Abstract
Glutamate, a key neurotransmitter in the vertebrate retina, acts via ionotropic and metabotropic receptors. Retina expresses mRNA for all metabotropic glutamate receptors and proteins for all but mGluR3. Every retinal cell class expresses one or more of these receptors. In general, these receptors are present presynaptically and serve to modulate synaptic transmission. While mGluRs on the photoreceptor terminal act as autoreceptors to titer glutamate levels, those on horizontal cell processes seem to shape the light response. Similarly, autoreceptors on bipolar axon terminals modulate glutamate release and the receptors on amacrine and ganglion cells modulate feedforward signals by modulating K+ or Ca2+ current to fine tune light responses. Since most of the mGluR sub-types are present in amacrine and ganglion cells that belong to many cell types, the pathways downstream of mGluRs are highly diverse with primarily modulatory effects. An exception to most mGluRs which have modulatory function is mGluR6 because it plays a key role in the feedforward transmission from photoreceptors to ON bipolar cells and is also required for the correct localization of the synaptic proteins in the dendritic tips. In humans, mutations in the gene encoding mGluR6 cause autosomal recessive night blindness. In addition, mGluRs appear to play a trophic role in development and after retinal damage, suggesting potential future therapeutic implications.
In retina, glutamate serves as the main, or perhaps only, feedforward neurotransmitter. It is released by photoreceptors onto horizontal and bipolar cells and by bipolar cells onto amacrine and ganglion cells. This feedforward pathway from photoreceptors to bipolar cells to ganglion cells is heavily modulated by horizontal and amacrine cells that use numerous molecular mechanisms to provide feedback onto the various pathways. It is also modulated significantly by autoreceptors that are primarily metabotropic glutamate receptors. Eight metabotropic glutamate receptors are known, and all proteins but one appear to be expressed in the retina (Table 1 and Fig. 1). In general, these receptors are found presynaptically and serve to modulate synaptic transmission. However, one receptor, mGluR6, stands out because it plays a key role in the feedforward transmission from photoreceptors to a particular class of bipolar cells. Here we will review the localization and possible functions of the metabotropic glutamate receptors found in each class of retinal cell. Summary of functional pathways is given in Fig. 2.
Table 1. Expression and localization of mGluRs in retinas of different species.
References for the expression and localization of mGluRs in the cell types of the retina.
| Receptor | Molecular species | Photoreceptors | Horizontal cells | Bipolar cells | Amacrine cells | Ganglion cells |
|---|---|---|---|---|---|---|
| mGluR1 | mRNA | H95 (rat) | H95; T00 (rat) | |||
| Protein | CP99*1; KG01; SG06*2 (cat and chicken) | SG06; J07 (chicken and goldfish) | K97*3; SG06*4; J07 (rat, chicken and goldfish) | K97; CP99*5; KG01; SG06 (rat, cat and chicken) | KG01; CP99 (chicken and cat) | |
| mGluR2 | mRNA | O93; H95 (rat) | O93; H95*5; T00 (rat) | |||
| Protein | CP99*6; J07*6 (cat, goldfish) | K96*7; CP99*8 (rat and cat) | ||||
| mGluR3 | mRNA | HC 07 (primate) | T00 (rat) | |||
| Protein | ||||||
| mGluR4 | mRNA | H95 (rat) | A94; H95; T00 (rat) | |||
| Protein | K96; Q07*13 (rat and mouse) | K96 (rat) | ||||
| mGluR5 | mRNA | H95 (rat) | H95 (rat) | T00 (rat) | ||
| Protein | KG01; SG06*2 (chicken) | KG01; SG06 (checken) | K97*3; SG06*4 (rat and chicken) | K97; KG01; SG06 (rat and chicken) | KG01 (chicken) | |
| mGluR6 | mRNA | N93; A94; H95 (rat) | T00*9 (rat) | |||
| Protein | N94; VM97; V00 (rat and monkey);*10 | |||||
| mGluR7 | mRNA | A94 (rat); H95 | A94; H95 (rat) | A94; H95*5; T00 (rat) | ||
| Protein | J07 (goldfish) | B96*11; J07*5 (rat and goldfish) | B96; Q07*13 (rat and mouse) | |||
| mGluR8 | mRNA | D95 (mouse) | D95 (mouse) | D95 (mouse) | D95; T00 (mouse and rat) | |
| Protein | K99; KB02 (mouse and rat) | KB02 (mouse and rat) | K99; KB02; Q07*12, 13 (rat and mouse) | K99; KB02 (rat and mouse) |
Species tested is in parenthesis.
The starred numbers refer to the information listed below.
- receptor was found only in rods;
– receptor was found in cones but not rods;
- receptor was found in rod bipolar cells and rarely in cone bipolar cells;
– receptor was found postsynaptic to cones but not rods.
- receptor was found only in a few cells of this class, postsynaptic to cone bipolar cells;
– antibody recognized both mGluR2 and 3;
- receptor was found specifically in cholinergic amacrine cells;
- receptor was found postsynaptic to cone bipolar cells and in A17 cells postsynaptic to rod bipolar cells;
- mRNA was found only during development and after axotomy;
- numerous articles reported expression of this receptor in ON bipolar cells; only the originals are cited;
- receptor was found in cone bipolar, but not rod bipolar cells;
– receptor was found in several types of amacrine cells, including the OFF but not the ON Chat.
localization of mGluR4, 7, 8 were punctate in IPL; puncta of different receptors did not colocalize, but no discrimination between amacrine or ganglion cells was made.
Fig. 1. Localization of mGluRs in the retinal circuits of the rodent retina.
