Abstract
We describe a method to facilitate electrophoretic separation of high molecular weight RNA species, such as ribosomal RNAs and their precursors, on agarose-formaldehyde gels. Two alternative “pK-matched” buffer systems were substituted for the traditionally used MOPS-based conductive media. The key advantages include shortened run times, a five-fold reduction in formaldehyde concentration, a significantly improved resolution of long RNAs, and consistency in separation. The new procedure has a streamlined work flow that helps minimize errors and is broadly applicable to agarose gel electrophoresis of RNA samples and their subsequent analysis by Northern blotting.
Keywords: RNA, electrophoresis, pre-rRNA, rRNA biogenesis, Northern blotting
Many RNA species in a cell are transcribed as precursor molecules that undergo post-transcriptional processing into shorter mature forms. For example, during ribosome assembly, a large pre-rRNA transcript (13.4–13.9 kb in human and mouse) is processed into three rRNAs, 18S (1.9 kb), 5.8S (0.16 kb) and 28S rRNAs (4.7 kb) through a series of distinct intermediates [1]. The ability to distinguish RNAs by their molecular size is critical for the analysis of rRNA transcription and processing. Agarose gel electrophoresis in the presence of formaldehyde has been an indispensable technique for the analysis of RNA and is a prerequisite for Northern blotting [2,3]. However, one frequently encountered problem with standard RNA electrophoresis procedures is the poor separation of large cellular RNA species, such as rRNA precursors. Here, we describe a method that provides consistent results with a wide range of RNAs and particularly improves the separation of long RNAs on a gel. Moreover, our procedure reduces time and effort for the gel analysis of RNA, while also helping to minimize errors in the preparation of electrophoresis media and samples.
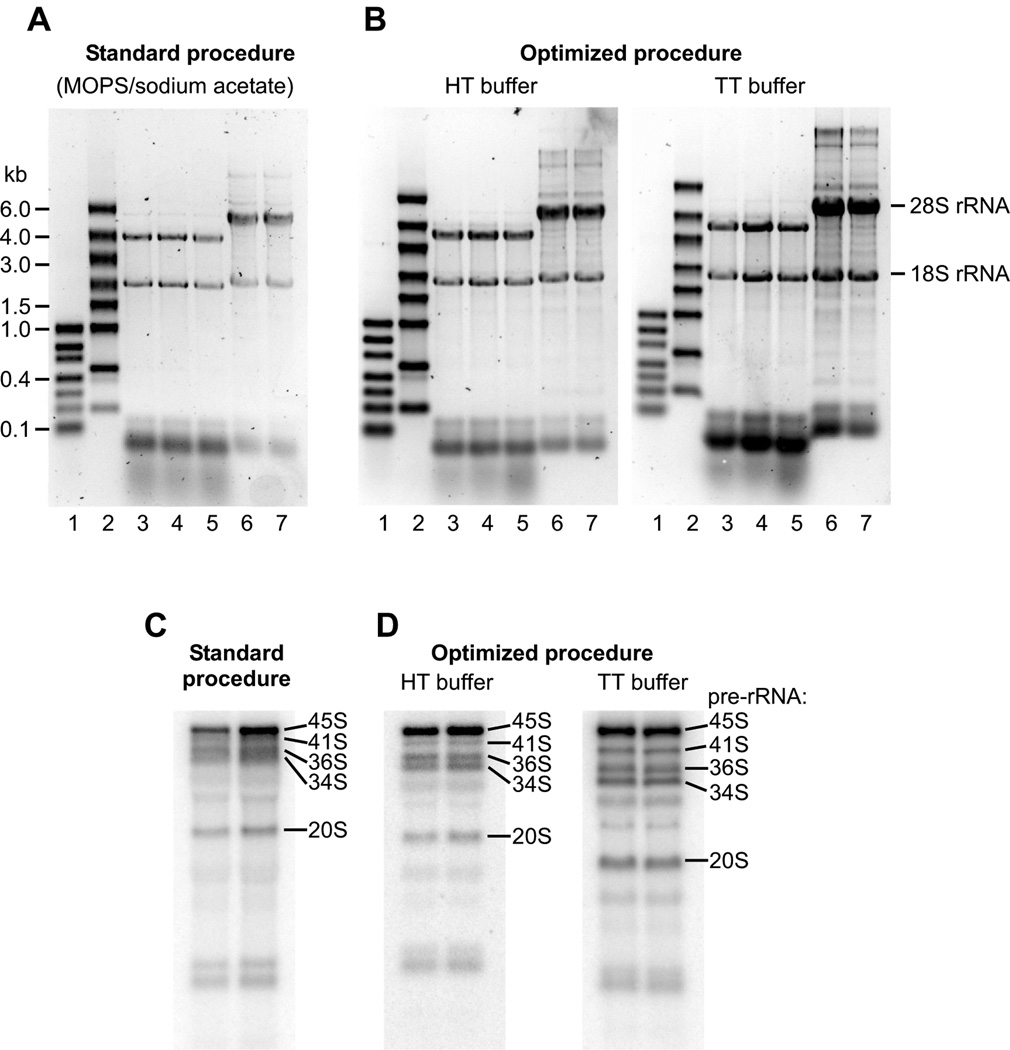
The commonly used procedures for agarose-formaldehyde gel electrophoresis of RNA traditionally use the combination of 3-(N-morpholino)propanesulfonic acid (MOPS) and sodium acetate as the conductive medium [2,3]. In our studies of rRNA maturation, large rRNA precursors were often difficult to separate using standard electrophoresis protocols, prompting us to evaluate other types of conductive media. Weak acid/weak base pairs with close pKa values, termed pK-matched buffers, were previously shown to provide an increased electrophoretic stability and good separation of DNA fragments [4]. When testing pK-matched buffers developed by Liu et al. [4], we observed a significant improvement in the resolution of large RNA species on a gel. Two buffer combinations that we found most useful for RNA electrophoresis were Hepes/triethanolamine and Tricine/triethanolamine (Table 1). These buffers will be referred to hereafter as HT and TT (the latter was also referred to as Tri/Tri in Ref. [4]). Fig. 1 demonstrates electrophoretic