Abstract
Objectives
Recently developed genotyping tools allow better understanding of Trichomonas vaginalis population genetics and epidemiology. These tools have yet to be applied to T vaginalis collected from HIV+ populations, where understanding the interaction between the pathogens is of great importance due to the correlation between T vaginalis infection and HIV transmission. The objectives of the study were twofold: first, to compare the genetic diversity and population structure of T vaginalis collected from HIV+ women with parasites from reference populations; second, to use the genetic markers to perform a case study demonstrating the usefulness of these techniques in investigating the mechanisms of repeat infections.
Methods
Repository T vaginalis samples from a previously described treatment trial were genotyped at 11 microsatellite loci. Estimates of genetic diversity and population structure were determined using standard techniques and compared with previously reported estimates of global populations. Genotyping data were used in conjunction with behavioural data to evaluate mechanisms of repeat infections.
Results
T vaginalis from HIV+ women maintain many of the population genetic characteristics of parasites from global reference populations. Although there is evidence of reduced diversity and bias towards type 1 parasites in the HIV+ population, the populations share a two-type population structure and parasite haplotypes. Genotyping/behavioural data suggest that 36% (12/33) of repeat infections in HIV+ women can be attributed to treatment failure.
Conclusions
T vaginalis infecting HIV+ women is not genetically distinct from T vaginalis infecting reference populations. Information from genotyping can be valuable for understanding mechanisms of repeat infections.
INTRODUCTION
With >248 million new infections each year, trichomoniasis is the most common non-viral sexually transmitted infection worldwide.1 This high prevalence is troubling given the significant association of Trichomonas vaginalis (TV) infections with increased risk of HIV-1 acquisition and transmission.2–5
Trichomoniasis is frequently diagnosed among HIV+ women, where prevalence ranges from 18% to 36%.6–9 HIV+ women also tend to experience higher rates of repeat T vaginalis infection (ie, diagnosis of TV during follow-up exams after metronidazole (MTZ) treatment) than HIV− women (~30%6–10 vs 5% to 8%9–12). Repeat infections can result from treatment failure, lack of adherence to treatment regimens or reinfection by new or untreated sexual partners.9 Aetiology of a T vaginalis repeat infection typically relies on patient self-report. Despite attempts to encourage accurate reporting,13,14 erroneous questionnaire responses may still be a confounding factor. New T vaginalis-specific genetic markers15,16 allow us to augment self-reports with genetic profiles of parasites isolated from sequential infections to more accurately determine the source of reinfection. When applied to T vaginalis isolates from around the world, these markers revealed extensive genetic diversity15–18 that assorts into a two-cluster population structure.16,17 Cluster type 1 parasites harbour T vaginalis virus (TVV) significantly more frequently than type 2 parasites, and type 2 parasites may be more resistant to MTZ.17 Type 1 and type 2 parasites were detected in near-equal frequencies in all but a few regions globally.17 Whether this diversity and population structure are maintained within HIV+ populations was unknown. The purpose of this study was to (1) evaluate the diversity and population structure of the parasites isolated from HIV+ women and (2) differentiate between sources of recurrent infection by using genotyping to compare parasites from repeat infections.
MATERIALS AND METHODS
Origin of samples
We used repository samples from a treatment trial published by Kissinger et al19 Briefly, 270 HIV+ women undergoing routine gynaecological examination at public HIV/reproductive health outpatient clinics in New Orleans (LA), Houston (TX) and Jackson (MS) who tested positive for T vaginalis by culture were enrolled in the study, randomly assigned to one of two MTZ treatment regimens and provided with MTZ to deliver to their sex partner(s). The participants were scheduled to return 6–12 days after completing medication doses for a test of care (TOC) visit. Any woman who tested positive for T vaginalis at the TOC visit was considered an early repeat infection. Women who tested negative for T vaginalis at the TOC visit or who did not complete a TOC visit were scheduled for a follow-up visit at 3 months after enrolment. Testing was also performed at 3 months for some patients diagnosed with T vaginalis at the TOC visit and at 6 months for some patients with a positive diagnosis at either the TOC visit or 3-month visit. At each study visit, participants were asked to take a survey conducted using audio computer-assisted self-interview (ACASI) format that asked detailed information about participant treatment adherence, delivery of treatment to partner(s) and sexual exposure to baseline or new partners.