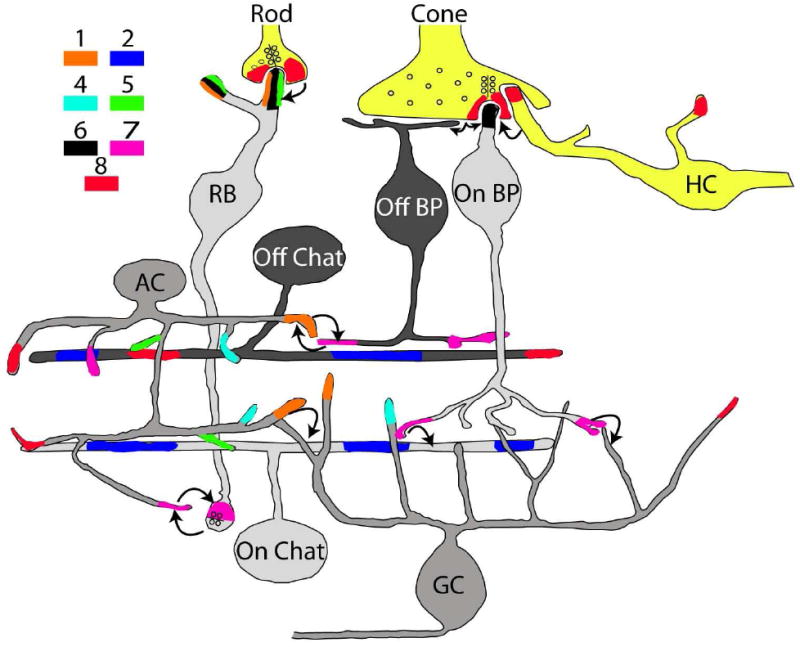
All but mGluR3 proteins are expressed in this retina. A single cell type often expresses more than one mGluR. However, localization of mGluRs has often been assigned to a class rather than a single type of cell, so many of the seemingly co-expressed receptors may segregate to cell types. The rod bipolar cell (RB) likely represents a single cell type; the OFF cone bipolar cell (Off BP) and the ON cone bipolar cell (On BP) each contains 4-5 types. The cholinergic amacrine cells (Chat) have two subtypes (ON and OFF). The prototypic amacrine cell (AC) and the ganglion cell (GC) in this scheme represent about 20 types of ON and OFF for each cell class. Lightly shaded cells are ON cells (depolarize to light) while darkly shaded cells are OFF (hyperpolarize). AC and GC are shaded in a medium tone to indicate that they represent both ON and OFF types. Photoreceptors (Rod and cone) and horizontal cells (HC) are colored yellow because, although they hyperpolarize to light, they are neither ON nor OFF. Colored areas at the various terminals indicate localization of mGluRs and the color key shows which particular type of mGluR. Arrows designate general information transfer via feedforward and feedback synapses.
Fig. 2. Similarity and diversity of specific mGluR pathways in the retina.
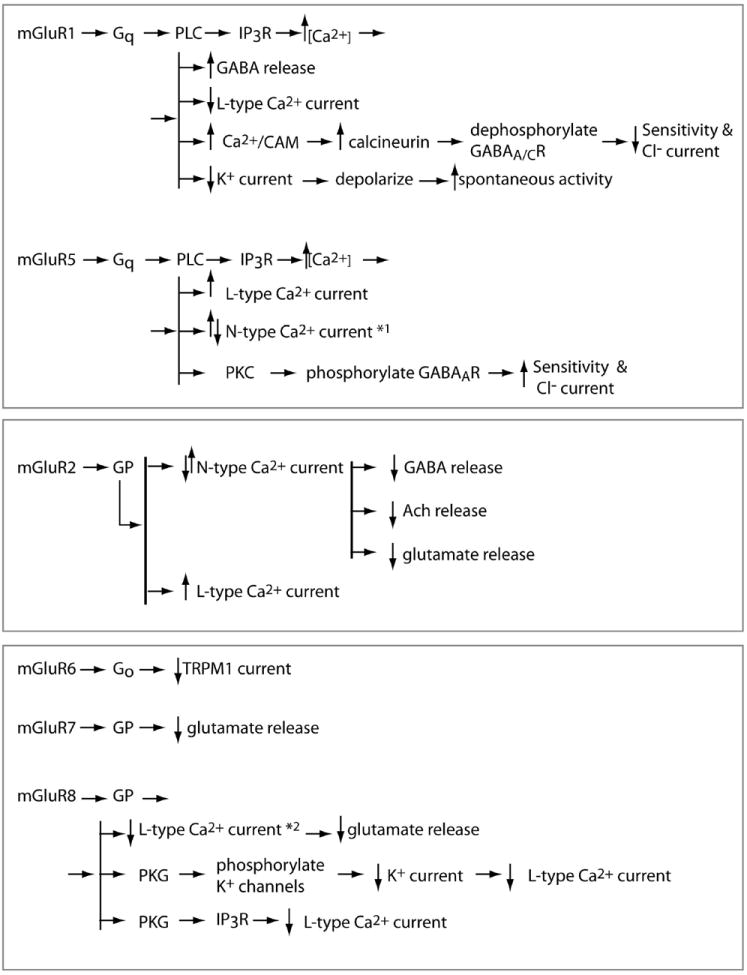
All mGluRs modulate either neurotransmitter release or channel current. When the receptor is located presynaptically, it acts as an autoreceptor that decreases glutamate release. When it is located postsynaptically, it acts to either increase or decrease neurotransmitter release, depending on the type and/or state of the cell. Correspondingly, modulation of the channel current can go either way, typically via phosphorylation or dephosphorylation. *1: this effect was attributed to group I and not necessarily to mGluR5. *2: modulation of the L-type Ca+2 current was specifically shown as a shift of its activation curve. PLC, phospholipase C; IP3R, inositol 1,4,5-tri-phosphate receptor; PKC, protein kinase C; GP, an unidentified G-protein; PKG, cGMP-dependent protein kinase. Arrows indicate either a direct or indirect effect.
1. The first synaptic layer: synapses from photoreceptors to second order cells
There exist two classes of photoreceptor: rods that are responsible for night vision, and cones that are responsible for daylight vision. These photoreceptors transduce light energy into voltage changes and transmit this information forward to horizontal cells and to two classes of bipolar cells, the ON class that depolarizes to light and the OFF class that hyperpolarizes to light. The number of horizontal cell or bipolar cell types vary greatly between species, but the basic structure of the synaptic complex remains similar: a presynaptic photoreceptor terminal houses synaptic ribbons that define the synaptic release sites, and each such site lies presynaptic to a triad. The triad consists of two lateral elements belonging to horizontal cell processes, and one central element belonging to the dendrite of an ON bipolar cell (Fig. 1). Dendrites of OFF bipolar cells contact the terminals some 0.5-1 μm away from the ribbon site. While the photoreceptor terminal is clearly presynaptic and the bipolar dendrite is clearly postsynaptic, the horizontal cell processes are both pre- and postsynaptic.
1.1 Presynaptic mGluRs in photoreceptors
A number of studies report that metabotropic glutamate autoreceptors modulate transmitter release from photoreceptors. However, the details differ somewhat, especially between higher and lower vertebrates. In higher vertebrates, studies have concentrated on rat rods. Calcium imaging from isolated rods has shown that glutamate, as well as the specific agonists of group III mGluRs [L-2-amino-4-phosphonobutyrate (L-AP4) and L-serine-O-phosphate], but not the agonists for other glutamate receptors, decreases intracellular calcium concentration 1. This mGluR-evoked effect depends on extracellular calcium and is mediated by a pertussis toxin-sensitive G-protein, possibly transducin (Gt), whose main function is to mediate phototransduction in the outer segment. The immediate down-stream effector for this G-protein is unknown, but clearly a decrease in calcium concentration will tend to reduce glutamate release. Understanding the exact function of such feedback in image processing requires understanding its time constant and sensitivity. Nonetheless, it is clear that this feedback will help to regulate the glutamate concentration in the cleft and thus may be crucial for reducing toxicity.
