care patients with at-risk drinking (≥4 drinks on at least one occasion in past 30 days) at baseline; patients enrolled August 2007-May 2010
P-values not given for each ethnic group because ethnicity was tested as a three-level variable
During prior 30 days at baseline
We computed effect sizes and 95% confidence intervals of contrasts involving NumDD in two ways. Cohen’s d provides ease of interpretation. Conventionally, d = 0.2, 0.5 and 0.8 indicate small, medium and large effects [31]. Incidence risk ratios (IRR, the ratio of rates in two treatments [41]) take into account the non-normal NumDD distribution.
We present information descriptively on post-treatment follow-up assessments.
RESULTS
Participants
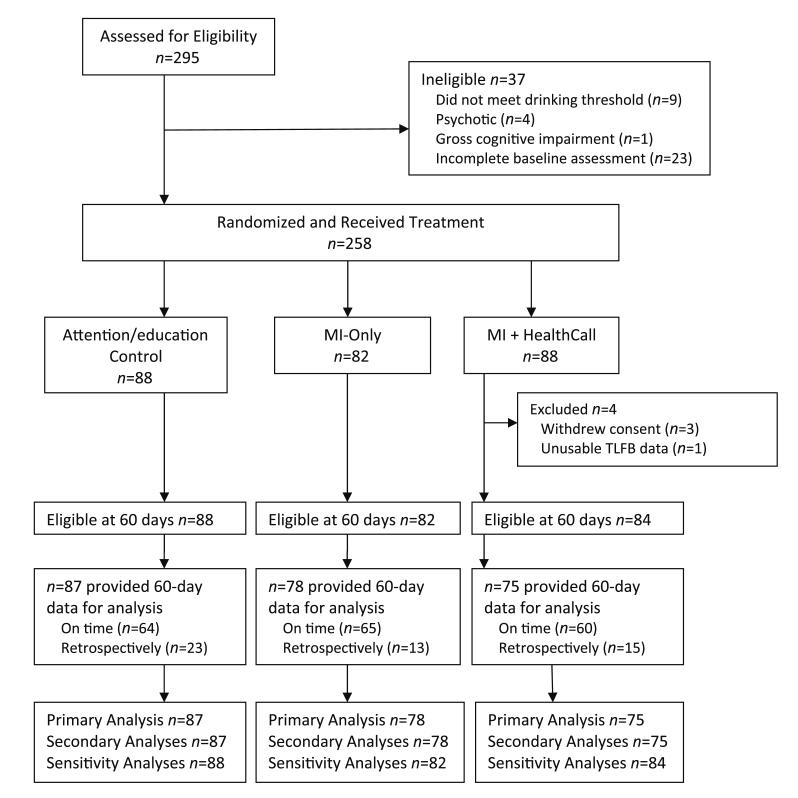
Of 295 patients referred to the study (Fig. 2), 258 met inclusion criteria and were randomized: MI+HealthCall (n = 88), MI-only (n = 82) and advice/education (n = 88). Three patients withdrew consent and one had unusable TLFB data, all from MI+HealthCall. Of the remaining 254 patients, 240 (94.5%) provided TLFB end-of-treatment data, 189 at 60 days and 51 retrospectively during subsequent evaluations [Table S3 shows number of patients (Ns), by subgroup, across all time-points]. Availability of 60-day data did not differ by any patient characteristic shown in Table 1, including baseline drinking levels, but did differ by treatment arm [control: 98.9% (one missing); MI-only: 95.1% (four missing); MI+HealthCall: 89.3% (nine missing); χ2 = 7.7, d.f. = 2, P = 0.02].
Figure 2.
Flow of study participants. Study of HealthCall enhancement of motivational interviewing for drinking reduction: New York City HIV primary care patients with at-risk drinking (≥4 drinks on at least one occasion in past 30 days) at baseline; patients enrolled August 2007–May 2010
Table 1 presents baseline sample characteristics. Patients drank a mean of 7.0 drinks per drinking day (6.8, 6.6 and 7.5 in controls, MI-only and MI+HealthCall), and drank on average 31.8% of the 30 days prior to baseline (31.9%, 30.2% and 33.5% in controls, MI-only and MI+HealthCall). Treatment groups did not differ on these or other (e.g. demographic) variables. Approximately half had current DSM-IV alcohol dependence.
