Abstract
Metabolic syndrome is defined as a set of coexisting metabolic disorders that increase an individual’s likelihood of developing type 2 diabetes, cardiovascular disease and stroke. Medicinal plants, some of which have been used for thousands of years, serve as an excellent source of bioactive compounds for the treatment of metabolic syndrome because they contain a wide range of phytochemicals with diverse metabolic effects. In order for botanicals to be effectively used against metabolic syndrome, however, botanical preparations must be characterized and standardized through the identification of their active compounds and respective modes of action, followed by validation in controlled clinical trials with clearly defined endpoints. This review assesses examples of commonly known and partially characterized botanicals to describe specific considerations for the phytochemical, preclinical and clinical characterization of botanicals associated with metabolic syndrome.
Keywords: Botanical, cardiovascular disease, diabetes, hypertension, insulin resistance, phytochemical, obesity
Introduction
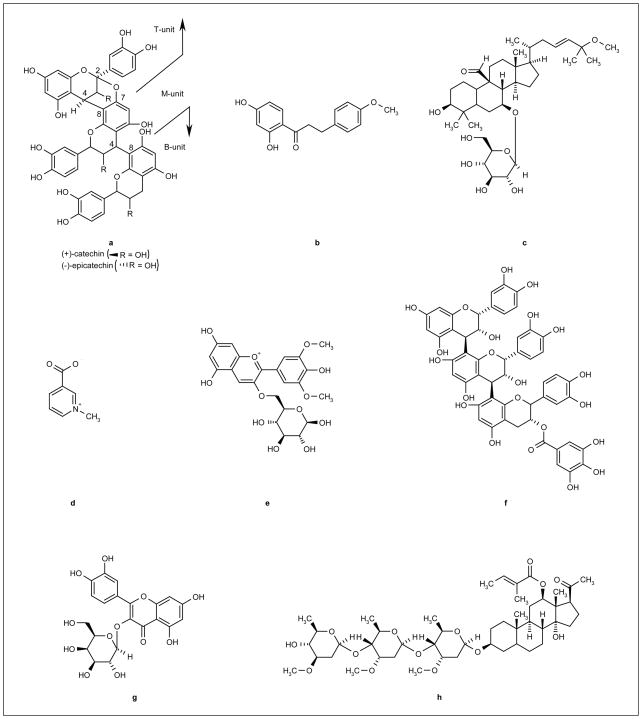
Metabolic syndrome is defined as a cluster of conditions or metabolic disorders, including hypertension, hyperglycemia/insulin resistance, excess abdominal fat and dyslipidemia, coexisting in an individual and leading to an increased risk of type 2 diabetes, cardiovascular disease and stroke [1,2]. Metabolic syndrome is of substantial concern, as the incidences of both type 2 diabetes and cardiovascular disorders have reached epidemic proportions worldwide. Unlike acute diseases, such as those caused by pathogens, metabolic syndrome is a complex, progressive disorder that can develop over many years and can vary between individuals both in terms of its extent and characteristics. Botanicals may serve as effective agents for the treatment or prevention of metabolic syndrome because they often contain diverse collections of biologically active compounds (Figure 1) with multiple mechanisms of action that may potentiate each other’s activity or have a synergistic effect, providing greater benefit than a single chemical entity. Thus, it is possible that the complexity of the disease may be addressed with a treatment or prophylactic strategy involving these complex compounds. More than 1200 different plants have been reported in the treatment of diabetes, many of which may also target other risk factors associated with metabolic syndrome, including hypertension and hypercholesterolemia [3]. The significant role that plant-derived therapeutics have played in both traditional and modern healthcare systems is strikingly evident in that medicinal plant preparations have been used for thousands of years, and can be traced as the source compounds in more than 25% of currently marketed pharmaceutical products [4,5].
Figure 1. Structures of botanical compounds used in the treatment of metabolic syndrome.
(a) Polyphenol type-A polymer (cinnamon), which consists of terminal- (T), midde- (M) and base- (B) units; (b) 2′,4′-dihydroxy-4- methoxydihydrochalcone (Russian tarragon); (c) cucurbitane (bitter melon); (d) trigonelline (fenugreek); (e) malvidin-3-glucoside (lowbush blueberry); (f) trimeric procyanidin (grape seed); (g) hyperoside (hawthorn); (h) P57-AS3 pregnane glycoside (Hoodia).
Despite the widespread use of putatively antidiabetic, antihypertensive and cholesterol-lowering botanical supplements, few botanicals have been evaluated effectively in appropriately controlled clinical trials using well-characterized agents. This review highlights the challenges associated with characterizing herbal products, focusing on botanicals that have commonly been associated with the treatment of metabolic syndrome (Table 1). These challenges include the consistency of the source material and the need for standardized preparations, as well as the identification of active components and mechanisms of action. In addition to these concerns, the complex and progressive aspects of metabolic syndrome create challenges for the design of trials, as patients often receive prescription drug treatment simultaneously with the test agent that may interfere with the analysis of the data obtained.
Table 1.
Selected plant-derived therapeutics and their effects on disease risks associated with metabolic syndrome.