In lower vertebrates, studies have been performed on several species, and the key finding from higher vertebrates, that group III mGluRs provide autofeedback in photoreceptors, holds true for these species as well. A nice study performed in newt retina showed that mGluR group III agonists shift the cone calcium activation curve to higher voltages via a G-protein cascade, and thereby reduce the presynaptic calcium current. This in turn reduces the light-off-activated inward current in second order cells (tested in horizontal cells and OFF bipolar cells) 2. Interestingly, unlike mammalian rods, newt rods are not affected by L-AP4. Similar feedback was observed in carp retina where L-AP4’s effect was evaluated by quantifying the frequencies and amplitudes of mEPSPs recorded from a cone horizontal cell 3. L-AP4 reduces mEPSP frequency, indicating a presynaptic effect and a reduced Ca2+ influx in the cones. In this case, the reduced Ca2+ current does not result from direct modulation of the Ca2+ conductance, but from modulation of the K+ conductance. Group III receptors contribute to autofeedback also in salamander, however group II receptors play the major role 4,5.
The best candidate for the group III receptor in photoreceptor terminals is mGluR8. Its mRNA has been shown in photoreceptors 6 and the protein has been localized to rat rod and cone terminals at their presynaptic membrane 1,7,8. Although a newer study in mouse did not find mGluR8, nor did it find mGluR4, or mGluR7 in the outer plexiform layer 9, it is possible that the antibodies used by Quraishi et al are not as sensitive as those used by Koulen et al.
A major problem with studying lower vertebrates is that the appropriate reagents (such as antibodies, agonists, or antagonists) needed to study their receptors have not been developed, so experimenters must assume that the receptors behave similarly to their homologous mammalian counterparts. Although specific examples of different pharmacological profiles have been illustrated 2, such assumptions are often justified because recordings from retinal cells in lower vertebrates are relatively easy and reliable, allowing more thorough experimental protocols.
1.2 Postsynaptic mGluRs in horizontal cells
Immunocytochemical experiments show that rat horizontal cells express mGluR8 8, but there is no corroborating physiological evidence. Interestingly, electron microscopy shows that only one of the two horizontal cell processes in a triad stains for mGluR8. This suggests that the cone’s input at the two horizontal cell processes (each originating from a different horizontal cell) may be modulated differently. The contribution of mGluRs to the signal processing in horizontal cells has been elaborately studied in lower vertebrates, particularly in catfish. Consistent with the findings in rat, in catfish, an agonist for mGluR group III, L-AP4, but not for groups I and II, reduces the current of an inwardly rectifying K+ channel (IRk+) 10. This response is dependent on phosphorylation by a cGMP-dependent kinase. In other studies IRk+ current is shown to contribute to the dark resting potential in horizontal cells, and modulation of this current by glutamate is shown to speed up the hyperpolarizing light response 11. In mudpuppy, L-AP4 affects a horizontal cell’s responses to spot versus annulus stimulation differently. This differential result has been interpreted to indicate an increase of the apparent electrical coupling between horizontal cells 12. Since this coupling may be a consequence of gap junction phosphorylation by mGluR-activated kinase, the group III mGluRs (possibly mGluR8) located on horizontal cells may serve to shape the light response of these cells.
Another study on catfish horizontal cells found yet another effect. In this study, agonists for mGluR groups I and III increase the sustained calcium current 13. An attempt to identify the receptors gave positive staining for mGluR1α, mGluR5, and mGluR6 14. In chick horizontal cells, positive immunostaining is seen for mGluR1 and mGluR5 15. However, since tissue lacking these receptors cannot be tested, these immunostainings may be nonspecific.
1.3 Postsynaptic mGluRs in bipolar cells
1.3.1 ON bipolar cells
ON bipolar cells comprise nearly three-quarters of all bipolar cells, and they hyperpolarize to glutamate released continuously during darkness and depolarize to light onset. Experiments with the glutamate analog L-AP4 have demonstrated the existence of a novel type of glutamate receptor in these cells 16. This receptor was shown to couple to a G protein cascade 17. Within a few years the receptor was cloned and termed mGluR6, a member of the group III metabotropic glutamate receptor family 18. Evidence that supports the key role of mGluR6 in the light signaling of ON bipolar cells includes its localization, the effect of its deletion in a mouse model, and the effect of spontaneous mutations in mice and humans.
1.3.1a. mGluR6 is restricted to the dendritic tips of ON bipolar cells and is required for their light response
Immunohistochemistry and electron microscopic studies localize mGluR6 specifically to the dendritic tips of all types of ON bipolar cells 19-21. A study conducted in primate retina showed that, within the dendritic tips of ON cone bipolar cells, mGluR6 clusters about 400 nm away from the release site and not directly under it, placing these receptors at a distance similar to that of OFF cone bipolar dendritic receptors 20. This restricted expression of mGluR6 is developmentally regulated as these receptors are expressed throughout the bipolar soma early in retinal development, gradually increase in the outer plexiform layer (OPL) around the time of eye opening (day 14), and are finally confined to the OPL by day 28 19.
The first direct evidence for the critical role of mGluR6 in ON bipolar cells came from a study showing that mice lacking mGluR6 lack the ERG b-wave, the wave that indicates ON bipolar cell activity 22. Consistent with this data, spontaneous mutations in the mouse Grm6 gene (nob3 and nob4) lead to negative ERG waveforms and visual behavior abnormalities 23. Similarly, in human, certain mutations in the GRM6 gene lead to the autosomal recessive complete form of congenital stationary night-blindness (arCSNB) 24,25.
1.3.1b. The mGluR6 cascade and its dual role in the ON bipolar cell pathway
A key feature of the photoreceptor to ON bipolar synapse is the reversal of the signal sign from light-induced hyperpolarization in photoreceptors to depolarization in ON bipolar cells. Crucial to this ‘sign inverting’ synapse is mGluR6 and its cascade (Fig. 3). mGluR6 couples strongly to Gαo, the G protein that is critical for the ON bipolar cascade 26-28. Activation of mGluR6 by glutamate produces activated Gαo, particularly Gαo1 29, and this in turn leads to closure of TRPM1, a non-selective cation channel that is presumably constitutively active 30,31. Gαo2, another splice variant of Gαo, improves the sensitivity of the response 32. Several other proteins that appear to play a modulatory or scaffolding role in this cascade include: Gß5, RGS11, RGS7, R9AP, Ret-PCP2, nyctalopin, and possibly Ret-RGS1 (Fig 3) 33-35.
Fig. 3. Protein complex and mGluR6 cascade in ON bipolar cells.