Exposure to HealthCall in the MI+HealthCall arm
Among patients in MI+HealthCall, the median IVR call rate was 64.4%. This was 63.3% and 68.3% in patients without and with alcohol dependence, respectively (Wilcoxon test P = 0.90) after excluding incarcerated or hospitalized days (3.7% and 4.3% of the days in non-dependent and dependent patients; P = 0.25).
Primary analysis: treatment effect on NumDD
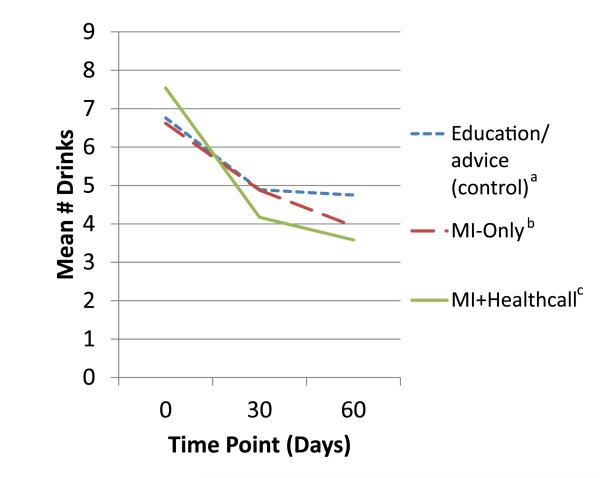
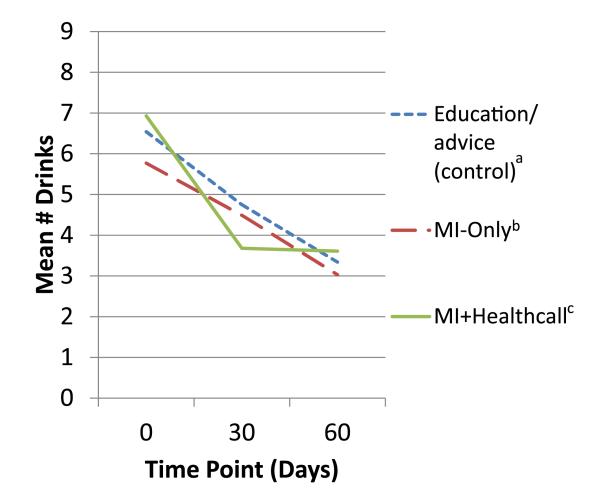
In the full sample, the generalized linear model showed a significant effect of treatment on 60-day NumDD (Table 2). Means (Fig. 3) and SDs at 60 days were lower than at baseline: 4.75 (3.22) in controls; 3.94 (2.90) in MI-only, 3.58 (1.81) for MI+HealthCall. Group contrasts showed a trend-level difference between MI-only versus control, a significant difference between MI+HealthCall and control, and no difference between MI-only and MI+HealthCall. Table 3 shows effect sizes and 95% confidence intervals. NumDD was 1.38 times greater in control than MI+HealthCall, a moderate effect (d = 0.44); other contrasts were small and non-significant.
Table 2.
Treatment effects on drinking at end-of-treatment (60 days): primary, secondary and sensitivity analyses.
| Contrasts at 60 days |
||||||||
|---|---|---|---|---|---|---|---|---|
| Model | Overall Model Significance | Control vs MI | Control vs MI+HealthCall | MI vs MI+HealthCall | ||||
| Primary Analysis | X2, df | P-value | X2 , df | P-value | X2 , df | P-value | X2 , df | P-value |
| Treatment effect on NumDDa | 9.11, 2 | 0.01 | 3.24, 1 | 0.07 | 9.00, 1 | <0.01 | 1.37, 1 | 0.24 |
| Secondary Analyses | F test, Numc df, Dend df | P-value | T test, df | P-value | T test, df | P-value | T test, df | P-value |
| Interaction of treatment × alcohol dependence on NumDDa |
3.63, 2, 165 | 0.03 | ||||||
| Among alcohol dependent patients | - | 1.70, 165 | 0.09 | 4.14, 165 | <0.01 | 2.22, 165 | 0.03 | |
| Among non-alcohol dependent patients | - | 0.38, 165 | 0.71 | −0.05, 165 | 0.96 | −0.41, 165 | 0.68 | |
| X2 , df | P-value | X2 , df | P-value | X2 , df | P-value | |||
| Treatment effect on PDAb | F=0.66, df=2 | 0.72 | 0.63, 1 | 0.43 | 0.05, 1 | 0.83 | 0.31, 1 | 0.58 |
| Sensitivity Analyses |
Test Statistics, dfs
(Range for imputed models) |
P-values
(Range for imputed models) |
X2 , df | P-value | X2 , df | P-value | X2 , df | P-value |
| Treatment effect on NumDDa; multiple imputation |
X2=6.21−10.8, df=2 | 0.04 − <0.01e | 2.73,1 | 0.10 | 8.28,1 | <0.01 | 1.37,1 | 0.24 |
| Interaction of treatment × alcohol dependence on NumDDa; multiple imputation |
F=2.86−4.83 Numc df=2, Dend df=l72−178 |
0.06−<0.01f | T test, df | P-value | T test, df | P-value | T test, df | P-value |