| Botanical | Scientific name | Therapeutic effect | Reference |
|---|---|---|---|
| Cinnamon | Cinnamomum cassia and Cinnamomum verum | ↑IS; ↓G; ↓BP; ↓LDL; ↓TG | [6–17] |
| Russian tarragon | Artemisia dracunculus | ↑IS; ↓G | [18–25] |
| Bitter melon | Momordica charantia | ↑IS; ↓G; ↓LDL; ↓TG | [26–37] |
| Fenugreek | Trigonella foenum-graecum | ↑IS; ↓G; ↓LDL; ↓TG | [38–40] |
| Lowbush blueberry | Vaccinium angustifolium | ↓BP; ↓G; ↓W | [42–51] |
| Grape seed | Vitis vinifera | ↓BP; ↓LDL | [52–58] |
| Hawthorn | Crataegus laevigata, Crataegus monogyna, Crataegus curvisepala (Crataegus oxyacantha) and Crataegus tanacetifolia | ↓BP; ↓LDL | [59–68] |
| Hoodia | Hoodia gordonii | ↓W | [69–71] |
BP blood pressure, G blood glucose, IS insulin sensitivity, TG triglycerides, W body weight
Cinnamon: Cinnamomum cassia (L.) D. Don and Cinnamomum verum J. Presl (Lauraceae)
Cinnamon has been used historically for the treatment of diabetes, and many cinnamon products are available as dietary supplements for the maintenance of blood glucose levels. Polyphenol type-A polymers (Figure 1a), considered to be the active components of cinnamon, have demonstrated insulin mimetic activity in vitro [6]. Cinnamon extracts also improved insulin signaling in murine models of diabetes [7]. Six clinical trials have been completed using cinnamon; however, the results have not been entirely consistent, probably as a result of the differences in selection criteria (ie, the severity of type 2 diabetes), patient population (numbers ranging from 23 to 79), cinnamon source (varying species from various geographic locations used), dose (1.5 to 6 g/day)and selected clinical endpoints (ie, fasting blood glucose, HbA1c or blood lipid levels) [8].
For example, although HbA1c levels are reliable in the evaluation of long-term blood glucose control, the levels change slowly, thus requiring treatment periods of longer than 30 days for effects to be observed. In a randomized, uncontrolled trial of patients (n = 109) with diabetes who were on a diet and exercise regimen, treatment with cinnamon (1 g) for 90 days lowered HbA1c levels in the test group (0.83%) relative to the control group (0.37%) [9]. The decrease observed was small but statistically significant and, therefore, medically as even small decreases in HbA1c are associated with significant reductions in diabetes comorbidities. In another uncontrolled trial in a Pakistani population with type 2 diabetes, significant reductions in both blood glucose and LDL cholesterol levels were observed in patients treated with 1 to 6 g of cinnamon daily for 40 days [10,11]; however, trials conducted under similar treatment conditions in a US population of adolescents with type 1 diabetes and in patients with mild type 2 diabetes identified no effect of cinnamon on blood glucose parameters [12,13]. It is probable that patient population characteristics, such as genetics, diet, age and disease severity, played a major role in the contradictory outcomes observed in these trials.
Cinnamon has demonstrated positive effects on other markers of metabolic syndrome in addition to blood glucose. The antihypertensive action of cinnamon extract was successfully demonstrated in both spontaneously hypertensive rats [14] and patients with metabolic syndrome (22 men and women treated with a 500-mg extract daily for 12 weeks) [15]. Moreover, triglyceride-lowering effects with cinnamon were observed in Wistar rats fed a high-fructose diet [16] and in some clinical trials. For example, in the trial in Pakistani patients with type 2 diabetes, as outlined in the previous paragraph, serum triglycerides, LDL-cholesterol and total cholesterol were all significantly lowered [10], although these effects were not corroborated by results from a trial conducted in a less severely diabetic European population administered a comparable dose of cinnamon [17].
Russian tarragon: Artemisia dracunculus L. (Asteraceae)
In 2006, an ethanolic extract of Russian tarragon was demonstrated to have antidiabetic properties. This extract exhibited a hypoglycemic effect in both streptozotocin-induced and genetically diabetic KKAy murine models [18]. Bioactivity-guided fractionation of the extract led to the isolation of six bioactive compounds, including 4,5-di-O-caffeolquinic acid, davidigenin, 6-demethoxy-capillarison and 2′,4′-dihydroxy-4-methoxydihydrochalcone (Figure 1b) as aldose reductase inhibitors [19], and 2′,4′-dihydroxy-4-methoxydihydrochalcone, 2′,4′-dihydroxy-4′-methoxydihydrochalcone and sakuranetin as protein tyrosine phosphatase-1B inhibitors [20]. In addition, 6-demethoxycapillarisin and 2′,4′-dihydroxy-4-methoxydihydrochalcone inhibited the gene expression for a primary enzyme involved with hepatic glucose output, PEPCK (phosphoenol pyruvate carboxykinase), in liver cell cultures [21].
Studies in murine and human muscle cell cultures demonstrated that the antidiabetic effects of Russian tarragon extract are mediated through the insulin signaling pathway, which becomes disrupted as a result of insulin resistance [22]. Insulin resistance, the central pathophysiological feature of metabolic syndrome, is itself a complex metabolic disorder, but it can be measured directly using the hyperinsulinemic euglycemic clamp in both animals and humans. The clamp technique is a metabolic procedure whereby the individual/animal receives a steady-state intravenous insulin infusion, as well as an intravenous glucose infusion, which is gradually increased until a normal blood glucose level is maintained and, thus, the maximum rate of glucose assimilation is achieved. Low levels of glucose infusion needed for euglycemia signify high insulin resistance. Although other methods are available to evaluate insulin resistance, such as the use of oral glucose tolerance tests or intravenous tolerance tests, the clamp is considered to be the gold standard because measurement is direct and does not rely on mathematical models.
A pilot trial was conducted to evaluate the clinical effect of Russian tarragon extract (6 g/day) on insulin resistance using the euglycemic clamp in non-diabetic, obese, insulin-resistant patients [23]. Assessment of the abundance of three bioactive compounds (davidigenin, chalcone and sakuranetin) in blood plasma by liquid chromatography-mass spectrometry (LC-MS) demonstrated significant increases in the Russian tarragon-treated group relative to baseline. At the conclusion of the trial, insulin sensitivity was increased in the Russian tarragon-treated individuals when compared with baseline values, while no change was noted between the placebo group and baseline. In addition, no change in body weight or body fat composition was observed between treatment groups. This trial provided the first evidence that specific bioactive compounds from a botanical extract of Russian tarragon with notable in vitro effects can be identified in the plasma of individuals responding to treatment with the extract [24].