separation of RNA in the traditional system using the MOPS/sodium acetate conductive medium [2] and in the new system based on the HT and TT buffers. RNA samples prepared according to Table 1 were denatured by heating at 70°C for 5 min, cooled to room temperature and loaded on a gel. In all cases, gels were run for 2 h at 6 V/cm in 1×running buffer (V/cm is defined as applied voltage divided by interelectrode distance in cm). This resulted in comparable separation of RNA ladders (Fig. 1A, B, lanes 1–2) and mature 18S/28S rRNAs in samples of total cellular RNA (lanes 3–7). Northern analysis of the RNA following electrophoretic separation revealed poor resolution and smearing of pre-rRNA species above 6 kb in the traditional protocol with the MOPS/sodium acetate buffer (Fig. 1C). By contrast, large pre-rRNAs were well resolved and appeared as sharp bands in gels run with HT and TT buffers (Fig. 1D). The TT buffer particularly improved the separation of large pre-RNAs by enhancing the relative mobility of bands in the top area of the gel (Fig 1D, right panel).
TABLE 1.
Comparative composition of the traditional and optimized RNA electrophoresis systems.
| Traditional system | Optimized system | ||
|---|---|---|---|
| Conductive media, stock concentration | MOPS/NaOAca, 10× | HTb, 50× | TTb, 50× |
| Composition of the stock solution | 0.2M MOPS-NaOH (pH 7.0), 20 mM NaOAc, 10 mM EDTA |
1.5M Hepes, 1.5M triethanolaminec |
1.5M Tricine, 1.5M triethanolaminec |
| Running buffer (1×) | 20 mM MOPS-NaOH (pH 7.0), 2 mM NaOAc, 1 mM EDTA |
30 mM Hepes, 30 mM triethanolamine |
30 mM Tricine, 30 mM triethanolamine |
| Formaldehyde concentration in gel | 2.2M | 0.4M | |
| Sample composition | RNA, 50% formamide, 2.7M formaldehyde, 1×running buffer, 5% glycerol, 1 mM EDTA, 0.025% bromophenol blue, 0.025% xylene cyanol FF |
RNA, 50% formamide 0.4 M formaldehyde 1×running buffer, 0.5 mM EDTA, 0.02% bromophenol blue |
|
Traditional recipe, prepared according to [2].
pK-matched buffers, originally described by [4].
To prepare 50×stock solution, start by pouring the required amount of triethanolamine in a beaker placed on a balance (note that triethanolamine is liquid). Next, add Hepes (or Tricine) and high-quality deionized water to ~0.9 of the final volume, dissolve reagents completely using a magnetic stirrer and bring to the final volume with deionized water. pH of the buffers (HT, 7.6±0.2; TT, 7.9±0.2) is established automatically and should not be adjusted. Wrong pH indicates an error in buffer preparation (e.g., using salts of Hepes and triethanolamine instead of free acid/base). With high-quality reagents, filtering freshly prepared 50×stock solutions through sterile 0.2 µm high-protein binding filters (e.g., nylon) is normally sufficient to guard against trace amounts of RNases. If desired, the stock solutions can be additionally autoclaved at 121°C for 15 min. A slight darkening that may occur after this does not affect buffer performance.