| Among alcohol dependent patients | - | 1.61, 119 | 0.11 | 3.88,134 | <0.01 | 1.99,110 | 0.05 | |
| Among non-alcohol dependent patients | - | 0.56, 159 | 0.58 | 0.35,156 | 0.73 | 0.88, 158 | 0.38 | |
| Treatment × alcohol dependence × time |
F=3.45 Numc df=2, Dend df=136 |
0.03 | ||||||
| Among Alcohol Dependent | - | 1.45, 136 | 0.15 | 3.70, 136 | <0.01 | 2.04, 136 | 0.04 | |
| Among Non-Alcohol Dependent | - | 0.22, 136 | 0.83 | −0.01, 136 | 0.99 | −0.22, 136 | 0.82 | |
NumDD = mean number of drinks per drinking day, prior 30 days
PDA = percent days abstinent, prior 30 days
Num = Numerator
Den = Denominator
10 out of 10 P-values from imputed datasets were P<0.05
8 out of 10 P-values from imputed datasets were P<0.05
Figure 3.
Mean drinks per drinking day to end of treatment (60 days), by treatment group, among whole sample. aEducation/advice (control) n: 0 days = 88; 30 days = 87; 60 days = 87. bMotivational interviewing (MI)-only n: 0 days = 82; 30 days = 78; 60 days = 78. cMI+HealthCall n: 0 days = 84; 30 days = 78; 60 days = 75
Table 3.
Effect sizes of treatment group contrasts at the end of treatment (60 days).
| Control versus MI | Control versus MI+HealthCall |
MI versus MI+HealthCall |
|
|---|---|---|---|
| Incidence risk ratio | Effect size (95% confidence interval) | ||
| Whole sample | 1.21 (0.98−1.48) | 1.38 (1.12−1.70) | 1.14 (0.91−1.42) |
| Alcohol-dependent | 1.25 (0.97−1.60) | 1.71 (1.33−2.21) | 1.37 (1.04−1.82) |
| Non-alcohol-dependent | 1.06 (0.78−1.43) | 0.99 (0.73−1.35) | 0.94 (0.68−1.28) |
| Cohen’s d | |||
| Whole sample | 0.26 (0.00−0.63) | 0.44 (0.07−0.81) | 0.15 (0.00−0.53) |
| Alcohol-dependent | 0.26 (0.00−0.79) | 0.90 (0.38−1.42) | 0.56 (0.02−1.10) |
| Non-alcohol-dependent | 0.17 (0.00−0.68) | 0.14 (0.00−0.67) | 0.33 (0.00−0.87) |
MI = motivational interviewing.
Secondary analysis: interaction effect of treatment × alcohol dependence on NumDD
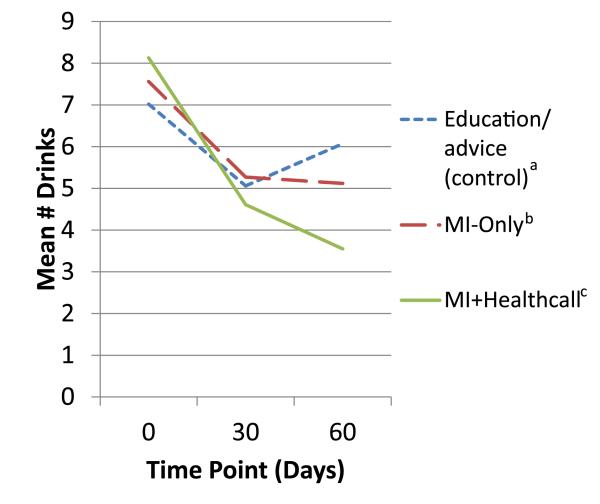
In the full sample, the generalized linear model showed a significant interaction of treatment and alcohol dependence on 60-day NumDD (Table 2). Among patients with alcohol dependence, means (Fig. 4) and SD at 60 days were 6.07 (3.58) in controls; 5.12 (3.81) in MI-only, 3.55 (1.60) for MI+HealthCall. Contrasts showed a trend-level difference between MI-only and control, while MI+HealthCall was significantly different from control and from MI-only. Effect sizes among alcohol-dependent patients were small for MI-only versus control, medium for MI+HealthCall versus MI-only and large for MI+HealthCall versus control. Among patients without alcohol dependence, means (Fig. 5) and SD at 60 days were 3.34 (2.01) in controls; 3.03 (1.43) in MI-only and 3.61 (2.09) for MI+HealthCall. No contrast was significant; effects were small (Table 3).