Further preclinical and clinical research using precise techniques, such as the hyperinsulinemic euglycemic clamp, is required to determine if the whole body effect of the Russian tarragon extract on glucose metabolism is directed at the muscle level or toward other parameters such as hepatic glucose production. In either case, an improvement in muscle sensitivity or a reduction in glucose output from the liver will ultimately result in lower blood glucose levels. Rigorous toxicological studies using Russian tarragon extract have demonstrated that it has no toxic effects and does not induce hypoglycemia in non-diabetic animals [25]. Russian tarragon extract is, therefore, safe and well characterized, with clearly identified bioactive components and potential mechanisms of action, as well as positive clinical activity and some pharmacokinetic data. Based on these data, further development of the extract is warranted for use as an insulin sensitizer and a botanical therapeutic for the treatment and prevention of metabolic syndrome.
Bitter melon: Momordica charantia L. (Cucurbitaceae)
Bitter melon is a popular plant of Asian origin that has been used to treat diabetes-related conditions and has been evaluated in several clinical trials (for a review, see reference [26]). The results of trials with bitter melon are inconsistent, with approximately half displaying positive effects. These inconsistencies are likely a result of deficiencies in trial design, low statistical powering, insufficient clinical endpoints (ie, non-fasting blood glucose measurements), and variations in the botanical preparations tested. The largest trial with bitter melon was conducted in patients (n = 100) with type 2 diabetes who had comparable baseline criteria and who underwent a 3-day wash-out period of oral medications [27]. The patients fasted overnight prior to consuming a drink of freshly prepared bitter melon fruit. A mean reduction of 18% in both fasting and subsequent post-prandial blood glucose levels was observed [27]. Despite certain design flaws (ie, single treatment), the data were statistically significant, perhaps as a result of the large population size and/or the use of fresh juice, which may have resulted in a greater effect than other preparations.
One of the most significant variables in the different clinical trials with bitter melon is the preparation of the test material, which has included fresh fruit juice, fresh whole fruit, dried whole fruit, dried seedless fruit, seeds, aqueous extract, methanolic extract or tablets [26,28,29]. Such variation could have a profound effect on the phytochemical content of the preparation, as well as the bioavailability of active compounds therein. Bitter melon fruit is reported to contain a collection of cucurbitane-type triterpenoids (Figure 1c), steroidal saponins called charantins, insulin-like peptides and alkaloids, all of which are associated with hypoglycemic activity [26].
In addition to blood glucose regulation, data from multiple animal models have demonstrated the positive effects of bitter melon in the treatment of various diabetes complications, including nephropathy [30], neuropathy and enteropathy [31], insulin resistance and cataract development [32], while positive effects on other markers of metabolic syndrome, including reduced LDL-cholesterol and triglyceride levels, have also been demonstrated in rat models [33–37].
Fenugreek: Trigonella foenum-graecum L. (Fabaceae)
Fenugreek is a leguminous herb commonly cultivated in India and Northern Africa, and its seeds are used worldwide as a cooking ingredient and a spice. Fenugreek seeds contain high amounts of protein and fiber and have well-documented hypoglycemic and hypocholesterolemic effects in animals (mice, rats, rabbits and dogs) and humans [38]. The hypoglycemic effects of fenugreek seeds are attributed to their high neutral and soluble fiber content, which slows gastric emptying, thus decreasing post-prandial blood glucose levels. The seeds also contain other gastrointestinally active compounds, such as the alkaloid trigonelline (Figure 1d), which potentially reduces glycosuria, and steroidal saponins, which may contribute to delayed gastric emptying [38].
Despite many studies assessing the effect of fenugreek in both animals and humans, the results obtained have been inconsistent, potentially as a result of differences in the study design, choice of experimental endpoints and method of presentation of the test substance (ie, in food or by gavage). In a clinical trial, food formulated to contain 10% fenugreek seed powder improved glucose tolerance by > 20% in both diabetic and non-diabetic patients (total n = 6) after 2 weeks [39]. In another trial, patients (n = 24) with diabetes were treated with hot-water-soaked fenugreek seeds (10g/day) for 8 weeks, resulting in reductions in fasting blood glucose, triglycerides and VLDL-cholesterol (by 25, 30 and 31%, respectively) [40]. Unlike most of the botanicals discussed in this review, fenugreek was generally effective only when administered in large doses (10 to 20 g/day), because of its direct effect on digestive processes (ie, delayed gastric emptying). Less direct effects of fenugreek may include stimulation of digestive enzymes, such as chymotrypsin, and pancreatic activity [38].
Lowbush blueberry: Vaccinium angustifolium Aiton (Ericaceae)
Lowbush blueberries are rich in polyphenols, which have a high antioxidant capacity, exceeding that of vitamin C or E by 4- to 5-fold [41], and are beneficial in a variety of medical conditions. Preparations from blueberry leaves lowered blood glucose levels in animal models (including depancreatized dogs), as well as in humans [42]. One of the compounds believed to be responsible for the hypoglycemic activity of blueberries in humans is myrtillin (delphinidin-3-O-glucoside), an anthocyanin, or red pigment, present in larger quantities in the berry than in the leaf. In 2009, an anthocyanin-enriched preparation from blueberries was demonstrated to have a significant hypoglycemic effect in diabetic rodents, lowering blood glucose by as much as 51% relative to the control group, when formulated with a bioenhancing agent [43]. In this study, the most abundant anthocyanin, malvidin-3-O-glucoside (Figure 1e), had a hypoglycemic effect when tested alone, whereas myrtillin was less effective [43].
Blueberry extracts have significantly different effects, depending on the formulation, that are likely to be related to their pharmacokinetic and, thus, bioavailability properties. Blueberry juice decreased hyperglycemia and attenuated adiponectin secretion in genetically diabetic mice when mixed with drinking water, but only when biotransformed using the Serratia vaccinii bacterium [44]. In addition, blueberry anthocyanin preparations administered with drinking water exhibited anti-obesogenic activity in dietary-induced obese mice, but the effect was not observed with berry preparations formulated within mouse food [45]. These results confirm that bioavailability is an important consideration for the administration of any botanical.