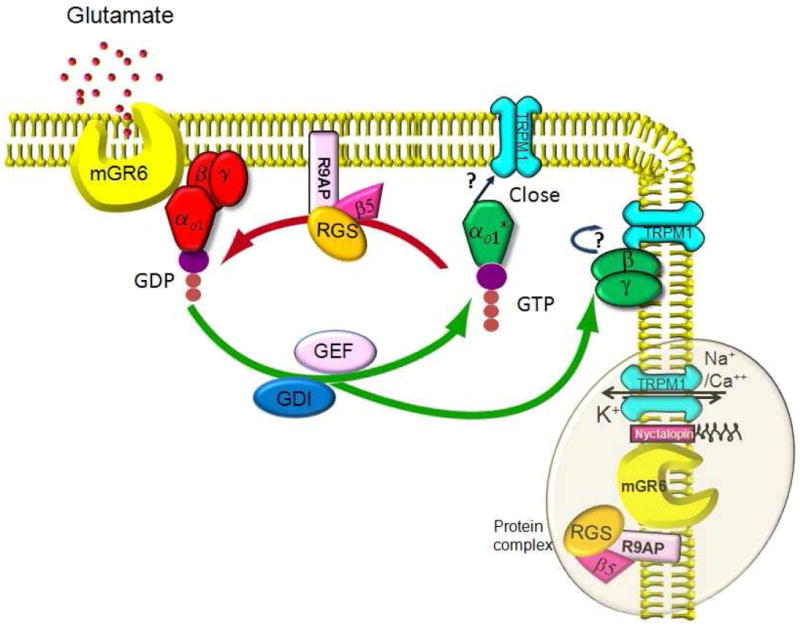
Glutamate released by photoreceptors binds to mGluR6 receptors on the dendritic tips of ON bipolar cells and activates the G protein Go. The α subunit of this G protein is activated by binding to GTP and dissociates from the βγ partners. Either α or βγ closes the TRPM1 channel and hyperpolarizes the cell. Several molecules modulate this cascade. These include Ret-PCP2 which may act as a GEFs (guanine nucleotide exchange factor) or GDI (guanine nucleotide dissociation inhibitor); RGS (regulator of G-protein signaling) including RGS7, RGS11, and possibly Ret-RGS; Gβ5, an atypical G protein β subunit; and R9AP, a transmembrane protein associated with anchoring. mGluR6 associates with the RGS11/Gß5/R9AP heterotrimer and helps stabilize the complex at the dendritic tips. mGluR6 also makes a complex with a scaffolding protein, nyctalopin, which interacts with TRPM1 and thus may help to bring about a fast response. These protein complexes (represented in the oval) are localized to the dendritic tips.
Besides playing a direct role in initiating the signaling cascade by binding to glutamate, mGluR6 also has an important function in the post-synaptic localization of several cascade proteins. mGluR6 interacts with the heterotrimeric complex of RGS11/Gß5/R9AP and is required for the proteolytic stability of this complex 34. mGluR6 also interacts with a scaffolding protein, nyctalopin, that interacts with TRPM1 36. Thus, it is conceivable that this mGluR6/ nyctalopin/ TRPM1 ternary complex leads to the compartmentalization of cascade elements to the dendritic tips of the ON bipolar cells, enabling the fast kinetics of signal transmission. The exact mechanism and role of synaptic activity in this process awaits further research.
1.3.1c. Targeting of mGluR6 to the ON bipolar dendritic tips
Given that mGluR6 is positioned ideally to sense glutamate released by photoreceptors and since it is responsible for the correct targeting of certain signaling complexes, it is important to understand the mechanism that targets mGluR6 itself. Localization and surface expression of many neurotransmitter receptors are governed by the receptor’s interaction with proteins that have pdz domains. These proteins help concentrate the transduction cascade components into tight complexes that enable a fast response. They may also play an important role in receptor cycling and thereby may regulate synaptic strength and neuronal function. Two pdz domain-containing proteins, GRIP and syntenin, have been shown to interact with mGluR6 in in vitro assays 37. However, whether or not they coexist with mGluR6 in ON bipolar cells and play a role in the ON pathway remains to be seen. In contrast, other pdz domain-containing proteins, such as Homer1 and ProSap family members, have been seen at the ON bipolar dendritic tips, but no interactions between these proteins and mGluR6 are known 38.
The specific targeting of mGluR6 alters after photoreceptor degeneration or when mutations silence the signaling from photoreceptors to bipolar cells. For example, during the early stages of photoreceptor degeneration in rats with retinal dystrophy, mGluR6 expression is diffuse and is no longer restricted to the dendritic tips 19. Similarly, mutations in bassoon, a presynaptic protein present on synaptic ribbons, or in presynaptic calcium channels (Cacna1f) result in reduced immunostaining for several postsynaptic proteins including mGluR6. In contrast, mutations of the ON bipolar cascade elements (TRPM1, RGS7, RGS11, nyctalopin, or Gαo) do not significantly affect the localization of mGluR6 27,31,34,35,39. Taken together, these studies suggest that the specific postsynaptic distribution of mGluR6 in the dendritic tips requires a signal from intact photoreceptors and that disruption of the photoreceptor to bipolar synaptic complex hinders the normal localization of mGluR6.
1.3.1d. mGluR6 deletion or mutation leads to molecular and functional defects in the ON bipolar cell
Under light microscopy, the general structure of the retina and the ON bipolar cells in mGluR6 knockout mice appear normal 22,40. However, ultrastructural analysis of nob4, a mouse with a mutation in mGluR6, shows a reduced number of invaginating rod bipolar dendrites 34. Moreover, expression of the cascade elements that normally localize at the dendritic tips is affected. For example, in nob4 mouse, Gß5, RGS7, RGS11, R9AP, and TRPM1 are either down-regulated or redistributed 34,41. In mGluR6-null mice, although this ultrastructural analysis has not been done, several proteins, including Gß5, RGS11, and TRPM1, fail to concentrate in the dendritic tips of the ON bipolar cell (unpublished data). Further abnormalities are found in the dendrites of the rod and ON cone bipolar cells as they contain ectopic synaptic ribbons. Moreover, the dendrites of ON cone bipolar cell express mGluR7, a receptor that is typically restricted to the axon terminals 42. At the functional level, mice lacking mGluR6 lack the ERG b-wave, and have impaired pupillary responses, optokinetic nystagmus, and behavioral responsiveness to light 43.
1.3.1e. Genomic organization and splice variants
The human mGluR6 gene consists of 16,742 bp that encompass 10 exons separated by 9 introns, and it encodes for an 877 amino acid protein44. A 9.5 kb upstream genomic region comprises the regulatory elements important for cell-specific and developmentally-regulated expression of the protein 45. Rodent and human retina also express alternatively spliced variants of mGluR6 that code for truncated isoforms. The alternate splice variant in mouse and rat (mGluR6b), and the two splice variants in human (hmGlu6b, hmGlu6c) are predicted to code for proteins containing 545, 508, 425 and 405 amino acids, respectively 46. The predicted truncated protein in these species contains the N-terminus extracellular domain of the receptor, and this could be secreted to act as a physiological regulator of glutamate-dependent synaptic signaling. However, its role in the ON bipolar cascade is yet to be elucidated.