Figure 4.
Mean drinks per drinking day to end of treatment (60 days), by treatment group, alcohol-dependent patients. aEducation/advice (control) n: 0 days = 41; 30 days = 41; 60 days = 41. bMotivational interviewing (MI)-only n: 0 days = 39; 30 days = 37; 60 days = 36. cMI+HealthCall n: 0 days = 43; 30 days = 39; 60 days = 38
Figure 5.
Mean drinks per drinking day to end of treatment (60 days), by treatment group, non-alcohol-dependent patients. aEducation/advice (control) n: 0 days = 47; 30 days = 46; 60 days = 46. bMotivational interviewing (MI)-only n: 0 days = 43; 30 days = 41; 60 days = 42. cMI+HealthCall n: 0 days = 41; 30 days = 39; 60 days = 37
Secondary analysis: treatment effect on PDA
Mean PDA for the full sample was 80.5% at 60 days [means (SD): controls 78.9% (22.5); MI-only 82.55 (25.3); and MI+HealthCall 80.3 (23.7)]. PDA did not differ by treatment (Table 2).
Sensitivity analysis 1: treatment effect on NumDD using multiple imputation
In the full sample, the generalized linear model showed significant treatment effects on 60-day NumDD across all imputation data sets (Table 2). The test statistic from the data set with observed values fit well within the range of test statistics from the imputed models. Contrasts showed no difference between MI-only and control or MI-only and MI+HealthCall, but a significant difference between MI+HealthCall and control.
Sensitivity analysis 2: interaction effect of treatment and alcohol dependence on NumDD using multiple imputation
In the full sample, the generalized linear model for the interaction of treatment by alcohol dependence on 60-day NumDD showed P-values of <0.05 for eight of the 10 imputed data sets, with remaining P-values ≤ 0.06 (Table 2). The test statistic from the data set with observed values fit well within the range of test statistics from the imputed data sets. Among alcohol-dependent patients, contrasts showed no difference between MI-only and control, with P < 0.01 for MI+HealthCall versus control and P = 0.05 for MI+HealthCall versus MI-only. Among patients without alcohol dependence, no contrast was significant.
Sensitivity analysis 3: time × treatment × alcohol dependence
The generalized linear mixed effects model showed a significant three-way interaction of time × treatment × alcohol dependence (Table 2). Contrasts indicated that MI-only did not differ from control, but MI+HealthCall differed significantly from control and from MI-only.
Post-treatment follow-up
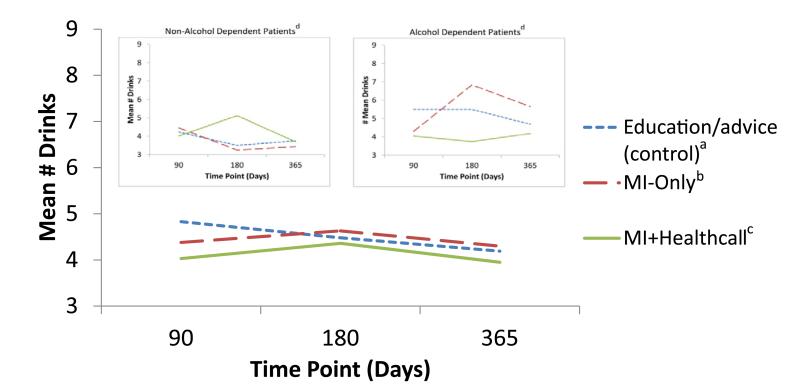
Overall, at 3, 6 and 12 months (Fig. 6), NumDD remained lower than at baseline in all groups, with group differences no longer significant by 12 months (P = 0.44). Among non-alcohol-dependent patients (Fig. 6, inset 1), groups did not differ on NumDD. Among alcohol-dependent patients, those in MI+HealthCall (Fig. 6, inset 2) continued to drink less than others by 12 months, although group differences were no longer significant.