In addition to their antiglycemic and anti-obesogenic effects, blueberries have also been investigated for their hypotensive potential. Salt-sensitive, spontaneously hypertensive stroke-prone rats (SHRSP) were fed a diet enriched with 3% blueberry powder for 8 weeks [46]. Blood pressure was significantly reduced at weeks 4 and 6 in the blueberry-treated group compared with the control group (by 19 and 30%, respectively) [46]. Blueberry extracts have been observed to increase vasodilation consistently in rat models through an endothelium-mediated pathway involving nitric oxide (NO) metabolism and the production/activity of COX-derived products [47,48].
The hypotensive effects of blueberry components have not been sufficiently investigated. The anthocyanins cyanidin and delphinidin increased endothelial NO synthase (eNOS) activity significantly in HUVEC cultures [49], while cyanidin-3-glucoside upregulated eNOS expression and NO release in bovine arterial endothelial cells [50]. However, lowbush blueberry fruits also contain particularly high levels of procyanidin components (colorless condensed tannins composed of flavonoid polymers), which are known to play a significant role in the hypotensive action of other botanicals [see the Grape seed: Vitis vinifera L. (Vitaceae) section]; however, the cardioprotective effects of such components isolated from blueberries have not yet been evaluated rigorously.
The clinical efficacy of blueberries as botanical extracts or functional food has also not been investigated extensively. Mixed berries were evaluated for their cardioprotective effects in a randomized, single-blind, placebo-controlled, intervention clinical trial lasting 8 weeks, in which middle-aged volunteers taking no medications consumed two portions of berries per day [51]. Daily portions consisted of 100 g of whole bilberries and the nectar of 50 g of crushed lingonberries every other day, with a puree of 100 g of blackcurrants or strawberries and 0.7 dl of chokeberry or raspberry juice on alternating days. Relative to the control group, moderate berry consumption decreased systolic blood pressure (SBP) only slightly (−1.5 mmHg); however, a significant inhibition of platelet activation (11%), and an increase in serum HDL-cholesterol concentration (5.2%) were observed in the group consuming the berries [51]. The rich polyphenol composition of blueberries relative to the berries used in this trial, combined with the in vivo and in vitro effects of blueberries, suggest that rigorous future clinical investigations using blueberry preparations are warranted.
Grape seed: Vitis vinifera L. (Vitaceae)
Grape seed extract (GSE) is a rich botanical source of polyphenols (approximately 90% of which are procyanidins and 7% other polyphenol compounds) [52], and has become popular for the treatment and prevention of heart disease and other disorders. In vivo, the hypotensive effects of GSE were demonstrated in spontaneously hypertensive rats fed a 0.5% GSE-supplemented diet with basal (0.6%) or high (8.0%) NaCl, leading to a reduction in mean arterial pressure of 10 and 26 mmHg, respectively, compared with non-supplemented groups [52].
In a 2009, in a clinical trial in patients (n = 9) with metabolic syndrome, two doses of standardized GSE (150 and 300 mg/day) caused equally significant hypotensive effects on SBP (−11 mmHg for both doses versus −2 mmHg for placebo) and diastolic blood pressure (DBP; −6 to −7 mmHg for GSE versus −4 mmHg for placebo) after 4 weeks of treatment [53]. However, as a result of the small sample size and the prehypertensive status of the participants (SBP = 120 to 139 mmHg) [54], the hypotensive potential of GSE in patients with stage 1 or stage 2 hypertension remains unclear. Large trials in overtly hypertensive participants using GSE as an adjuvant therapy with pharmaceutical agents could be used to evaluate the hypotensive effects of GSE, but the results of combined therapy may not be relevant to the independent effects of GSE.
Several in vitro studies have demonstrated endothelium-dependent relaxation (EDR) as the primary mechanism of action for GSE [55–57]. Interestingly, through bioassay-guided fractionation of crude GSE using the rat aortic vasorelaxation model, increasing EDR activity was demonstrated to correlate with increased concentrations of procyanidin oligomerization [56,57]. This finding suggests that highly polymerized polyphenols (Figure 1f), although not studied extensively in other botanicals, may play a significant role in the hypotensive activities of GSEs [56,57].
GSEs also have therapeutic potential as LDL-cholesterol and triglyceride-lowering agents. In Wistar rats, grape seed procyanidins were demonstrated to correct dyslipidemia associated with a high-fat diet by repressing hepatic genes that control lipogenesis and VLDL assembly [58]. Clinical evidence is needed to support the theory that GSEs can correct dyslipidemia in humans, but the overwhelming evidence that GSEs successfully targets several risk factors associated with metabolic syndrome merits additional research in this field.
Hawthorn: Crataegus laevigata (Poir.) DC., Crataegus monogyna Jacq., Crataegus curvisepala Lindm. (Crataegus oxyacantha L.) and Crataegus tanacetifolia (Lam.) Pers. (Rosaceae)
The beneficial cardiovascular effects of hawthorn leaves, flowers and fruits, particularly in the treatment of cardiac failure, are recognized in both Eastern and Western medicine. In fact, hawthorn has been so successful in clinical trials associated with heart-related symptoms that leaf and flower extracts have been approved by the German Commission E for use in patients with stage II heart failure, as defined by the New York Heart Association [59].
Data from a placebo-controlled, double-blind clinical trial in an Iranian population demonstrated that both SBP and DBP were significantly hypertensive women and men (n = 92) administered a hydroalcoholic extract of Crataegus curvisepala leaves and flowers three times daily for > 3 months [60]. Moreover, in a separate trial, hawthorn was demonstrated to be a safe and effective complementary therapy in patients with metabolic disorders who were taking other drugs. More specifically, mildly hypertensive patients with type 2 diabetes who were taking prescription glycemic, lipidemic and/or hypotensive drugs in addition to 1200 mg of hawthorn extract per day exhibited greater reductions in blood pressure compared with placebo (SBP: −2.6 and +0.5 mmHg, and DBP: −3.6 and −0.8 mmHg for the hawthorn-treated and placebo groups, respectively [61]).