The study was approved by the institutional review boards of the clinic sites, and all women gave written informed consent before randomisation.
Genotyping T vaginalis from HIV+ women
DNA extracted from T vaginalis-positive cultures was sent to New York University for genotyping. The DNA samples were subjected to whole genome amplification using a REPLI-g Mini Kit (Qiagen), following the manufacturer’s protocol, and genotyped in duplicate at 11 microsatellite loci as previously described.15,20 Samples where multiple alleles were detected at two or more microsatellite loci were classified as mixed, that is, consisting of multiple parasite genotypes in the same infection.17
T vaginalis global, national and regional reference datasets
T vaginalis genotypes from previously published studies17,20,21 were used to establish a global reference dataset with which the population genetic metrics of T vaginalis isolated from HIV+ women were compared. This dataset comprises isolates from the USA (N=137), Mexico (N=11), Chile (N=14), Italy (N=12), South Africa (N=18), Mozambique (N=1), Australia (N=13), Papua New Guinea (PNG) (N=30) and India (N=1) and represents samples from both symptomatic and asymptomatic women seeking medical care in a range of clinical settings (total N=237). The HIV status of the patients infected with the global reference samples is unknown.
To allow for comparisons at national and regional levels, we used subsets of the global dataset: the national dataset comprises samples from patients within the USA (N=137), and the regional dataset, a subset of the national dataset, included samples from patients attending southeastern USA clinics (New Orleans, Louisiana, USA and Atlanta, Georgia, USA; N=37).
Population genetic analyses
Population genetic analyses were conducted as previously described.17 Briefly, microsatellite allelic richness was estimated using ADZE.22 Arlequin3.523 was used to test for Fst between populations and to estimate genetic diversity (expected heterozygosity (HE)). STRUCTURE 2.324 was used to determine T vaginalis population structure. Simulations were run 10 times each for six K values (K=1–6) with a burn-in period of 5×105 iterations followed by 105 iterations. The number of populations was inferred by plotting the log probability of the data (Ln P(D)) for each k value, followed by clusteredness calculations.22
Classification of T vaginalis reinfections in an HIV+ population
Genotyping data and patient responses to questions in ACASI surveys were used to categorise a repeat infection as (i) treatment failure, (ii) incomplete treatment, (iii) reinfection by baseline partner, (iv) infection by exposure to a new partner or (v) possible new infection. The definitions of these categories can be found in online supplementary appendix 1.
Statistical analyses
Statistical analyses were performed using the software package JMP Genomics. Contingency analyses were used to test for associations between categorical data, using χ2 statistical tests. Fisher’s exact tests are reported for 2×2 categorical analyses, and likelihood ratios are reported for all other categorical analyses.
RESULTS
Genotyping T vaginalis parasites from HIV+ women
To compare the diversity of T vaginalis isolates from HIV+ women with that of global isolates, we genotyped samples collected from 139 HIV+ women using a panel of 11 microsatellite markers (see online supplementary appendix 2). We successfully genotyped 165 samples from 123 patients at ≥6 loci, detecting 13 mixed infections (7.9%). We inferred the haplotypes of two of these mixed infections using genotype data from their subsequent infections and excluded the remaining mixed infections from further analysis. Of the remaining 154 samples, 25 were collected from the same patient at different clinic visits and had identical haplotypes, suggesting that these were persistent infections. To prevent over-representation of these non-incident infections, only the initial infection was included in the population genetic analyses. Ultimately, genotypes from 131 samples collected from 112 patients were analysed.
Demographics of the HIV+ hosts and T vaginalis parasites used in population genetic analyses
Of the 131 samples from 112 HIV+ women, 60 (46%) originated from Houston, 41 (31%) from Jackson and 30 (23%) from New Orleans (table 1, see online supplementary appendix 2). Average CD4 count at the time of the first T vaginalis diagnosis was 369.77 cells/mm3 (SD=±282), and the mean and median viral loads were 73 516 copies/ml (SD=±165 013) and 2299 copies/ml (IQR=309–68 366), respectively. The large difference between the mean viral load and median viral load resulted from a high proportion of patients having very low viral levels, while a few had very high viral loads.
Table 1.