Figure 6.
Mean drinks per drinking day during follow-up period, by treatment group. aEducation/advice (control) n: 90 days = 86; 180 days = 86; 360 days = 87. bMotivational interviewing (MI)-only n: 90 days = 77; 180 days = 77; 360 days = 76. cMI+HealthCall n: 90 days = 75; 180 days = 75; 360 days = 74. dSee Table S3 for number of patients (Ns), by treatment group, at all follow-up time-points
DISCUSSION
In heavy-drinking HIV primary care patients, among whom drinking reduction is important to health and survival, this randomized trial of advice/education control, MI-only and MI+HealthCall for drinking reduction showed a significant main effect for treatment, and an interaction of treatment by alcohol dependence on end-of-treatment NumDD. Among non-dependent patients, NumDD decreased similarly in all groups. In alcohol-dependent patients receiving MI+HealthCall, end-of-treatment NumDD was significantly lower than among those in MI-only or control. Therefore, among HIV patients with alcohol dependence, MI-only was not sufficient for significant drinking reduction, while MI+HealthCall was feasible and appeared effective in helping HIV-infected alcohol-dependent patients to make clinically important reductions in drinking quantities. Post-treatment follow-up NumDD remained lower than baseline in all treatment groups. While, by 12 months among alcohol-dependent patients, treatment differences in drinking were no longer significant, NumDD remained lowest in the MI+HealthCall group.
Concerning mechanisms of HealthCall effects, IVR calls may facilitate self-monitoring and remind patients of drinking-reduction goals and motives. Feedback graphs may contribute important summary information for patients (the pilot study [11] indicated that the graphs made a strong impression on patients) and inform patient–counselor discussions of drinking. HealthCall may also have increased self-efficacy, to be explored in future work. Another explanation of HealthCall effects is simply more intervention time (i.e. higher dose) than the other arms. Given our aim to increase intervention with little increase in staff time, this is not a disadvantage. To determine whether HealthCall effects were due to time/attention or more specific elements would require another treatment arm testing IVR calls without alcohol content. Regardless, such information would not change the clinically important aspect of HealthCall, which is the extension of patient engagement in intervention with few demands on clinic staff.
We did not find treatment effects on our secondary outcome, PDA. Methodologically, NumDD appears a better measure than PDA, given closer correspondence of NumDD than PDA to daily IVR-reported drinking among MI+HealthCall patients (Table S2). Substantively, lack of effects on PDA may be because all patients received intervention to decrease drinking, but only patients receiving MI focused on quantity (setting, achieving and maintaining the drinking goal). Future HealthCall versions may strengthen effects on PDA by reinforcing abstinent days, during IVR calls and counselor meetings.
This study was initiated before reviews highlighted differences in brief intervention effects among alcohol-dependent and non-dependent primary care patients [8,12]. While our results suggested an overall treatment effect, the secondary analysis suggested by Saitz’s review [8] indicated sharp differences by dependence status. Among non-dependent patients, all interventions were helpful, including drinking-reduction advice and general health education, analogous to usual clinic care. In contrast, alcohol-dependent patients appeared to need more; those in the control arm rebounded by end-of-treatment, while those in MI-only differed from control weakly or not at all, depending on the analysis. In contrast, those in MI+HealthCall drank less at end-of-treatment, regardless of the analysis. These results address important gaps in knowledge about brief intervention in HIV primary care for alcohol-dependent patients.
The drinking eligibility criterion, ≥4 drinks, was one drink below the NIAAA risk level defined for men. This level was selected because patients were ill with a condition often accompanied by liver damage, and because a single eligibility criterion for men and women was easier for clinic staff to remember. Nevertheless, screening produced a sample whose average baseline drinking was much higher than four drinks. Thus, even among non-dependent patients, drinking was greater than medically advisable, warranting intervention.
We did not compensate patients for calling HealthCall because HIV primary care clinics are unlikely to do so. While future studies could explore whether compensation increases calling, importantly, the study shows that urban minority HIV patients will use HealthCall, and that even imperfect use (~65% of calls made) appears to offer a benefit in reducing drinking quantity among those with alcohol dependence.
Inaccurate drinking reports often involve underreporting due to many factors, including forgetting. After 60 days of externally structured self-monitoring, patients using HealthCall may be less likely to forget and underreport their drinking, resulting in more accurate TLFB reports than other patients. If so, then the apparent HealthCall effects reported above may be underestimated and thus conservative.