Hawthorn preparations may lower blood pressure through several mechanisms, including endothelium-mediated vasodilation through eNOS activation [62], ACE inhibition [63] and antioxidant activity [64]. Further research is required to define the chemical components responsible for the cardioprotective effects of hawthorn, although polyphenols, mainly flavonoids and procyanidins, are considered to have a key role. The isolated flavonoid hyperoside (100 mg/kg) (Figure 1g) exhibited enhanced antihypertensive activity compared with crude aqueous leaf extract of Crataegus tanacetifolia when administered to L-NAME (Nω-nitro-L-arginine-methyl ester)-induced hypertensive rats by gavage in a 4-week study [65]. In a separate study, the procyanidin fraction of hawthorn extract caused endothelium-dependent relaxation in isolated rat aortas [66].
Hawthorn extract exhibited positive effects on dyslipidemia by preventing the increase of triglycerides and LDL- and VLDL-cholesterol levels in hyperlipidemic rats [67], as well as by preventing cholesterol accumulation in the livers of atherogenic rats by suppressing cholesterol biosynthesis and promoting cholesterol degradation [68]. The preclinical, clinical and phytochemical characterization of this plant’s ability to reduce several metabolic risk factors, as well as the extensive use of hawthorn extracts in the treatment of cardiovascular disease, suggest that this extract would be an effective botanical therapeutic for metabolic syndrome.
Hoodia: Hoodia gordonii (Masson) Sweet ex Decne. (Apocynaceae)
Obesity is a key factor of metabolic syndrome and insulin resistance that can be addressed using a variety of approaches, often involving a reduction in caloric intake and/or an increase in energy expenditure. Diet and exercise alone, however, are often unsuccessful as a result of poor compliance. Hoodia is a botanical that was reported to have an anorexic effect in animal models, and is now widely marketed as an appetite-suppressant component in many diet products. A steroidal pregnane glycoside, P57-AS3 (Figure 1h), was purified from Hoodia and validated as an anorectic compound, demonstrating that it may act centrally through hypothalamic modulation [69]. However, more than 30 pregnane glycosides have been identified as components of decreased among Hoodia extracts, each of which may play a role in the activity of this botanical [70]. Moreover, no published clinical evidence of the appetite-suppressing effects of Hoodia is available, raising concerns about its safety and effective dose range. In addition, major concerns have been raised regarding the adulteration of Hoodia products currently available on the market, as many products reported to contain Hoodia do not actually contain this botanical [71]. The anti-obesogenic activity of plant-derived therapeutics is valuable against the risk factors associated with metabolic syndrome, but reservations regarding the safety and standardization of weight-loss products continue to be an issue preventing botanicals from having a truly beneficial role in disease prevention.
Conclusion
Metabolic syndrome is of high priority among medical and research communities worldwide, as it comprises a cluster of risk factors requiring improved treatment and prevention strategies. Because the relevant risk factors for metabolic syndrome are biologically inter-related in terms of their effect on disease progression, it would be useful to develop treatment and prevention strategies that address multiple risk factors at once. As demonstrated by the botanicals described in this review, plant extracts often contain natural active components that act upon numerous biological targets, providing an opportunity to simultaneously correct multiple defects associated with metabolic syndrome, in contrast to single-target drugs. Increased understanding of the effects of botanical therapeutics may advance their progress in the treatment and prevention of various metabolic diseases, so that eventually they may become effective therapies for metabolic syndrome. Characterization of the active components within botanicals, combined with an understanding of their safety, efficacy and mechanisms of action, are required to enable standardization of therapeutic preparations. Once standardized preparations are generated, it will be important to conduct well-designed, controlled clinical trials to demonstrate the true potential of these preparations for disease treatment and prevention. Moreover, trials must be stratified to measure differences in disease severity, age, gender and genetic variation of sample populations in order to clearly evaluate botanicals for the categorical treatment of metabolic syndrome.
Acknowledgments
Research performed by the authors is supported by a grant from Phytomedics Inc and NIH Center for Dietary Supplements Research on Botanicals and Metabolic Syndrome (Grant # 1-P50 AT002776-01).
References
•• of outstanding interest
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Simmons RK, Alberti KG, Gale EA, Colagiuri S, Tuomilehto J, Qiao Q, Ramachandran A, Tajima N, Brajkovich Mirchov I, Ben-Nakhi A, Reaven G, et al. The metabolic syndrome: Useful concept or clinical tool? Report of a WHO expert consultation. Diabetologia. 2010;53(4):600–605. doi: 10.1007/s00125-009-1620-4. [DOI] [PubMed] [Google Scholar]