Patient demographics and population genetic matrices for Trichomonas vaginalis parasites isolated from an HIV+ host population and from global, national and regional reference populations
| HIV+ Pop | Global reference population | National reference population | Regional reference population | Statistical test | |
|---|---|---|---|---|---|
| Isolates | N=131 | N=213 | N=113 | N=37 | |
| Patients | N=112 | N=76† | N=68† | N=26† | |
| Pt. demographics | |||||
| Avg. age | 39.3 | 31* | 30* | 25* | Wilcoxon |
| Ethnicity | * | ** | χ2 | ||
| Black non-Hispanic | 93% | 73% | 80% | 88% | |
| White non-Hispanic | 4% | 12% | 13% | 4% | |
| Hispanic | 3% | 5% | 6% | 8% | |
| Native American | <1% | 0 | 0% | 0% | |
| Other | <1% | 10% | 1% | 0% | |
| Population pair-wise fst | |||||
| HIV+ Pop vs: | |||||
| Global reference pop | 0.03* | ||||
| National reference pop | 0.03* | NS | |||
| Regional reference pop | 0.02** | NS | NS | ||
| Allelic richness (for N=10) | 3.68 | 4.02 | 3.98 | 4.08 | t test |
| Expected heterozygosity | 0.63 | 0.71* | 0.70* | 0.72** | t test |
| Per cent type 1 parasites | 75% | 52%* | 52%* | 57%† | χ2 |
HIV population differs at p≤0.001 level.
HIV population differs at p≤0.05 level.
Demographic information was not available for all patients. Only one isolate was collected from each patient.
NS, no significant population structure.
Haplotypes are shared among HIV+ and global reference host populations
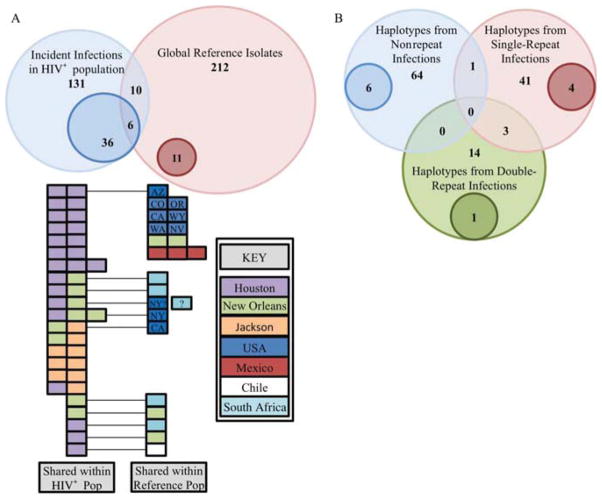
We found 17 cases, encompassing 36 isolates (36/131), of possible shared haplotypes within the HIV+ population, whereas within the global reference dataset we found only five cases of shared haplotypes (figure 1A). We found 12 haplotypes that were shared between the HIV+ and global populations. The isolates from HIV+ women clearly have more instances of shared haplotypes, although whether this difference is related to host HIV status or to deeper sampling of HIV+ women from a more limited geographical area cannot be determined without further sampling.
Figure 1.
(A) Venn diagram representing the distribution of unique and shared haplotypes among the HIV+ population and the global reference population. The light blue and light red circles represent the number of isolates comprising the HIV+ population and the global reference population, respectively. The darker blue and red circles within the light circles indicate the number of isolates that have haplotypes that are shared by multiple Trichomonas vaginalis strains within the population. The numbers within the overlapping regions indicate the number of isolates that have haplotypes that were detected in those categories (eg, there are six haplotypes that are shared among multiple HIV+ hosts and hosts from the global reference population). The colour-coded boxes below indicate the regions in which each of the shared haplotypes was identified. The isolates shared between HIV+ populations and reference populations are connected to their counterparts with a black line. (B) Venn diagram representing the relationship of parasite haplotypes among non-repeat, single-repeat and double-repeat infections. The light blue, light red and light green circles indicate the number of haplotypes among the non-repeat infections, single-repeat infections and double-repeat infections, respectively. The darker blue, red and green circles within the light circles represent the haplotypes that are shared by multiple T vaginalis strains from the same category of repeat infection. The numbers within the overlapping regions indicate the number of haplotypes shared between these groups (eg, there are three haplotypes that were isolated from both women with double-repeat infections and from women with single-repeat infections).