1.3.1f. ON bipolar cells also express Group I mGluRs
Although mGluR6 is the dominant glutamate receptor found in bipolar cells, other metabotropic receptors have been seen. Rod bipolar cells in rat and mouse, and cone bipolar cells in chicken express mGluR1a and mGluR5a in their dendrites 47-49. ON mixed-input bipolar cell processes in fish also express mGluR1a and there, the receptor appears to serve a modulatory role 50,51. However, it is not known if Gαo1 mediate the response, or if these receptors couple to Gαo2 or Gαi2, G-proteins whose mRNA has been seen in bipolar cells 28,29. Furthermore, the downstream effectors or function is still unclear.
1.3.2. OFF bipolar cells
This class of bipolar cell senses glutamate mainly via AMPA/Kainate receptors. Joselevitch et al. (2007) reported that goldfish OFF bipolar dendrites stain for mGluR7, and Koulen et al. (2002) found that about 1 out of 10 rat OFF bipolar dendrites expresses mGluR8a.
2. The second synaptic layer: synapses from bipolar cells to third order cells
The second synaptic layer contains numerous and diverse types of synapses that connect about 10 types of bipolar cells to about 20 types of amacrine and 20 types of ganglion cells. Similar to the photoreceptor, the bipolar axon terminal uses a presynaptic ribbon that coordinates glutamate release onto postsynaptic amacrine and ganglion cells at a structure called a “dyad”. This synaptic layer also contains numerous types of feedback synapses from amacrine cells onto bipolar and ganglion cells. Thus, in this layer, bipolar cell axon terminals are clearly presynaptic, ganglion cell dendrites are postsynaptic, but amacrine cell processes can be presynaptic, postsynaptic, or both in the same compartment (Fig. 1).
2.1 Presynaptic mGluRs in bipolar cells
As with the autofeedback system of the photoreceptor, mGluRs, particularly those from group III, serve as autoreceptors at the synapse from the bipolar axon terminal to the ganglion cells. Support for this comes from work on salamander retina where recordings were taken from OFF ganglion cells to avoid the inevitable effect of modulating the mGluR6 of ON bipolar cells 4,52. These studies showed that L-AP4 reduces the frequency of spontaneous mEPSPs and even reduces the response evoked by turning off the light. However, the autofeedback in these bipolar cells is complicated by the precise parameters of the light stimulus. L-AP4 does not affect the response to turning off a dim or a strong light, but only a middle intensity light. Furthermore, L-AP4 may enhance the response to turning off a short light stimulus, but not to turning off a long light stimulus. Though a precise function for glutamate autoreceptors cannot be concluded at this time, the general function is clear. Autoreceptors reduce glutamate release from bipolar cell terminals, perhaps to shape the light response and to protect the retina from toxic glutamate levels.
The receptor at the bipolar cell axon terminal is most likely mGluR7. The mRNA for this receptor was found in rat bipolar cells 53,54. Moreover, the protein was localized to the bipolar cell presynaptic membrane, a staining that disappeared in the mGluR7-null retina 55. Examining mGluR7 localization in bipolar cells led to three interesting observations: (i) mGluR7 localizes to ON and OFF cone bipolar cells, but not to rod bipolar cells; (ii) the receptor localizes to only one side of the dyad, i.e., it opposes only one of the two postsynaptic dendrites; and (iii) when ON cone bipolar cells lose mGluR6, their dendrites gain mGluR7 staining near the abnormally present ectopic ribbons 42. The localization of mGluR7 to only one side may have far reaching consequences in signal transmission, because the two postsynaptic elements may get input that is only partially rather than completely correlated. Although mGluR7 localization to the bipolar cell axon terminals is quite certain, it does not fit the physiological finding that the presynaptic receptor is more sensitive than mGluR7 is known to be 4. However, it is possible that receptors in native tissue have different sensitivities than those in cell lines.
2.2 Pre- and postsynaptic mGluRs in amacrine cells
There are over 20 types of amacrine cells in the retina, and they house most of the mGluR types (Table 1 and Fig. 1). This suggests that mGluRs play a diverse role in signal processing, but difficulty in identifying amacrine cell types constrains current knowledge of their function. The action of mGluRs on amacrine cells has mostly been studied in lower vertebrates and chick. A common outcome of stimulating amacrine cells with group I agonists is a slow increase of intracellular calcium, most likely via the PLC-IP3 pathway. Examples to the contrary are given below. The main effect of agonists of the other groups is also a decrease in release, but probably by a different chemical pathway (Fig. 2). A short review of mGluRs in amacrine cells was published recently 56. Some examples of the physiological effects mediated via mGluRs in amacrine cells are given below.
2.2a. Effect of Group I mGluRs
Goldfish ON bipolar cells have particularly large axon terminal, making them an easy target for studying synaptic properties. These bipolar cells, as all bipolar cells in all species, form reciprocal synapses with GABAergic amacrine cells. Recordings from these cells showed that when they release glutamate over a long time period, the GABAergic feedback that the reciprocal amacrine cell synapse exerts on them is enhanced 57. This effect of glutamate was attributed to mGluR1, which presumably stimulates PLC, increases intracellular calcium, and hence increases GABA release. This enhanced inhibition may contribute to slow adaptation.
Elevation of calcium was also seen in chick amacrine cells in culture 58, but the downstream effects are diverse. In some cases, subsequent to release from stores, mGluR5 further elevates calcium by increasing the L-type calcium current 59. And while mGluR5 tends to elevate calcium, mGluR1 reduces this elevation 15. Pathways further diverge because calcium has multiple effects in cells, so the downstream effects of calcium elevation are different in different cells.
Recordings from isolated calmodulin-positive wide field amacrine cells in white bass showed that glutamate or mGluR1 agonists reduce the response of these cells to applied GABA. This effect is slow, and is likely mediated via calcium as follows: mGluR1 → Gq → PLC → IP3R → Ca2+ → Ca/calmodulin → calcineurin → dephosphorylates GABAA receptor and reduces its sensitivity 60. Rats display a similar modulation of the GABAC receptor 61. In contrast, mGluR5 in cultured amacrine cells enhances GABAA receptor current, probably due to phosphorylation by protein kinase C 58.
2.2b. Effect of Group II mGluRs
A unique and interesting type of amacrine cell is the cholinergic cell (also called the “starburst”). Starburst amacrine cells are divided into two subtypes, the ON and the OFF. Starburst cells draw much attention in the retina field because they release both GABA and acetylcholine; they respond preferentially to light or dark bars that move centrifugally; and they are an essential component of the circuit that constructs directional selectivity in the directional selective ganglion cells 62. Relevant to mGluRs, these amacrine cells express mGluR2 40,63,64. When a group II mGluR agonist is applied to the retina, the directional selective ganglion cell loses its directional selectivity, and this is probably due to inhibition of GABA release. When a group II antagonist is applied, this cell’s response to its preferred direction is reduced 65. It appears therefore, that glutamate not only provides the major light signal to the starburst and ganglion cells (via ionotropic receptors), but that it also modulates the starburst output (via mGluR2) to further increase the ganglion cell’s response to the preferred direction.