Our finding of decreased drinking among non-dependent patients after very brief intervention agrees with earlier studies [8,12] and does not require replication. However, the MI+HealthCall effects among alcohol-dependent patients could represent an important advance, as their drinking quantities were substantially lower than the other groups at the end of treatment, a crucial difference among those with HIV. Therefore, further study focused on alcohol-dependent HIV primary care patients is needed to replicate these findings and to determine if improvements in HealthCall could strengthen its effects, during treatment and beyond.
Our trial was conducted in an urban setting with largely minority patients, some Spanish-speaking only. The minimal exclusion criteria and high proportion of patients with end-of-treatment data support generalizability. The counselors’ backgrounds were similar to personnel often found in such clinics, further enhancing generalizability. Alcohol-dependent patients reduced drinking through counseling that took only ~40 minutes prior to the 60-day assessment and interaction with an IVR system designed to extend the intervention in a feasible manner in time-pressured, resource-strapped HIV clinics. Future analyses will investigate whether results are moderated by demographic characteristics, or mediated by motivation or self-efficacy. Future studies should investigate replicability in alcohol-dependent patients, use of different technological platforms to strengthen HealthCall’s effects and tests in other settings. While maintaining significant MI+HealthCall differences throughout the 12-month follow-up would be preferable, this does not minimize the importance of the sharp decline in drinking during treatment among alcohol-dependent HIV patients, given their risks for liver-related illness and mortality. Expectations of brief interventions to have longer-lasting effects may be unrealistic, but an advantage of MI+HealthCall is that with little additional counselor involvement, patients could use HealthCall again as a booster if they found their improvements slipping, or wanted to make further gains. The flexibility and low cost of HealthCall (our platform cost <US$11 000), its ease of use in multiple languages, ubiquitous telephone access across national and class boundaries [17,42], and our successful implementation by personnel without previous research experience, all suggest promise for HealthCall-type enhancements of brief intervention to reduce drinking among HIV-infected alcohol-dependent individuals, a clinically important goal.
Supplementary Material
Appendix S1 Reducing Excess Drinking in HIV Primary Care Patients
Table S1 Mean (standard deviation) drinks per drinking day at 60 days, as assessed by time-line follow-back (TLFB) collected on time from patients at their 60-day assessment and data collected retrospectively among patients who missed their 60-day appointment
Table S2 Interactive voice response (IVR) versus time-line follow-back (TLFB) drinking information
Table S3 Number of patients with data at each time-point in the study, by timing of data collection: on time or retrospectively (at a later assessment)
Acknowledgements
D.S.H. conceived the study and obtained the funding. D.S.H., E.A. and A.O.L. designed the study, with assistance from M.W. E.A. acquired the data. M.P., S.A. and E.G. analyzed the data, which were interpreted by D.S.H., E.A., A.O.L., M.W., M.P. and S.A. D.H., E.A., R.W. and E.G. drafted the manuscript. All authors approved the final version. The findings and conclusions in this report are those of the authors and do not necessarily represent the official view of the Centers for Disease Control and Prevention. This study was supported by R01AA014323, K05AA014223 and the New York State Psychiatric Institute.
Footnotes
Clinical trial registration
This trial is registered with ClinicalTrials.gov, NCT00371969.