Population genetic indices of T vaginalis parasites from HIV+ women versus the global dataset
To determine whether parasites from the HIV+ and global isolates constitute distinct populations, we calculated the population pair-wise fixation index (FST), a measure of population differentiation due to genetic structure. FST can range from 0 (no difference in allele frequencies between populations) to 1 (complete differentiation). Significant FST was detected for the HIV+ isolates versus global, national and regional populations (0.03, 0.03 and 0.02, respectively), but the low values suggest that the HIV+ population, while distinct, is not highly differentiated (table 1).
Similarly, we compared the diversity metrics of HIV+ and global populations. We found no significant difference between allelic richness values estimated for a population size of 10 at any population level (table 1). We did, however, find significant difference in HE (table 1), with HIV+ population diversity being intermediate compared with the global dataset (see online supplementary appendix 3). Isolates from New Orleans, Jackson and Houston clinics have HE values of 0.65, 0.65 and 0.62, respectively, higher than those of isolates from southern Africa and comparable with those of isolates from Mexico, Italy and PNG (see online supplementary appendix 3).
T vaginalis from HIV+ women maintain a two-type population structure
Using a Bayesian clustering model implemented in STRUCTURE 2.2,23 we confirmed that the previously described two-cluster population structure is maintained in HIV+ isolates.17 HIV+ samples were assigned to the same parasite types when analysed alone or combined with the global dataset. Of 113 HIV+ T vaginalis parasites genotyped, 75% were assigned to type 1, a higher frequency than that found in global, national and regional reference datasets (table 1).
Differentiating treatment failure from reinfection in MTZ-treated HIV+ women with repeat T vaginalis infections
Non-repeat infections and repeat infections rarely share haplotypes
In our analyses of repeat infections, we used a subset of 149 T vaginalis samples from 107 different patients (see online supplementary appendix 2). The samples comprise 107 baseline infections, 11 infections diagnosed at the TOC visit, 19 at the 3-month follow-up visit and 12 at the 6-month visit. Of these, 72 patients were successfully treated and had no repeat infections. Among the remaining patients, 25 were diagnosed with T vaginalis for second time at a follow-up visit (single-repeat infection), and 10 were diagnosed two additional times (two-repeat infections). The 72 non-repeat infections comprised as few as 64 unique haplotypes, seven of which appear to be shared (figure 1B). All but one of the shared haplotypes (Isolate 184-B) were found exclusively within women with non-repeat infections.
Among the 45 non-persistent infections identified among single-repeat infections, we identified as few as 41 different haplotypes, eight of which appear to be shared (figure 1B). All haplotypes except Isolate 184-B were found exclusively among repeat infections (either single repeats or two repeats). Among the two-repeat infections, we detected 16 non-persistent infections comprising 14 haplotypes, with four shared haplotypes similarly found only among repeat infections.
Determining the source of reinfection
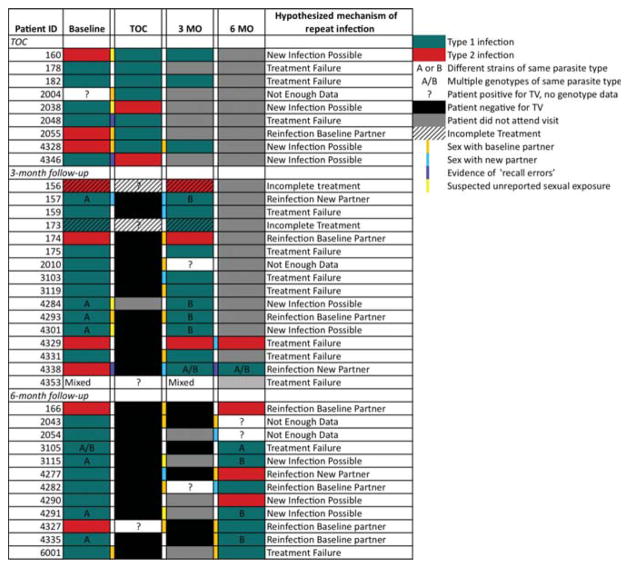
Of the 42 repeat infections genotyped, patient-reported behavioural data were available for 37, 33 of which were complete datasets. Among these, we detected 12 cases of treatment failure (36%), two cases of incomplete treatment (6%), seven cases of reinfection by a baseline/untreated partner (21%) and three cases of reinfection by exposure to a new partner (9%) (figure 2). Genotyping clarified two ambiguous calls. Patient information alone would have led to categorising one repeat infection (#3103) as being due to exposure to a new partner, but since both infections have the same genotype, it was likely due to treatment failure. In the second case, a patient had sexual contact with the baseline partner and a new partner. Again, because the genotypes matched across both diagnoses, we were able to rule out infection by the new partner. The remaining nine cases (27%) were likely new infections due to the detection of new genotypes at follow-up visits. While it is possible that the new genotype was present initially at undetectable levels and became more prevalent after drug treatment, inaccurate self-reporting cannot be discounted as a cause.