Fig. 1.
(A, B) SYBR Gold stained gels of RNA separated with the standard and optimized electrophoresis procedures as detailed in the text. We used a 7.5×10 cm mini-gel horizontal system (C.B.S. Scientific) to run a 1% agarose gel at 100 V (6 V/cm) for 2 h; bromophenol blue migrated ~7 cm in all cases. Marker lanes (1–2) contained commercial RNA ladders (Thermo Fisher Scientific #SM1833 and #SM1823). Lanes 3–5, total RNA extracted from Saccharomyces cerevisiae cells. Lanes 6–7, total RNA from mouse 3T3 fibroblasts. Equal amounts of RNAs were loaded on all gels. rRNA bands look less intense in the standard protocol because of a lower efficiency of SYBR Gold staining in the MOPS buffer, as compared with HT and TT buffers; RNA ladders were premixed with 0.0125% ethidium bromide. (C, D) Northern hybridization of mouse rRNA precursors separated on gels as described in panels A and B. RNA was hybridized [9] with probe ITS1-1c [10] and visualized using a Typhoon 8600 Phosphorimager (GE Healthcare Life Sciences). Positions of 45S (12.8 kb), 41S (8.8 kb), 36S (6.9 kb), 34S (6.3 kb) and 20S (2.9 kb) pre-rRNAs are indicated.
An increased separation of high molecular size fragments of double-stranded DNA was not noted during electrophoresis in pK-matched buffers [4]. The improvement in resolution observed in electrophoresis of RNA may therefore reflect a better ability of the HT and TT buffers to control migration of RNA molecules during separation under denaturing conditions. For instance, components of the buffers might interact with RNA and play a role in maintaining RNA molecules in a denatured state. A higher buffering capacity [4] and balanced ionic composition of these buffers may also help to prevent ion exhaustion in gel areas where large RNA molecules migrate, thereby contributing to their separation according to molecular size.
There are other advantages of HT- and TT-based electrophoresis media as compared with the traditionally used MOPS/sodium acetate. When used at 30 mM as a running buffer, HT and TT permitted separation of even the largest cellular pre-rRNAs within 2 h on a mini-gel (Fig. 1B, D). To analyze multiple samples simultaneously, we also routinely use larger sized (12×14cm) gels. Application of a higher voltage to run these gels at 4–6 V/cm produces well-separated RNAs in 3 h or less and obviates the necessity for buffer recirculation. By comparison, commonly used protocols for agarose-formaldehyde gel electrophoresis call for 4 to 5-hour runs with recirculation of the MOPS/sodium acetate buffer [2]. Both HT and TT buffers can be conveniently prepared as a 50×stock solution (Table 1), which does not precipitate during storage and is not conducive to microbial growth. Furthermore, unlike photosensitive MOPS solutions [2], these buffers are stable under ambient light conditions. We observed no changes in the performance of the 50×HT and TT solutions stored in clear glass bottles on a laboratory shelf at room temperature for up to a year.
Because the HT and TT buffers allowed rapid electrophoretic separation of long RNAs, we next tested whether we could reduce the amount of formaldehyde used in the procedure. Formaldehyde serves primarily as a denaturing agent for RNA during agarose gel electophoresis. An additional useful property of formaldehyde is its inhibitory effect on RNases [5], which helps maintain RNA integrity during separation and gel handling. High concentrations (2.2 M) of formaldehyde typically used in RNA electrophoresis protocols were thought to ensure complete denaturation of GC-rich regions in large RNAs [6]. Others suggested that the reason for the high concentrations was to counteract the diffusion of formaldehyde out of gels during long runs [2, 3]. Testing different formaldehyde concentrations with RNA samples run in HT and TT buffers showed that 0.4 M formaldehyde in both gel and loading dye was sufficient to obtain well-separated pre-rRNAs (Fig. 1B, D), indicating that this concentration provides adequate denaturation of the large RNA molecules. However, it is important to tightly cover the gel casting assembly with plastic wrap during agarose solidification and only submerge gels in running buffer immediately before samples are loaded to prevent formaldehyde losses from the gels. The five-fold reduction in formaldehyde concentration in our system (Table 1) helps reduce exposure to this toxic chemical. As an additional benefit, we find that low-formaldehyde gels can be used directly for blotting to nylon membranes in 10×SSC, unlike high-formaldehyde gels that need to be incubated in water prior to transfer [2].