Declarations of interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- 1.Rosenthal E, Salmon-Ceron D, Lewden C, Bouteloup V, Pialoux G, Bonnet F, et al. Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC Joint Study Group Network (Mortavic 2005 study in collaboration with the Mortalite 2005 survey, ANRS EN19) HIV Med. 2009;10:282–9. doi: 10.1111/j.1468-1293.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- 2.Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377:1198–209. doi: 10.1016/S0140-6736(10)62001-6. [DOI] [PubMed] [Google Scholar]
- 3.DeLorenze GN, Weisner C, Tsai AL, Satre DD, Quesenberry CP., Jr. Excess mortality among HIV-infected patients diagnosed with substance use dependence or abuse receiving care in a fully integrated medical care program. Alcohol Clin Exp Res. 2011;35:203–10. doi: 10.1111/j.1530-0277.2010.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strauss SM, Tiburcio NJ, Munoz-Plaza C, Gwadz M, Lunievicz J, Osborne A, et al. HIV care providers’ implementation of routine alcohol reduction support for their patients. AIDS Patient Care STDS. 2009;23:211–8. doi: 10.1089/apc.2008.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strauss SM, Munoz-Plaza CE, Tiburcio NJ, Gwadz M. Barriers and facilitators in implementing ‘prevention for positives’ alcohol-reduction support: the perspectives of directors and providers in hospital-based HIV care centers. J Assoc Nurses AIDS Care. 2012;23:30–40. doi: 10.1016/j.jana.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metsch LR, Pereyra M, Colfax G, Dawson-Rose C, Cardenas G, McKirnan D, et al. HIV-positive patients’ discussion of alcohol use with their HIV primary care providers. Drug Alcohol Depend. 2008;95:37–44. doi: 10.1016/j.drugalcdep.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Miller WR, Baca C, Compton WM, Ernst D, Manuel JK, Pringle B, et al. Addressing substance abuse in health care settings. Alcohol Clin Exp Res. 2006;30:292–302. doi: 10.1111/j.1530-0277.2006.00027.x. [DOI] [PubMed] [Google Scholar]
- 8.Saitz R. Alcohol screening and brief intervention in primary care: absence of evidence for efficacy in people with dependence or very heavy drinking. Drug Alcohol Rev. 2010;29:631–40. doi: 10.1111/j.1465-3362.2010.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papas RK, Sidle JE, Gakinya BN, Baliddawa JB, Martino S, Mwaniki MM, et al. Treatment outcomes of a Stage 1 cognitive–behavioral trial to reduce alcohol use among HIV-infected outpatients in western Kenya. Addiction. 2011;106:2156–66. doi: 10.1111/j.1360-0443.2011.03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd Guilford Press; New York: 2002. [Google Scholar]
- 11.Aharonovich E, Hatzenbuehler ML, Johnston B, O’Leary A, Morgenstern J, Wainberg ML, et al. A low-cost, sustainable intervention for drinking reduction in the HIV primary care setting. AIDS Care. 2006;18:561–8. doi: 10.1080/09540120500264134. [DOI] [PubMed] [Google Scholar]
- 12.Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, Brief Intervention, and Referral to Treatment (SBIRT): toward a public health approach to the management of substance abuse. Subst Abuse. 2007;28:7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- 13.Apodaca TR, Longabaugh R. Mechanisms of change in motivational interviewing: a review and preliminary evaluation of the evidence. Addiction. 2009;104:705–15. doi: 10.1111/j.1360-0443.2009.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moyer A, Finney JW, Swearingen CE, Vergun P. Brief interventions for alcohol problems: a meta-analytical review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97:279–92. doi: 10.1046/j.1360-0443.2002.00018.x. [DOI] [PubMed] [Google Scholar]
- 15.Whitlock EP, Polen MR, Green CA, Orleans T, Klein J. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:557–68. doi: 10.7326/0003-4819-140-7-200404060-00017. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan WA. Can the ubiquitous power of mobile phones be used to improve health outcomes in developing countries? Global Health. 2006;2:9. doi: 10.1186/1744-8603-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–45. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 18.Oake N, Jennings A, van Walraven C, Forster AJ. Interactive voice response systems for improving delivery of ambulatory care. Am J Manag Care. 2009;15:383–91. [PubMed] [Google Scholar]
- 19.David P, Buckworth J, Pennell ML, Katz ML, Degraffinreid CR, Paskett ED. A walking intervention for postmenopausal women using mobile phones and interactive voice response. J Telemed Telecare. 2012;18:20–25. doi: 10.1258/jtt.2011.110311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naylor MR, Naud S, Keefe FJ, Helzer JE. Therapeutic Interactive Voice Response (TIVR) to reduce analgesic medication use for chronic pain management. J Pain. 2010;11:1410–9. doi: 10.1016/j.jpain.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham J, Tomcavage J, Salek D, Sciandra J, Davis DE, Stewart WF. Postdischarge monitoring using interactive voice response system reduces 30-day readmission rates in a case-managed Medicare population. Med Care. 2012;50:50–7. doi: 10.1097/MLR.0b013e318229433e. [DOI] [PubMed] [Google Scholar]
- 22.Griffin JM, Hulbert EM, Vernon SW, Nelson D, Hagel EM, Nugent S, et al. Improving endoscopy completion: effectiveness of an interactive voice response system. Am J Manag Care. 2011;17:199–208. [PubMed] [Google Scholar]
- 23.Zhao W, Weng Y, Wu Q, Palesch Y. Quantitative comparison of randomization designs in sequential clinical trials based on treatment balance and allocation randomness. Pharm Stat. 2012;11:39–48. doi: 10.1002/pst.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reitan R, Wolfson D. The Halstead–Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. 2nd Neuropsychology Press; Tucson, AZ: 1992. [Google Scholar]
- 25.National Institute on Alcohol Abuse and Alcoholism How to Cut Down on Your Drinking [online] 1996 Available at: http://pubs.niaaa.nih.gov/publications/handout.htm (accessed 6 June 2011). Archived at http://www.webcitation.org/6EjFlwb8J on 26 February 2013.