- 3.Marles RJ, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2:137–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 4.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70(3):461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 5.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66(7):1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 6••.Anderson RA, Broadhurst CL, Polansky MM, Schmidt WF, Khan A, Flanagan VP, Schoene NW, Graves DJ. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J Agric Food Chem. 2004;52(1):65–70. doi: 10.1021/jf034916b. Identified the primary bioactive component of cinnamon using activity-guided fractionation and evaluated multiple biological activities of the component in vitro. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104(1–2):119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 8.Dugoua JJ, Seely D, Perri D, Cooley K, Forelli T, Mills E, Koren G. From type 2 diabetes to antioxidant activity: A systematic review of the safety and efficacy of common and cassia cinnamon bark. Can J Physiol Pharmacol. 2007;85(9):837–847. doi: 10.1139/Y07-080. [DOI] [PubMed] [Google Scholar]
- 9••.Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1c in patients with type 2 diabetes: A randomized, controlled trial. J Am Board Fam Med. 2009;22(5):507–512. doi: 10.3122/jabfm.2009.05.080093. A controlled clinical trial demonstrating that cinnamon treatment can lower HbA1c levels. [DOI] [PubMed] [Google Scholar]
- 10.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 11.Anderson RA. Chromium and polyphenols from cinnamon improve insulin sensitivity. Proc Nutr Soc. 2008;67(1):48–53. doi: 10.1017/S0029665108006010. [DOI] [PubMed] [Google Scholar]
- 12.Altschuler JA, Casella SJ, MacKenzie TA, Curtis KM. The effect of cinnamon on A1c among adolescents with type 1 diabetes. Diabetes Care. 2007;30(4):813–816. doi: 10.2337/dc06-1871. [DOI] [PubMed] [Google Scholar]
- 13.Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabetes Care. 2007;30(9):2236–2237. doi: 10.2337/dc07-0098. [DOI] [PubMed] [Google Scholar]
- 14.Preuss HG, Echard B, Polansky MM, Anderson R. Whole cinnamon and aqueous extracts ameliorate sucrose- induced blood pressure elevations in spontaneously hypertensive rats. J Am Coll Nutr. 2006;25(2):144–150. doi: 10.1080/07315724.2006.10719525. [DOI] [PubMed] [Google Scholar]
- 15.Ziegenfuss TN, Hofheins JE, Mendel RW, Landis J, Anderson RA. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J Int Soc Sports Nutr. 2006;3:45–53. doi: 10.1186/1550-2783-3-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin B, Polansky MM, Anderson RA. Cinnamon extract regulates plasma levels of adipose-derived factors and expression of multiple genes related to carbohydrate metabolism and lipogenesis in adipose tissue of fructose-fed rats. Horm Metab Res. 2010;42(3):187–193. doi: 10.1055/s-0029-1242746. [DOI] [PubMed] [Google Scholar]
- 17.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, Hahn A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36(5):340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 18.Ribnicky DM, Poulev A, Watford M, Cefalu WT, Raskin I. Antihyperglycemic activity of tarralin, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13(8):550–557. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Logendra S, Ribnicky DM, Yang H, Poulev A, Ma J, Kennelly EJ, Raskin I. Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus. Phytochemistry. 2006;67(14):1539–1546. doi: 10.1016/j.phytochem.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 20••.Wang ZQ, Ribnicky D, Zhang XH, Raskin I, Yu Y, Cefalu WT. Bioactives of Artemisia dracunculus L enhance cellular insulin signaling in primary human skeletal muscle culture. Metabolism. 2008;57(7 Suppl 1):S58–S64. doi: 10.1016/j.metabol.2008.04.003. Identified the active components of Russian tarragon extract using activity-guided fractionation, and demonstrated that the extract and its components directly alter insulin signaling in cultures of human skeletal muscle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Govorko D, Logendra S, Wang Y, Esposito D, Komarnytsky S, Ribnicky D, Poulev A, Wang Z, Cefalu WT, Raskin I. Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line. Am J Physiol Endocrinol Metab. 2007;293(6):E1503–E1510. doi: 10.1152/ajpendo.00420.2007. [DOI] [PubMed] [Google Scholar]
- 22.Cefalu WT. Inflammation, insulin resistance, and type 2 diabetes: Back to the future? Diabetes. 2009;58(2):307–308. doi: 10.2337/db08-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Ribnicky DM, Poulev A, Rood J, Raskin I, Cefalu WT. Plasma bioactives of Artemisia dracunculus L. and insulin sensitivity in obese, insulin-resistant human subjects: A pilot trial. J Complement Alternative Med. 2010 in press. Presents a clinical trial in which the bioavailability of the active compounds from Russian tarragon extract was assessed. Improved insulin sensitivity was demonstrated with extract treatment using the euglycemic hyperinsulinemic clamp technique. [Google Scholar]
- 24.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 25.Ribnicky DM, Poulev A, O’Neal J, Wnorowski G, Malek DE, Jager R, Raskin I. Toxicological evaluation of the ethanolic extract of Artemisia dracunculus L. for use as a dietary supplement and in functional foods. Food Chem Toxicol. 2004;42(4):585–598. doi: 10.1016/j.fct.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Leung L, Birtwhistle R, Kotecha J, Hannah S, Cuthbertson S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): A mini review. Br J Nutr. 2009;102(12):1703–1708. doi: 10.1017/S0007114509992054. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad N, Hassan MR, Halder H, Bennoor KS. Effect of Momordica charantia (Karolla) extracts on fasting and postprandial serum glucose levels in NIDDM patients. Bangladesh Med Res Counc Bull. 1999;25(1):11–13. [PubMed] [Google Scholar]
- 28.Grover JK, Yadav SP. Pharmacological actions and potential uses of Momordica charantia: A review. J Ethnopharmacol. 2004;93(1):123–132. doi: 10.1016/j.jep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 29.Rathi SS, Grover JK, Vats V. The effect of Momordica charantia and Mucuna pruriens in experimental diabetes and their effect on key metabolic enzymes involved in carbohydrate metabolism. Phytother Res. 2002;16(3):236–243. doi: 10.1002/ptr.842. [DOI] [PubMed] [Google Scholar]
