Abstract
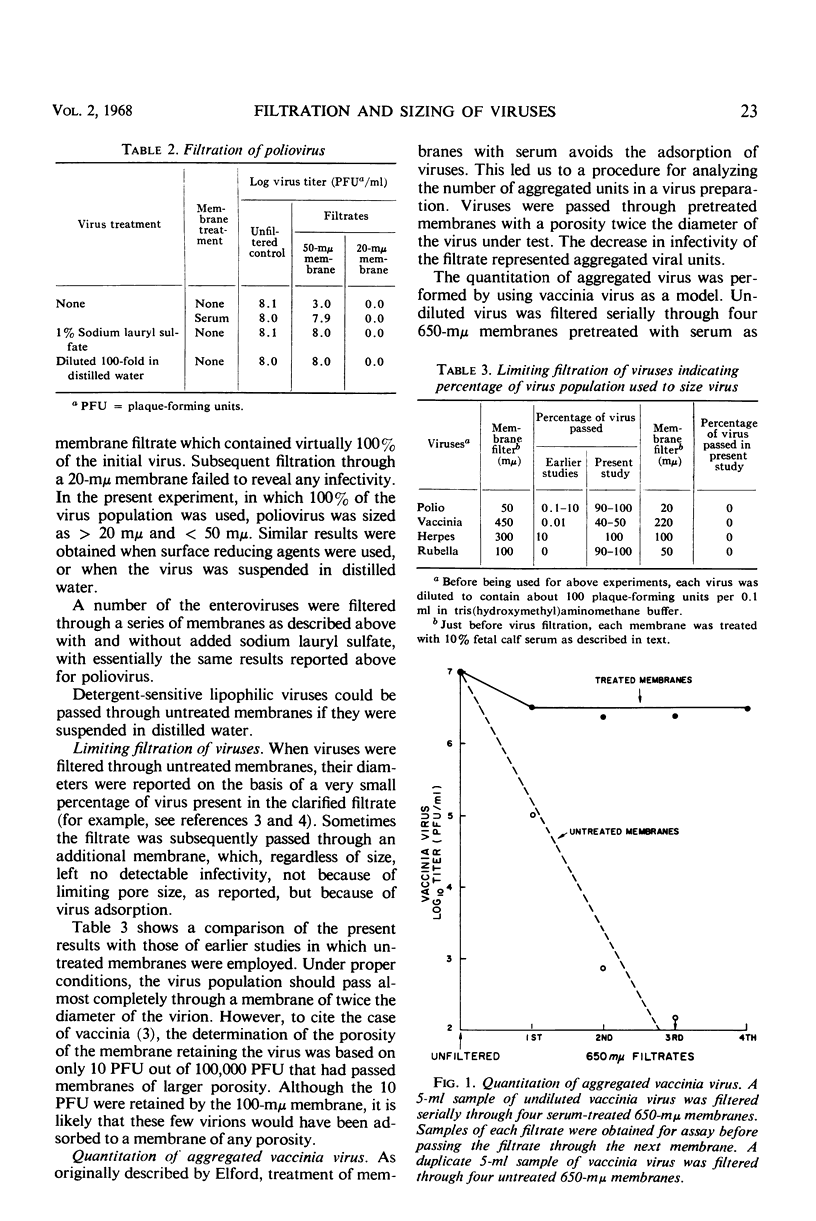
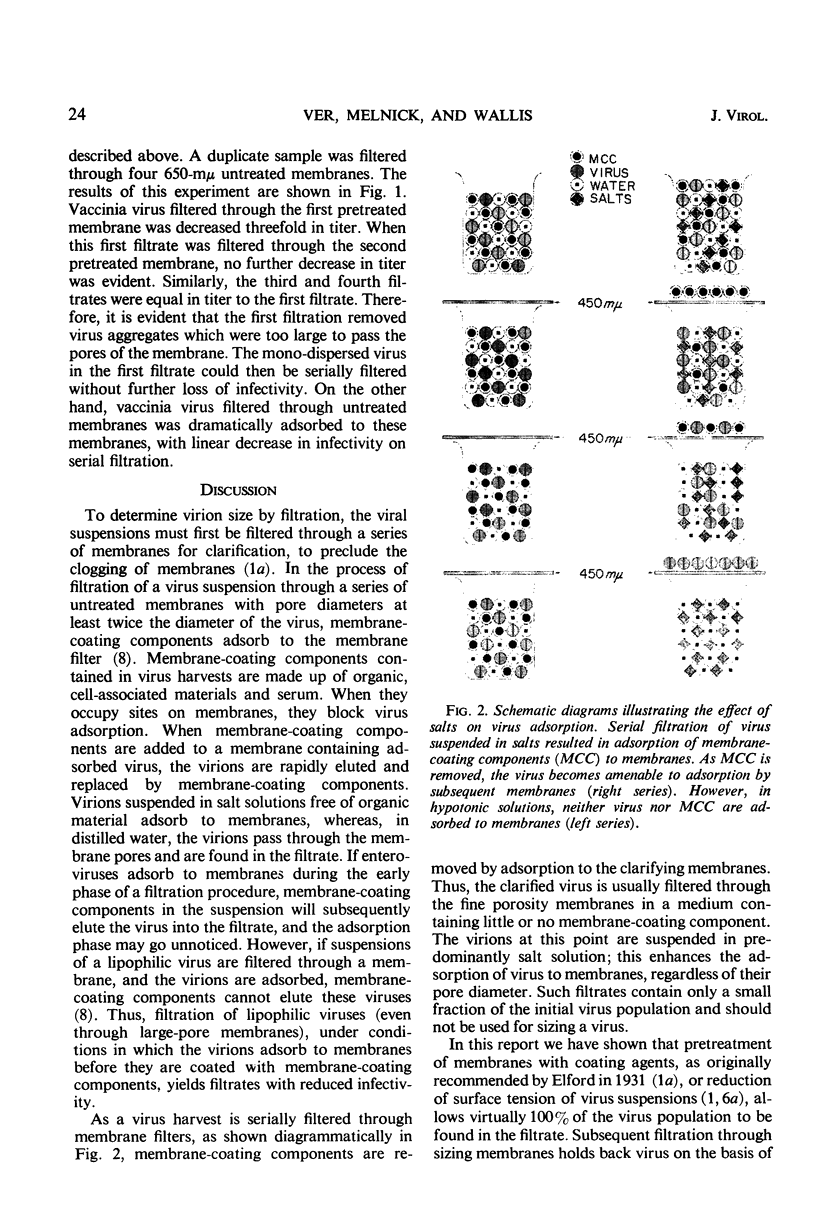
Untreated membrane filters retain viruses by adsorption, as well as by physical restriction which occurs when the pore diameter of the filter is smaller than that of the virus particle. As originally recommended by Elford, membranes had to be pretreated with proteinaceous material to preclude virus adsorption. However, coating materials that prevent adsorption of certain viruses do not necessarily prevent adsorption of other viruses. In contrast to proteins, salts enhance virus adsorption. Viruses treated with sodium lauryl sulfate to reduce the surface tension, or purified viruses in distilled water, are not adsorbed to membranes. A procedure is recommended by which viruses may be passed through membranes with a porosity twice the diameter of the virus. Such filtrates, which contain 50 to 100% of the initial virus concentration, should be used for sizing viruses by subsequent filtration through smaller pores. The determination of virus size would then be based on the major population of particles in the virus suspension. In the past, as little as 0.1 to 0.001% of the initial virus population was the basis for size determination, because more than 99.9% of the virus was often lost by adsorption to membranes during the clarifying procedures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARTWRIGHT S. F., THORNE H. V. Some applications of detergents to the study of the virus of foot-and-mouth disease. J Gen Microbiol. 1959 Feb;20(1):61–77. doi: 10.1099/00221287-20-1-61. [DOI] [PubMed] [Google Scholar]
- GIVAN K. F., ROZEE K. R., RHODES A. J. INCIDENCE OF RUBELLA ANTIBODIES IN FEMALE SUBJECTS. Can Med Assoc J. 1965 Jan 16;92:126–128. [PMC free article] [PubMed] [Google Scholar]