- 26.Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat. 2005;28:19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Grant BF, Harford TC, Dawson DA, Chou PS, Pickering RP. The Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS): reliability of alcohol and drug modules in a general population sample. Drug Alcohol Depend. 1995;39:37–44. doi: 10.1016/0376-8716(95)01134-k. [DOI] [PubMed] [Google Scholar]
- 28.Hasin D, Carpenter KM, McCloud S, Smith M, Grant BF. The Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS): reliability of alcohol and drug modules in a clinical sample. Drug Alcohol Depend. 1997;44:133–41. doi: 10.1016/s0376-8716(97)01332-x. [DOI] [PubMed] [Google Scholar]
- 29.Chatterji S, Saunders JB, Vrasti R, Grant BF, Hasin D, Mager D. Reliability of the alcohol and drug modules of the Alcohol Use Disorder and Associated Disabilities Interview Schedule—Alcohol/Drug-Revised (AUDADIS-ADR): an international comparison. Drug Alcohol Depend. 1997;47:171–85. doi: 10.1016/s0376-8716(97)00088-4. [DOI] [PubMed] [Google Scholar]
- 30.Sobell L, Sobell M. Alcohol Timeline Followback (TLFB) Users Manual. Addiction Research Foundation; Toronto, Canada: 1995. [Google Scholar]
- 31.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 32.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33.van Buuren S, Groothuis-Oudshoorn K. MICE: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 34.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–77. [PubMed] [Google Scholar]
- 35.Houck PR, Mazumdar S, Koru-Sengul T, Tang G, Mulsant BH, Pollock BG, et al. Estimating treatment effects from longitudinal clinical trial data with missing values: comparative analyses using different methods. Psychiatry Res. 2004;129:209–15. doi: 10.1016/j.psychres.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Liu G, Gould AL. Comparison of alternative strategies for analysis of longitudinal trials with dropouts. J Biopharm Stat. 2002;12:207–26. doi: 10.1081/bip-120015744. [DOI] [PubMed] [Google Scholar]
- 37.Siddique J, Brown CH, Hedeker D, Duan N, Gibbons RD, Miranda J, et al. Missing data in longitudinal trials—Part B, analytic issues. Psychiatr Ann. 2008;38:793–801. doi: 10.3928/00485713-20081201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Revicki DA, Gold K, Buckman D, Chan K, Kallich JD, Woolley JM. Imputing physical health status scores missing owing to mortality: results of a simulation comparing multiple techniques. Med Care. 2001;39:61–71. doi: 10.1097/00005650-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Tang L, Song J, Belin TR, Unutzer J. A comparison of imputation methods in a longitudinal randomized clinical trial. Stat Med. 2005;24:2111–28. doi: 10.1002/sim.2099. [DOI] [PubMed] [Google Scholar]
- 40.Smolkowski K, Danaher BG, Seeley JR, Kosty DB, Severson HH. Modeling missing binary outcome data in a successful web-based smokeless tobacco cessation program. Addiction. 2010;105:1005–15. doi: 10.1111/j.1360-0443.2009.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilbe JM. Negative Binomial Regression. 2nd Cambridge University Press; Cambridge, UK: 2011. [Google Scholar]
- 42.Shacham E, Stamm K, Overton ET. Can you hear me now? Limited use of technology among an urban HIV-infected cohort. AIDS Care. 2009;21:1000–6. doi: 10.1080/09540120802612832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Reducing Excess Drinking in HIV Primary Care Patients
Table S1 Mean (standard deviation) drinks per drinking day at 60 days, as assessed by time-line follow-back (TLFB) collected on time from patients at their 60-day assessment and data collected retrospectively among patients who missed their 60-day appointment
Table S2 Interactive voice response (IVR) versus time-line follow-back (TLFB) drinking information
Table S3 Number of patients with data at each time-point in the study, by timing of data collection: on time or retrospectively (at a later assessment)