- 30.Grover JK, Vats V, Rathi SS, Dawar R. Traditional Indian anti-diabetic plants attenuate progression of renal damage in streptozotocin induced diabetic mice. J Ethnopharmacol. 2001;76(3):233–238. doi: 10.1016/s0378-8741(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 31.Grover JK, Rathi SS, Vats V. Amelioration of experimental diabetic neuropathy and gastropathy in rats following oral administration of plant (Eugenia jambolana, Mucuna pruriens and Tinospora cordifolia) extracts. Indian J Exp Biol. 2002;40(3):273–276. [PubMed] [Google Scholar]
- 32.Rathi SS, Grover JK, Vikrant V, Biswas NR. Prevention of experimental diabetic cataract by Indian ayurvedic plant extracts. Phytother Res. 2002;16(8):774–777. doi: 10.1002/ptr.1064. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed I, Lakhani MS, Gillett M, John A, Raza H. Hypo-triglyceridemic and hypocholesterolemic effects of anti-diabetic Momordica charantia (karela) fruit extract in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 2001;51(3):155–161. doi: 10.1016/s0168-8227(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 34.Chaturvedi P. Role of Momordica charantia in maintaining the normal levels of lipids and glucose in diabetic rats fed a high-fat and low-carbohydrate diet. Br J Biomed Sci. 2005;62(3):124–126. doi: 10.1080/09674845.2005.11732698. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes NP, Lagishetty CV, Panda VS, Naik SR. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Complement Altern Med. 2007;7:29. doi: 10.1186/1472-6882-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayasooriya AP, Sakono M, Yukizaki C, Kawano M, Yamamoto K, Fukuda N. Effects of Momordica charantia powder on serum glucose levels and various lipid parameters in rats fed with cholesterol-free and cholesterol-enriched diets. J Ethnopharmacol. 2000;72(1–2):331–336. doi: 10.1016/s0378-8741(00)00259-2. [DOI] [PubMed] [Google Scholar]
- 37.Senanayake GV, Maruyama M, Shibuya K, Sakono M, Fukuda N, Morishita T, Yukizaki C, Kawano M, Ohta H. The effects of bitter melon (Momordica charantia) on serum and liver triglyceride levels in rats. J Ethnopharmacol. 2004;91(2–3):257–262. doi: 10.1016/j.jep.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasan K. Fenugreek (Trigonella foenum-graecum): A review of health beneficial physiological effects. Food Rev Intl. 2006;22(2):203–224. [Google Scholar]
- 39.Gopalpura PB, Jayanthi C, Dubey S. Effect of Trigonella foenum-graecum seeds on the glycemic index of food: A clinical evaluation. Int J Diab Dev Countries. 2009;27(2):41–45. [Google Scholar]
- 40.Kassaian N, Azadbakht L, Forghani B, Amini M. Effect of fenugreek seeds on blood glucose and lipid profiles in type 2 diabetic patients. Int J Vitam Nutr Res. 2009;79(1):34–39. doi: 10.1024/0300-9831.79.1.34. [DOI] [PubMed] [Google Scholar]
- 41.Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 2003;6(4):291–299. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 42.Watson EM. Some observations on the effect of blueberry leaf extract in diabetes mellitus. Can Med Assoc J. 1928;19(2):166–171. [PMC free article] [PubMed] [Google Scholar]
- 43••.Grace MH, Ribnicky DM, Kuhn P, Poulev A, Logendra S, Yousef GG, Raskin I, Lila MA. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16(5):406–415. doi: 10.1016/j.phymed.2009.02.018. Provided evidence of hypoglycemic activity in mice with a blueberry anthocyanin-rich extract using a bioenhancing formulation, identifying the anthyocyanin with the greatest activity. The effectiveness of the bioenhancing agent suggested that the bioavailability of the compounds in blueberry extracts is generally low, but can be altered depending on the presentation of the compounds (ie, in food or by gavage) to the mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vuong T, Benhaddou-Andaloussi A, Brault A, Harbilas D, Martineau LC, Vallerand D, Ramassamy C, Matar C, Haddad PS. Antiobesity and antidiabetic effects of biotransformed blueberry juice in KKA(y) mice. Int J Obes (Lond) 2009;33(10):1166–1173. doi: 10.1038/ijo.2009.149. [DOI] [PubMed] [Google Scholar]
- 45.Prior RL, Wilkes SE, Rogers TR, Khanal RC, Wu X, Howard LR. Purified blueberry anthocyanins and development of obesity in mice fed an obesogenic high-fat diet. J Agric Food Chem. 2010;58(7):3970–3976. doi: 10.1021/jf902852d. [DOI] [PubMed] [Google Scholar]
- 46••.Shaughnessy KS, Boswall IA, Scanlan AP, Gottschall-Pass KT, Sweeney MI. Diets containing blueberry extract lower blood pressure in spontaneously hypertensive stroke-prone rats. Nutr Res. 2009;29(2):130–138. doi: 10.1016/j.nutres.2009.01.001. The first in vivo demonstration of the hypotensive potential of lowbush blueberry extract. [DOI] [PubMed] [Google Scholar]
- 47.Kalea AZ, Clark K, Schuschke DA, Klimis-Zacas DJ. Vascular reactivity is affected by dietary consumption of wild blueberries in the Sprague-Dawley rat. J Med Food. 2009;12(1):21–28. doi: 10.1089/jmf.2008.0078. [DOI] [PubMed] [Google Scholar]
- 48.Kalea AZ, Clark K, Schuschke DA, Kristo AS, Klimis-Zacas DJ. Dietary enrichment with wild blueberries (Vaccinium angustifolium) affects the vascular reactivity in the aorta of young spontaneously hypertensive rats. J Nutr Biochem. 2010;21(1):14–22. doi: 10.1016/j.jnutbio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 49.Lazze MC, Pizzala R, Perucca P, Cazzalini O, Savio M, Forti L, Vannini V, Bianchi L. Anthocyanidins decrease endothelin-1 production and increase endothelial nitric oxide synthase in human endothelial cells. Mol Nutr Food Res. 2006;50(1):44–51. doi: 10.1002/mnfr.200500134. [DOI] [PubMed] [Google Scholar]