2.2c. Effect of Group III mGluRs
Examples of Group III mGluRs in amacrine cells are few. In one example, recordings from OFF and ON-OFF ganglion cells show that applying mGluR8-specific agonist, DCPG, greatly reduces their OFF response, but only slightly affects the ON response of ON or ON-OFF ganglion cells 9. This effect appears presynaptic to ganglion cells, i.e., it occurs either on bipolar cell terminals or on amacrine cells. However, because mGluR8 is probably not localized to ON bipolar cells, DCPG most likely affects the ganglion cells through the amacrine cells. This agrees with the observation in other systems, including chick cultured amacrine cells, that application of L-AP4 suppresses the calcium current and consequently transmitter release 66.
2.3 Postsynaptic mGluRs in ganglion cells
Ganglion cells collectively (i.e., different types and different species) express most of the mGluR proteins (see Table 1). Experiments designed to investigate the mGluRs in these cells without interference from the network generally use acutely cultured or isolated ganglion cells. In Xenopus, group I mGluRs reduce voltage-activated calcium current, in particular the L-type 67. In mouse, however, this group (and possibly also group III) may enhance or reduce N channel current 68. In rat, mGluR1 decreases K+ current; this in turn increases the input resistance of the cells, depolarizes them and increases their spontaneous activity 69. Calcium is also elevated, but since TTX blocks this elevation, this elevation must be due to the depolarization.
Group II (specifically mGluR2) generally acts to increase low voltage-activated calcium current salamander, 70, rat, 71, but it may reduce N-type calcium current 72. In salamander, Group III reduces L-type calcium current, an effect that interestingly appears to be mediated by the IP3 receptor 70.
3. mGluRs and retinal disorders
Certain mutations in the GRM6 gene (encoding mGluR6 protein) lead to autosomal recessive congenital stationary night blindness (arCSNB) that is characterized by complete loss of night vision 25. This defect is consistent with the absence of the ERG b-wave under dark adapted conditions in mice lacking mGluR6. In families with arCSNB, a total of 10 disease-causing sequence variations have been observed in the GRM6 gene. These include mis-sense, frame shift and non-sense mutations 73. The mis-sense mutations (P46L, G58R, G150S, I405T, C522Y, and E781K) in the GRM6 gene have been analyzed in HEK cells 73 or in neuronal cultures 74, and most of them seem to affect the trafficking of the mGluR6 receptor to the plasma membrane. One mutation in rat mGluR6 (E775K, corresponding to E781 in human) produces an inability of mGluR6 to couple to Gαo, but it does not alter its cell surface expression or its affinity for glutamate.
GRM8, the gene for the mGluR8 protein, is considered to be a strong positional candidate for retinitis pigmentosa 10 (RP10). This is because the GRM8 gene is an exceptionally large (~1Mb) gene that maps to the same region as the mutation that causes RP10, and because it is expressed in retinal photoreceptors 75 (see Table 1).
A few studies have suggested that mGluRs play some trophic or neuro-protective role; however, evidence for this role is rather indirect. First, in juvenile rats, subcutaneous administration of DL 2-amino-3-phosphono-propionic acid (AP3), a mGluR antagonist, has retinotoxic effects 76. The authors suggest that glutamate triggers a number of second messenger cascades that may be vital for growth, development and survival of the neurons, and these are inhibited by DL-AP3. However, this study does not conclusively demonstrate the mechanistic basis of the effect, i.e., whether the effect is mediated via a generalized expression of DL-AP3 sensitive receptors throughout the retina or via their expression in cells that play a supportive role for developing retina. Second, in rat retina, activation of mGluRs reduces the toxic effect of N-methyl-D-aspartate (NMDA) 77. Third, neuronal injuries lead to adaptive changes in mGluR expression that resemble the pattern in development. Developing ganglion cells express transcripts for all types of mGluRs, and several of these downregulate in adulthood. Upon neuronal injury or ganglion cell axotomy, this developmental downregulation appears to reverse. For example, mGluR6 mRNA, which is not present in adult RGCs, reappears after axotomy; similarly, mGluR1 and -7 are more abundant in axotomized retina than in control 78.
4. Concluding remarks
While the function of mGluR6 is known, largely due to its crucial and direct role in the ON bipolar cell cascade, the precise role of other mGluRs is only beginning to be elucidated. This understanding will greatly benefit from newly developed specific ligands and novel approaches to study receptor biology. The large diversity of mGluRs in retina, with almost all cell types expressing these receptors at the pre- and/ or post-synaptic sites, suggests multitude of functions in governing responses to glutamate, ranging from feedforward signaling to modulatory or fine-tuning responses. These multitude of functions most likely also occur in development and in diseased tissues. Consequently, better understanding of the mGluR pathways and their role in development and disease in retina is expected to help design therapeutic strategies for retinal diseases in future.