- Hsiung G. D. Use of ultrafiltration for animal virus grouping. Bacteriol Rev. 1965 Dec;29(4):477–486. doi: 10.1128/br.29.4.477-486.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKMAN P. D., BUESCHER E. L., ARTENSTEIN M. S., MCCOWN J. M., MUNDON F. K., DRUZD A. D. STUDIES OF RUBELLA. I. PROPERTIES OF THE VIRUS. J Immunol. 1964 Oct;93:595–607. [PubMed] [Google Scholar]
- Rawls W. E., Desmyter J., Melnick J. L. Rubella virus neutralization by plaque reduction. Proc Soc Exp Biol Med. 1967 Jan;124(1):167–172. doi: 10.3181/00379727-124-31692. [DOI] [PubMed] [Google Scholar]
- Sharp D. G., Kim K. S. Multiplicity reactivation and radiation survival of aggregated vaccinia virus. Calculation of plaque titer based on MR and particle aggregation seen in the electron microscope. Virology. 1966 Jul;29(3):359–366. doi: 10.1016/0042-6822(66)90211-x. [DOI] [PubMed] [Google Scholar]
- THORNE H. V., BURROWS T. M. Aerosol sampling for the virus of foot-and-mouth disease and the measurement of virus penetration through aerosol filters. J Hyg (Lond) 1960 Dec;58:409–417. doi: 10.1017/s0022172400038559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYTELL A. A., NEUMAN R. E. A medium free of agar, serum and peptone for plaque assay of herpes simplex virus. Proc Soc Exp Biol Med. 1963 Jun;113:343–346. doi: 10.3181/00379727-113-28362. [DOI] [PubMed] [Google Scholar]
- WALLIS C., MELNICK J. L., BIANCHI M. Factors influencing enterovirus and reovirus growth and plaque formation. Tex Rep Biol Med. 1962;20:693–702. [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Concentration of enteroviruses on membrane filters. J Virol. 1967 Jun;1(3):472–477. doi: 10.1128/jvi.1.3.472-477.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Virus aggregation as the cause of the non-neutralizable persistent fraction. J Virol. 1967 Jun;1(3):478–488. doi: 10.1128/jvi.1.3.478-488.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]