- 50••.Xu JW, Ikeda K, Yamori Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension. 2004;44(2):217–222. doi: 10.1161/01.HYP.0000135868.38343.c6. Identified for the first time the cell signaling pathway that is activated by a common anthocyanin to upregulate eNOS expression in vitro. [DOI] [PubMed] [Google Scholar]
- 51.Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, Mattila P, Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr. 2008;87(2):323–331. doi: 10.1093/ajcn/87.2.323. [DOI] [PubMed] [Google Scholar]
- 52.Peng N, Clark JT, Prasain J, Kim H, White CR, Wyss JM. Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005;289(3):R771–R775. doi: 10.1152/ajpregu.00147.2005. [DOI] [PubMed] [Google Scholar]
- 53••.Sivaprakasapillai B, Edirisinghe I, Randolph J, Steinberg F, Kappagoda T. Effect of grape seed extract on blood pressure in subjects with the metabolic syndrome. Metabolism. 2009;58 (12):1743–1746. doi: 10.1016/j.metabol.2009.05.030. Presents data demonstrating a significant hypotensive effect with grape seed extract with treatment among prehypertensive individuals. [DOI] [PubMed] [Google Scholar]
- 54.Jones DW, Hall JE. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and evidence from new hypertension trials. Hypertension. 2004;43(1):1–3. doi: 10.1161/01.HYP.0000110061.06674.ca. [DOI] [PubMed] [Google Scholar]
- 55.Aldini G, Carini M, Piccoli A, Rossoni G, Facino RM. Procyanidins from grape seeds protect endothelial cells from peroxynitrite damage and enhance endothelium-dependent relaxation in human artery: New evidences for cardioprotection. Life Sci. 2003;73(22):2883–2898. doi: 10.1016/s0024-3205(03)00697-0. [DOI] [PubMed] [Google Scholar]
- 56.Fitzpatrick DF, Bing B, Maggi DA, Fleming RC, O’Malley RM. Vasodilating procyanidins derived from grape seeds. Ann NY Acad Sci. 2002;957:78–89. doi: 10.1111/j.1749-6632.2002.tb02907.x. [DOI] [PubMed] [Google Scholar]
- 57.Fitzpatrick DF, Fleming RC, Bing B, Maggi DA, O’Malley RM. Isolation and characterization of endothelium-dependent vasorelaxing compounds from grape seeds. J Agric Food Chem. 2000;48(12):6384–6390. doi: 10.1021/jf0009347. [DOI] [PubMed] [Google Scholar]
- 58••.Quesada H, del Bas JM, Pajuelo D, Díaz S, Fernandez-Larrea J, Pinent M, Arola L, Salvadó MJ, Bladé C. Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver. Int J Obes (Lond) 2009;33(9):1007–1012. doi: 10.1038/ijo.2009.136. Demonstrated that grape seed procyanidins correct dyslipidemia in vivo, and suggested the molecular mechanisms of action involved. [DOI] [PubMed] [Google Scholar]
- 59.Pittler MH, Guo R, Ernst E. Hawthorn extract for treating chronic heart failure. Cochrane Database Syst Rev. 2008;(1):CD005312. doi: 10.1002/14651858.CD005312.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asgary S, Naderi GH, Sadeghi M, Kelishadi R, Amiri M. Antihypertensive effect of Iranian Crataegus curvisepala Lind.: A randomized, double-blind study. Drugs Exp Clin Res. 2004;30(5–6):221–225. [PubMed] [Google Scholar]
- 61••.Walker AF, Marakis G, Simpson E, Hope JL, Robinson PA, Hassanein M, Simpson HC. Hypotensive effects of hawthorn for patients with diabetes taking prescription drugs: A randomised controlled trial. Br J Gen Pract. 2006;56(527):437–443. Presents a controlled clinical trial assessing herb-drug interactions, demonstrating that hawthorn is safe to use in combination with pharmaceutical drugs as an adjunct therapy for hypertension. [PMC free article] [PubMed] [Google Scholar]
- 62.Brixius K, Willms S, Napp A, Tossios P, Ladage D, Bloch W, Mehlhorn U, Schwinger RH. Crataegus special extract WS 1442 induces an endothelium-dependent, NO-mediated vasorelaxation via eNOS-phosphorylation at serine 1177. Cardiovasc Drugs Therhypotensive. 2006;20(3):177–184. doi: 10.1007/s10557-006-8723-7. [DOI] [PubMed] [Google Scholar]
- 63.Lacaille D, Franck U, Wagner H. Search for potential angiotensin converting enzyme (ACE)-inhibitors from plants. Phytomedicine. 2001;8(1):47–52. doi: 10.1078/0944-7113-00003. [DOI] [PubMed] [Google Scholar]
- 64.Tadi VM, Dobri S, Markovi GM, Dordevi SM, Arsi IA, Menkovi NR, Stevi T. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J Agric Food Chem. 2008;56 (17):7700–7709. doi: 10.1021/jf801668c. [DOI] [PubMed] [Google Scholar]
- 65.Kocyildiz ZC, Birman H, Olgac V, Akgun-Dar K, Melikoğlu G, Meriçli AH. Crataegus tanacetifolia leaf extract prevents L-NAME-induced hypertension in rats: A morphological study. Phytother Res. 2006;20(1):66–70. doi: 10.1002/ptr.1808. [DOI] [PubMed] [Google Scholar]
- 66.Kim SH, Kang KW, Kim KW, Kim ND. Procyanidins in Crataegus extract evoke endothelium-dependent vasorelaxation in rat aorta. Life Sci. 2000;67(2):121–131. doi: 10.1016/s0024-3205(00)00608-1. [DOI] [PubMed] [Google Scholar]
- 67.Shanthi S, Parasakthy K, Deepalakshmi PD, Devaraj SN. Hypolipidemic activity of tincture of Crataegus in rats. Indian J Biochem Biophys. 1994;31(2):143–146. [PubMed] [Google Scholar]
- 68.Rajendran S, Deepalakshmi PD, Parasakthy K, Devaraj H, Devaraj SN. Effect of tincture of Crataegus on the LDL-receptor activity of hepatic plasma membrane of rats fed an atherogenic diet. Atherosclerosis. 1996;123(1–2):235–241. doi: 10.1016/0021-9150(96)05813-3. [DOI] [PubMed] [Google Scholar]
- 69.MacLean DB, Luo LG. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: Studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004;1020(1–2):1–11. doi: 10.1016/j.brainres.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 70.Shukla YJ, Pawar RS, Ding Y, Li XC, Ferreira D, Khan IA. Pregnane glycosides from Hoodia gordonii. Phytochemistry. 2009;70(5):675–683. doi: 10.1016/j.phytochem.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 71.Avula B, Wang YH, Pawar RS, Shukla YJ, Smillie TJ, Khan IA. A rapid method for chemical fingerprint species, related genera, and dietary supplements using UPLC-UV-MS. J Pharm Biomed Anal. 2008;48(3):722–731. doi: 10.1016/j.jpba.2008.07.005. [DOI] [PubMed] [Google Scholar]