References cites
- 1.Koulen P, Liu J, Nixon E, Madry C. Invest Ophthalmol Vis Sci. 2005;46(1):287. doi: 10.1167/iovs.04-0963. [DOI] [PubMed] [Google Scholar]
- 2.Hosoi N, Arai I, Tachibana M. J Neurosci. 2005;25(16):4062. doi: 10.1523/JNEUROSCI.2735-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirasawa H, Shiells R, Yamada M. J Gen Physiol. 2002;119(1):55. doi: 10.1085/jgp.119.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgs MH, Romano C, Lukasiewicz PD. Neuroscience. 2002;115(1):163. doi: 10.1016/s0306-4522(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 5.Higgs MH, Lukasiewicz PD. Vis Neurosci. 2002;19(3):275. doi: 10.1017/s0952523802192054. [DOI] [PubMed] [Google Scholar]
- 6.Duvoisin RM, Zhang C, Ramonell K. Journal of Neuroscience. 1995;15:3075. doi: 10.1523/JNEUROSCI.15-04-03075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koulen P, Kuhn R, Wassle H, Brandstatter JH. Proceedings of the National Academy of Sciences USA. 1999;96(17):9909. doi: 10.1073/pnas.96.17.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koulen P, Brandstatter JH. Invest Ophthalmol Vis Sci. 2002;43(6):1933. [PubMed] [Google Scholar]
- 9.Quraishi S, Gayet J, Morgans CW, Duvoisin RM. J Comp Neurol. 2007;501(6):931. doi: 10.1002/cne.21274. [DOI] [PubMed] [Google Scholar]
- 10.Dixon DB, Copenhagen DR. J Neurosci. 1997;17(23):8945. doi: 10.1523/JNEUROSCI.17-23-08945.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong CJ, Werblin FS. J Neurophysiol. 1995;74(6):2258. doi: 10.1152/jn.1995.74.6.2258. [DOI] [PubMed] [Google Scholar]; Tachibana M. J Physiol. 1985;358:153. doi: 10.1113/jphysiol.1985.sp015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong CJ, McReynolds JS. Vision Res. 1989;29(5):541. doi: 10.1016/0042-6989(89)90040-0. [DOI] [PubMed] [Google Scholar]
- 13.Linn CL, Gafka AC. J Neurophysiol. 1999;81(2):425. doi: 10.1152/jn.1999.81.2.425. [DOI] [PubMed] [Google Scholar]
- 14.Gafka AC, Vogel KS, Linn CL. Neuroscience. 1999;90(4):1403. doi: 10.1016/s0306-4522(98)00512-0. [DOI] [PubMed] [Google Scholar]
- 15.Kreimborg KM, Lester ML, Medler KF, Gleason EL. Journal of Neurochemistry. 2001;77:452. doi: 10.1046/j.1471-4159.2001.00225.x. [DOI] [PubMed] [Google Scholar]
- 16.Slaughter MM, Miller RF. Science. 1981;211:182. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]; Shiells RA, Falk G, Naghshineh S. Nature. 1981;294(5841):592. doi: 10.1038/294592a0. [DOI] [PubMed] [Google Scholar]
- 17.Shiells RA, Falk G. Proceedings of the Royal Society (London) B. 1990;242:91. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]; Nawy S, Jahr CE. Nature. 1990;346:269. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- 18.Nakajima Y, et al. Journal of Biological Chemistry. 1993;268(16):11868. [PubMed] [Google Scholar]
- 19.Nomura A, et al. Cell. 1994;77:361. doi: 10.1016/0092-8674(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 20.Vardi N, Duvoisin RM, Wu G, Sterling P. Journal of Comparative Neurology. 2000;423:402. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Vardi N, Morigiwa K. Visual Neuroscience. 1997;14:789. doi: 10.1017/s0952523800012736. [DOI] [PubMed] [Google Scholar]
- 22.Masu M, et al. Cell. 1995;80:757. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- 23.Maddox DM, et al. J Physiol. 2008;586(Pt 18):4409. doi: 10.1113/jphysiol.2008.157289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, et al. Mol Vis. 2009;15:2094. [PMC free article] [PubMed] [Google Scholar]; Zeitz C, et al. Invest Ophthalmol Vis Sci. 2009;50(12):5919. doi: 10.1167/iovs.09-3548. [DOI] [PubMed] [Google Scholar]
- 25.Zeitz C, et al. Invest Ophthalmol Vis Sci. 2005;46(11):4328. doi: 10.1167/iovs.05-0526. [DOI] [PubMed] [Google Scholar]; Dryja TP, et al. Proc Natl Acad Sci U S A. 2005;102(13):4884. doi: 10.1073/pnas.0501233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawy S. Journal of Neuroscience. 1999;19(8):2938. doi: 10.1523/JNEUROSCI.19-08-02938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhingra A, et al. Journal of Neuroscience. 2000;20:9053. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian L, Kammermeier PJ. Vis Neurosci. 2006;23(6):909. doi: 10.1017/S0952523806230268. [DOI] [PubMed] [Google Scholar]
- 29.Dhingra A, et al. Journal of Neuroscience. 2002;22(12):4878. doi: 10.1523/JNEUROSCI.22-12-04878.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen Y, et al. J Neurosci. 2009;29(19):6088. doi: 10.1523/JNEUROSCI.0132-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Morgans CW, Brown RL, Duvoisin RM. Bioessays. 2010;32(7):609. doi: 10.1002/bies.200900198. [DOI] [PMC free article] [PubMed] [Google Scholar]; Morgans CW, et al. Proc Natl Acad Sci U S A. 2009;106(45):19174. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]; Koike C, et al. Cell Calcium. 2010;48(2-3):95. doi: 10.1016/j.ceca.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Koike C, et al. Proc Natl Acad Sci U S A. 2010;107(1):332. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okawa H, et al. J Gen Physiol. 2010;136(4):443. doi: 10.1085/jgp.201010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao A, Dallman R, Henderson S, Chen CK. J Neurosci. 2007;27(51):14199. doi: 10.1523/JNEUROSCI.4934-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; Morgans CW, et al. Eur J Neurosci. 2007;26(10):2899. doi: 10.1111/j.1460-9568.2007.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mojumder DK, Qian Y, Wensel TG. J Neurosci. 2009;29(24):7753. doi: 10.1523/JNEUROSCI.1794-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xu Y, et al. J Neurosci. 2008;28(36):8873. doi: 10.1523/JNEUROSCI.0812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen FS, et al. Invest Ophthalmol Vis Sci. 51(2):686. doi: 10.1167/iovs.09-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jeffrey BG, et al. Vis Neurosci. 27(1-2):9. doi: 10.1017/S0952523809990319. [DOI] [PMC free article] [PubMed] [Google Scholar]; Masuho I, Celver J, Kovoor A, Martemyanov KA. J Biol Chem. 2010;285(7):4781. doi: 10.1074/jbc.M109.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dhingra A, Fina M, Vardi N. Society of Neuroscience. 2004 [Google Scholar]
- 34.Cao Y, et al. J Neurosci. 2009;29(29):9301. doi: 10.1523/JNEUROSCI.1367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, et al. Invest Ophthalmol Vis Sci. 2010;51(2):1121. doi: 10.1167/iovs.09-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearring JN, et al. J Neurosci. 2010;31(27):10060. doi: 10.1523/JNEUROSCI.1014-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cao Y, Posokhova E, Martemyanov KA. J Neurosci. 2011;31(32):11521. doi: 10.1523/JNEUROSCI.1682-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirbec H, et al. J Biol Chem. 2002;277(18):15221. doi: 10.1074/jbc.C200112200. [DOI] [PubMed] [Google Scholar]