Figure 2.
Strain and audio computer-assisted self-interview survey data for repeat Trichomonas vaginalis infections. Parasite types for isolates collected at baseline, test of care, 3 months and 6 months are indicated by green (type 1) or red (type 2) shading. In cases where repeated infections were assigned to the same parasite type, the letters A and B are used to indicate differences in parasite haplotype. Self-reported behavioural events of importance to the source of reinfection are indicated by colour coding of columns between clinical visits.
T vaginalis genetic type and clinical treatment failure
We next sought to determine whether the T vaginalis genetic type influences the outcome of reinfections caused by treatment failure. We found type 1 parasites in 10 of 12 treatment failures, with one of the remaining being type 2 and the other being a mixed infection that was maintained in both samples from the patient. For the nine ‘New Infection Possible’ samples, the majority changed from a type 1 haplotype to a different type 1 haplotype (4/9, 44%), three changed from a type 1 haplotype to a type 2 haplotype and two changed from a type 2 haplotype to a type 1 haplotype.
Interpreting these changes is challenging as the sample size is small and the high frequency of the type 1 parasites that remain after MTZ treatment likely reflects type 1 prevalence within the population. We find no difference in the proportion of the types that were cleared or that underwent treatment failure. In addition, we find no difference in the proportion of type 1 and type 2 parasites at baseline visit, the TOC visit or at the 3-month follow-up or 6-month follow-up. Thus, we find no evidence that parasite genetic type influences whether an infection is likely to be cured by drug treatment.
Association of T vaginalis genetic type with viral load
To determine if parasite type plays a role in the association of T vaginalis with increased HIV viral load in the seminal and cervicovaginal compartments of HIV-infected patients, 24 25 we compared the viral loads of patients diagnosed with type 1 (N=82) or type 2 (N=28) infections. Type 1 parasites were associated with higher HIV viral loads than type 2 parasites (median: 4475.5 vs 400, Wilcoxon; p=0.01). After stratifying for antiretroviral therapy (ART) use, this relationship held only among patients currently receiving ART (type 1: N=51, type 2: N=20; median: 2850 vs 86; Wilcoxon; p=0.0022).
DISCUSSION
Trichomoniasis is implicated in increasing sexual transmission of HIV up to twofold,2,26 making parasite–virus–host interactions a topic of great significance in controlling HIV/AIDS. We used genetic markers and information about the global population structure of T vaginalis to investigate the association of HIV coinfection with changes in the genetic diversity and distribution of the two-type population structure of the parasite. We genotyped T vaginalis genomes isolated from female patients attending public outpatient HIV clinics and compared their genetic diversity and population structure with those of isolates from patients of unknown HIV status. Because these isolates were collected without consideration of the HIV status of the patient, we expect the number of HIV+ individuals sampled to reflect the prevalence of HIV infections within the sample’s population.
We determined that the two-type population structure derived from global datasets16,17 is maintained in T vaginalis parasites found in HIV+ women, albeit with reduced diversity in HIV+ isolates, which might be expected from the limited geographical distribution and ethnic diversity of the patients involved in the study. The genetic diversity of T vaginalis parasites from HIV+ women attending the clinics is comparable with that of the global reference population, suggesting that they are not abnormally homogeneous. However, we detect small but significant FST difference between the HIV+ and reference populations at global, national and regional levels. This difference can most readily be attributed to the bias towards type 1 parasites particular to the HIV+ population. Although this could be an artefact of the low geographical diversity of the HIV− population, it is interesting that this same trend is only detected in southern Africa (see online supplementary appendix 3) where we expect a higher prevalence of HIV in the reference population. Uncovering a type 1 bias in HIV+ women in other geographical areas would lend support to a hypothesis that type 1 parasites have increased fitness in comparison with type 2 within the context of HIV infection.