In agreement with reports indicating that EDTA is not required during DNA electrophoresis [8], we found that its presence in the buffer did not visibly affect separation of RNA in both HT and TT systems. However, we observed an abnormal migration of some RNA samples during electrophoresis when EDTA was omitted from the loading dye, presumably due to the presence of residual metal ions interfering with RNA denaturation. Therefore, EDTA was included in the loading dye, but not in the running buffer (Table 1). Our tests have also shown that glycerol in the loading dye is unnecessary because samples containing 50% formamide have a sufficient density to be underlayed into wells of a horizontal agarose gel. A convenient way to prepare such samples for electrophoresis is to start by dissolving RNA in 100% formamide, which has an additional benefit of protecting RNA from the degradation by RNases [7]. The following procedure reduces the number of pipetting steps and time required for sample preparation as compared with previously described procedures [2,3].
To prepare loading dye, add 50×HT or TT buffer stock solution (Table 1), 0.5M EDTA (pH 8.0) and bromophenol blue to deionized water to the final concentrations of 2.1× electrophoresis buffer, 1 mM EDTA, 0.04% bromophenol blue. Filter through a sterile 0.2 µm syringe filter. The loading dye prepared in advance can be stored at −20°C for at least one year.
When ready to proceed with electrophoresis, pipet the required amounts of RNA dissolved in formamide into microtubes or PCR strips.
Prepare the sufficient amount of the 2×loading master mix by combining 14 volumes of the loading dye with 1 volume of 37% formaldehyde. (Loading dye mixed with formaldehyde is not stable upon storage and must be used within a few hours.)
Add freshly prepared 2×master mix to each RNA sample (1 volume of master mix to 1 volume of RNA in formamide). Close tubes tightly, mix the contents, spin briefly in a microcentrifuge, heat at 70°C for 5 min to denature RNA, cool to room temperature and load samples into wells of a gel.
In conclusion, the method described here provides an easier way to run agarose-formaldehyde gels and helps to resolve long RNAs. The entire procedure requires fewer reagents and manipulations compared with traditional protocols, works for different gel formats and, in our experience, is sufficiently robust to work even in novices' hands. The HT and TT buffers are two alternative buffer systems that produce a good overall separation quality of RNA. Because of a typically higher cost of Tricine compared with Hepes, the TT formulation may be primarily useful in situations when an improved resolution of very large (>5 kb) RNAs is desired. The HT buffer makes a good all-around conductive medium for the separation of a wide range of RNA species.
Acknowledgements
We thank members of the Pestov laboratory for their helpful comments and suggestions. This work was supported by the NIH grant GM074091.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mullineux S, Lafontaine DLJ. Mapping the cleavage sites on mammalian pre-rRNAs: where do we stand? Biochimie. 2012;94:1521–1532. doi: 10.1016/j.biochi.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Sambrook J, Russel DW. Molecular cloning: a laboratory manual. 3rd ed. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 3.Brown T, Mackey K, Du T. Current protocols in molecular biology. Unit 4.9. John Wiley & Sons, Inc.; 2004. Analysis of RNA by northern and slot blot hybridization. [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Li X, Sommer SS. pK-matched running buffers for gel electrophoresis. Anal Biochem. 1999;270:112–122. doi: 10.1006/abio.1999.4064. [DOI] [PubMed] [Google Scholar]
- 5.Jonsson N, Lagerstedt S. The effect of formaldehyde containing fixatives on ribonuclease activity, Zeitschrift für Zellforschung und mikroskopische Anatomie Abt. Histochemie. 1959;1:251–256. [Google Scholar]
- 6.Lehrach H, Diamond D, Wozney JM, Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977;16:4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- 7.Chomczynski P. Solubilization in formamide protects RNA from degradation. Nucleic Acids Res. 1992;20:3791–3792. doi: 10.1093/nar/20.14.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brody JR, Kern SE. History and principles of conductive media for standard DNA electrophoresis. Anal Biochem. 2004;333:1–13. doi: 10.1016/j.ab.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 9.Pestov DG, Lapik YR, Lau LF. Current protocols in cell biology. Unit 22.11. John Wiley & Sons, Inc.; 2008. Assays for ribosomal RNA processing and ribosome assembly. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Pestov DG. 5'-end surveillance by Xrn2 acts as a shared mechanism for mammalian pre-rRNA maturation and decay. Nucleic Acids Res. 2011;39:1811–1822. doi: 10.1093/nar/gkq1050. [DOI] [PMC free article] [PubMed] [Google Scholar]