- 38.Brandstatter JH, Dick O, Boeckers TM. J Comp Neurol. 2004;475(4):551. doi: 10.1002/cne.20194. [DOI] [PubMed] [Google Scholar]
- 39.Gregg RG, et al. J Neurophysiol. 2007;98(5):3023. doi: 10.1152/jn.00608.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ball SL, et al. Vis Neurosci. 2003;20(3):267. doi: 10.1017/s0952523803203059. [DOI] [PubMed] [Google Scholar]
- 40.Tagawa Y, et al. Journal of Neuroscience. 1999;19(7):2568. doi: 10.1523/JNEUROSCI.19-07-02568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgans CW, et al. Eur J Neurosci. 2007;26(10):2899. doi: 10.1111/j.1460-9568.2007.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsukamoto Y, et al. J Neurosci. 2007;27(23):6261. doi: 10.1523/JNEUROSCI.5646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ishii M, et al. Cell Tissue Res. 2009;338(3):355. doi: 10.1007/s00441-009-0880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwakabe H, Katsuura G, Ishibashi C, Nakanishi S. Neuropharmacology. 1997;36(2):135. doi: 10.1016/s0028-3908(96)00167-0. [DOI] [PubMed] [Google Scholar]; Takao M, et al. Neuroscience. 2000;97(4):779. doi: 10.1016/s0306-4522(00)00053-1. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto T, et al. Eur J Neurosci. 1997;9(6):1226. doi: 10.1111/j.1460-9568.1997.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 45.Ueda Y, et al. Journal of Neuroscience. 1997;17(9):3014. doi: 10.1523/JNEUROSCI.17-09-03014.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valerio A, et al. Neuroreport. 2001;12(12):2711. doi: 10.1097/00001756-200108280-00024. [DOI] [PubMed] [Google Scholar]; Vardi T, et al. Journal of Histochemistry & Cytochemistry. 2011 in press. [Google Scholar]
- 47.Koulen P, Kuhn R, Wassle H, Brandstatter JH. Journal of Neuroscience. 1997;1723(6):2200. doi: 10.1523/JNEUROSCI.17-06-02200.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sen M, Gleason E. Vis Neurosci. 2006;23(2):221. doi: 10.1017/S0952523806232073. [DOI] [PubMed] [Google Scholar]
- 49.Dyka FM, May CA, Enz R. J Neurochem. 2004;90(1):190. doi: 10.1111/j.1471-4159.2004.02474.x. [DOI] [PubMed] [Google Scholar]
- 50.Yazulla S, Studholme KM. J Neurocytol. 2001;30(7):551. doi: 10.1023/a:1016512617484. [DOI] [PubMed] [Google Scholar]; Klooster J, Studholme KM, Yazulla S. J Comp Neurol. 2001;441(2):155. doi: 10.1002/cne.1404. [DOI] [PubMed] [Google Scholar]
- 51.Joselevitch C, Klooster J, Kamermans M. Cell Tissue Res. 2007;330(3):389. doi: 10.1007/s00441-007-0496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Awatramani GB, Slaughter MM. Journal of Neuroscience. 2001;21(2):741. doi: 10.1523/JNEUROSCI.21-02-00741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akazawa C, et al. Neuroscience Letters. 1994;171:52. doi: 10.1016/0304-3940(94)90602-5. [DOI] [PubMed] [Google Scholar]
- 54.Hartveit E, Brandstatter JH, Enz R, Wassle H. Eur J Neurosci. 1995;7(7):1472. doi: 10.1111/j.1460-9568.1995.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 55.Brandstatter JH, et al. J Neurosci. 1996;16(15):4749. doi: 10.1523/JNEUROSCI.16-15-04749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gleason E. Vis Neurosci. 2011:1. [Google Scholar]
- 57.Vigh J, Li GL, Hull C, von Gersdorff H. Neuron. 2005;46(3):469. doi: 10.1016/j.neuron.2005.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffpauir BK, Gleason EL. Journal of Neurophysiology. 2002;88:1766. doi: 10.1152/jn.2002.88.4.1766. [DOI] [PubMed] [Google Scholar]
- 59.Sosa R, et al. Journal of Neurochemistry. 2002;81:973. doi: 10.1046/j.1471-4159.2002.00883.x. [DOI] [PubMed] [Google Scholar]
- 60.Vigh J, Lasater EM. Eur J Neurosci. 2003;17(11):2237. doi: 10.1046/j.1460-9568.2003.02652.x. [DOI] [PubMed] [Google Scholar]
- 61.Euler T, Wassle H. J Neurophysiol. 1998;79(3):1384. doi: 10.1152/jn.1998.79.3.1384. [DOI] [PubMed] [Google Scholar]
- 62.Yoshida K, et al. Neuron. 2001;30:771. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- 63.Koulen P, et al. European Journal of Neuroscience. 1996;8:2177. doi: 10.1111/j.1460-9568.1996.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 64.Cai W, Pourcho RG. J Comp Neurol. 1999;407(3):427. doi: 10.1002/(sici)1096-9861(19990510)407:3<427::aid-cne10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 65.Jensen RJ. Brain Res. 2006;1122(1):86. doi: 10.1016/j.brainres.2006.08.119. [DOI] [PubMed] [Google Scholar]
- 66.Caramelo OL, Santos PF, Carvalho AP, Duarte CB. J Neurosci Res. 1999;58(4):505. doi: 10.1002/(sici)1097-4547(19991115)58:4<505::aid-jnr4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 67.Akopian A, Witkovsky P. Vis Neurosci. 1996;13(3):549. doi: 10.1017/s0952523800008221. [DOI] [PubMed] [Google Scholar]
- 68.Rothe T, Bigl V, Grantyn R. Pflugers Arch. 1994;426(1-2):161. doi: 10.1007/BF00374684. [DOI] [PubMed] [Google Scholar]
- 69.Yu J, Daniels BA, Baldridge WH. J Neurophysiol. 2009;102(6):3728. doi: 10.1152/jn.00650.2009. [DOI] [PubMed] [Google Scholar]
- 70.Shen W, Slaughter MM. Journal of Physiology. 1998;510(3):815. doi: 10.1111/j.1469-7793.1998.815bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robbins J, Reynolds AM, Treseder S, Davies R. Mol Cell Neurosci. 2003;23(3):341. doi: 10.1016/s1044-7431(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 72.Zhang C, Schmidt JT. J Neurophysiol. 1999;82(6):2947. doi: 10.1152/jn.1999.82.6.2947. [DOI] [PubMed] [Google Scholar]
- 73.Zeitz C, et al. Hum Mutat. 2007;28(8):771. doi: 10.1002/humu.20499. [DOI] [PubMed] [Google Scholar]
- 74.Beqollari D, Betzenhauser MJ, Kammermeier PJ. Mol Pharmacol. 2009;76(5):992. doi: 10.1124/mol.109.058628. [DOI] [PubMed] [Google Scholar]
- 75.Scherer SW, et al. Genomics. 1997;44(2):232. doi: 10.1006/geno.1997.4842. [DOI] [PubMed] [Google Scholar]
- 76.Price MT, et al. Neuropharmacology. 1995;34(8):1069. doi: 10.1016/0028-3908(95)00065-e. [DOI] [PubMed] [Google Scholar]
- 77.Siliprandi R, et al. Neuroreport. 1994;5(10):1227. doi: 10.1097/00001756-199406020-00017. [DOI] [PubMed] [Google Scholar]
- 78.Tehrani A, Wheeler-Schilling TH, Guenther E. Invest Ophthalmol Vis Sci. 2000;41(1):314. [PubMed] [Google Scholar]
- 79.Hanna MC, Calkins DJ. Mol Vis. 2007;13:2194. [PubMed] [Google Scholar]
- 80.Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Neuroscience. 1993;53(4):1009. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]