Genotypic markers can be very useful in determining the source of repeat T vaginalis infections in women, particularly in cases where multiple sources of repeat infection are possible or self-reported behavioural data may be flawed. Here, we have demonstrated that a panel of 11 microsatellite markers can assist in differentiating recrudescent infections (reappearing after being quiescent) from infections resulting from contact with sexual partners. The significant proportion of samples that genotypically appear to be new infections despite patients reporting no contact with sexual partners highlights the need for objective, reproducible tools for understanding the epidemiology of repeat infections. While a number of these cases are likely to be attributed to inaccurate self-reporting, it is also important to acknowledge and quantify the sensitivity limits of markers when detecting multiple genotypes in a single infection. Despite previously demonstrating that microsatellite markers are effective in detecting mixed infections,17,20 the actual prevalence of mixed infections will always be underestimated,27 and the field will benefit from further studies elucidating the likelihood of missing minor genotypes in mixed infections and the frequency with which inaccurate behavioural data is reported.
Finally, the association that we detected between type 1 T vaginalis infections and increased HIV viral loads in patients on ART may be of clinical importance. Further studies to verify this trend will be important, as will investigating the role that TVV may have in this association. Because of the high prevalence of TVV in type 1 parasites, it will be necessary to discriminate between the contributions of parasite genetics, of TVV infection and to understand how they may work in concert to influence HIV viral loads.
Supplementary Material
Key messages.
Population genetic analyses indicate that T vaginalis infecting HIV+ women is not genetically distinct from T vaginalis infecting reference populations, although there is evidence of population differentiation.
Genotyping methods can be valuable sources of information for understanding mechanisms of repeat infections.
The increased HIV viral load associated with T vaginalis infection may be specific to type 1 parasites.
Acknowledgments
We thank our collaborators for their isolates and expertise and Dr Steven Sullivan for his editing talents.
Funding This work was funded in part by US National Institutes of Health (NIH) grant 5R21AI083954-02 to JMC and (NIH/NIAID) U19 AI61972 to PK. MDC was partially supported by NIH training grant 5T32AI007180-28.
Footnotes
Contributors MDC, PK, NS, DHM and JMC conceived and designed the experiments; MDC performed the experiments; MDC and NS analysed the data; PK, NS and DM contributed reagents/materials/analysis tools; and MDC and JMC wrote the paper with comments and suggestions provided by PK, NS and DHM.
Competing interests None.
Patient consent Obtained.
Ethics approval This study was approved by the institutional review boards of the clinical sites (HIV Outpatient Clinic, NOAIDS, Crossroads Clinic, Thomas St Clinic, Northwest Clinic).
Provenance and peer review Commissioned; externally peer reviewed.
References
- 1.World Health Organization Department of Reproductive Health and Research. . Prevalence and incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis: methods and results used by WHO to generate 2005 estimates. Geneva, Switzerland: WHO Press; 2011. [accessed 1 Aug 2011]. http://www.who.int/reproductivehealth/publications/rtis/9789241502450/en/index.html. [Google Scholar]
- 2.McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 3.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 4.Mavedzenge SN, Pol BV, Cheng H, et al. Epidemiological synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African women. Sex Transm Dis. 2010;37:460–6. doi: 10.1097/OLQ.0b013e3181cfcc4b. [DOI] [PubMed] [Google Scholar]
- 5.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–65. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cu-Uvin S, Ko H, Jamieson DJ, et al. Prevalence, incidence, and persistence or recurrence of trichomoniasis among human immunodeficiency virus (HIV)-positive women and among HIV-negative women at high risk for HIV infection. Clin Infect Dis. 2002;34:1406–11. doi: 10.1086/340264. [DOI] [PubMed] [Google Scholar]
- 7.Magnus M, Clark R, Myers L, et al. Trichomonas vaginalis among HIV-Infected women: are immune status or protease inhibitor use associated with subsequent T. vaginalis positivity? Sex Transm Dis. 2003;30:839–43. doi: 10.1097/01.OLQ.0000086609.95617.8D. [DOI] [PubMed] [Google Scholar]
- 8.Niccolai LM, Kopicko JJ, Kassie A, et al. Incidence and predictors of reinfection with Trichomonas vaginalis in HIV-infected women. Sex Transm Dis. 2000;27:284–8. doi: 10.1097/00007435-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Kissinger P, Secor WE, Leichliter JS, et al. Early repeated infections with Trichomonas vaginalis among HIV-positive and HIV-negative women. Clin Infect Dis. 2008;46:994–9. doi: 10.1086/529149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Der Pol B, Williams JA. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. J Infect Dis. 2005;192:2039–44. doi: 10.1086/498217. [DOI] [PubMed] [Google Scholar]
- 11.Kissinger P, Schmidt N, Mohammed H, et al. Patient-delivered partner treatment for Trichomonas vaginalis infection: a randomized controlled trial. Sex Transm Dis. 2006;33:445–50. doi: 10.1097/01.olq.0000204511.84485.4c. [DOI] [PubMed] [Google Scholar]
- 12.Crosby R, DiClemente RJ, Wingood GM, et al. Predictors of infection with Trichomonas vaginalis: a prospective study of low income African-American adolescent females. Sex Transm Infect. 2002;78:360–4. doi: 10.1136/sti.78.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kissinger P, Rice J, Farley T, et al. Application of computer-assisted interviews to sexual behavior research. Am J Epidemiol. 1999;149:950–4. doi: 10.1093/oxfordjournals.aje.a009739. [DOI] [PubMed] [Google Scholar]
- 14.Tideman RL, Chen MY, Pitts MK, et al. A randomised controlled trial comparing computer-assisted with face-to-face sexual history taking in a clinical setting. Sex Transm Infect. 2007;83:52–6. doi: 10.1136/sti.2006.020776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad M, Zubacova Z, Dunn LA, et al. Microsatellite polymorphism in the sexually transmitted human pathogen Trichomonas vaginalis indicates a genetically diverse parasite. Mol Biochem Parasitol. 2010;175:30–8. doi: 10.1016/j.molbiopara.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelius DC, Robinson DA, Muzny CA, et al. Genetic characterization of Trichomonas vaginalis isolates by use of multilocus sequence typing. J Clin Microbiol. 2012;50:3293–300. doi: 10.1128/JCM.00643-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad MD, Gorman AW, Schillinger JA, et al. Extensive genetic diversity, unique population structure and evidence of genetic exchange in the sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis. 2012;6:e1573. doi: 10.1371/journal.pntd.0001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prokopi M, Chatzitheodorou T, Ackers JP, et al. A preliminary investigation of microsatellite-based genotyping in Trichomonas vaginalis. Trans R Soc Trop Med Hyg. 2011;105:479–81. doi: 10.1016/j.trstmh.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Kissinger P, Mena L, Levison J, et al. A randomized treatment trial: single versus 7-day dose of metronidazole for the treatment of Trichomonas vaginalis among HIV-infected women. J Acquir Immune Defic Syndr. 2010;55:565–71. doi: 10.1097/QAI.0b013e3181eda955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brotman RM, Bradford LL, Conrad M, et al. Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis. 2012;39:807–12. doi: 10.1097/OLQ.0b013e3182631c79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin DH, Zozaya M, Lillis RA, et al. Unique vaginal microbiota which include an unknown Mycoplasma-like organism are associated with Trichomonas vaginalis infection. J Infect Dis. doi: 10.1093/infdis/jit100. Published Online First: 4 Apr 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg NA, Mahajan S, Ramachandran S, et al. Clines, clusters, and the effect of study design on the inference of human population structure. PLoS Genet. 2005;1:e70. doi: 10.1371/journal.pgen.0010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–59. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price MA, Zimba D, Hoffman IF, et al. Addition of treatment for trichomoniasis to syndromic management of urethritis in Malawi: a randomized clinical trial. Sex Transm Dis. 2003;30:516–22. doi: 10.1097/00007435-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Wang CC, McClelland RS, Reilly M, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis. 2001;183:1017–22. doi: 10.1086/319287. [DOI] [PubMed] [Google Scholar]
- 26.Laga M, Alary M, Nzila N, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet. 1994;344:246–8. doi: 10.1016/s0140-6736(94)93005-8. [DOI] [PubMed] [Google Scholar]
- 27.Balmer O, Tanner M. Prevalence and implications of multiple-strain infections. Lancet Infect Dis. 2011;11:868–78. doi: 10.1016/S1473-3099(11)70241-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.